Abstract
Background
Using resting state functional magnetic resonance imaging (RS-fMRI) we explored: 1) pre- to post-operative changes in functional connectivity in default mode, salience, and central executive networks after total knee arthroplasty (TKA) with general anesthesia, and 2) the contribution of cognitive/brain reserve metrics these resting state functional declines.
Methods
Individuals age 60 and older electing unilateral total knee arthroplasty (TKA; n=48) and non-surgery peers with osteoarthritis (n=45) completed baseline cognitive testing, baseline and post-surgery (post-baseline, 48 hour post-surgery) brain MRI. We acquired cognitive and brain estimates for premorbid (vocabulary, reading, education, intracranial volume) and current (working memory, processing speed, declarative memory, ventricular volume) reserve. Functional network analyses corrected for pain severity and pain medication.
Results
The surgery group declined in every functional network of interest (p < 0.001). Relative to non-surgery peers, 23% of surgery participants declined in at least one network and 15% of the total TKA sample declined across all networks. Larger preoperative ventricular volume and lower scores on preoperative metrics of processing speed and working memory predicted default mode network (DMN) connectivity decline. Premorbid cognitive and premorbid brain reserve did not predict decline.
Conclusion
Within 48 hours after surgery, at least one fourth of the older adult sample showed significant functional network decline. Metrics of current brain status (ventricular volume), working memory, and processing speed predicted the severity of default mode network connectivity decline. These findings demonstrate the relevance of preoperative cognition and brain integrity on acute postoperative functional network change.
Keywords: magnetic resonance imaging, orthopedics, dementia, cognitive reserve, cognitive dysfunction, efficiency, anesthesia
Introduction
Total knee arthroplasty (TKA) is performed primarily in older adults and an increasing number of individuals are electing to receive this surgical procedure. Neuronal insults from perioperative stressors are concerning given the heightened risk for mild cognitive impairment and dementia with aging [1]. Although anesthesia and surgery can cause long lasting cognitive deficits, acute and adverse postoperative outcomes such as delirium and postoperative cognitive decline can also occur [2–5]. Risk factors include lower premorbid cognitive reserve (lower reading level or fewer formal education years), increased age, lower preoperative brain integrity (e.g., enlarged ventricular volume), and lower preoperative executive and memory functions [3, 6–11]. The type and extent of acute functional neuronal activity change after TKA in older adults are unknown, however. Resting functional magnetic resonance imaging (RS-fMRI) provides us with a powerful method to bridge gaps in research knowledge.
In RS-fMRI, blood oxygen level dependent (BOLD) fluctuations from different brain regions are harnessed to yield insights into intrinsically organized networks called resting state networks (RSNs). Each RSN consists of multiple functionally related but spatially separated brain regions. Although RSNs have been investigated relative to age-related and neurological disorders [12], the application of RSNs to surgical studies are still in an early stage. Researchers to date investigated the utility of RS-fMRI in neurosurgery, particularly for tumor resection, (e.g., [13, 14]) and cardiac surgery (e.g., [15]) patients. Browndyke and colleagues [15] demonstrated preliminary findings of a link between RSN connectivity and postoperative cognitive dysfunction, however they did not assess network connectivity in the acute postoperative stage or risk factors for this decline. There are also resting state functional MRI network connectivity changes during active anesthesia [16–20]. Functional MRI brain networks may also change throughout active episodes of delirium with the changes resolving once delirium ends [21]. These studies indicate network connectivity changes during anesthesia or altered mental status but gaps in the literature remain, particularly regarding type of network change and predictors of changes around the acute perioperative period.
Given that functional brain connectivity analyses have not been applied to large samples of older adults electing a common surgery such as TKA, this sample is ideal to study due to their typically lower health comorbidities; knee replacements are often performed largely for quality of life reasons rather than urgent health issues. Preoperative brain and cognitive factors affecting acute RSN connectivity changes after this commonly elected orthopedic surgery under general anesthesia are unknown.
For the current investigation, we prospectively assessed older adults electing TKA for pre–to post–TKA acute brain changes in three prominent RSNs: 1) default mode network (DMN), 2) salience network (SN), and 3) central executive network (CEN). Each network has distinct interconnected regions and functionalities. The DMN (precuneus/posterior cingulate cortex, medial prefrontal cortex, and lateral inferior parietal cortex) is most active at rest and less active during demanding cognitive tasks [22, 23]. The DMN, in addition to its known function in mediating self-referential processes, is also considered important for memory consolidation, and is affected in individuals with neurological disorders such as Alzheimer’s disease [24–26]. In contrast, DMN connectivity can increase with certain interventions such as exercise [27] or anticholinesterase inhibitors [28]. The SN (dorsal anterior cingulate cortex, bilateral anterior insula) and the CEN (bilateral dorsolateral prefrontal cortex, inferior parietal lobule) also show changes with cognitive impairment [29, 30]. Both SN and CEN are active during cognitively demanding tasks.
Of the three RSNs, we hypothesized older adults electing TKA would show a particular decline from preoperative to acute 48 hour postoperative DMN connectivity due its sensitivity to neuronal changes and blood flow disruption. A non-surgery group of older adults matched on age and education was also prospectively recruited to assess expected amounts of change with repeat RSN assessment. We then explored the predictive value of premorbid brain and cognitive reserve estimates (intellectual estimate; total intracranial volume) versus current brain/cognitive status (ventricular volume; working memory, processing speed, declarative memory) in the TKA sample’s RSN decline.
Methods
The University of Florida Institutional Review Board in Gainesville, Florida approved this study. All participants were appropriately informed and signed consents. The study was conducted in accordance to principles of the Declaration of Helsinki.
Participants
Participants in the total knee arthroplasty (TKA) group were recruited through University of Florida orthopedic clinics, screened for dementia via a telephone interview (TICS; [31, 32]), and enrolled between 2013 and May of 2016 as part of an ongoing federally funded investigation. Participants in the non-surgery group were recruited through University of Florida orthopedic clinics, community mailings, and locally posted fliers. Non-surgery participants were selected through a yoked review process to match individual surgery participants on age, education, sex, and ethnicity/race. Non-surgery participants had to abstain from surgery for at least one year. Both groups were recruited over the same time frame and were tested and scanned at the same time intervals. All participants met the following inclusion/exclusion criteria: 1) aged 60 or older, 2) English as primary language, 3) have osteoarthritis or comparable joint pain, 4) have intact activities of daily living, and 5) have baseline neuropsychological testing unsupportive for dementia criteria per Diagnostic and Statistical Manual of Mental Disorders – Fifth Edition [33]. Additional exclusion criteria included: any other major surgery within the study timeline, history of head trauma/neurodegenerative illness, documented learning or seizure disorder, less than a sixth-grade education, substance abuse in the last year, major cardiac disease, chronic medical illness known to induce encephalopathy, implantable device precluding an MRI, and an unwillingness to complete the MRI. Two neuropsychologists reviewed the baseline data to confirm that test scores met the expected ranges for non-demented individuals.
Procedures
Participants completed a phone cognitive screening [32] and a comprehensive history/systems interview to confirm inclusion/exclusion criteria, followed by an in-person comorbidity rating [34], activities of daily living [35], cognitive testing, and brain MRI. The same examiner completed testing for all participants. Trained raters blind to group condition scored all behavioral data.
Anesthesia and Surgery Protocol
Protocols were standardized, with surgery participants receiving intravenous midazolam (1–4 mg) given for anxiety. Patients then received continuous femoral (CFNB) and single-injection subgluteal sciatic nerve blocks with 20 mL and 30 mL, respectively, of 0.5% ropivacaine as a bolus injection. The CFNB was continued with ropivacaine 0.2% at an infusion rate of 10 mL per hour. No opioids were added. Propofol, fentanyl, and rocuronium were used for anesthesia induction and intubation. Patients were ventilated with an air/oxygen mixture to maintain an end-tidal carbon dioxide at 35 ± 5 mm; anesthesia was maintained with inhaled isoflurane and intravenous fentanyl and rocuronium.
