Abstract
Background
Laparoscopic thermal ablation is a common alternative to surgical resection in treating hepatic tumors, particularly in those located in difficult-to-reach locations. The aim of this study was to compare the safety and long-term efficacy of laparoscopic radiofrequency ablation (RFA) and microwave ablation (MWA) in treating hepatocellular carcinoma (HCC).
Method
From February 2009 to May 2015, data from patients with HCC nodules who had undergone either laproscopic MWA or laparoscopic RFA were examined. Complications, complete ablation rates, local tumor progression (LTP) rates, disease-free survival and cumulative survival rates were compared between the two treatment groups.
Results
A total of 154 patients with HCC (60 MWA and 94 RFA) were treated via laparoscopic approach. Major complication rates were found to be 1% and 2% in the RFA group and the MWA group, respectively (p=0.747). Complete ablation rates were 95% for both treatment groups (p=0.931) and LTP rates were 21.2% for RFA and 8.3% for MWA (p=0.034). Disease-free survival rates at 5 years were 19% in the RFA group and 12% in the MWA group, respectively (p=0.434), while cumulative survival rates at 5 years were 50% in the RFA group and 37% in the MWA group, respectively (p=0.185).
Conclusion
Laparoscopic RFA and MWA appear to be safe in the treatment of early-stage HCC. The local tumor progression rates was lower in the laparoscopic MWA compared to the laparoscopic RFA group but their respective overall survival and disease-free survival rates remained similar.
INTRODUCTION
Treatment options for hepatocellular carcinoma (HCC) currently includes liver transplant, surgical resection, thermal ablation, trans-arterial chemoembolization (TACE), and targeted agent such as sorafenib [1]. Among these options, ultrasound (US)-guided radiofrequency ablation (RFA) is the most widely-utilized technique for treating small HCCs < 3 cm in diameter [2–4]. Due to its percutanous approach, radiofrequency ablation can treat small tumors with less blood loss and fewer complications compared to surgical resection [5–9]. One limitation of RFA is its inability to heat tumors larger than 3 cm in size or around nearby blood vessels that may act as a heat-sink [10–12]. Incomplete ablations in these circumstances may lead to high rates of local tumor progression. Physicians have begun addressing these deficiencies by using high-powered microwave ablation (MWA) systems, which utilize an electric field to heat tissue. This penetrating electric field can create larger ablation zones and heat tumor tissue to hotter temperatures compared to RFA [13,14].
Despite the benefits associated with percutaneous ablation, there are sitll HCC cases that are not amenable to a direct percutaneous approach due to the location of the nodule, particularly if it is near the capsule or diaphragm [15–18]. An alternative method of treating these nodules is to utilize a laparoscopic approach [19], which allows the physician to grossly examine the tumor spread, improve tumor staging via intra-operative ultrasound (IOUS) and identify safer insertion paths to treat tumors located in difficult location [20–23]. Laparoscopic treatment strategies with MWA or RFA have only recently been adopted to reduce complication rates [16, 19]. Long-term clinical results from laparoscopic MWA treatment of HCC have not yet been characterized in the literature. The purpose of this retrospective comparative study between laparoscopic MWA and RFA for HCC was to compare their respective technical success, complication rates and rates of LTP, as well as survival rates.
Materials and methods
Institutional Review Board approval and written consent from all patients were obtained prior to the beginning of this retrospective study. All patients with HCC were treated with either laparoscopic MWA or RFA as determined by our multidisciplinary tumor board from February 2009 to May 2015. The Barcelona-Clinic Liver Cancer (BCLC) criteria was utilized as a broad framework for deciding appropriate treatment strategies [3]. Inclusion criteria for ablation therapies were a single lesion with a diameter >5 cm or two to three lesions < 3 cm in diameter, unresectable due to risk of complications, Child-Pugh class A cirrhosis (selected patients with Child-Pugh class B cirrhosis), early recurrence after surgical resection or percutaneous RFA or patient’s desire to decline surgery [16, 19]. Radiofrequency ablation was dominant ablation modality at the initiation of the study period but microwave ablation became the preferred ablation modality in the later stages of the study as MWA safety data became known. The patient exclusion criteria for this study included: severe impairment of coagulation tests (platelets < 40.000 and/or International Normalized Ratio (INR) > 1.2); superficial lesions adjacent to abdominal viscera that could be easily displaced during the laparoscopic maneuvers; deeply-seeded lesions that were not amenable to percutaneous approaches (i.e. not visible at US or proximal to primary biliary or portal tributaries); complete portal vein thrombosis or pre-existing severe liver disease (class C according to the Pugh-Child classification) [16, 19].
