Abstract
Background
Among all in-breast tumor recurrences (IBTR) following breast conserving therapy (BCT), some comprise metachronous new primaries (NP) while others are true recurrences (TR). Establishing this distinction remains a challenge.
Methods
We studied 3932 women who underwent BCT for stage I-III breast cancer from 1998-2008. Of these, 115 (2.9%) had an IBTR. Excluding patients with inoperable/unresectable recurrences or simultaneous distant metastases, 81 patients with isolated IBTR comprised the study population. An IBTR was categorized as a NP rather than a TR if it included an in situ component. The log-rank test and Kaplan-Meier method were used to evaluate disease-free (DFS) and overall survival (OS). Univariate and multivariate analyses were performed with Cox proportional hazards regression models.
Results
At a median of 64.5 months from IBTR diagnosis, 28 of 81 patients had DFS events. Five-year DFS was 43.1% in the TR group (p = 0.0001) versus 80.3% in the NP group. Five-year OS was 59.7% in the TR group versus 91.7% among those with NP (p = 0.0011). On univariate analysis, increasing tumor size, high grade, positive margins, lymphovascular invasion, node involvement, lack of axillary surgery, chemotherapy, radiation therapy, and IBTR type (TR vs. NP) were significantly associated with worse DFS. Controlling for tumor size and margin status, TR remained significantly associated with lower DFS (HR = 3.717, 95%CI 1.607 – 8.595, p = 0.002).
Conclusion
Presence of an in situ component is associated with prognosis among patients with IBTR following BCT and may be useful in differentiating TR and NP.
Introduction
Isolated locoregional recurrence following breast conserving therapy (BCT) arises in a minority of patients with localized disease1–5. Advances in treatment and screening have yielded favorable outcomes for early-localized breast cancer, with contemporary studies suggesting a recurrence risk below 5% in select populations6–9. Despite these advances in treating primary disease, locoregional recurrences remain a clinical challenge, with ten-year distant metastasis-free survival estimates ranging between 36 and 65% and ten year overall survival estimated between 39 and 64.5%7,10–12.
While chest wall and regional nodal recurrences likely arise from the primary tumor, in-breast tumor recurrence (IBTR) after BCT may represent a true recurrence (TR) or metachronous new primary (NP). There is no universally accepted method of determining which recurrences stem from the primary tumor and which have arisen de novo. Most studies base this determination on anatomic distance from the original primary in addition to a host of other features such as change in histologic type, hormone receptor status, and nuclear grade13–20. Studies that use these factors to differentiate TR and NP have shown variable differences in prognosis between these entities, and it remains unclear whether management should be driven by these classifiers.
An alternate method of classifying recurrences may be to consider the presence of an in situ component21,22. Invasive breast tumors often develop from in situ lesions, such as ductal carcinoma in situ (DCIS) and, indeed, the majority of primary invasive ductal cancers have an accompanying intraductal component23–26. Genetic and molecular studies have shown that invasive cancers and their accompanying intraductal components share underlying similarities, suggesting that the two are related in origin27–29. Given the progression from intraductal to invasive carcinoma, invasive recurrences presenting with an intraductal component are likely to have evolved de novo from previously existing DCIS or normal tissue rather than from cells of the primary invasive tumor that survived despite treatment. Likewise, recurrences with no intraductal component may be more likely to represent a true recurrence of primary invasive cancer rather than a new primary tumor. Here, we sought to evaluate the prognostic significance of a classification system defined by the presence or absence of an in situ component adjacent to invasive in-breast tumor recurrence of breast cancer.
Patients and Methods
Study Population
Between 1998 and 2008, 3932 consecutive female patients with stage I-III invasive breast cancer were treated with BCT (breast conserving surgery and radiation therapy) at our institution. Patient data were prospectively collected in a multidisciplinary computerized database. Patients were excluded if they received neoadjuvant chemotherapy, had inflammatory breast cancer, or were not assessed for estrogen receptor (ER), progesterone receptor (PR), or HER2-neu status. One hundred and sixty-two (4.1%) patients had an invasive locoregional recurrence as the first site of failure. One hundred and fifteen (2.9%) of these were IBTRs. After exclusion of 18 patients with simultaneous distant metastases (within two months of recurrence diagnosis), 7 patients with follow-up of less than two months from diagnosis of recurrence, 2 patients with lack of pathology reports, and 7 patients who did not have breast surgery, 81 patients with isolated, invasive IBTR treated with surgery comprised the study population. An institutional review board waiver was received for this retrospective study.
