Abstract
Rumination has been shown to increase negative affect and is highly associated with increased duration of depressive episodes. Previous research has shown that enhanced elaborative processing of negative stimuli is often associated with depression and trait rumination. We hypothesized that engaging in rumination would result in sustained elaborative processing of negative information, as measured by late positive potential (LPP) asymmetry, regardless of depression. Participants were experimentally induced to engage in ruminative- or distraction-oriented thoughts and subsequently viewed negative, positive, and neutral images. Our results showed a very specific right-dominant frontal and parietal LPP to negative, but not neutral or positive, pictures in the rumination condition only that was not correlated with any measures of trait rumination or depression symptoms. This suggests that state rumination alone may lead to an enhanced, sustained processing of negative material that is typically associated with depression.
Keywords: Rumination, event-related potentials, late positive potential
In the presence of negative mood, individuals can employ a wide range of cognitive responses and emotion regulation strategies. Certain strategies, such as distraction and reappraisal, are considered effective emotion regulation techniques (Brans, Koval, Verduyn, Lim, & Kuppens, 2013). In contrast, ruminative responses worsen depressed mood (Park, Goodyer, & Teasdale, 2004; Genet & Siemer, 2012) and increase the duration of depressive episodes (Lyubomirsky & Nolen-Hoeksema, 1995). Moreover, a small number of studies have shown that experimental induction of rumination can increase self-reported negative mood (McLaughlin, Borkovec, & Sibrava, 2007; Nolen-Hoeksema & Morrow, 1993; Park et al., 2004). Previous research has shown that trait rumination is associated with sustained reactions to emotional stimuli (Siegle, Steinhauer, Carter, Ramel, & Thase, 2003; Siegle, Steinhauer, Thase, Stenger, & Carter, 2002), which is consistent with findings that sustained processing of negative stimuli is associated with depression (Koster, De Raedt, Goeleven, Franck, & Crombez, 2005; Larson, Nitschke, & Davidson, 2007; Taubitz, Robinson, & Larson, 2013). Due to the highly interconnected nature of rumination and depression, it has been difficult thus far to isolate what specific role state rumination may have in the processing of negative material. However, it seems likely that if experimental inductions of rumination can lead to greater negative affect, then state rumination, regardless of depression, can lead to the same sustained response to negatively valenced information.
Electrophysiological indices of emotional processing, such as frontal electroencephalographic (EEG) asymmetry and event-related potentials (ERPs), are frequently used to measure reactions to discrete affective stimuli. Studies examining EEG alpha asymmetry in response to affective stimuli have found greater relative right frontal activity in response to negative stimuli and greater relative left frontal activity to positive stimuli (see Coan & Allen, 2004, for a review). Relative right frontal alpha asymmetry at rest has been linked with sustained processing of unpleasant stimuli (Jackson et al., 2003), and similarly, relative left frontal alpha hypoactivation has been observed in individuals with major depressive disorder (Henriques & Davidson, 1991; for a review, see Davidson, Pizzagalli, Nitschke, & Putnam, 2002). Although less well characterized, there is also evidence that parietal regions show similar patterns of right lateralization in participants with heightened negative affect (Nitschke, Heller, Palmieri, & Miller, 1999).
An ERP component frequently used in emotion research, the late positive potential (LPP), is elicited by affective and motivationally relevant stimuli (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; for a review, see Hajcak, Weinberg, MacNamara, & Foti, 2011) and is considered to reflect elaborative processing of emotional content (Cuthbert et al., 2000; Hajcak et al., 2011). Although typically maximal over centro-parietal midline sites, the emotion-elicited LPP has also been documented at frontal midline sites (Foti & Hajcak, 2008; Hajcak, Dunning, & Foti, 2007; Hajcak & Nieuwenhuis, 2006; MacNamara, Foti, & Hajcak, 2009; Woodcock, Yu, Liu, & Han, 2013) and as a frontal asymmetry. Frontal LPP asymmetry has been shown to index emotional responding and shows right dominance to negative stimuli (Cunningham, Espinet, DeYoung, & Zelazo, 2005; Zhang, Zhou, & Oei, 2011). Parietal asymmetries of the LPP (sometimes including the P3 for a P3/LPP complex) have also shown a similar right lateralized response to negative stimuli (Cacioppo, Crites, & Gardner, 1996; Zhang & Zhou, 2014).