Procedure
All patients underwent intraoperative US examination (Aloka Alfa 10; Aloka Co, Tokyo) by surgeons trained in US techniques and ablations [16, 19]. The laparoscopic ultrasound (LUS) probe had a flexible tip and dimensions of 10 mm in diameter and 50 cm in length [24–25]. For all ultrasound-guided RFAs [26], a 200-W, 480 KHz monopolar radiofrequency generator (Valleylab, Boulder, CO, USA) was used with power settings at 140 watts and a mean ablation time of 19.6 ± 8.6 minutes (median: 18; range: 12–25 minutes). An insulated 18-gauge water-cooled tip antenna was inserted into the tumor under ultrasound guidance. All microwave ablations [26] utilized a 2.45 MHz microwave generator (AMICA-GEN, HS Hospital Service SpA, Aprilia, Italy) with power settings at a median of 60 Watts (range: 50–70 W), and a mean ablation time of 10.9 ± 5.3 minutes (median: 10; range: 7–13.5 minutes).
Pre- and post-treatment evaluation
Preoperative assessment included an US study of the liver and a triple-phase spiral contrast-enhanced CT scan to confirm HCC diagnosis and location. In selected cases where CT or US imaging was equivocal, magnetic resonance imaging (MRI) of the liver was utilized.
Postoperative mortality was defined as occurrence of death within 90 days after treatment. We used the Common Toxicity Criteria of the National Cancer Institute and the Dindo-Clavien classification of surgical complications to index pain and postoperative morbidity, respectively [27–28]. Ultrasound and CT scans were performed at 1 and 3-months to evaluate treatment response and complications. Afterwards, the post-treatment response was evaluated with CT every 6 months. A single, experienced radiologist with 10 years of expertise in ablation techniques and images reviewed all CT/MRI exams.
Technical outcome and oncologic response were defined using the International Working Group on image-guided tumor ablation [29] standardized definitions. Technical success was defined by the tumor lesion being completely covered by the ablation zones at the 1-month follow-up exams with contrast-enhanced imaging. LTP was defined by the reappearance of tumor foci within the edge of the ablation zone. We defined intra-hepatic recurrence to include LTP but also extending to the boundaries of the entire liver and not limited to the original ablation zone [29].
Patients who did not show a complete local response after the first ablation session immediatley underwent either additional ablation sessions or TACE. Patients with LTP or intrahepatic recurrence were treated with appropriate therapies following the EASL and the AASLD guidelines [3, 4].
Statistical analysis
Kaplan Meier curves, which included overall survival, disease-free survival, intra-hepatic recurrences and LTP curves, were created and compared using the log-rank test. Characterization of baseline patient characteristics between RFA and MWA groups were done using the Mann-Whitney U test and the Wilcoxon matched pairs test. Data distributed in a normal distribution were expressed as mean ± standard deviation; if data were non-parametric, median and interquartile range values (IR) were reported. Comparisons of proportions were done using two-sided Pearson’s χ2 test or Fisher’s exact test, depending on sample size. The association of each parameter with survival and recurrence rates was estimated via univariate analysis. Signicant parameters with p-values less than 0.05 were then included in a multivariate analysis. For each parameter analyzed in the multivariate analyses, p-values, hazard ratio and 95% confidence intervals (CI) were obtained. All analysis was performed on commercial statistical software (Statistica-Mac, Statsoft, Tulsa OK, USA).