Tumor Characteristics
Information on multifocality, lymphovascular invasion (LVI), size, and histology was gathered from pathological reports of biopsy and surgical specimens. All pathology slides were reviewed at a single institution. An IBTR was determined to be a NP if it had an in situ component (ductal or lobular carcinoma in situ) within or directly adjacent to the invasive tumor component and a TR if the specimen was invasive only (Figure 1). Biologic subtype was approximated as luminal A (ER/PR+, HER2−, Grade 1/2), luminal B (ER/PR+, HER2-, Grade 3), luminal-HER2 (ER/PR+, HER2+), HER2 (ER/PR−, HER2+), or triple negative (ER/PR-, HER2-). ER and PR status was determined by immunohistochemistry (IHC), with tumors with 1% nuclear staining or more classified as positive30. HER2 status was considered positive if IHC was 3+ or FISH was amplified31. Equivocal HER2 was grouped as negative for analysis.
Figure 1.
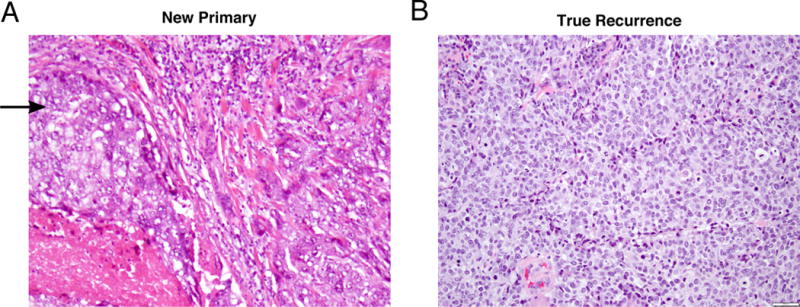
Pathology samples of in-breast tumor recurrences. A. 200X magnified view of infiltrating ductal carcinoma adjacent to ductal carcinoma in situ (arrow). B. 200X magnified view of infiltrating ductal carcinoma with no in situ component.
Definition of End-points
The primary endpoint of this study was disease-free survival (DFS) after diagnosis of IBTR. DFS events included second locoregional recurrence, distant recurrence, death attributable to any cause, and second primary non-breast invasive cancer, as defined by Hudis et al.32. Overall survival (OS), distant metastasis-free survival (DMFS), and second locoregional recurrence (LRR)-free survival (2nd LRR-FS) were analyzed as secondary endpoints. Locoregional recurrence was defined as invasive recurrence in the ipsilateral breast, chest wall, and/or regional nodes.
Statistical Analysis
The Fisher’s exact or chi-squared tests and Welch’s t-test were used to compare categorical and continuous variables, respectively, for patient and tumor characteristics of patients who had NP and TR. Kaplan-Meier method was used to calculate DFS, OS, DMFS, and second LRR-FS, and the log-rank test was used to compare survival curves. Univariate and multivariate analyses were performed using Cox regression analysis. All p-values were two-sided, with values <0.05 considered significant.
Results
Patient and Treatment Characteristics
A total of 81 patients with IBTR after BCT were analyzed. At initial diagnosis of primary breast cancer, all patients received breast conserving surgery and adjuvant whole breast radiotherapy. Fifty-two percent of patients received hormonal therapy, and 65% of patients received chemotherapy. In most patients, radiation therapy was delivered to the whole breast using tangential fields at a median dose of 46.8 Gy (interquartile range[IQR] 46.8 – 50.4 Gy). Sixty-five patients received a boost to the tumor bed at a median dose of 10.0 Gy (IQR 9.5 – 14.0 Gy). Three patients were treated with partial breast irradiation, and six (7.4%) received regional nodal irradiation. There were no significant differences in primary tumor treatment characteristics between TR and NP.
Patient and IBTR treatment characteristics are summarized in table 1. The median age at IBTR diagnosis was 58 (range 36–87). Median follow-up from diagnosis of recurrence was 64.5 months (range 12.8 – 177.1). The median time interval from definitive surgery of the primary tumor to diagnosis with IBTR was 53.9 months (range 7.3 - 161.1). There were no significant differences in age or time to recurrence between patients with TR and those with NP.
Table 1.