The current study sought to directly test the hypothesis that state rumination can enhance elaborative processing of negative stimuli. To test this, we experimentally induced rumination or distraction (Nolen-Hoeksema & Morrow, 1993) and then measured frontal LPP asymmetry in response to negative, neutral, and positive images from the International Affective Pictures System (IAPS; Lang, Bradley, & Cuthbert, 1999). We were particularly interested in the effect of rumination versus distraction on LPP asymmetry in response to negative images. We predicted that rumination would lead to a heightened emotional response to the negative stimuli, as indexed by a stronger right frontal LPP to negative images in the rumination group.
Method
Participants
Sixty-one right-handed undergraduate students from the University of Wisconsin–Milwaukee participated. All participants provided informed consent and received course credit for their participation. Data from 11 subjects were excluded from the data set: 5 due to excessive artifact (greater than 40% rejected trials) and 6 due to computer malfunctions. The final sample included 50 subjects (22 males; age, M = 21.65 years, range = 18–38 years).
Stimuli and Procedure
Participants first performed a task that induced either rumination or distraction (based on the procedure by Nolen-Hoeksema & Morrow, 1993) and subsequently viewed a series of positive, negative, and neutral IAPS pictures (Lang et al., 1999) while EEG was recorded. Twenty-five participants were induced to ruminate and 25 were induced to distract.
For the rumination and distraction inductions, participants were instructed to focus on a statement presented on the screen and advance to the next when they had fully contemplated the current statement. All statements were adapted from Nolen-Hoeksema and Morrow (1993; see also Lyubomirsky & Nolen-Hoeksema, 1995). Participants in the rumination condition were given 45 internally oriented, affectively neutral statements (e.g., “Think about the kind of person you think you should be” or “Think about why you react the way you do”) while participants in the distraction condition were given 45 externally focused, affectively neutral scenarios (e.g., “Think about the layout of the local shopping center” or “Think about the shape of a cello”). Participants reviewed the statements at their own pace for 8 min and then were instructed to move on to the next portion of the experiment.
The stimuli used for picture viewing included 120 IAPS images: 40 negative (mean valence = 2.07, arousal = 6.31), 40 positive (mean valence = 7.16, arousal = 6.07), and 40 neutral (mean valence = 4.95, arousal = 2.63). A paired samples t test of arousal ratings showed that positive and negative stimuli were not significantly different (ps = .084). Ratings were taken from the published norms (see Appendix for images used). Participants viewed four blocks of 30 trials with 10 pictures of each valence per block. Each image was on the screen for 6 s, followed by a 3.5- to 6.5-s intertrial interval consisting of a blank screen.
After completion of the experiment, participants filled out the Mood and Anxiety Symptom Questionnaire (MASQ; Watson et al., 1995) and General Behavior Inventory (GBI; Depue et al., 1981) to test for depression symptoms and the Response Styles Questionnaire (RSQ; Nolen-Hoeksema, 1991) to assess trait rumination.
EEG Acquisition and Preprocessing
Continuous EEG data were collected using a 64-channel Neuroscan Quickcap and SynAmps2 amplifier (Compumedics USA, Charlotte, NC). Electrophysiological recordings were sampled at 1,000 Hz and analyzed offline using Scan version 4.2. After ocular artifact reduction, using the procedure by Gratton, Coles, and Donchin (1983), epochs (−200 to 2,000 ms) with amplitudes exceeding ±150μV were automatically rejected. Epochs were manually reviewed for artifact. Prestimulus interval baseline correction was performed, and all trials were rereferenced to the average of the two mastoid sensors and bandpass filtered (0.1–30 Hz) before averaging. An average left frontal electrode was computed by taking the mean of sensors F1, F3, F5, and F7 for each time point in the epoch, and an average right frontal electrode was similarly created using sensors F2, F4, F6, and F8. Mean amplitudes for the frontal LPP for each picture valence were computed from the 800- to 1,800-ms poststimulus onset time period. This later window was selected for the frontal LPP because the topography of the LPP shifts to include more frontal sites at later latencies (Foti & Hajcak, 2008). We had originally intended to examine more sustained LPP responses (Hajcak, Dunning, & Foti, 2009); however, visual inspection of our grand averaged waveforms (one method recommended in a recent ERP guidelines paper for identifying component time windows, Keil et al., 2014) revealed that the LPP was more abbreviated than many studies1 (e.g., Hajcak et al., 2009) and was best captured by this 800- to 1,800-ms window (see Figure 1). Visual inspection also indicated an early P3/LPP complex evident 300–800 ms after stimulus onset in parietal regions. To assess asymmetry in this early P3/LPP window, we created a left parietal electrode (mean of sensors P1, P3, P5, and P7) and right parietal electrode (mean of sensors P2, P4, P6, and P8).