RESULTS
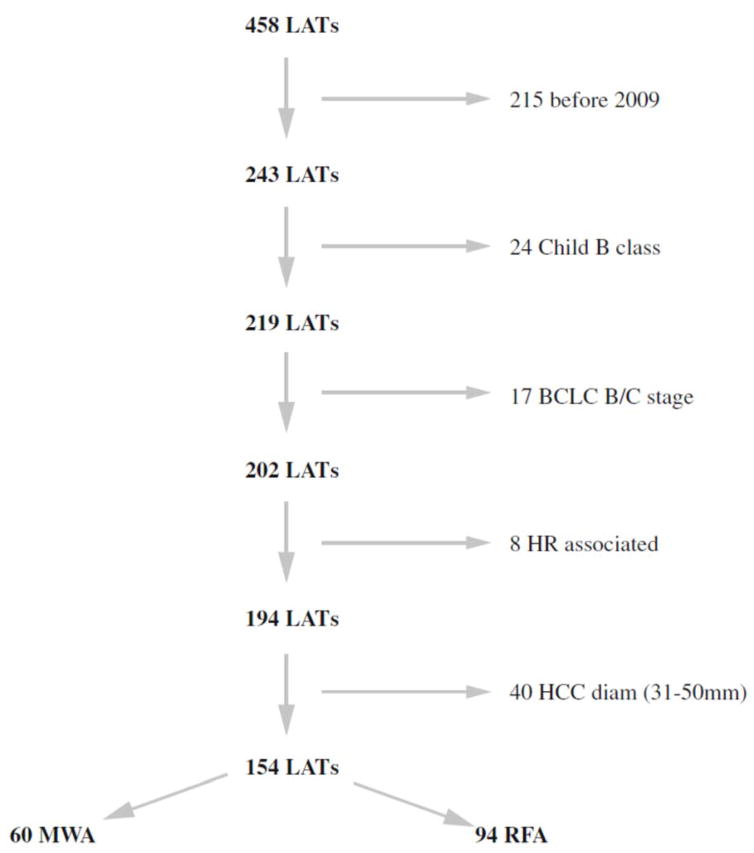
A total of 458 consecutive patients were identified to have undergone laparoscopic ablation for HCC at our institution (Figure 1). Among this cohort, 215 patients were excluded because they were treated before February 2009, the year MWA procedures began at our institution. Additional patients were excluded due to Child-Pugh class B liver function (n = 24), BCLC B/C stage (n = 17), surgical resection in association with an ablation therapy (n = 8) or nodule diameter greater than 3 cm in diameter (n = 40). After the exclusion process, there were 154 patients available for analysis (MWA: 60 and RFA: 94).
Figure 1.
Study flow chart. One hundred and fifty-four patients were finally included in the study. BCLC: Barcelona Clinic Liver Cancer, HR: hepatic resection, HCC: hepatocellular carcinoma, LATs: laparoscopic ablation therapies, MWA: microwave ablation, RFA: radiofrequency ablation
The baseline characteristics of patients allocated to MWA or RFA are described in Table 1 and electronic supplementary Table 1. A significant difference in the maximum diameter of HCC nodules between MWA and RFA (21.5+5.3 mm vs 19.2+5 mm; p=0.008). The inclusion/exclusion criteria for laparoscopic approach is listed in detail in electronic supplementary Table 2. The conversion rate to an open approach during the laparoscopic access was 0%. There were no perioperative deaths at 90 days. The mean postoperative hospital stay was 4.2+1.4 days after RFA and 4.4+1.8 after MWA (p=0.4993). Transient post-procedural pain (Grade 1–2) was observed in 6% (6/94) of patients after RFA and 18% (11/60) of patients after MWA (p=0.021). Pain was relieved with a short course of non-steroidal anti-inflammatory medication. Six patients (6%) after RFA and 5 (8%) after MWA had a low-grade fever lasting 12–72 h after the procedure, all of which self-resolved. The overall morbidity rate was 19% after RFA and 23% after MWA (p=0.747) (Table 2). In one patient after MWA, a pleural effusion required a pleuric drainage (Dindo-Clavien class 3A). In one patient after RFA, a wall hematoma at the trocar access formed, requiring a surgical hemostasis (Dindo-Clavien class 3B).
Table 1.