Patient and Salvage Treatment Characteristics by IBTR Subtype
| Total | TR | NP | ||||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | p value | ||
| No. of Patients | 81 | 27 | 54 | |||||
|
| ||||||||
| Age at IBTR | ≤45 | 15 | 19 | 5 | 19 | 10 | 19 | 0.99 |
| >45 | 66 | 81 | 22 | 81 | 44 | 81 | ||
| Median (yrs) | 58.2 | 60.1 | 55.6 | 0.15 | ||||
| Range (yrs) | 36.6 | 87.6 | 36.8 | 87.6 | 36.6 | 83.3 | ||
|
| ||||||||
| Time to Recurrence | median (months) | 53.9 | 58.2 | 53.2 | 0.96 | |||
| range (months) | 7.3 – 161.1 | 9.1 – 161.1 | 7.3 – 144.9 | |||||
|
| ||||||||
| Follow Up | median (months) | 64.5 | 49.3 | 69.4 | 0.08 | |||
| range (months) | 12. 8 – 177.1 | 15.4 – 177.1 | 12.8 – 171.0 | |||||
|
| ||||||||
| Salvage Breast Surgery | Mastectomy | 73 | 90 | 21 | 78 | 52 | 96 | 0.01 |
| BCS | 8 | 10 | 6 | 22 | 2 | 4 | ||
|
| ||||||||
| Axillary Surgery | Axillary Dissection | 15 | 19 | 5 | 19 | 10 | 19 | 0.14 |
| SLNB | 41 | 51 | 10 | 37 | 31 | 57 | ||
| Nonea | 25 | 31 | 12 | 44 | 13 | 24 | ||
|
| ||||||||
| Margin Status (Breast) | Negative | 76 | 94 | 23 | 85 | 53 | 98 | 0.04 |
| Positive | 5 | 6 | 4 | 15 | 1 | 2 | ||
|
| ||||||||
| Salvage Chemotherapy | Yes | 44 | 54 | 17 | 63 | 27 | 50 | 0.35 |
| No | 37 | 46 | 10 | 37 | 27 | 50 | ||
|
| ||||||||
| Salvage Hormonal Therapy | Yes | 42 | 52 | 18 | 67 | 24 | 44 | 0.07 |
| No | 39 | 48 | 9 | 33 | 30 | 56 | ||
|
| ||||||||
| Salvage Radiation Therapy | Yes | 6 | 7 | 4 | 15 | 2 | 4 | 0.092 |
| No | 75 | 93 | 23 | 85 | 52 | 96 | ||
|
| ||||||||
| Salvage Trastuzumab | Yes | 5 | 45 | 0 | 0 | 5 | 50 | 0.99 |
| HER2+ Only, n = 11 | No | 6 | 55 | 1 | 100 | 5 | 50 | |
IBTR: in-breast tumor recurrence; TR: true recurrence; NP: new primary; BCS: breast conserving surgery; SLNB: sentinel lymph node biopsy
After IBTR, 73 (90%) patients were treated with mastectomy and 8 (10%) were treated with repeat breast conserving surgery (BCS). Five (6%) of 81 patients had positive margins, with TR patients more likely to have positive margins than NP. Forty-four (54%) patients were treated with chemotherapy, 42 (52%) with hormonal therapy, 6 (7%) with reirradiation (median 56 Gy; all external beam within 3–9 years of the first radiation course; no major reirradiation toxicities were observed), and 5 of 11 HER2 positive patients were treated with trastuzumab. TR were more likely to have been treated with partial mastectomy than NP (p = 0.01). There were no other significant differences in treatment between TR and NP, though TR trended towards being more often treated with hormone therapy and radiation therapy (Table 1).
Recurrent and Primary Tumor Characteristics
Seventy-three (90%) tumors were found in the breast only, while 8 patients (3 TR and 5 NP) additionally had involvement of the axilla (Table 2). TR tended to be larger than NP (median size 1.25 vs. 0.7 cm, p = 0.02). There were no significant differences between TR and NP in grade, multifocality, LVI, node involvement, histologic type, or receptor subtype.
Table 2.