Figure 1.

Grand average waveforms for participants in the rumination condition (n = 25) and distraction condition (n = 25) at frontal and parietal sites. Grand average waveforms for each of the three picture valences for the frontal cluster (F1, F3, F5, F7 and F2, F4, F6, F8) for the (A) rumination and (B) distraction inductions, as well as for the parietal cluster (P1, P3, P5, P7 and P2, P4, P6, P8) for the (C) rumination and (D) distraction inductions. Analysis windows are indicated by dotted lines. See the online article for the color version of this figure.
Results
Frontal LPP Asymmetry
We ran a 2 (Mood Induction: rumination, distraction) × 3 (Picture Valence: positive, negative, neutral) × 2 (Hemisphere: right frontal, left frontal) mixed-model analysis of variance (ANOVA) with Mood Induction as a between-subjects factor and Picture Valence and Hemisphere as within-subjects factors.2 This ANOVA did not yield any significant main effects or two-way interactions (all p < .30). However, there was a trend for a Mood Induction × Picture Valence × Hemisphere three-way interaction, F(2, 47) = 2.429, p = .099, . Although this three-way interaction was nonsignificant, this trend was consistent with our a priori hypotheses, and as such, we conducted the relevant follow-up comparisons.
We tested our a priori hypothesis that participants induced to ruminate would have greater right frontal LPP amplitudes to negative images with planned comparisons. Because each of these comparisons was performed three times (once per valence), Bonferroni correction was performed on all the reported p values by multiplying the initial value by the number of comparisons. Including all 50 subjects across conditions, paired t tests comparing left and right mean frontal LPP amplitudes for each picture valence revealed a significant hemispheric difference for negative pictures only, with right frontal LPP amplitude (M = 3.89, SD = 3.72) greater than left (M = 2.62, SD = 3.67) for the negative images, t(49) = 2.581, p < .05, Cohen’s d = 0.75, 95% confidence interval (CI) [0.28, 2.25]. To better understand how this difference occurred as a function of induction condition, the same analysis was performed separately for the rumination and distraction groups. Consistent with our hypothesis, participants induced to ruminate exhibited significantly greater right (M = 4.62, SD = 3.98) than left (M = 2.17, SD = 4.17) frontal LPP amplitudes to negative, t(24) = 3.60, p < .01, Cohen’s d = 1.039, 95% CI [1.05, 3.85], but not neutral or positive images (all p > .55; see Figures 1 and 2). Participants induced to distract did not show significant hemispheric differences for any of the picture valences (all uncorrected p > .38), indicating that the right over left effect for negative images was driven by the rumination group.
Figure 2.