Demographic and clinical characteristics of all patients enclosed in the study
| MWA (n=60) | RFA (n=94) | P value | |
|---|---|---|---|
| Male sex | 43 (72%) | 69 (73%) | 0.813 |
|
| |||
| Age (years) (median; IR) | 70±8.3 (69.9; 66–76) | 69±9.0 (71; 65–76) | 0.634 |
|
| |||
| Cirrhosis etiology | 0.898 | ||
| HCV | 40 (67%) | 63 (67%) | |
| HBV | 9 (15%) | 16 (17%) | |
| Other | 11 (18%) | 15 (16%) | |
|
| |||
| Child-Pugh class A | 60 (100%) | 94 (100%) | NS |
|
| |||
| MELD score (median; IR) | 8.7±1.9 (8; 7–9) | 9.2±2.9 (9; 7–10) | 0.209 |
|
| |||
| BCLC [3] | 0.558 | ||
| A1 | 16 (27%) | 28 (30%) | |
| A2 | 12 (20%) | 19 (20%) | |
| A3 | 7 (12%) | 17 (18%) | |
| A4 | 25 (41%) | 30 (32%) | |
|
| |||
| Esophageal varices | 22 (37%) | 41 (44%) | 0.138 |
|
| |||
| Tumor location: | |||
| Deep intrahepatic location | 37 (62%) | 64 (68%) | 0.350 |
|
|
|||
| Adjacent to hepatic structures or other viscerae | 22 (37%) | 33 (35%) | 0.844 |
|
| |||
| Adjacent to major vessels | 4 (7%) | 11 (12%) | 0.304 |
|
| |||
| Lesions number: 1 | 34 (57%) | 63 (67%) | 0.256 |
| 2–3 | 26 (43%) | 31 (33%) | |
|
| |||
| Charlson’s index ≤3 | 24 (40)% | 32 (34)% | 0.373 |
|
| |||
| HCC segment: | 0.247 | ||
| II/III/IV/V | 27 (46%) | 36 (38%) | |
| I/VI/VII/VIII | 33 (54%) | 58 (62%) | |
|
| |||
| HCC lesion diameter (mm) (Median, IR) | 21.5±5.3 (22; 17.5–25) | 19.2±5 (20; 15–22) | 0.008 |
|
| |||
| IOUS° MI HCC | 28 (47%) | 44 (47%) | 0.986 |
|
| |||
| Hemoglobin (g/dl) (Median, IR) | 13.6±1.8 (13.8, 9.3 – 16.4) | 13.5±1.7 (13.7, 8.9 – 16.5) | 0.730 |
|
| |||
| Platelet count (x103/mm3) (Median, IR) | 107±48 (97.5; 71–131) | 110±51 (105.5; 67–140) | 0.710 |
|
| |||
| Total bilirubin (mg/dl) (Median, IR) | 1.15±0.48 (1.11; 0.8–1.35) | 1.20±0.53 (1.11; 0.85–1.5) | 0.493 |
|
| |||
| Serum albumin (g/l) (Median, IR) | 3.94±0.48 (3.96; 3.6–4.2) | 3.85±0.50 (3.79, 3.5–4.2) | 0.278 |
|
| |||
| Prothrombin time (INR) (Median, IR) | 1.11±0.12 (1.07; 1.04–1.17) | 1.14±0.24 (1.1; 1.04–1.2) | 0.330 |
|
| |||
| AST (U/L) (Median, IR) | 74.3±59.2 (61; 35–101) | 68.9±56.4 (52; 34–87) | 0.569 |
|
| |||
| α-fetoprotein (ng/ml) (median, IR) | 99±260 (9.1; 3.9–66.7) | 75±192 (7.3; 3.2 –30) | 0.523 |
IR: interquartile range
IOUS: intraoperative ultrasound
MI: microinvasive
Table 2.