Patterns of IBTR by Subtype
| Total | TR | NP | ||||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | P value | ||
| Location | Breast Only | 73 | 90 | 24 | 89 | 49 | 91 | 0.35 |
| Breast and Axilla | 7 | 9 | 2 | 7 | 5 | 9 | ||
| Breast, Axilla, and IMN | 1 | 1 | 1 | 4 | 0 | 0 | ||
|
| ||||||||
| Tumor Size | median (cm) | 0.9 | 1.25 | 0.7 | 0.022 | |||
| range (cm) | 0.009 – 4.3 | 0.1 – 3.8 | 0.009 – 4.3 | |||||
|
| ||||||||
| T Stage | T1 | 70 | 86 | 23 | 85 | 47 | 87 | 0.99 |
| T2 | 9 | 11 | 3 | 11 | 6 | 11 | ||
| Unspecified | 2 | 2 | 1 | 4 | 1 | 2 | ||
|
| ||||||||
| Multifocality | Yes | 24 | 30 | 8 | 30 | 16 | 30 | 0.99 |
| No | 57 | 70 | 19 | 70 | 38 | 70 | ||
|
| ||||||||
| Grade | I/II | 22 | 27 | 6 | 22 | 16 | 30 | 0.71 |
| III | 52 | 64 | 19 | 70 | 33 | 61 | ||
| Unspecified | 7 | 9 | 2 | 7 | 5 | 9 | ||
|
| ||||||||
| LVI | Yes | 17 | 21 | 7 | 26 | 10 | 19 | 0.56 |
| No | 62 | 77 | 19 | 70 | 43 | 80 | ||
| Unspecified | 2 | 2 | 1 | 4 | 1 | 2 | ||
|
| ||||||||
| Histology | Ductal | 71 | 88 | 24 | 89 | 47 | 87 | 0.962 |
| Lobular | 7 | 9 | 2 | 7 | 5 | 9 | ||
| Other | 3 | 4 | 1 | 4 | 2 | 4 | ||
|
| ||||||||
| Change in Histology | Yes | 6 | 7 | 0 | 0 | 6 | 11 | 0.17 |
| (vs. Primary Tumor) | No | 75 | 93 | 27 | 100 | 48 | 89 | |
|
| ||||||||
| Receptor Subtype | Luminal A | 20 | 25 | 6 | 22 | 14 | 26 | 0.45 |
| Luminal B | 23 | 28 | 10 | 37 | 13 | 24 | ||
| Luminal-HER | 6 | 7 | 1 | 4 | 5 | 9 | ||
| HER | 5 | 6 | 0 | 0 | 5 | 9 | ||
| Triple Negative | 25 | 31 | 9 | 33 | 16 | 30 | ||
| Luminal A/B (Unknown Grade) | 2 | 2 | 1 | 4 | 1 | 2 | ||
|
| ||||||||
| Change in Receptor Subtype | Yes | 27 | 33 | 8 | 30 | 19 | 35 | 0.44 |
| No | 54 | 67 | 19 | 70 | 35 | 65 | ||
|
| ||||||||
| ER Discordance | Same | 69 | 85 | 25 | 93 | 44 | 81 | 0.33 |
| (vs. Primary Tumor) | Changed: − to + | 5 | 6 | 1 | 4 | 4 | 7 | |
| Changed: + to − | 2 | 2 | 1 | 4 | 1 | 2 | ||
| Unspecified | 5 | 6 | 0 | 0 | 5 | 9 | ||
|
| ||||||||
| PR Discordance | Same | 61 | 75 | 21 | 78 | 40 | 74 | 0.39 |
| (vs. Primary Tumor) | Changed: − to + | 6 | 7 | 2 | 7 | 4 | 7 | |
| Changed: + to − | 9 | 11 | 4 | 15 | 5 | 9 | ||
| Unspecified | 5 | 6 | 0 | 0 | 5 | 9 | ||
|
| ||||||||
| HER2 Discordance | Same | 65 | 80 | 24 | 89 | 41 | 76 | 0.32 |
| (vs. Primary Tumor) | Changed: − to + | 4 | 5 | 1 | 4 | 3 | 6 | |
| Changed: + to − | 6 | 7 | 2 | 7 | 4 | 7 | ||
| Unspecified | 6 | 7 | 0 | 0 | 6 | 11 | ||
TR: true recurrence; NP: new primary; SLNB: sentinel lymph node biopsy; IMN: internal mammary node; LVI: lymphovascular invasion
In comparing primary tumor characteristics, TR and NP exhibited similar grade, multifocality, histology, and subtype. However, TR tended to be larger (median size 1.7 vs. 1.15 cm, p = 0.01) and more frequently had LVI (41% vs. 17%, p = 0.03). Four of 27 TR patients had positive margins in primary surgery, 3 of which were invasive tumor and 1 of which was DCIS. NP were more likely have margins positive with DCIS, with all 5 of 54 patients with positive margins being positive with DCIS rather than invasive tumor (p = 0.03).
Outcomes after IBTR
Twenty-eight of 81 patients had a DFS event including 14 second LRR, 8 distant metastases, 4 deaths, and 2 primary non-breast or contralateral breast cancers. The 5-year DFS was 43.1% (95% CI 23.0 – 62.1) for patients with TR versus 80.3% (95% CI 66.4 – 88.9) for those with NP (p = 0.0001; Figure 2). Similarly, OS, DMFS, and second LRR-free survival differed between the two sets of patients (Figure 2). Five-year OS was 59.7% (95% CI 36.4 – 76.9) for patients with TR and 91.7% (95% CI 79.2 – 96.8) for patients with NP. Five-year 2nd LRR-FS for TR and NP patients was 40.1% (95% CI 19.6 – 60.6) and 87.9% (95% CI 75.0 – 94.4), respectively. Five-year DMFS for TR and NP patients was 57.9% (95% CI 35.2 – 75.2), and 84.3% (95% CI 70.9 – 91.9), respectively.