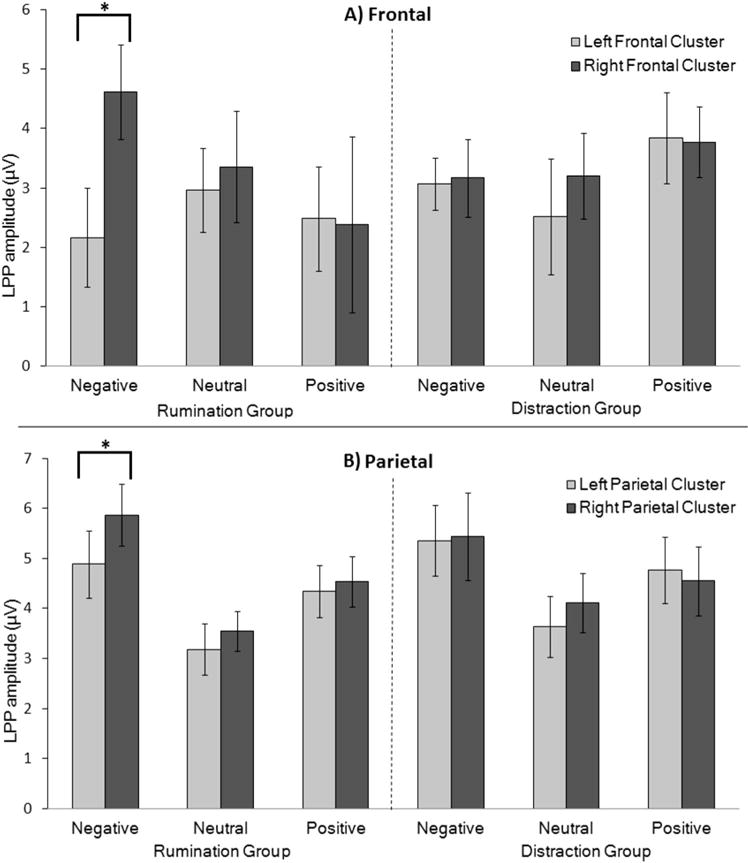
Mean (± standard error of the mean) late positive potential (LPP) amplitude for participants in the rumination and distraction conditions in (A) frontal sensors during an 800- to 1,800-ms time window and (B) parietal sensors during a 300- to 800-ms time window. Significant differences were found between left and right frontal LPP amplitude and parietal P3/LPP only for negative images in the rumination condition (p < .05).
To assess whether this hemispheric asymmetry differed between picture valences, we also performed paired t tests comparing valences for right-minus-left (R-L) amplitude differences. In the rumination group, there was a significant difference between negative and neutral valences prior to Bonferroni correction, with the R-L asymmetry greater for negative (M = 2.45, SD = 3.40) than for neutral images (M = 0.39, SD = 3.27), t(24) = 2.501, p < .05, Cohen’s d = 0.72, 95% CI [0.35, 3.76], but after correction, this was no longer significant (ps = .12). There were no significant differences between picture valences for the distraction group. We also compared R-L asymmetry for the rumination versus distraction groups for each valence. As expected, the R-L difference for negative images was greater in the rumination group (M = 2.45, SD = 3.40) than in the distraction group (M = 0.97, SD = 3.22), t(48) = 2.51, p < .05, Cohen’s d = 0.73, 95% CI [0.46, 4.23]. No significant differences were found between groups for positive or neutral images (all uncorrected p > .47).
Parietal LPP Asymmetry
The same 2 × 3 × 2 mixed-model ANOVA was performed on the parietal sensors, but did not yield a significant three-way interaction (ps = .12). However, due to our expectation that the LPP asymmetry would also be present in more posterior sites, we performed the relevant a priori comparisons. Across rumination and distraction groups, the right compared to left mean amplitude for each picture valence yielded a trending effect postcorrection in negative pictures only, with right parietal P3/LPP amplitude (M = 5.65, SD = 3.51) greater than left (M = 5.12, SD = 3.28), t(49) = 2.239, p = .09, Cohen’s d = 0.64, 95% CI [0.05, 1.0]. To examine the effect as a function of mood induction condition, we performed these t tests separately for the rumination and distraction groups. As with the frontal LPP, a significant effect was found for negative pictures in the rumination group, with right parietal P3/LPP amplitude (M = 5.87, SD = 3.07) greater than left (M = 4.88, SD = 3.34), t(24) = 3.307, p < .01, Cohen’s d = 1.35, 95% CI [0.37, 1.59]. No right versus left valence effects were found in the distraction group (all uncorrected p > .18).
Potential LPP Associations With Symptom Reports
To determine whether the group induction results were affected by underlying depression symptoms or trait rumination, we correlated the GBI Depression, the MASQ General Distress Depression, and RSQ Rumination subscales with each of the frontal and parietal LPP asymmetry measures for each valence. None of these correlations reached significance either across or within mood induction groups (all p > .18).3 Additionally, to make sure there were not significant differences between induction groups on any of these self-report measures, we performed t test comparisons on each measure, none of which were significant (all p > .16).