Total knee replacement surgery was done in a standard manner for all patients by the same surgeon. A tourniquet was used for all cases set to 250mm Hg and elevated prior to incision and deflated just prior to closure. Bony preparation was done by intramedullary instrumentation for the femoral side and extramedullary for the tibial side. The anterior and posterior cruciate ligaments were sacrificed for all patients and implants were fixed to the bone using bone cement.
Neuroimaging
Structural and resting state functional MRI was conducted both pre- and post-operatively within 48 hours after surgery or a pseudosurgery (for non-surgery peers) date. Delirium was assessed 24 hours post-operatively with the Confusion Assessment Method [36].
MRI Acquisition
All participants received neuroimaging (3T Siemens Verio; 8 channel head coil). T1-weighted and resting state fMRI were acquired. T1-weighted images were acquired with the following parameters: TR: 2500ms; TE: 3.77ms; 176 sagittal 1mm3 slices, 1 mm isotropic resolution; 256×256×176 matrix, 7/8 phase partial Fourier, total acquisition time: 9:22. Resting state fMRI were acquired with participants’ eyes closed and with the following parameters: TR: 2000ms; TE: 30ms; 36 transverse slices; 3.5 mm3 isotropic voxel size, 225×225×126 matrix, GRAPPA, total acquisition time: 7:38. To avoid surgery participants having potential difficulties in fixating on a cross during the postoperative resting state we opted for an eyes closed condition.
fMRI Data Preprocessing
Resting state fMRI data were preprocessed according to the following steps. The first six functional scans were discarded to eliminate transients. The remaining fMRI images were preprocessed using SPM (www.fil.ion.ucl.ac.uk/spm/). Slice timing correction was performed to compensate for acquisition delays across slices. Motion artifacts of timing corrected images were estimated and corrected by realigning all functional images to the first image. No participants were rejected due to excessive motion according to predetermined criteria [37, 38]. All the motion corrected functional images were co-registered to the T1 structural image, which were then normalized to the standard MNI152 T1 template, and resampled with 3mm×3mm×3mm resolution. Functional images in the MNI template space were spatially smoothed with an 8 mm full width at half maximum (FWHM) isotropic Gaussian kernel.
fMRI Regions of Interest Selection
Group independent component analysis (ICA) implemented in the GIFT Toolbox (http://icatb.sourceforge.net/) was applied to the preprocessed resting state fMRI data, combining pre-surgery and post-surgery scans of all 92 participants. The optimal number of independent components was determined to be 33 by the GIFT ICA algorithm. Four components corresponding to three resting state networks (RSN) of interest, namely, default mode network (DMN), salience network (SN), and central executive network (CEN), were automatically identified by a spatial template-matching technique [39]. DMN and SN were each represented by a single ICA component whereas CEN was represented by two ICA components lateralized to the left and right hemisphere respectively. For each of the four ICA components, individual component maps were subjected to a one-sample t-test to obtain a group level t-value map. Eleven regions of interest (ROIs) were selected from group level t-value maps, including medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC), and bilateral angular gyrus (AG) of the DMN; dorsal anterior cingulate cortex (ACC) and bilateral anterior insula (IN) of the SN; and bilateral dorsolateral prefrontal cortex (DLPFC) and bilateral inferior parietal lobule (IPL) of the CEN. A given ROI representing a brain region was defined to be a sphere of 5 mm in radius centered at the local maximum t-value of that region.
Functional Connectivity Analysis
Resting state fMRI (RS-fMRI) time series were extracted from all the voxels in each spherical ROI after regressing out nine nuisance signals, including 6 movement variables and 2 averaged signals representing white matter and cerebrospinal fluid, and a global signal. The time series were bandpass filtered between 0.01 and 0.1 Hz with a finite impulse response (FIR) filter. The filtered signals from each ROI were averaged across voxels to yield one signal for each ROI. A motion censoring procedure, “motion scrubbing” [40], was further applied on the BOLD signals to reduce the potential adverse effects of movements on functional connectivity. Within each RSN, functional connectivity between each ROI pair was measured by the cross correlation between the two scrubbed BOLD signals. Network level functional connectivity of each RSN was also computed by averaging the cross correlation values across all ROI pairs within that network. For each participant, both preoperative and postoperative resting state functional connectivity metrics were calculated.
Medication, Pain, and RSN
The magnitude of functional connectivity coefficients have been shown to be related to pain [41, 42] and morphine administration [43]. Using a published conversion algorithm, morphine equivalent dosages (MED) were calculated for each patient relative to the post-surgery scan [44]. The MED was considered potentially active if the most recent dose was within six hours prior to the post-surgery MRI. Pain assessment ratings (0–100; 100=worst) were acquired before the resting state functional MRI. In order to reduce the effects of those potentially confounding variables on functional connectivity values, we controlled pre-surgery functional connectivity for pre-surgery pain using linear regression to create unstandardized residual values. For post-surgery MRI functional connectivity we controlled for both post-surgery pain and active MED in the surgery group and post-surgery pain in the non-surgery group.
Reliable Change Index (RCI) of pre-post TKA network functional connectivity
The postoperative network functional connectivity changes for all networks of interest were quantified using a reliable change index (RCI). RCI values are typically calculated for repeated neuropsychological assessment [45] to provide estimates of clinically meaningful (statistically abnormal) change relative to expected change (from a comparison non-surgery group); we applied statistical methods of RCIs to correct functional connectivity changes within the surgery group for observed changes within the non-surgery group. RCI values were calculated using the equation . T2 is the pain and MED controlled post-surgery resting state network connectivity value, T1 is the pain controlled pre-surgery network connectivity value, M2 is the non-surgery group pain controlled post-pseudosurgical network connectivity mean, M1 is the non-surgery group’s pre-pseudosurgical pain controlled mean network connectivity value, and SED is the non-surgery group’s standard error of difference, which was calculated following published procedures [46]:
SD1 is the non-surgery group’s preoperative network connectivity standard deviation, SD2 is the non-surgery group’s post-surgery network connectivity standard deviation, and r12 is the Pearson correlation coefficient between preoperative and post-surgery resting state network connectivity values in the non-surgery group. The resulting RCI values reflect the magnitude of post-surgery resting state connectivity change from pre-surgery baseline, controlling for observed non-surgery functional connectivity test-retest reliability.
Difference-in-Differences Analysis
We also modeled the surgery effect (relative to the non-surgery group) on network connectivity using a Difference-in-Differences (DiD) estimator analysis [47]. Network connectivity changes were predicted based on age (to account for possible age interactions with network connectivity), TICS (to account for baseline global cognition), group status (surgery, non-surgery), time (pre and post), and group*time.
Cognitive and Brain Variables of Interest
All baseline measures were acquired within one week prior to the surgery/pseudosurgery.
Premorbid Brain Reserve
Brain reserve is a concept referring to the ability of someone’s brain to physiologically cope with injury, neurodegenerative pathology, and age-related changes without that individual manifesting clinical symptoms of deficit or decline [48, 49]. Premorbid brain reserve was operationalized as total intracranial volume (TICV; [50]). TICV was estimated by FreeSurfer maskvol, which has been shown to have strong positive correlation to gold standard manual TICV masks (r = 0.94; cross correlation coefficient = 0.92, p<0.01; [51]).
Current Brain Integrity
Neuroanatomical brain integrity prior to the surgery was operationalized using ventricular volume. T1 images were processed with FreeSurfer (Version 6.0; [52, 53]). Larger lateral ventricular volume is a risk factor for dementia and has been used in calculations of brain reserve [54]. Left and right volumes (including temporal horn) were summed to create total ventricular volume. For each participant, total ventricle volume was divided by total intracranial volume (FreeSurfer maskvol). We then calculated the natural log transformation to normalize the ventricle volume distribution (pre-transformation Shapiro-Wilk p < 0.001, post-transformation p = 0.734).