Summary of complications according to type of technique, radiofrequency (RFA) or microwave ablation (MWA)
| MWA (n=60) | RFA (n=94) | |||||
|---|---|---|---|---|---|---|
| Dindo-Clavien classification of complications | ||||||
| Complications | 1 | 2 | 3a | 1 | 2 | 3b |
| Ascites | 2 | 1 | 0 | 2 | 1 | 0 |
| Encephalopathy | 2 | 0 | 0 | 2 | 0 | 0 |
| Jaundice | 3 | 0 | 0 | 5 | 0 | 0 |
| Wall hematoma | 3 | 0 | 0 | 1 | 2 | 1 |
| Intraperitoneal bleeding | 0 | 1 | 0 | 0 | 0 | 0 |
| Pleural effusion | 0 | 0 | 1 | 0 | 0 | 0 |
| Arhythmia | 0 | 1 | 0 | 1 | 1 | 0 |
| Hematuria | 0 | 0 | 0 | 1 | 1 | 0 |
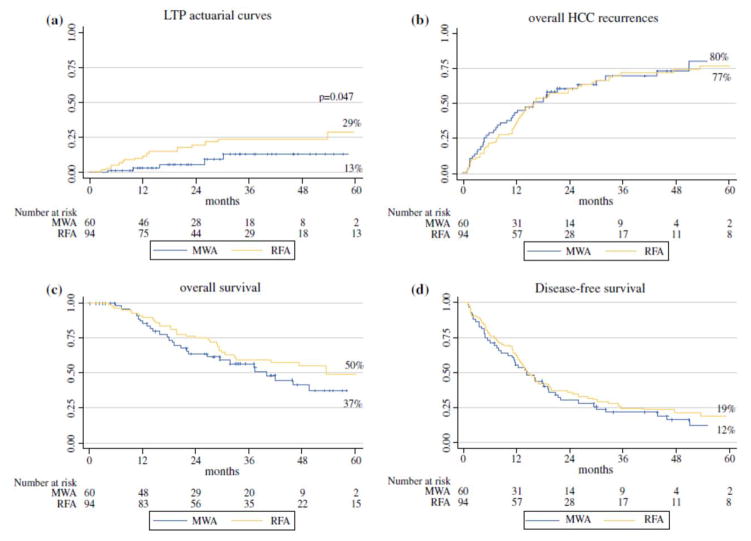
The mean follow-up period of the patients was 31+19.5 months (median: 26.9; range: 15–45.8 months). Technical success was achieved in 95% (89/94) of patients treated with RFA and 95% (57/60) of patients treated with MWA (p=0.931). There was a significantly higher rate of LTP incidence in RFA patients compared to MWA patients (21.2% vs 8.3%; p=0.034) as well as their respective Kaplan-Meier curves (p=0.047) (Figure 2A). Sixteen patients had recurrences that needed to be re-treated (3 MWA, 5 RFA and 8 TACE), while 2 patients refused the new treatment, 1 patient had liver insufficiency and 1 patient showed multiple lesions (15 patients obtained a complete ablation after the re-treatment, while one patient submitted to TACE showed an incomplete necrosis). All five patients with LTP after MWA were succesfully re-treated (1 MWA, 2 RFA and 2 TACE). In the RFA group, there was an LTP rate of 21.2% (20/94), with lesions near a major vessel showing a significantly higher LTP rate (45%; 5/11) than lesions that were not near major vessel (18%; 15/83; p=0.037). Within the MWA group, there was an LTP rate of 8.3% (5/60), with all LTP occuring away from major vessels (9%; 5/56). No recurrences occurred in the MWA group near major vessels (0%; 0/4).
Figure 2.
Local tumor progression rate [A], and intra-hepatic hepatocellular carcinoma (HCC) recurrence rate [B] according to radiofrequency ablation (RFA) or microwave ablation (MWA) (p=0.047 and p=N.S., respectively).
Overall survival [C] and disease-free survival curves [D] of patients with hepatocellular carcinoma (HCC) according to radiofrequency ablation (RFA) or microwave ablation (MWA) (p=N.S.)
The cumulative intra-hepatic tumor recurrence rates in the MWA group at 1-, 3- and 5-years were 39%, 69% and 80%, respectively, whereas in the RFA group, they were 33%, 68% and 77%, respectively (p=0.679) (Figure 2B). The two groups demonstrated similar rates of overall survival (p=0.185) (Figure 2C) and cumulative disease-free survival (p=0.434) (Figure 2D). The causes of death included HCC progression in 17 patients (28%), hepatic failure in 7 patients (12%), and other extrahepatic causes in 4 patients (7%) in the MWA group, while in the RFA group, causes of death included HCC progression in 18 patients (19%), hepatic failure in 9 patients (10%) and other extrahepatic causes in 10 patients (11%) (p=0.400).
DISCUSSION
Laparoscopic approaches for thermal ablation provides access to difficult tumors, particularly if the tumors are located underneath the liver capsule, adjacent gallbladder, or diaphragm [18, 19]. In this study, we compared the efficacy, complication rates and long-term outcomes between laparoscopic MWA and RFA procedures. Our results demonstrate that both laparoscopic approaches are associated with a near-complete technical success and low complication rates, similar to previously-reported percutaneous RFA results in treating HCC nodules [30, 31]. No significant differences were found between ablation groups in terms of recurrence rates, disease-free survival rates and overall survival rates. However, our study revealed a lower incidence of LTP in the MWA group compared to the RFA group (8% vs. 21%, p=0.034).