Early ERP Windows
Although our hypothesis centered on the LPP, 3 (Valence) × 2 (Mood Induction) ANOVAs were also run on early ERP components, including an anterior N1 (75–125 ms), a posterior N1 (140–180 ms), anterior and posterior P2 (150–275 ms), and early frontal LPP (500–800 ms). Each analysis was run on both midline and asymmetry measures, and all pairwise effects were Bonferroni corrected. There were no significant effects using midline (Fz or Pz) or asymmetrical (F1–F7 vs. F2–F8 or P1–P7 vs. P2–P8) sites in either N1 window (all p > .12). The anterior P2 midline analysis (at Fz) yielded a significant main effect of Valence (F = 3.313, p < .05, ), with positive images (M = −2.32, SD = 0.41) eliciting a greater P2 amplitude than neutral (M = −2.98, SD = 0.38, Cohen’s d = 1.63, 95% CI [0.17, 1.34]), but no differences between other valences (all p > .15). The early frontal LPP asymmetry yielded a main effect of Valence (F = 10.71, p < .001, ), with the LPP to negative images (M = 0.06, SD = 4.23) greater than neutral (M = −1.41, SD = 4.0, p < .01, Cohen’s d = 0.93, 95% CI [0.56, 2.36]) and positive (M = 0.43, SD = 3.86) greater than neutral (p < .001, Cohen’s d = 1.27, 95% CI [1.02, 2.71]), but no significant difference between positive and negative (p > 0.39). Thus, although there were some valence-based effects, none of these earlier components were modulated by the rumination versus distraction induction.
Discussion
Previous findings have shown that trait rumination and depressive symptoms are correlated with an enhanced, sustained response to negative material (Larson et al., 2007; Siegle et al., 2002). The present study sought to test that state rumination alone can cause this enhancement in response to negative stimuli. Our results supported our a priori hypothesis that participants induced to ruminate would have a greater relative right LPP. Also consistent with our hypothesis, this right-dominant LPP occurred only for negative images in the rumination group, with no LPP differences in the positive or neutral images or in the distraction group. As might be expected given the temporal topography of the LPP, this rumination-potentiated right hemisphere bias shifted from posterior to anterior sites over time. It should be noted that the LPP response in our picture paradigm was not as sustained as that observed in prior work using pictures drawn from the same IAPS set (Foti & Hajcak, 2008; Hajcak & Nieuwenhuis, 2006; cf. Morriss et al., 20131). It is possible that the prepicture rumination and distraction inductions led to this truncation, although it is difficult to make strong conclusions about the cause of the shortened LPP. However, as it stands, our findings support the notion that rumination enhances emotional processing of negative stimuli as measured by frontal LPP asymmetry.
These results are consistent with research implicating right hemisphere dominance in the sustained processing of negative stimuli (Grimm et al., 2008; Jackson et al., 2003) and associating enhanced processing of negative information with rumination (McLaughlin et al., 2007; Siegle et al., 2002; Siegle et al., 2003). We supply corroborating evidence that rumination may lead to an enhanced, right-dominant response to negative material and extend Siegle and colleagues’ (2003) trait rumination findings through the experimental induction of a ruminative state.
Recent investigations into the effects of emotion and cognition on the LPP lend further insight into why the purposeful induction of a ruminative state could modulate LPPs elicited by affective pictures. Several studies by Hajcak and colleagues (Foti & Hajcak, 2008; MacNamara et al., 2009) indicate that the LPP is responsive to the context in which an emotional stimulus is presented. For example, Foti and Hajcak (2008) found that the LPP response to unpleasant pictures was significantly greater when preceded by a negative stimulus description over a neutral one. They have also shown that the frontal LPP to emotional stimuli is reduced during cognitive reappraisal (Hajcak & Nieuwenhuis, 2006). Given that the LPP to unpleasant stimuli can be influenced by the context in which the stimulus is presented or by the participant’s cognitive and/or emotional state, it follows that state rumination could modulate the LPP. It is interesting to note that reappraisal, an effective emotion regulation strategy, attenuated the LPP response, whereas rumination, a generally maladaptive and ineffective strategy, led to an enhanced LPP response to negative stimuli.