Premorbid Cognitive Reserve
Cognitive reserve is a concept similar to brain reserve to partially explain why there is not a direct relationship between clinical symptoms (e.g., memory) and some factor (e.g., neurodegenerative pathology) that should affect function; higher cognitive reserve is related to psychosocial and experiential factors (e.g., greater educational attainment) and genetic factors (e.g., childhood intelligence) [55]. Premorbid cognitive reserve was operationalized as a global composite of three metrics shown to be resistant to neurodegenerative disease processes and are considered estimates of premorbid intelligence [56]: word reading ability (Wide Range Achievement Test; total words correctly read; [57]), vocabulary knowledge (Wechsler Abbreviated Scale of Intelligence; WASI; Vocabulary Subtest; [58]), and years of education.
Preoperative Cognitive Domains of Interest
We completed a comprehensive cognitive assessment to assess the a priori hypothesis that preoperative current cognitive abilities in the domains of working memory, processing speed, and declarative memory would explain a portion of post-surgery RSN decline. These domains are key elements of cognitive vulnerability after surgery [11, 59] and are also associated with DMN connectivity in early dementia groups [26]. These domains of interest were based on a standardized composite of clinical neuropsychological measures:
Processing Speed
Stroop Word Reading (Stroop Color Word Test, total correct in 45 seconds; [60]), Digit Symbol subtest from the Wechsler Adult Intelligence Scale, 3rd edition (raw score in 120 seconds; WAIS-III; [61]), Trail Making Test, Part A [62];
Working Memory
Digit Span Backward Span from the WAIS-III (longest backward span), Spatial Span Backward from the Wechsler Memory Scale, 3rd edition (WMS-III; [63]), Letter-Number Sequencing from the WAIS-III;
Declarative Memory – measures of verbal recall and recognition of learned information
Logical Memory II (delay recall) from the WMS-III (total score), Hopkins Verbal Learning Test – Revised (HVLT) delay recall and recognition [64].
Global memory and cognition was operationalized as a mean composite of the three cognitive domains.
Statistical Analyses
Using an independent samples t-test statistic we compared groups on cognitive baseline/pre-surgery measures that had been standardized on published test norms. Paired t-test analyses assessed pre- to post-operative functional connectivity values regressed for pain and morphine dose. We examined the mean of the RSN first and, if significant, then examined each RSN network independently. To assess the frequency of decline in our surgery sample, we converted individual network functional connectivity RCI scores to z scores (using non-surgery peer data as “normative”). We used a conservative cut-point of ≤1.96 standard deviations to define a rate of clinically significant RSN decline. Based on this model, we expect a baseline of 2.5% of any group to show decline; a percent > 2.5% within our surgery sample indicates a significant portion of our sample experienced change in RSN after surgery [45]. We then used Pearson Product Moment Correlations to examine associations between brain/cognitive predictor variables of interest and the RSNs, using the difference between post-surgery connectivity and pre-surgery connectivity (i.e., not the RCI scores). A post hoc linear regression analysis examined the portion of the variance explained by the variables of interest. We also conducted a post hoc removal of surgery participants meeting comprehensive criteria for any form of mild cognitive impairment (MCI; [65]) and repeated the correlational analyses between brain/cognitive predictor variables of interest and the RSNs.
Results
Participants
A total of 174 surgery patients were referred by the study surgeon (HP) and contacted for study inclusion. Of these, 63 agreed to consider the study with 51 meeting inclusion and exclusion criteria and completing baseline neuropsychological assessment and MRI. Data from three surgery participants were excluded due to presence of pre-existing silent strokes (1 participant) and MRI post-surgery scanner complications (2 participants). For non-surgery orthopedic peers, a total of 84 participants were screened with 48 enrolled. Of the 48, two non-surgery peers were withdrawn due to concerns for a learning disorder identified during neuropsychological testing and one was excluded for a missing RS-fMRI sequence. A total of 45 completed the baseline assessment and pre-post pseudosurgery imaging sessions.
Participant Characteristics
The final groups of participants (surgery n = 48; non-surgery n = 45) were not statistically different in age, years of education, sex, lateral ventricular volume, or TICV (Table 1). Although non-surgery participants were selected to match surgery participants demographically and via a thorough screening process, surgery participants scored lower than the non-surgery peers on the test of reading (WRAT), a general cognitive screener, and on the declarative memory and processing speed composites (Table 1). There was no statistical group difference in baseline pre-surgery pain level while in the MRI scanner during the time of the resting state sequence; however, post-surgery comparisons showed the surgery group had significantly higher pain than non-surgery peers (p < 0.05). Days between baseline/pre-surgery MRI and post-surgery MRI were not statistically different by group (Table 1; p = 0.18). Although four surgery participants were identified with delirium lasting less than one day no participants had evidence of delirium at the time of the post-surgery MRI; all participants were included in analyses.
Table 1.
Demographic summary and baseline characteristics for surgery and control participants
| Demographic | Surgery (n = 48) Mean±SD or %(n) |
Control (n = 45) Mean±SD or %(n) |
|---|---|---|
|
| ||
| Age | 67.81±5.62 (range: 60–84) | 69.02±5.81 (range: 60–83) |
| Education | 15.21±2.79 (range: 11–23) | 16.29±2.67 (range: 9–24) |
| Sex (M:F) | 20:28 | 21:24 |
| TICS | 37.38±3.81 (range: 26–47)* | 38.60±3.12 (range: 32–45) |
| Pre-morbid Cognitive Reserve | 0.56±0.52 (range: −0.49–1.62)* | 1.00±0.48 (range: −0.60–1.77) |
| Working Memory | 0.51±0.73 (range: −1.18–2.20) | 0.74±0.80 (range: −1.10–2.55) |
| Processing Speed | −0.05±0.55 (range: −1.27–1.29)* | 0.22±0.68 (range: −1.74–1.76) |
| Declarative Memory | 0.21±0.74 (range: −1.42–1.36)* | 0.81±0.79 (range: −1.33–1.82) |
| Ventricle Volume | 29669.13±14542.14 (range: 9452.6–67240) | 29339.57±14497.10 (range: 9065.8–69274.7) |
| TICV | 1.54×106±1.47×105 (range: 1.29×106–1.89×106) | 1.56×106±1.46×105 (range: 1.27×106–1.89×106) |
| PreMRI Pain | 11.90±18.02 (range: 0–65) | 5.70±11.95 (range: 0–50) |
| PostMRI Pain | 36.08±22.02 (range: 2–100)* | 5.77±8.94 (range: 0–40) |
| MED | 10.68±10.67 (range: 0–37.50) | -- |
| PrePost MRI day span | 8.50±5.68 (range: 3–41) | 7.40±3.14 (range: 2–21) |
Indicates group difference p < 0.05.
TICS: Telephone Interview for Cognitive Status [31, 32]. Pre-morbid Cognitive Reserve: Composite of Vocabulary (from the Wechsler Abbreviated Scale of Intelligence) and Wide Range Achievement Test. Working Memory: Composite score of Digit Span backward, Letter-Number Sequencing, and Spatial Span backward. Processing Speed: Composite score of Digit Symbol total, Stroop word reading, and Trail Making Test Part A total time. Declarative Memory: Composite score of HVLT delay recall and recognition and WMS Logical Memory delay recall. Composite scores are based on published normative samples. Ventricle Volume: sum of left and right lateral ventricle volumes in mm3. TICV = Total Intracranial Volume in mm3. PreMRI Pain: 0–100 pain score acquired just before the pre-surgery resting state fMRI scan. PostMRI Pain: 0–100 pain score acquired just before the post-surgery resting state fMRI scan. MED: Morphine Equivalence Dose calculated using CDC guidelines [44]. PrePost MRI day span: time in days between pre and post MRI scans.