With regards to the safety of both laparoscopic MWA or RFA, the present study found no perioperative mortality and a low incidence of postoperative complications: 19% after RFA and 23% after MWA with major complications (Dindo-Clavien classes 3A and 3B) rates less than 2%. These values are comparable to the safety profiles found in previous clinical ablation studies [2, 31]. A recent systematic review comparing both ablation modalities (radiofrequency and microwave) also reported similar data for both techniques with low rates of complications: major complication rates associated with RFA and MWA was 4.1% and 4.6%, respectively [32]. In our study, only two cases (1.3%) required an invasive procedure (pleural drainage and surgical wound hemostasis) and eight cases (5.2%) required further pharmacological treatment or blood transfusions.
Current literature concerning complete ablation and LTP rates of MWA versus RFA are controversial [33, 34]. In our study, a lower incidence of LTP was observed in the MWA group compared with the RFA group. MWA systems create larger margins and overcome the heat-sink effect, shutting off vessels that can be related to LTP [36]. In our experience, LTP rates increases from 18% to 45% if the lesion is near to a major vessel after RFA, while no LTP was registered for these lesions after MWA. Direct clinical trial results comparing LTP rates among patients undergoing MWA and RF results are equivocal, with many studies treating larger tumors with MWA and smaller tumors with RFA [37, 38]. Since the size of the nodule is a well-known factor associated with LTP, there are questions about the generalizability of these previous results [30,39].
Survival outcomes after RFA for HCC can be improved by minimizing the risk for LTP. We found that LTP was significantly higher in the RFA group than in the MWA group but had similar overall survival rates. Survival rates may have been related to our protocol of immediate treatment after LTP detection with repeat RFA, MWA or TACE: only 4 patients had no follow-up treatment and another patient showed an incomplete necrosis after TACE. A retrospective study of 171 patients who underwent RFA for HCC that met the Milan criteria had similar conclusions: cumulative survival rates were significantly higher in patients without LTP than in those with LTP (p<0.001) [40].
There were limitations to our study design. The retrospective, unblinded nature of our analysis may have introduced bias to the results, especially since RFA procedures were performed earlier in the study while MWA procedures were performed predominantly in the later points of the study. At our institute, we prefer to use MWA if the HCC nodule diameter is >20 mm. This decision to treat tumors between 2–3 cm in size with MWA and those < 2 cm with RFA presents a potential bias in the reported results that could influence overall survival. However, overall HCC recurrences were not statistically significant and, above all, LTP rates of the RFA patients were higher than that of the MWA patients, despite their smaller nodule. As previously described, repeated treatments received by patients at HCC recurrence could represent a potential confounder for interpreting survival data.
CONCLUSION
Our study showed that laparoscopic ablations with both RFA or MWA can be a safe and effective alternative in patients with HCC located in difficult-to-reach locations. There were no mortalities in either group and no difference in rates of post-operative complications. The LTP rates in patients treated with MWA were lower than those treated with RFA. However, this finding had little impact on overall survival and intra-hepatic recurrence rates, which were similar between each group. A multi-center, prospective, randomized controlled trials is warranted to confirm these results.
Supplementary Material
Synopsis.
This study showed that laparoscopic MWA or RFA can be a safe and effective alternative treatment in patients with HCC located in difficult-to-reach locations. Laparoscopic MWA was associated with significantly less local tumor progression compared to laparoscopic RFA. However, laparoscopic MWA and RFA had similar rates of long-term overall survival.