Our findings also extend recent theories integrating cognition and affect in the scope of rumination and depression. Koster, De Lissnyder, Derakshan, and De Raedt (2011) proposed an impaired disengagement hypothesis of rumination, which suggests that poor attentional disengagement from self-reflective information leads to more prolonged ruminative state, subsequently contributing to negative affect and depression. Additionally, Joormann, Levens, and Gotlib (2011) hypothesize that impaired cognitive control for negative information is associated with depression. Our findings support these notions, given that sustained processing of negative stimuli may be resulting from ineffective attentional disengagement from or cognitive inflexibility regarding the negative material.
Potentially the most interesting aspect of our results is that the sustained, elaborative processing of negative material typically associated with depression (Larson et al., 2007) can be replicated in the absence of depression through rumination induction. However, further research should specifically control for depression symptoms or directly compare depressed and nondepressed groups, as we did not select participants based on depressive symptoms and cannot assume that there would not be differences between controls and depressed individuals. Given the potential for variability between groups, future researchers may wish to perform this manipulation with each participant experiencing both distraction and rumination inductions. It should also be mentioned that although our results are in line with what we would expect from an effective rumination induction, a limitation of this study is that we did not perform a behavioral check to ensure that the inductions were successful. Our results point to the ability of state rumination to cause an enhanced right-lateralized LPP response to negative stimuli, reflecting the sustained, elaborative processing of unpleasant material. We propose that this effect can be directly tied to rumination in the absence of depression symptoms and suggest that future studies examine how these effects could potentially differ when comparing depressed and control groups.
Acknowledgments
This research was supported in part by a grant from the University of Wisconsin–Milwaukee.
Appendix: List of IAPS Images Used
Unpleasant: 2353, 2370, 3015, 3030, 3060, 3063, 3110, 3150, 3168, 3170, 3180, 3181, 3230, 3261, 3266, 3350, 3500, 3351, 6212, 6230, 6251, 6312, 6313, 6360, 6540, 6550, 6560, 6570, 9050, 9140, 9250, 9252, 9253, 9405, 9421, 9433, 9569, 9810, 9910, 9921
Pleasant: 1650, 1722, 2216, 4250, 4290, 4599, 4607, 4640, 4641, 4659, 4665, 4670, 4680, 5260, 5270, 5450, 5460, 5470, 5621, 5626, 5629, 7330, 7501, 8034, 8040, 8080, 8090, 8116, 8161, 8170, 8185, 8190, 8210, 8300, 8340, 8400, 8470, 8490, 8496, 8531
Neutral: 2190, 2221, 2381, 3440, 2480, 2620, 2840, 2850, 2870, 2890, 5120, 5130, 5390, 5500, 5510, 5530, 7000, 7004, 7006, 7009, 7010, 7020, 7025, 7034, 7035, 7040, 7050, 7090, 7110, 7160, 7175, 7185, 7187, 7217, 7234, 7235, 7490, 7491, 7705, 7950
Footnotes
The somewhat truncated LPP responses we observed are not completely unprecedented. The time course evident in our data is extremely similar to a recently published study examining LPPs to emotional pictures embedded in an additional task (Morriss, Taylor, Roesch, & van Reekum, 2013).
To examine whether individual differences in rumination or depression could account for our findings, we tried modeling the MASQ General Distress Depression, GBI Depression, or RSQ Rumination as covariates in the model, but none were significant. Thus, no covariates were used in the final model.
The only correlation that was a trend (p = .07) occurred between GBI Depression and left frontal LPP during viewing of neutral images. Because of a lack of support for why this relationship should be valid, it was not further investigated.