Resting state fMRI
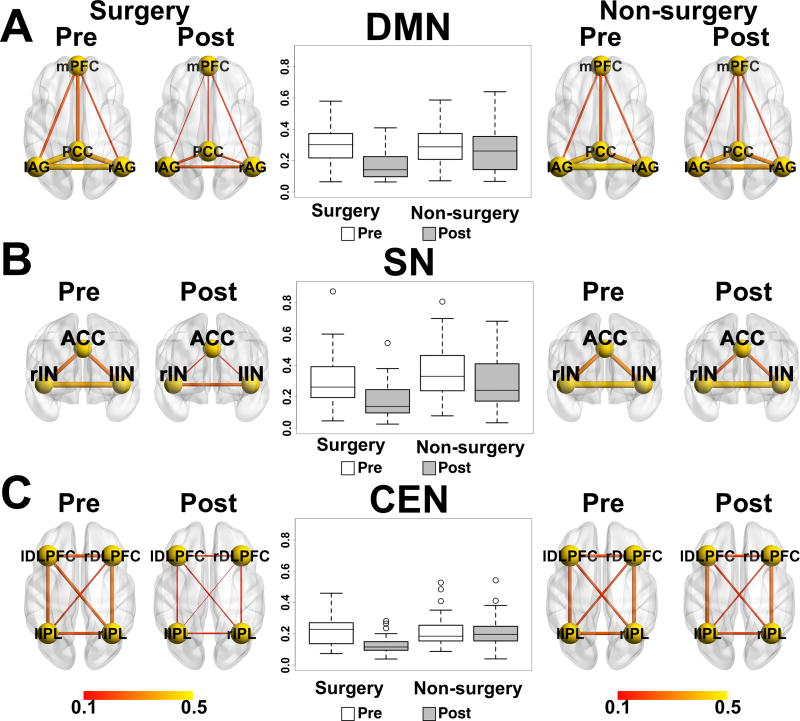
As shown in Figure 1 and Table 2, the DMN comprised mPFC, PCC and bilateral AG. The SN comprised ACC and bilateral IN. The CEN comprised bilateral DLPFC and bilateral IPL (all p values <1e-8 FWE-corrected). Groups were examined separately for RSN preoperative to postoperative changes.
Figure 1.
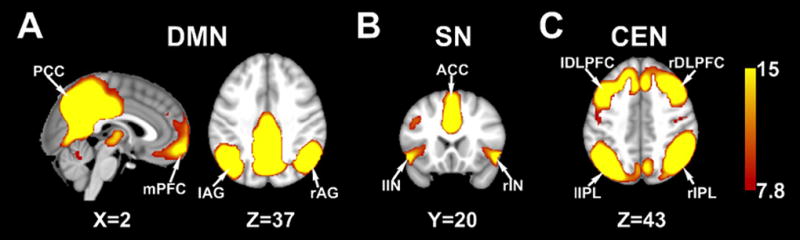
Resting state networks: A) DMN, B) SN, and C) CEN (P<1e-8 FWE Corrected). Combining pre and post scans from 48surgery participants and 45 non-surgery peers.
Table 2.
Resting State functional Magnetic Resonance Imaging (RS-fMRI) network Montreal Neurological Institute (MNI) coordinates and associated Brodmann areas
| ROI | MNI | BA | |||
|---|---|---|---|---|---|
|
| |||||
| X | Y | Z | |||
| DMN | |||||
| PCC | −6 | −51 | 27 | 23 | |
| mPFC | 3 | 54 | −18 | 11 | |
| lAG | −42 | −63 | 36 | 39 | |
| rAG | 51 | −57 | 33 | 39 | |
| SN | |||||
| ACC | 6 | 12 | 36 | 24/32 | |
| lIN | −39 | 15 | −3 | 48 | |
| rIN | 45 | 18 | −3 | 47/48 | |
| CEN | |||||
| lDLPFC | −30 | 12 | 51 | 9 | |
| lIPL | −45 | −60 | 48 | 7/39 | |
| rDLPFC | 45 | 33 | 27 | 45 | |
| rIPL | 36 | −63 | 45 | 7/39 | |
DMN = Default Mode Network, PCC = Posterior Cingulate Cortex, mPFC = Medial Prefrontal Cortex, lAG = Left Angular Gyrus, rAG = Right Angular Gyrus, SN = Salience Network, ACC = Anterior Cingulate Cortex, lIN = Left Insula, rIN = Right Insula, CEN = Central Executive Network, lDLPFC = Left Dorsolateral Prefrontal Cortex, lIPL = Left Inferior Parietal Lobe, rDLPFC = Right Dorsolateral Prefrontal Cortex, rIPL = Right Inferior Parietal Lobe. MNI = Montreal Neurological Institute. BA = Brodmann Area
Surgery group
The surgery group showed a significant change in the mean of the three RSNs (mean RSN; t(46) = 7.53; p < 0.001) even after controlling for pain and MED. Each of the individual network connectivity values in the surgery group also decreased (Figure 2; DMN: t(46) = 5.52, p < 0.001; SN: t(46) = 5.25, p < 0.001; CEN: t(46) = 6.49, p < 0.001). A post hoc analysis removing participants with MCI yielded similar results (DMN: t(42) = 4.61, p < 0.001; SN: t(42) = 4.94, p < 0.001; CEN: t(42) = 5.83, p < 0.001). Network connectivity changes were not correlated with time spent under anesthesia (p values > 0.67) or number of prior surgeries (p values > 0.12).
Figure 2.
Pre and post surgery functional connectivity for surgery and non-surgery participants within three resting state networks: A) DMN, B) SN, and C) CEN.
Non-surgery group
Network connectivity values did not significantly change (p values all > 0.05; Figure 2).
Difference-in-Differences Analysis
Using a linear regression Difference-in-Differences (DiD) model (see Supplementary Figure 1) we further confirmed the relevance of the surgery intervention on network connectivity change scores (corrected for pain and morphine equivalent dose). For the DMN, group*time (t = −2.636, p = 0.009) and age regardless of group status (t = −2.695, p = 0.008) were the only significant predictors; all other p values (TICS, group, and time) > 0.118. For the SN, group*time was the only significant predictor (t = −1.977, p = 0.05) with age demonstrating trend significance (t = −1.889, p = 0.061); all other p values > 0.187. For the CEN, group*time was the only significant predictor (t = −2.976, p = 0.003); all other p values > 0.133.
Reliable change of pre-post connectivity
Using the mean network connectivity change scores from the non-surgery peers and setting a significant cut point of −1.96, six (12.5%) surgery participants had DMN connectivity decline (1/6 also had CEN decline). Four additional participants (8.3%) had significant SN decline (1/4 also had CEN decline) and three (6.25%) had CEN decline (2/3 also had DMN or SN decline). Seven (14.6%) had decline across the mean of the three networks and 11 (22.9%) surgery participants had RSN connectivity decline in at least one network (Table 3). A post hoc analysis demonstrated individuals who reliably declined in network connectivity were not older than those who did not decline (non-decliners mean age = 67.83; decliners mean age = 67.67; p = 0.95). They were also not different in mean education (decliners = 16.57; non-decliners = 14.98; p = 0.16).
Table 3.
Surgery and control participants who had “reliable” RSN decline
| Subject | DMN RCI | SN RCI | CEN RCI | 3 Network Mean RSN RCI |
|
|---|---|---|---|---|---|
| Surgery | 9 | −0.47 | −2.59 | −1.06 | −1.99 |
| 15 | −3.48 | −1.48 | −1.11 | −2.7 | |
| 26 | −2.34 | 0.22 | −0.55 | −1.09 | |
| 30 | 0.05 | −2.09 | 0.38 | −0.96 | |
| 31 | −1.94 | −1.61 | −1.99 | −2.45 | |
| 35 | −1.52 | −2.57 | −0.62 | −2.27 | |
| 56 | −1.99 | −0.54 | −0.83 | −1.45 | |
| 71 | −2.65 | −1.38 | −1.84 | −2.57 | |
| 89 | −2.38 | 0.09 | −2.54 | −1.93 | |
| 113 | −1.47 | −3.15 | −2.27 | −3.18 | |
| 131 | −2.04 | −0.88 | −1.72 | −1.99 | |
|
|
|||||
| Control | 23 | −2.10 | −1.84 | −0.69 | −2.15 |
| 65 | −0.50 | 2.12 | −2.89 | −0.16 | |
Reliable Change Index (RCI) scores are z scores calculated using the non-surgery group as reference. Cells shaded gray are “reliably” declined scores (using a conservative cutoff of ≤ −1.96).