Figure 2.
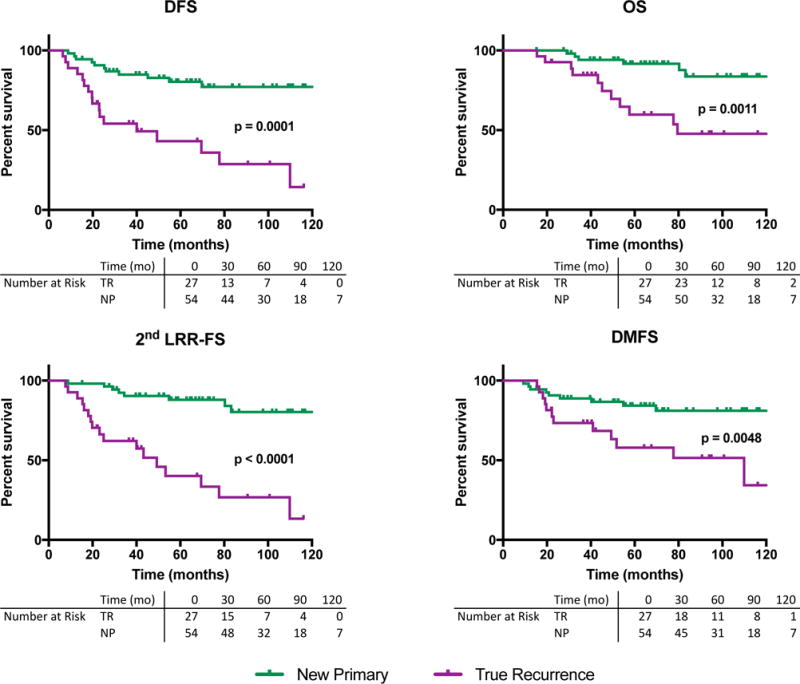
Kaplan-Meier Survival estimates for new primary (NP) and true recurrence (TR) when defined by presence or absence, respectively, of an in situ component. A. Disease Free Survival (DFS). B. Overall Survival (OS). C. Second-Locoregional Recurrence Free Survival (2nd LRR-FS). D. Distant Metastasis Free Survival (DMFS).
Univariate Analysis
The IBTR subtype (TR vs. NP) was strongly associated with worsened DFS on univariate Cox regression analysis (HR 4.751, 95% CI 2.203 – 10.246, p < 0.001), as seen in Table 3. Tumor size, grade, margin status, and LVI were also significantly associated with DFS, with decreased DFS found among larger tumors (HR 2.047 per cm, 95% CI 1.491 – 2.811, p < 0.001), high grade (HR 4.153, 95% CI 1.242 – 13.887, p = 0.021), positive margins (HR 3.338, 95% CI 1.152 – 9.674, p = 0.026), and LVI (HR 2.259, 95% CI 1.017 – 5.018, p = 0.045). Receipt of chemotherapy (HR 3.218, 95% CI 1.365 – 7.585, p = 0.008) and radiotherapy (HR 3.042, 95% CI 1.047 – 8.836, p = 0.041) were also associated with decreased DFS. Intrinsic subtype was also significant, primarily driven by worsened DFS in luminal B (HR 7.55, 95% CI 1.70 – 33.533, p = 0.008) and triple negative (HR 5.653, 95% CI 1.233– 25.913, p = 0.026) subtypes compared to luminal A.
Table 3.