Contributor Information
Kimberly L. Lewis, Department of Psychology, University of Wisconsin–MilwaukeeDepartment of Psychology, University of Chicago
Lauren E. Taubitz, Department of Psychology, University of Wisconsin–Milwaukee
Michael W. Duke, Department of Psychology, University of Wisconsin–Milwaukee
Elizabeth L. Steuer, Department of Psychology, University of Wisconsin–Milwaukee
Christine L. Larson, Department of Psychology, University of Wisconsin–Milwaukee
References
- Brans K, Koval P, Verduyn P, Lim YL, Kuppens P. The regulation of negative and positive affect in daily life. Emotion. 2013;13:926–939. doi: 10.1037/a0032400. http://dx.doi.org/10.1037/a0032400. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Crites SL, Jr, Gardner WL. Attitudes to the right: Evaluative processing is associated with lateralized late positive event-related brain potentials. Personality and Social Psychology Bulletin. 1996;22:1205–1219. http://dx.doi.org/10.1177/01461672962212002. [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67:7–50. doi: 10.1016/j.biopsycho.2004.03.002. http://dx.doi.org/10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Espinet SD, DeYoung CG, Zelazo PD. Attitudes to the right- and left: Frontal ERP asymmetries associated with stimulus valence and processing goals. NeuroImage. 2005;28:827–834. doi: 10.1016/j.neuroimage.2005.04.044. http://dx.doi.org/10.1016/j.neuroimage.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. http://dx.doi.org/10.1016/S0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annual Review of Psychology. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. http://dx.doi.org/10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Depue RA, Slater JF, Wolfstetter-Kausch H, Klein D, Goplerud E, Farr D. A behavioral paradigm for identifying persons at risk for bipolar depressive disorder: A conceptual framework and five validation studies. Journal of Abnormal Psychology. 1981;90:381–437. doi: 10.1037//0021-843x.90.5.381. http://dx.doi.org/10.1037/0021-843X.90.5.381. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience. 2008;20:977–988. doi: 10.1162/jocn.2008.20066. http://dx.doi.org/10.1162/jocn.2008.20066. [DOI] [PubMed] [Google Scholar]
- Genet JJ, Siemer M. Rumination moderates the effects of daily events on negative mood: Results from a diary study. Emotion. 2012;12:1329–1339. doi: 10.1037/a0028070. http://dx.doi.org/10.1037/a0028070. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography & Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. http://dx.doi.org/10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, Northoff G. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: An fMRI study in severe major depressive disorder. Biological Psychiatry. 2008;63:369–376. doi: 10.1016/j.biopsych.2007.05.033. http://dx.doi.org/10.1016/j.biopsych.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Dunning JP, Foti D. Neural response to emotional pictures is unaffected by concurrent task difficulty: An event-related potential study. Behavioral Neuroscience. 2007;121:1156–1162. doi: 10.1037/0735-7044.121.6.1156. http://dx.doi.org/10.1037/0735-7044.121.6.1156. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Dunning JP, Foti D. Motivated and controlled attention to emotion: Time-course of the late positive potential. Clinical Neurophysiology. 2009;120:505–510. doi: 10.1016/j.clinph.2008.11.028. http://dx.doi.org/10.1016/j.clinph.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affective & Behavioral Neuroscience. 2006;6:291–297. doi: 10.3758/cabn.6.4.291. http://dx.doi.org/10.3758/CABN.6.4.291. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, Foti D. ERPs and the study of emotion. In: Luck SJ, Kappenman ES, editors. The Oxford handbook of event-related potential components. New York, NY: Oxford University Press; 2011. pp. 441–472. [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. Journal of Abnormal Psychology. 1991;100:535–545. doi: 10.1037//0021-843x.100.4.535. http://dx.doi.org/10.1037/0021-843X.100.4.535. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Mueller CJ, Dolski I, Dalton KM, Nitschke JB, Urry HL, Davidson RJ. Now you feel it, now you don’t: Frontal brain electrical asymmetry and individual differences in emotion regulation. Psychological Science. 2003;14:612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x. http://dx.doi.org/10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- Joormann J, Levens SM, Gotlib IH. Sticky thoughts: Depression and rumination are associated with difficulties manipulating emotional material in working memory. Psychological Science. 2011;22:979–983. doi: 10.1177/0956797611415539. http://dx.doi.org/10.1177/0956797611415539. [DOI] [PubMed] [Google Scholar]