DMN RCI = Default Mode Network Reliable Change Index. SN RCI = Salience Network Reliable Change Index. CEN RCI = Central Executive Network Reliable Change Index. 3 Network Mean RSN RCI = mean of DMN, SN, and CEN resting state networks (RSN) Reliable Change Index.
For comparison, one (2.2%) non-surgery participant had significant DMN decline, one (2.2%) had significant CEN decline, and one (2.2%; the same individual with significant DMN decline) had significant decline across all three networks. In total, two (4.4%) non-surgery participants had reliable RSN connectivity decline in one or more network (Table 3).
Predictors of pre-post TKA connectivity change
Premorbid brain and cognitive reserve
Premorbid cognitive reserve metrics (WRAT, Vocabulary), TICV, and age did not associate with network connectivity change (all p values > 0.15).
Current brain integrity and memory/cognitive efficiency
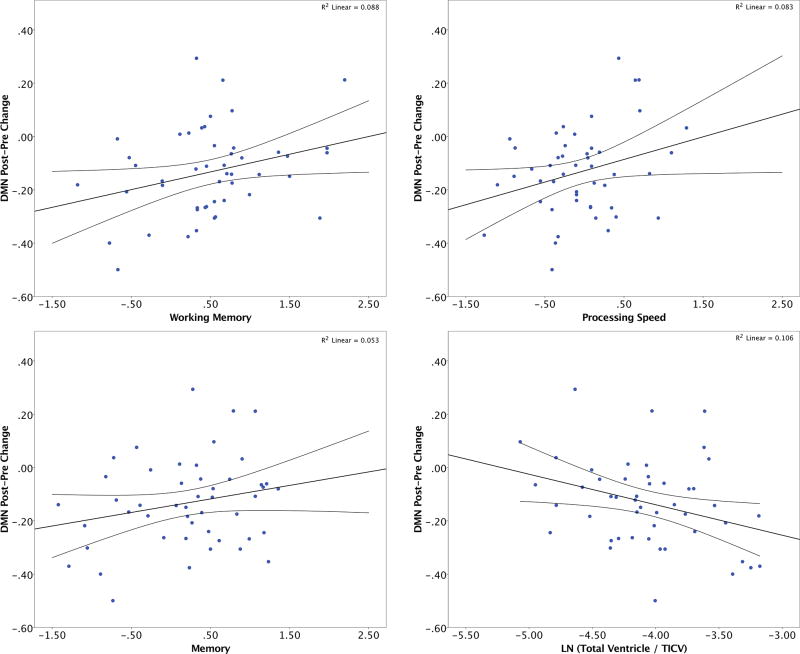
Global memory/cognitive efficiency associated with DMN connectivity change (r = 0.35, p = 0.02; lower scores showed greater decline). This association was primarily driven by working memory and processing speed (Figure 3; working memory r = 0.30, p = 0.04; processing speed r = 0.29, p = 0.048; declarative memory r = 0.23, p = 0.12). Current brain integrity also associated with DMN change (r = −0.34, p = 0.02; individuals with larger ventricle volumes showed greater decline).
Figure 3.
Current pre-surgical cognition (working memory, processing speed, and declarative memory) and brain reserve (lateral ventricle volume) relationships with DMN Reliable Change scores in the surgery group (n = 48).
In a post hoc analysis excluding participants with MCI (n = 6), significant associations remained between current brain integrity (ventricle volume) and DMN decline (r = −0.33, p = 0.03) while cognitive associations to DMN were no longer significant (working memory, p = 0.21; processing speed, p = 0.13). A Fisher’s r-to-z transformation test assessing the significance of the difference between two correlation coefficients (e.g., DMN change and WMI in the full surgery sample versus DMN change and WMI in the sample excluding MCI participants [n = 6]) was not significant (p = 0.63). Brain and cognitive metrics did not significantly correlate with SN or CEN declines (all p values > 0.06).
Processing speed and ventricular volume explained 17.7% of variance in DMN decline (processing speed Beta = 0.258, p = 0.064; ventricle volume Beta = −0.31, p = 0.028). Adding working memory to the model explained an additional 1.0% of the variance (p = 0.47). This model does not change when including age (p = 0.602). The patterns were similar when excluding MCI participants (processing speed Beta = 0.31, p = 0.042; ventricle volume Beta = −0.385, p = 0.012; working memory and age did not change the model: p values > 0.743). Adding education to the models did not affect the results (p = 0.50).
Discussion
Our results show significant reductions in RS-fMRI connectivity for three major networks of interest (DMN, SN, CEN) in non-demented older adults electing unilateral total knee arthroplasty under general anesthesia. By using a novel application of reliable change statistics to functional MRI data, we show that 15% of surgery participants significantly declined across all three functional networks and an additional 8% declined in at least one network (i.e., 23% of the sample declined in one or more networks). Pain, opioid medication dose at the time of the postoperative MRI, estimates of premorbid cognitive function (vocabulary, reading ability, education), an estimate of brain reserve (total intracranial volume), and group differences in baseline global cognition did not significantly contribute to or explain network connectivity decline. Rather, an estimate of preoperative brain integrity (ventricular volume) and current cognitive function explained functional connectivity decline with this specific to the DMN.
Perioperative Changes in Resting State Functional Networks
The DMN plays a critical role in self-monitoring and self-referencing [66–69], episodic and autobiographical memory [70], semantic processing [71], and inhibitory control during tasks [72]. The DMN is important for memory consolidation and is reduced in individuals with Alzheimer’s disease [24–26]. The DMN also changes with administration of sevoflurane and propofol [16–20] and becomes weaker and/or more disorganized during maintenance [20].
The significance of the DMN in pre to postoperative cognitive change was reported by Browndyke and colleagues (2017) in their study of 12 patients electing cardiac surgery relative to 12 individuals with a history of coronary artery disease. These investigators report significant positive correlates between pre to postoperative DMN change and six-week postoperative decline in a global cognitive index score [15]. Although this needs replication and investigation with a larger sample size, it appears acute RS-fMRI connectivity decline (particularly DMN) has clinical relevance and that DNM activity could be an important prognostic marker for intervention. Our study complements Browndyke and colleagues’ recent work. In addition to demonstrating pre-postoperative DMN change in a separate surgery group (orthopedic surgery), we demonstrate within-group change for two other well-known functional networks known to have cognitive relevance (SN, CEN) [30], and highlight that intervention may exist prior to the surgical event - as preoperative brain/cognitive risk factors predict DMN drop.
Connectivity decrease was observed in two other key networks, the SN and CEN, although our cognitive and brain variables of interest did not explain a significant portion of this drop. The SN, a network involving the dorsal anterior cingulate cortex and bilateral anterior insula is a frontally dominant network involved in the detection of both external and internal salient events [73], conflict resolution [74], cognitive control [72], autonomic and affective information processing [73, 75], irrelevant information suppression [73, 76], and top-down attention control [30, 77]. The CEN, by comparison, involves the bilateral dorsolateral prefrontal cortex and inferior parietal lobules. The CEN is associated with information maintenance and manipulation (working memory), high order cognitive control [76, 78], rule-based problem solving and goal-directed decision making [12]. We encourage future research into the significance of these networks on type of cognitive change (memory versus executive) after surgery.
Preoperative cognition, brain integrity, and network connectivity
The current study adds to the growing body of literature suggesting that preoperative (current) cognitive and brain status are important considerations for older adults electing major surgery. Processing speed and working memory are markers of cognitive efficiency and typically decline with age [79–81]; they depend on rapid frontal-subcortical and frontal-parietal communication [82]. Ventricular volume is considered an indicator of brain tissue loss [83] and also relates to metrics of cognitive efficiency in normal aging and dementia [82]. These cognitive and brain variables explained 19% of the variance in DMN drop. By contrast, premorbid verbal cognitive reserve and TICV (which can serve as a marker of brain reserve and protect against cognitive decline in aging and neurodegeneration) [84] did not predict decline. Therefore, preoperative cognitive and brain status appears particularly relevant to acute physiological (DMN) change. Disruption of the DMN occurs in MCI and AD [26, 85–87]. Therefore, acute DMN disruption in combination with lower preoperative cognitive performance on working memory and processing speed might herald future development of cognitive decline or dementia. While the neurobiological mechanisms of the RSN connectivity declines remain unknown our results provide further evidence current cognitive status of older adults is an important consideration within surgical settings [11, 88]. This interpretation remains even after removing participants meeting comprehensive criteria for any form of prodromal dementia (MCI; [65]).