Footnotes
Authors disclosures
Roberto Santambrogio, M.D., Jason Chiang, M.D., PhD, Matteo Barabino, M.D., Franca Meloni3, M.D., Emanuela Bertolini, M.D., Fabio Melchiorre, M.D. and Enrico Opocher, M.D. have no conflicts of interest or financial ties to disclose. Funding for J.C. provided by: National Cancer Institute (F30 CA165548) and National Institute for General Medical Sciences (T32 GM008692)
References
- 1.Colombo M, Sangiovanni A. Treatment of hepatocellular caricnoma: beyond international guidelines. Liver Int. 2015;35(suppl 1):129–38. doi: 10.1111/liv.12713. [DOI] [PubMed] [Google Scholar]
- 2.Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 4.De Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56(suppl 1):S75–87. doi: 10.1016/S0168-8278(12)60009-9. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Zhang J, Liu X, Li X, Jiao B, Kang T. Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2012;27:51–58. doi: 10.1111/j.1440-1746.2011.06947.x. [DOI] [PubMed] [Google Scholar]
- 6.Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to Milan criteria. Ann Surg. 2010;252:903–12. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 7.Cho YC, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010;51:1284–90. doi: 10.1002/hep.23466. [DOI] [PubMed] [Google Scholar]
- 8.Kuang M, Xie XY, Huang C, et al. Long-term outcome of percutaneous ablation in very early-stage hepatocellular carcinoma. J Gastrointest Surg. 2011;15:2165–71. doi: 10.1007/s11605-011-1716-2. [DOI] [PubMed] [Google Scholar]
- 9.Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56:412–8. doi: 10.1016/j.jhep.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Ke S, Ding XM, Qian XJ, Zhou YM, Cao BX, Gao K, Sun WB. Radiofrequency ablation of hepatocellular carcinoma sized >3 and <5 cm: is ablative margin of more than 1 cm justified? World J Gastroenterol. 2013;14:7389–7398. doi: 10.3748/wjg.v19.i42.7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang HW. Influence of blood vessel on the thermal lesion formation during radiofrequency ablation for liver tumors. Med Phys. 2013;40:073303. doi: 10.1118/1.4811135. [DOI] [PubMed] [Google Scholar]
- 12.Lu DSK, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, Sayre J. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14:1267–1274. doi: 10.1097/01.rvi.0000092666.72261.6b. [DOI] [PubMed] [Google Scholar]
- 13.Lubner MG, Brace CL, Ziemlewicz TJ, Hinshaw JL, Lee FT. Microwave ablation of hepatic malignancy. Semin Intervent Radiol. 2013;30:56–66. doi: 10.1055/s-0033-1333654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang S, Yu J, Liang P, Yu X, Cheng Z, Han Z, Li Q. Percutaneous microwave ablation for hepatocellular carcinoma adjacent to large vessels: a long-term follow-up. Eur J radiol. 2014;83:552–558. doi: 10.1016/j.ejrad.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Chung MH, Wood TF, Tsioulias GJ, Rose DM, Bilchik AJ. Laparoscopic radiofrequency ablation of unresectable hepatic malignancies. A phase 2 trial. Surg Endosc. 2001;15:1020–6. doi: 10.1007/s00464-001-0026-2. [DOI] [PubMed] [Google Scholar]
- 16.Santambrogio R, Opocher E, Costa M, Cappellani A, Montorsi M. Survival and intra-hepatic recurrences after laparoscopic radiofrequency of hepatocellular carcinoma in patients with liver cirrhosis. J Surg Oncol. 2005;89:218–26. doi: 10.1002/jso.20204. [DOI] [PubMed] [Google Scholar]
- 17.Ballem N, Berber E, Pitt T, Siperstein A. Laparoscopic radiofrequency ablation of unresectable hepatocellular carcinoma: long-term follow-up. HPB. 2008;10:315–20. doi: 10.1080/13651820802247102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De la Serna S, Vilana R, Sanchez-Cabus S, et al. Results of laparoscopic radiofrequency ablation of HCC. Could the location of the tumor influence a complete response to treatment? A single European experience. HPB. 2015;17:387–93. doi: 10.1111/hpb.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santambrogio R, Barabino M, Bruno S, et al. Long-term outcome of laparoscopic ablation therapies for unresectable hepatocellular carcinoma: a single European center experience of 426 patients. Surg Endosc. 2015 doi: 10.1007/s00464-015-4468-3. (Pub online) [DOI] [PubMed] [Google Scholar]