- Keil A, Debener S, Gratton G, Junghöfer M, Kappenman ES, Luck SJ, Yee CM. Committee report: Publication guidelines and recommendations for studies using electroencephalography and magnetoencephalography. Psychophysiology. 2014;51:1–21. doi: 10.1111/psyp.12147. http://dx.doi.org/10.1111/psyp.12147. [DOI] [PubMed] [Google Scholar]
- Koster EHW, De Lissnyder E, Derakshan N, De Raedt R. Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clinical Psychology Review. 2011;31:138–145. doi: 10.1016/j.cpr.2010.08.005. http://dx.doi.org/10.1016/j.cpr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Koster EHW, De Raedt R, Goeleven E, Franck E, Crombez G. Mood-congruent attentional bias in dysphoria: Maintained attention to and impaired disengagement from negative information. Emotion. 2005;5:446–455. doi: 10.1037/1528-3542.5.4.446. http://dx.doi.org/10.1037/1528-3542.5.4.446. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system: Technical manual and affective ratings. Gainesville: Center for Research in Psychophysiology, University of Florida; 1999. [Google Scholar]
- Larson CL, Nitschke JB, Davidson RJ. Common and distinct patterns of affective response in dimensions of anxiety and depression. Emotion. 2007;7:182–191. doi: 10.1037/1528-3542.7.1.182. http://dx.doi.org/10.1037/1528-3542.7.1.182. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, Nolen-Hoeksema S. Effects of self-focused rumination on negative thinking and interpersonal problem solving. Journal of Personality and Social Psychology. 1995;69:176–190. doi: 10.1037//0022-3514.69.1.176. http://dx.doi.org/10.1037/0022-3514.69.1.176. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Foti D, Hajcak G. Tell me about it: Neural activity elicited by emotional pictures and preceding descriptions. Emotion. 2009;9:531–543. doi: 10.1037/a0016251. http://dx.doi.org/10.1037/a0016251. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Borkovec TD, Sibrava NJ. The effects of worry and rumination on affect states and cognitive activity. Behavior Therapy. 2007;38:23–38. doi: 10.1016/j.beth.2006.03.003. http://dx.doi.org/10.1016/j.beth.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Morriss J, Taylor ANW, Roesch EB, van Reekum CM. Still feeling it: The time course of emotional recovery from an attentional perspective. Frontiers in Human Neuroscience. 2013;7:201. doi: 10.3389/fnhum.2013.00201. http://dx.doi.org/10.3389/fnhum.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Palmieri PA, Miller GA. Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 1999;36:628–637. http://dx.doi.org/10.1111/1469-8986.3650628. [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Response Styles Questionnaire. Stanford University; Stanford, CA: 1991. Unpublished manuscript. [Google Scholar]
- Nolen-Hoeksema S, Morrow J. Effects of rumination and distraction on naturally occurring depressed mood. Cognition and Emotion. 1993;7:561–570. http://dx.doi.org/10.1080/02699939308409206. [Google Scholar]
- Park RJ, Goodyer IM, Teasdale JD. Effects of induced rumination and distraction on mood and overgeneral autobiographical memory in adolescent major depressive disorder and controls. Journal of Child Psychology and Psychiatry. 2004;45:996–1006. doi: 10.1111/j.1469-7610.2004.t01-1-00291.x. http://dx.doi.org/10.1111/j.1469-7610.2004.t01-1-00291.x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Carter CS, Ramel W, Thase ME. Do the seconds turn into hours? Relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cognitive Therapy and Research. 2003;27:365–382. http://dx.doi.org/10.1023/A:1023974602357. [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: Event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. http://dx.doi.org/10.1016/S0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Taubitz LE, Robinson JS, Larson CL. Modulation of the startle reflex across time by unpleasant pictures distinguishes dysphoric from non-dysphoric women. International Journal of Psychophysiology. 2013;87:124–129. doi: 10.1016/j.ijpsycho.2012.11.002. http://dx.doi.org/10.1016/j.ijpsycho.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. http://dx.doi.org/10.1037/0021-843X.104.1.3. [DOI] [PubMed] [Google Scholar]
- Woodcock KA, Yu D, Liu Y, Han S. The presence of a culturally similar or dissimilar social partner affects neural responses to emotional stimuli. Socioaffective Neuroscience & Psychology. 2013;3:20500. doi: 10.3402/snp.v3i0.20500. http://dx.doi.org/10.3402/snp.v3i0.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhou R. Individual differences in automatic emotion regulation affect the asymmetry of the LPP component. PLoS ONE. 2014;9:e88261. doi: 10.1371/journal.pone.0088261. http://dx.doi.org/10.1371/journal.pone.0088261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhou R, Oei TPS. The effects of valence and arousal on hemispheric asymmetry of emotion: Evidence from event-related potentials. Journal of Psychophysiology. 2011;25:95–103. http://dx.doi.org/10.1027/0269-8803/a000045. [Google Scholar]