Limitations
We acknowledge limitations in preoperative cognitive matching for patients relative to non-surgery peers. Despite our best attempts to match non-surgery peers to surgery patients on key demographics and general cognition (via the TICS) during a phone screening, our final statistical analyses of these groups showed differences in key neuropsychological measures assessing memory and frontal measures. We propose to the reader, however, that lower preoperative scores on declarative memory and processing speed measures are not entirely unexpected given orthopedic limitations in physical activity and pain, both of which associate with memory and processing speed abilities [89, 90]. Our participant profile also fits with growing literature showing many older adults electing surgery have preoperative cognitive impairment [91]. We also point out that as a group, the surgery participants’ scores for all included cognitive domains were in the average range relative to published norms. Further, a Difference-in-Differences model accounting for baseline global cognition (TICS) continued to demonstrate the strong effect of surgery on network connectivity changes pre to post-surgery.
We also recognize controversies in our RS-fMRI acquisition and RSN analyses. We used an eyes closed instead of eyes open condition. While there is not a clear answer regarding which method is better, some research indicates a slight increased reliability for eyes open and fixated on a cross [92]. A future study including an eyes open RS-fMRI condition might provide more reliable estimates of connectivity changes following surgery. In contrast, it might not be viable for patients to fixate on a cross on a computer screen post-surgery for a RS-fMRI so using an eyes closed condition might be preferable. In either case, the same condition needs to be used at both time points to enable pre-post comparison. For RSN analyses, we used a global signal regression (GSR). While there is no clear answer whether it is better or worse to use GSR, a known drawback of GSR is that it may adversely impact negative correlations between task-positive networks (e.g., CEN) and task-negative networks (e.g., DMN). In contrast, GSR is known to improve specificity of positive correlations [93]. In the present work we mainly focused on the positive within-network correlations and therefore chose to use GSR in our analyses.
Strengths of the study include the comprehensive cognitive assessment protocol, consideration for theoretical constructs of brain/cognitive reserve relative to current cognitive and brain status on RSN change, rigor of intraoperative protocol for anesthesia and surgical similarity, the similar timing of pre and post MRI for surgery and non-surgery peers, examination of acute brain change within 48 hours after surgery prior to potential confounds introduced by different recovery trajectories and rehabilitation procedures, and comparison of DMN drop relative to changes within two other established RSN networks.
There have been few studies assessing RS-fMRI changes following major surgery and most were focused on patients undergoing neurosurgery (e.g., [13, 14]). Outside surgical populations, disruption of these important RSNs correlates with cognitive deficits in numerous neurological and psychiatric disorders such as Alzheimer’s disease, MCI, multiple sclerosis, schizophrenia, ADHD, autism and depression [77, 85, 94–98]. Given the importance of RSNs for understanding neurological disorders, including Alzheimer’s disease, it is vital researchers continue to assess interactions between brain status, disease, MCI RSNs, and surgery. We also encourage future research on the mechanism of the functional connectivity changes after surgery. A review of effects of general anesthesia on the brain [99] raises some mechanistic possibilities, including impaired neurotrophin signaling and inflammation [100].
Conclusion
Brain and cognitive status metrics assessing brain integrity and cognitive efficiency are important considerations for acute pre to postoperative DMN decline. We now need additional studies examining preoperative cognitive and brain markers, why neuronal networks change, and whether network decline predicts later cognitive change. The study is relevant to researchers studying geriatric medicine in older adults and particularly those who might have prodromal Alzheimer’s disease or other preclinical neurodegenerative processes.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health RO1 NR014181; partially by UL1TR001427, KL2TR001429 and TL1TR001428. We wish to acknowledge the valuable time and efforts of the research participants, as well as staff Donna Weber and Kristi Ayers, associated graduate students Nadine Schwab, Samuel Crowley, Loren Hizel, and Elle Wiggins, and research assistants Carlos Hernaiz and Allison Choi.
Bibliography
- 1.Luck T, Then FS, Schroeter ML, Witte V, Engel C, Loeffler M, Thiery J, Villringer A, Riedel-Heller SG. Prevalence of DSM-5 mild neurocognitive disorder in dementia-free older adults: Results of the population-based life-adult-study. Am J Geriatr Psychiatry. 2017;25:328–339. doi: 10.1016/j.jagp.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International study of post-operative cognitive dysfunction. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 3.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 4.Abildstrom H, Rasmussen LS, Rentowl P, Hanning CD, Rasmussen H, Kristensen PA, Moller JT. Cognitive dysfunction 1–2 years after non-cardiac surgery in the elderly. ISPOCD group. International study of post-operative cognitive dysfunction. Acta Anaesthesiol Scand. 2000;44:1246–1251. doi: 10.1034/j.1399-6576.2000.441010.x. [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen LS, Johnson T, Kuipers HM, Kristensen D, Siersma VD, Vila P, Jolles J, Papaioannou A, Abildstrom H, Silverstein JH, Bonal JA, Raeder J, Nielsen IK, Korttila K, Munoz L, Dodds C, Hanning CD, Moller JT. Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol Scand. 2003;47:260–266. doi: 10.1034/j.1399-6576.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 6.Greene NH, Attix DK, Weldon BC, Smith PJ, McDonagh DL, Monk TG. Measures of executive function and depression identify patients at risk for postoperative delirium. Anesthesiology. 2009;110:788–795. doi: 10.1097/aln.0b013e31819b5ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith PJ, Attix DK, Weldon BC, Greene NH, Monk TG. Executive function and depression as independent risk factors for postoperative delirium. Anesthesiology. 2009;110:781–787. doi: 10.1097/aln.0b013e31819b5bc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shioiri A, Kurumaji A, Takeuchi T, Nemoto K, Arai H, Nishikawa T. A decrease in the volume of gray matter as a risk factor for postoperative delirium revealed by an atlas-based method. Am J Geriatr Psychiatry. 2016;24:528–536. doi: 10.1016/j.jagp.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Saczynski JS, Inouye SK, Kosar CM, Tommet D, Marcantonio ER, Fong T, Hshieh T, Vasunilashorn S, Metzger ED, Schmitt E, Alsop DC, Jones RN, Group SS. Cognitive and brain reserve and the risk of postoperative delirium in older patients: Analysis of data from a prospective observational study. Lancet Psychiatry. 2014;1:437–443. doi: 10.1016/S2215-0366(14)00009-1. [DOI] [PubMed] [Google Scholar]
- 10.Price CC, Garvan C, Hizel L, Lopez MG, Billings I, Frederic T. Delayed recall and working memory mmse domains predict delirium following cardiac surgery. J Alzheimers Dis. 2017:1–9. doi: 10.3233/JAD-170380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price CC, Tanner JJ, Schmalfuss I, Garvan CW, Gearen P, Dickey D, Heilman K, McDonagh DL, Libon DJ, Leonard C, Bowers D, Monk TG. A pilot study evaluating presurgery neuroanatomical biomarkers for postoperative cognitive decline after total knee arthroplasty in older adults. Anesthesiology. 2014;120:601–613. doi: 10.1097/ALN.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menon V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Vassal M, Charroud C, Deverdun J, Le Bars E, Molino F, Bonnetblanc F, Boyer A, Dutta A, Herbet G, Moritz-Gasser S, Bonafé A, Duffau H, de Champfleur NM. Recovery of functional connectivity of the sensorimotor network after surgery for diffuse low-grade gliomas involving the supplementary motor area. J Neurosurg. 2016:1–10. doi: 10.3171/2016.4.JNS152484. [DOI] [PubMed] [Google Scholar]