Cox Regression Analysis of DFS after IBTR
| Variable | Reference | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p value | Hazard Ratio | 95% CI | p value | ||
| True Recurrence | New Primary | 4.751 | 2.203 – 10.246 | <0.001 | 3.717 | 1.607 - 8.595 | 0.002 |
| Age at IBTR | (continuous) | 1.023 | 0.996 – 1.051 | 0.098 | |||
| Left-Sided | Right-Sided | 1.147 | 0.779 – 1.689 | 0.488 | |||
| Time to Recurrence | (continuous) | 0.997 | 0.985 – 1.008 | 0.547 | |||
| Tumor Size | (continuous) | 2.047 | 1.491 – 2.811 | <0.001 | 2.083 | 1.303 - 3.330 | 0.002 |
| Multifocality | Unifocal | 1.486 | 0.685 – 3.224 | 0.316 | |||
| Grade III | Grade I/II | 4.153 | 1.242 – 13.887 | 0.021 | |||
| Positive Margins | Negative Margins | 3.338 | 1.152 – 9.674 | 0.026 | 0.419 | 0.095 - 1.849 | 0.251 |
| LVI | no LVI | 2.259 | 1.017 – 5.018 | 0.045 | |||
| LN positive | Breast Only | 3.446 | 1.387 – 8.561 | 0.008 | |||
| Receptor Subtype | 0.053 | ||||||
| Luminal B | Luminal A | 7.55 | 1.700 – 33.533 | 0.008 | |||
| Luminal HER | Luminal A | 1.602 | 0.145 – 17.708 | 0.700 | |||
| HER | Luminal A | 2.42 | 0.203 – 24.751 | 0.510 | |||
| Triple Negative | Luminal A | 5.653 | 1.233 – 25.913 | 0.026 | |||
| Histology | 0.585 | ||||||
| Lobular | Ductal | 0.343 | 0.046 – 2.530 | 0.343 | |||
| Other | Ductal | 0.61 | 0.082 – 4.508 | 0.610 | |||
| BCS | Mastectomy | 2.465 | 0.925 – 6.529 | 0.071 | |||
| Axillary Surgery | 0.003 | ||||||
| Dissection | SLNB | 3.45 | 1.157 – 10.285 | 0.026 | |||
| None | SLNB | 5.187 | 2.004 – 13.425 | 0.001 | |||
| Chemotherapy | No Chemotherapy | 3.218 | 1.365 – 7.585 | 0.008 | |||
| Endocrine Therapy | No Endocrine Therapy | 0.934 | 0.444 – 1.967 | 0.858 | |||
| Radiation Therapy | No Radiation Therapy | 3.042 | 1.047 – 8.836 | 0.041 | |||
IBTR: in-breast tumor recurrence; LVI: lymphovascular invasion; LN: lymph node; BCS: breast conserving surgery; SLNB: sentinel lymph node biopsy
Multivariate Analysis
In order to adjust for potential confounders, multivariate analysis was performed. Tumor size and margin status were the only two variables which significantly differed between TR and NP and had significant associations with DFS on univariate analysis. When these two variables were included with IBTR subtype (TR versus NP) in multivariate analysis, IBTR subtype remained independently associated with DFS (HR 3.717, 95% CI 1.607 – 8.595, p = 0.002). Tumor size also remained independently associated with DFS (HR 2.083 per cm, 95% CI 1.303 – 3.330, p = 0.002). Margin status was not significantly associated on multivariate analysis despite its association on univariate analysis.
Discussion
These results suggest that in-breast tumor recurrences after breast conserving therapy have an improved DFS when an in situ component is associated with the invasive recurrence. When defined by the presence of adjacent in situ carcinoma, NP tumors in our cohort did not exhibit a longer time to “recurrence”, but were detected at smaller sizes. This may reflect a difference in the natural history of TR and NP, with NP tumors progressing from de novo or non-excised in situ carcinoma rather than from residual primary invasive cancer.
Most prior studies have differentiated NP from TR with location as the main distinguishing criterion13–16,18–22,33–35. Under this classification schema, NPs have been shown to arise sooner after primary surgery and generally have better prognosis than TR in the tumor bed13,15,16,19. However, there continues to be a need for further exploration of how best to identify and treat true TR compared to NP. For instance, among studies that consider location of IBTR, it is unclear how far a tumor must be from the tumor bed to be considered a NP14,15,19. The likelihood of a NP developing near the tumor bed is also significant, especially in considering that most breast cancers arise in the upper outer quadrant36. Furthermore, when not combined with other classification criteria such as change in histologic type, location alone may not portend a statistically significant difference in prognosis14,37.
In our study, we explored an alternative method to distinguish NP versus TR – by the presence of an adjacent in situ component. Using this criterion, new NPs had significantly better outcomes than TR, with 5-year DFS of 43% and 80% for TR and NP, respectively. This difference in DFS outcome is similar to or greater than values reported in other studies, with 10-year distant disease free survival values reported between 26–56% for TR and 77–94% for NP. The difference in overall survival seen in our study is also similar to those reported in other classification schemes; 5-year OS was 60% and 92% for TR and NP, respectively, while prior studies have reported 10-year OS ranging from 46–76% for TR and 64–92% for NP13,15,16,19,21
When classifying NP by presence of an in situ component in our cohort, NP tended to be smaller than TR, which may support the hypothesis that these tumors progress from in situ carcinoma rather than more aggressive invasive carcinoma. They also tended to have less aggressive primary tumor characteristics and positive margins of DCIS rather than invasive tumor, further validating this hypothesis. However, there was no difference between TR and NP in time from primary tumor surgery to recurrence, contrary to our expectations. This may have contributed to TR having larger tumor size, while NP had a more indolent presentation. A longer time to recurrence was seen in two prior studies in a Japanese population that similarly defined NP by the presence of an intraductal component. Nishimura et al. categorized IBTR as NP if it included an intraductal component or if surgical margins were positive during treatment of the primary tumor. Compared to TR, NP had a longer mean time to recurrence (37 vs. 55 months, p = 0.031) and more favorable 5-year distant disease-free survival (93% vs. 61%, p = 0.0028), with a trend towards having a more favorable 5-year survival than TR (91% vs. 76%, p = 0.0627)21. In addition to shorter time to recurrence, we also expected true recurrences to more commonly have the same histology as the primary tumor. All six tumors that changed histologic type were classified as new primaries in our study.