- 20.Lo CM, Lai ECS, Liu CL, Fan ST, Wong J. Laparoscopy and laparoscopic ultrasonography avoid exploratory laparotomy in patients with hepatocellular carcinoma. Ann Surg. 1998;227:527–32. doi: 10.1097/00000658-199804000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim PN, Choi D, Rhim H, et al. Planning ultrasound for percutaneous radiofrequency ablation to treat (<3 cm) hepatocellular carcinomas detected on computed tomography or magnetic resonance imaging: a multicenter prospective study to assess factors affecting ultrasound visibility. J Vasc Interv Radiol. 2012;23:627–634. doi: 10.1016/j.jvir.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 22.Kim JE, Kim YS, Rhim H, et al. Outcomes of patients with hepatocellular carcinoma referred for percutaneous radiofrequency ablation at a tertiary center: analysis focused on the feasibility with the use of ultrasonography guidance. Eur J Radiol. 2011;79:e80–e84. doi: 10.1016/j.ejrad.2011.03.090. [DOI] [PubMed] [Google Scholar]
- 23.Rhim H, Lee MH, Kim YS, Choi D, Lee WJ, Lim HK. Planning sonography to assess the feasibility of percutaneous radiofrequency ablation of hepatocellular carcinomas. AJR. 2008;190:1324–1330. doi: 10.2214/AJR.07.2970. [DOI] [PubMed] [Google Scholar]
- 24.Machi J. Intraoperative and laparoscopic ultrasound. Surg Oncol Clin N Am. 1999;8:205–226. [PubMed] [Google Scholar]
- 25.Santambrogio R, Bianchi P, Pasta A, Palmisano A, Montorsi M. Ultrasound-guided interventional procedures fo the liver during laparoscopy. Technical considerations. Surg Endosc. 2002;16:349–54. doi: 10.1007/s004640090082. [DOI] [PubMed] [Google Scholar]
- 26.Santambrogio R, Opocher E. Diagnostic Laparoscoy and allied technology. In: Calise F, Casciola G, editors. Minimally Invasive Surgery of the Liver, Updates in Surgery. Springer-Verlag; Italia: 2013. pp. 83–94. [Google Scholar]
- 27.National Cancer Institute. Cancer therapy evaluation program: common toxicity evaluation manual, version 2.0. 1999 Jun 1; Available at: ctep.cancer.gov/reporting/ctc.html.
- 28.Dindo D, Demartines N, Clavien PA. Classification of surgical complications. A new proposal with evaluation in a cohort of 633 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed M. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. Radiology. 2014;273:241–260. doi: 10.1148/radiol.14132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation. Multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livraghi T, Meloni F, Solbiati L, Zanus G For the Collaborative Italian Group using AMICA system. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol. 2012;35:868–874. doi: 10.1007/s00270-011-0241-8. [DOI] [PubMed] [Google Scholar]
- 32.Bertot LC, Sato M, Tateishi R, Yoshida H, Koike K. Mortality and complications rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. Eur Radiol. 2011;21:2584–2596. doi: 10.1007/s00330-011-2222-3. [DOI] [PubMed] [Google Scholar]
- 33.Chinnaratha MA, Chuang MA, Fraser RJ, Woodman RJ, Wigg AJ. Percutaneous thermal ablation for primary hepatocellular carcinoma: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2015 Jun 25; doi: 10.1111/jgh.13028. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 34.Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1054–1063. doi: 10.4254/wjh.v7.i8.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lencioni R, De Baere T, Martin RC, Nutting CW, Narayanan G. Image-guided ablation of malignant liver tumors: reccomendations for clinical validation of novel thermal or non-thermal technologies – a Western perspective. Liver Cancer. 2015;4:208–2014. doi: 10.1159/000367747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dodd JD, Dodd NA, Lanctot AC, Glueck DA. Effect of variation of portal venous blood flow on radiofrequency and microwave ablations in a blood-perfused bovine liver model. Radiology. 2013;267:129–136. doi: 10.1148/radiol.12120486. [DOI] [PubMed] [Google Scholar]
- 37.Ohmoto K, Yoshioka N, Tomiyama Y, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2009;24:223–7. doi: 10.1111/j.1440-1746.2008.05596.x. [DOI] [PubMed] [Google Scholar]
- 38.Ding J, Jing X, Liu J, Wang Y, Wang F, Wang Y, Du Z. Comparison of two different thermal techniques for the treatment of hepatocelllular carcinoma. Eur J Radiol. 2013;82:1379–84. doi: 10.1016/j.ejrad.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 39.Chinnaratha MA, Sathananthan D, Pateria P, et al. High local recurrence of early-stage hepatocellular carcinoma after percutaneous thermal ablation in routine clinical practice. Eur J Gastroenterol Hepatol. 2015;27:349–54. doi: 10.1097/MEG.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi S, Kudo M, Chung H, et al. Initial treatment response is essential to improve survival in patients with hepatocellular carcinoma who underwent curative radiofrequency ablation therapy. Oncology. 2007;72(suppl 1):98–103. doi: 10.1159/000111714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.