- 14.Hart MG, Price SJ, Suckling J. Functional connectivity networks for preoperative brain mapping in neurosurgery. J of Neurosurg. 2016:1–10. doi: 10.3171/2016.6.JNS1662. [DOI] [PubMed] [Google Scholar]
- 15.Browndyke JN, Berger M, Harshbarger TB, Smith PJ, White W, Bisanar TL, Alexander JH, Gaca JG, Welsh-Bohmer K, Newman MF, Mathew JP. Resting-state functional connectivity and cognition after major cardiac surgery in older adults without preoperative cognitive impairment: Preliminary findings. J Am Geriatr Soc. 2017;65:e6–e12. doi: 10.1111/jgs.14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palanca BJA, Mitra A, Larson-Prior L, Snyder AZ, Avidan MS, Raichle ME. Resting state functional magnetic resonance imaging correlates of sevoflurane-induced unconsciousness. Anesthesiology. 2015;123:346–356. doi: 10.1097/ALN.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amico E, Gomez F, Di Perri C, Vanhaudenhuyse A, Lesenfants D, Boveroux P, Bonhomme V, Brichant J-F, Marinazzo D, Laureys S. Posterior cingulate cortex-related co-activation patterns: A resting state fMRI study in propofol-induced loss of consciousness. PLOS ONE. 2014;9:e100012. doi: 10.1371/journal.pone.0100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noirhomme Q, Soddu A, Lehembre R, Vanhaudenhuyse A, Boveroux P, Boly M, Laureys S. Brain connectivity in pathological and pharmacological coma. Front Sys Neurosci. 2010;4:160. doi: 10.3389/fnsys.2010.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudetz AG. General anesthesia and human brain connectivity. Brain Connectivity. 2012;2:291–302. doi: 10.1089/brain.2012.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riehl JR, Palanca BJ, Ching S. High energy brain dynamics during anesthesia-induced unconsciousness. Network Neurosci. 2017;0:1–24. doi: 10.1162/NETN_a_00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi S-H, Lee H, Chung T-S, Park K-M, Jung Y-C, Kim SI, Kim J-J. Neural network functional connectivity during and after an episode of delirium. Am J Psychiat. 2012;169:498–507. doi: 10.1176/appi.ajp.2012.11060976. [DOI] [PubMed] [Google Scholar]
- 22.Mohan A, Roberto AJ, Mohan A, Lorenzo A, Jones K, Carney MJ, Liogier-Weyback L, Hwang S, Lapidus KAB. The significance of the default mode network (DMN) in neurological and neuropsychiatric disorders: A review. Yale J Biol Med. 2016;89:49–57. [PMC free article] [PubMed] [Google Scholar]
- 23.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 24.Wu X, Li R, Fleisher AS, Reiman EM, Guan X, Zhang Y, Chen K, Yao L. Altered default mode network connectivity in Alzheimer’s disease—a resting functional MRI and Bayesian network study. Hum Brain Mapp. 2011;32:1868–1881. doi: 10.1002/hbm.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle Marcus E, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damoiseaux JS, Beckmann CF, Arigita EJS, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SARB. Reduced resting-state brain activity in the"default network” in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- 27.McFadden KL, Cornier M-A, Melanson EL, Bechtell JL, Tregellas JR. Effects of exercise on resting-state default mode and salience network activity in overweight/obese adults. Neuroreport. 2013;24:866–871. doi: 10.1097/WNR.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sole-Padulles C, Bartres-Faz D, Llado A, Bosch B, Pena-Gomez C, Castellvi M, Rami L, Bargallo N, Sanchez-Valle R, Molinuevo JL. Donepezil treatment stabilizes functional connectivity during resting state and brain activity during memory encoding in Alzheimer’s disease. J Clin Psychopharmacol. 2013;33:199–205. doi: 10.1097/JCP.0b013e3182825bfd. [DOI] [PubMed] [Google Scholar]
- 29.He X, Qin W, Liu Y, Zhang X, Duan Y, Song J, Li K, Jiang T, Yu C. Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer’s disease. Hum Brain Mapp. 2014;35:3446–3464. doi: 10.1002/hbm.22414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bressler SL, Menon V. Large-scale brain networks in cognition: Emerging methods and principles. Trends Cogn Sci. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Welsh KA, Breitner JCS, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Cog Behav Neurol. 1993;6:103. [Google Scholar]
- 32.Cook SE, Marsiske M, McCoy KJ. The use of the modified telephone interview for cognitive status (TICS-M) in the detection of amnestic mild cognitive impairment. J Geriatr Psych Neur. 2009;22:103–109. doi: 10.1177/0891988708328214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Association AP. Diagnostic and statistical manual of mental disorders. American Psychiatric Publishing; Washington, DC: 2013. [Google Scholar]
- 34.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 35.Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 36.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: The confusion assessment methoda new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 37.Wylie GR, Genova H, DeLuca J, Chiaravalloti N, Sumowski JF. Functional magnetic resonance imaging movers and shakers: Does subject-movement cause sampling bias? Hum Brain Mapp. 2012;35:1–13. doi: 10.1002/hbm.22150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long X, Zuo X, Kiviniemi V, Yang Y, Zou Q, Zhu C, Jiang T, Yang H, Gong Q, Wang L, Li K, Xie S, Zang Y. Default mode network as revealed with multiple methods for resting-state functional MRI analysis. J Neurosci Meth. 2008;171:349–355. doi: 10.1016/j.jneumeth.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 39.Franco AR, Pritchard A, Calhoun VD, Mayer AR. Interrater and intermethod reliability of default mode network selection. Hum Brain Mapp. 2009;30:2293–2303. doi: 10.1002/hbm.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, Wasan AD, Napadow V. Default mode network connectivity encodes clinical pain: An arterial spin labeling study. Pain. 2013;154:24–33. doi: 10.1016/j.pain.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuner R, Flor H. Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci. 2016;18:20–30. doi: 10.1038/nrn.2016.162. [DOI] [PubMed] [Google Scholar]
- 43.Khalili Mahani N, Zoethout RMW, Beckmann CF, Baerends E, de Kam ML, Soeter RP, Dahan A, van Buchem MA, van Gerven JMA, Rombouts SARB. Effects of morphine and alcohol on functional brain connectivity during “resting state”: A placebo-controlled crossover study in healthy young men. Hum Brain Mapp. 2012;33:1003–1018. doi: 10.1002/hbm.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dowell D, Haegerich TM, Chou R. Cdc guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heaton, et al. Detecting change: A comparison of three neuropsychological methods, using normal and clinical samples. Arch Clin Neuropsych. 2001;16:75–91. [PubMed] [Google Scholar]
- 46.Iverson GL. Interpreting change on the WAIS-III/WMS-III in clinical samples. Arch Clin Neuropsych. 2001;16:183–191. [PubMed] [Google Scholar]
- 47.Buckley J, Shang Y. Estimating policy and program effects with observational data: The"differences-in-differences” estimator. Prac Assess Res Eval. 2003;8:1–10. [Google Scholar]
- 48.Satz P. Brain reserve capacity on symptom onset after brain injury: A formulation and review of evidence for threshold theory. Neuropsychology. 1993;7:273–295. [Google Scholar]
- 49.Tate DF, Neeley ES, Norton MC, Tschanz JT, Miller MJ, Wolfson L, Hulette C, Leslie C, Welsh-Bohmer KA, Plassman B, Bigler ED. Intracranial volume and dementia: Some evidence in support of the cerebral reserve hypothesis. Brain Res. 2011;1385:151–162. doi: 10.1016/j.brainres.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bigler ED, Tate DF. Brain volume, intracranial volume, and dementia. Invest Radiol. 2001;36:539–546. doi: 10.1097/00004424-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Crowley S, Tanner JJ, Ramon D, Schwab NA, Hizel L, Price CC. Reliability and utility of manual and automated estimates of total intracranial volume. J Int Neuropsych Soc. doi: 10.1017/S1355617717000868. (In press) https://doi.org/10.1017/S1355617717000868. [DOI] [PMC free article] [PubMed]