These results must be interpreted in the context of the study design. A small number of patients underwent repeat BCT, an approach that was more frequently performed in TR than NP and has since been reported as a feasible alternative to salvage mastectomy, though with limited long-term data38,39. We further found that receipt of chemotherapy and radiation therapy was associated with a decline in DFS, likely due to confounding by indication. Differences in treatment, among other tumor characteristics, may impact the association of NP with favorable outcome, though multivariate analysis was used to control for this possibility. We included tumor size and margin status in this analysis due to their significant associations with DFS and significant differences between TR and NP, but other variables may also play a role in determining outcome. Finally, our study is limited by a lack of molecular assays which may best determine which “recurrences” truly share molecular identity with their primary lesions40,41.
Thus, when defined by the presence of an in situ component, NP have favorable outcomes when compared to TR. Consideration of an in situ component at the time of IBTR may add prognostic value to the assessment of subsequent risk when used in combination with location, histologic type, and receptor status. This characteristic may be particularly informative among patients whose primary tumor characteristics are unknown. Further validation is needed to validate these findings and better inform local and systemic management of IBTR.
Synopsis.
When defining in-breast tumor recurrence as a new primary or true recurrence based on the presence or absence of an in situ component, new primary tumors are independently associated with improved disease-free and overall survival.
Footnotes
Presented in part at the 2015 American Society for Radiation Oncology (ASTRO) Annual Meeting: San Antonio, Texas, October 21st, 2015.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Early Breast Cancer Trialists’ Collaborative G. Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–16. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24:2028–37. doi: 10.1200/JCO.2005.04.3273. [DOI] [PubMed] [Google Scholar]
- 3.Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27:2466–73. doi: 10.1200/JCO.2008.19.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clemons M, Danson S, Hamilton T, et al. Locoregionally recurrent breast cancer: incidence, risk factors and survival. Cancer Treat Rev. 2001;27:67–82. doi: 10.1053/ctrv.2000.0204. [DOI] [PubMed] [Google Scholar]
- 5.Haffty BG, Reiss M, Beinfield M, et al. Ipsilateral breast tumor recurrence as a predictor of distant disease: implications for systemic therapy at the time of local relapse. J Clin Oncol. 1996;14:52–7. doi: 10.1200/JCO.1996.14.1.52. [DOI] [PubMed] [Google Scholar]
- 6.van Laar C, van der Sangen MJ, Poortmans PM, et al. Local recurrence following breast-conserving treatment in women aged 40 years or younger: trends in risk and the impact on prognosis in a population-based cohort of 1143 patients. Eur J Cancer. 2013;49:3093–101. doi: 10.1016/j.ejca.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 7.Braunstein LZ, Niemierko A, Shenouda MN, et al. Outcome Following Local-Regional Recurrence in Women with Early-Stage Breast Cancer: Impact of Biologic Subtype. Breast J. 2015 doi: 10.1111/tbj.12371. [DOI] [PubMed] [Google Scholar]
- 8.Arvold ND, Taghian AG, Niemierko A, et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. 2011;29:3885–91. doi: 10.1200/JCO.2011.36.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Truong PT, Sadek BT, Lesperance MF, et al. Is biological subtype prognostic of locoregional recurrence risk in women with pT1–2N0 breast cancer treated with mastectomy? Int J Radiat Oncol Biol Phys. 2014;88:57–64. doi: 10.1016/j.ijrobp.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Voogd AC, van Oost FJ, Rutgers EJ, et al. Long-term prognosis of patients with local recurrence after conservative surgery and radiotherapy for early breast cancer. Eur J Cancer. 2005;41:2637–44. doi: 10.1016/j.ejca.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 11.Fredriksson I, Liljegren G, Arnesson LG, et al. Local recurrence in the breast after conservative surgery-a study of prognosis and prognostic factors in 391 women. Eur J Cancer. 2002;38:1860–70. doi: 10.1016/s0959-8049(02)00219-8. [DOI] [PubMed] [Google Scholar]