- 52.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 53.Fischl B. FreeSurfer. NeuroImage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coffey CE, Saxton JA, Ratcliff G, Bryan RN, Lucke JF. Relation of education to brain size in normal aging: Implications for the reserve hypothesis. Neurology. 1999;53:189–196. doi: 10.1212/wnl.53.1.189. [DOI] [PubMed] [Google Scholar]
- 55.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lezak MD. Neuropsychological assessment. Oxford University Press; Oxford ; New York: 2012. [Google Scholar]
- 57.Wilkinson GS, Robertson GJ Psychological Assessment Resources Inc. WRAT 4: Wide Range Achievement Test: Professional manual. Psychological Assessment Resources, Inc.; Lutz, FL: 2006. [Google Scholar]
- 58.Holdnack HA. Wechsler Abbreviated Scale of Intelligence. Harcourt Assessment; San Antonio, TX: 1999. [Google Scholar]
- 59.Price CC, Garvan CW, Monk TG. Type and severity of cognitive decline in older adults after noncardiac surgery. Anesthesiology. 2008;108:8–17. doi: 10.1097/01.anes.0000296072.02527.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Golden CJ, Freshwater SM. The Stroop color and word test: A manual for clinical and experimental uses. Stoelting; Chicago, IL: 2002. [Google Scholar]
- 61.Holdnack HA. Wechsler Adult Intelligence Scale, third edition (WAIS-III) The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 62.Tombaugh T. Trail Making Test A and B: Normative data stratified by age and education. Arch Clin Neuropsych. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 63.Holdnack HA. Wechsler Memory Scale, third edition (WMS-III) The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 64.Brandt J, Benedict RHB. Hopkins Verbal Learning Test--Revised: Professional manual. Psychological Assessment Resources; 2001. [Google Scholar]
- 65.Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, Delis DC. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriat Psychiat. 2009;17:368–375. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 67.Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 68.Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. J Cognitive Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 69.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. P Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- 71.Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, Sharp DJ. Salience network integrity predicts default mode network function after traumatic brain injury. P Natl Acad Sci USA. 2012;109:4690–4695. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 75.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 76.Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wen X, Liu Y, Yao L, Ding M. Top-down regulation of default mode activity in spatial visual attention. J Neurosci. 2013;33:6444–6453. doi: 10.1523/JNEUROSCI.4939-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Müller NG, Knight RT. The functional neuroanatomy of working memory: Contributions of human brain lesion studies. Neuroscience. 2006;139:51–58. doi: 10.1016/j.neuroscience.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 79.Babcock RL, Salthouse TA. Effects of increased processing demands on age differences in working memory. Psychol Aging. 1990;5:421–428. doi: 10.1037//0882-7974.5.3.421. [DOI] [PubMed] [Google Scholar]
- 80.Kail R, Salthouse TA. Processing speed as a mental capacity. Acta Psychol. 1994;86:199–225. doi: 10.1016/0001-6918(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 81.Salthouse TA, Toth J, Daniels K, Parks C, Pak R, Wolbrette M, Hocking KJ. Effects of aging on efficiency of task switching in a variant of the trail making test. Neuropsychology. 2000;14:102–111. [PubMed] [Google Scholar]
- 82.Ramirez J, McNeely AA, Scott CJ, Stuss DT, Black SE. Subcortical hyperintensity volumetrics in Alzheimer’s disease and normal elderly in the sunnybrook dementia study: Correlations with atrophy, executive function, mental processing speed, and verbal memory. Alzheimers Res Ther. 2014;6:49. doi: 10.1186/alzrt279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Madsen SK, Gutman BA, Joshi SH, Toga AW, Jack CR, Jr, Weiner MW, Thompson PM. Mapping dynamic changes in ventricular volume onto baseline cortical surfaces in normal aging, mci, and Alzheimer’s disease. Lect Notes Comput Sci. 2013;8159:84–94. doi: 10.1007/978-3-319-02126-3_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perneczky R, Wagenpfeil S, Lunetta KL, Cupples LA, Green RC, DeCarli C, Farrer LA, Kurz A, Group FtMS Head circumference, atrophy, and cognition: Implications for brain reserve in alzheimer disease. Neurology. 2010;75:137–142. doi: 10.1212/WNL.0b013e3181e7ca97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2008;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang K, Liang M, Wang L, Tian L, Zhang X, Li K, Jiang T. Altered functional connectivity in early Alzheimer’s disease: A resting-state fMRI study. Hum Brain Mapp. 2007;28:967–978. doi: 10.1002/hbm.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Binnewijzend MA, Schoonheim MM, Sanz-Arigita E, Wink AM, van der Flier WM, Tolboom N, Adriaanse SM, Damoiseaux JS, Scheltens P, van Berckel BN, Barkhof F. Resting-state fMRI changes in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2012;33:2018–2028. doi: 10.1016/j.neurobiolaging.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 88.Monk TG, Price CC. Postoperative cognitive disorders. Curr Opin Crit Care. 2011;17:376–381. doi: 10.1097/MCC.0b013e328348bece. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Middleton LE, Manini TM, Simonsick EM, Harris TB, Barnes DE, Tylavsky F, Brach JS, Everhart JE, Yaffe K. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171:1251–1257. doi: 10.1001/archinternmed.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brown SC, Glass JM, Park DC. The relationship of pain and depression to cognitive function in rheumatoid arthritis patients. Pain. 2002;96:279–284. doi: 10.1016/S0304-3959(01)00457-2. [DOI] [PubMed] [Google Scholar]
- 91.Culley DJ, Flaherty D, Reddy S, Fahey MC, Rudolph J, Huang CC, Liu X, Xie Z, Bader AM, Hyman BT, Blacker D, Crosby G. Preoperative cognitive stratification of older elective surgical patients: A cross-sectional study. Anesth Analg. 2016;123:186–192. doi: 10.1213/ANE.0000000000001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patriat R, Molloy EK, Meier TB, Kirk GR, Nair VA, Meyerand ME, Prabhakaran V, Birn RM. The effect of resting condition on resting-state fMRI reliability and consistency: A comparison between resting with eyes open, closed, and fixated. Neuroimage. 2013;78:463–473. doi: 10.1016/j.neuroimage.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murphy K, Fox MD. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage. 2017;154:169–173. doi: 10.1016/j.neuroimage.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JDE, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. P Natl Acad Sci USA. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Supekar K, Menon V, Rubin D, Musen M, Greicius MD. Network analysis of intrinsic functional brain connectivity in alzheimer's disease. PLOS Comput Biol. 2008;4:e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sorg C, Riedl V, Mühlau M, Calhoun VD, Eichele T, Läer L, Drzezga A, Förstl H, Kurz A, Zimmer C, Wohlschläger AM. Selective changes of resting-state networks in individuals at risk for alzheimer's disease. P Natl Acad Sci USA. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang J, Wang L, Zang Y, Yang H, Tang H, Gong Q, Chen Z, Zhu C, He Y. Parcellation-dependent small-world brain functional networks: A resting-state fMRI study. Hum Brain Mapp. 2009;30:1511–1523. doi: 10.1002/hbm.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fox MD, Greicius MD. Clinical applications of resting state functional connectivity. Front Sys Neurosci. 2010;4:1–13. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vutskits L, Xie Z. Lasting impact of general anaesthesia on the brain: Mechanisms and relevance. Nat Rev Neurosci. 2016;17:705–717. doi: 10.1038/nrn.2016.128. [DOI] [PubMed] [Google Scholar]
- 100.Berger M, Ponnusamy V, Greene N, Cooter M, Nadler JW, Friedman A, McDonagh DL, Laskowitz DT, Newman MF, Shaw LM, Warner DS, Mathew JP, James ML, FtM-PI The effect of propofol vs. Isoflurane anesthesia on postoperative changes in cerebrospinal fluid cytokine levels: Results from a randomized trial. Front Immunol. 2017;8 doi: 10.3389/fimmu.2017.01528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.