- 12.Alpert TE, Kuerer HM, Arthur DW, et al. Ipsilateral breast tumor recurrence after breast conservation therapy: outcomes of salvage mastectomy vs. salvage breast-conserving surgery and prognostic factors for salvage breast preservation. Int J Radiat Oncol Biol Phys. 2005;63:845–51. doi: 10.1016/j.ijrobp.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 13.Smith TE, Lee D, Turner BC, et al. True recurrence vs. new primary ipsilateral breast tumor relapse: an analysis of clinical and pathologic differences and their implications in natural history, prognoses, and therapeutic management. Int J Radiat Oncol Biol Phys. 2000;48:1281–9. doi: 10.1016/s0360-3016(00)01378-x. [DOI] [PubMed] [Google Scholar]
- 14.Krauss DJ, Kestin LL, Mitchell C, et al. Changes in temporal patterns of local failure after breast-conserving therapy and their prognostic implications. Int J Radiat Oncol Biol Phys. 2004;60:731–40. doi: 10.1016/j.ijrobp.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Komoike Y, Akiyama F, Iino Y, et al. Analysis of ipsilateral breast tumor recurrences after breast-conserving treatment based on the classification of true recurrences and new primary tumors. Breast Cancer. 2005;12:104–11. doi: 10.2325/jbcs.12.104. [DOI] [PubMed] [Google Scholar]
- 16.Fodor J, Major T, Polgar C, et al. Prognosis of patients with local recurrence after mastectomy or conservative surgery for early-stage invasive breast cancer. Breast. 2008;17:302–8. doi: 10.1016/j.breast.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Abd-Alla HM, Lotayef MM, Abou Bakr A, et al. Ipsilateral in-breast tumor relapse after breast conservation therapy: true recurrence versus new primary tumor. J Egypt Natl Canc Inst. 2006;18:183–90. [PubMed] [Google Scholar]
- 18.Yi M, Buchholz TA, Meric-Bernstam F, et al. Classification of ipsilateral breast tumor recurrences after breast conservation therapy can predict patient prognosis and facilitate treatment planning. Ann Surg. 2011;253:572–9. doi: 10.1097/SLA.0b013e318208fc2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang E, Buchholz TA, Meric F, et al. Classifying local disease recurrences after breast conservation therapy based on location and histology: new primary tumors have more favorable outcomes than true local disease recurrences. Cancer. 2002;95:2059–67. doi: 10.1002/cncr.10952. [DOI] [PubMed] [Google Scholar]
- 20.Panet-Raymond V, Truong PT, McDonald RE, et al. True recurrence versus new primary: an analysis of ipsilateral breast tumor recurrences after breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2011;81:409–17. doi: 10.1016/j.ijrobp.2010.05.063. [DOI] [PubMed] [Google Scholar]
- 21.Nishimura S, Takahashi K, Akiyama F, et al. Classification of ipsilateral breast tumor recurrence after breast-conserving therapy: new primary cancer allows a good prognosis. Breast Cancer. 2005;12:112–7. doi: 10.2325/jbcs.12.112. [DOI] [PubMed] [Google Scholar]
- 22.Sakai T, Nishimura S, Ogiya A, et al. Four types of ipsilateral breast tumor recurrence (IBTR) after breast-conserving surgery: classification of IBTR based on precise pathological examination. Pathol Int. 2015;65:113–8. doi: 10.1111/pin.12253. [DOI] [PubMed] [Google Scholar]
- 23.Silverberg SG, Chitale AR. Assessment of significance of proportions of intraductal and infiltrating tumor growth in ductal carcinoma of the breast. Cancer. 1973;32:830–7. doi: 10.1002/1097-0142(197310)32:4<830::aid-cncr2820320413>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 24.Fisher ER, Gregorio RM, Fisher B, et al. The pathology of invasive breast cancer. A syllabus derived from findings of the National Surgical Adjuvant Breast Project (protocol no 4) Cancer. 1975;36:1–85. doi: 10.1002/1097-0142(197507)36:1<1::aid-cncr2820360102>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Allred DC, Wu Y, Mao S, et al. Ductal carcinoma in situ and the emergence of diversity during breast cancer evolution. Clin Cancer Res. 2008;14:370–8. doi: 10.1158/1078-0432.CCR-07-1127. [DOI] [PubMed] [Google Scholar]
- 26.Abdel-Fatah TM, Powe DG, Hodi Z, et al. High frequency of coexistence of columnar cell lesions, lobular neoplasia, and low grade ductal carcinoma in situ with invasive tubular carcinoma and invasive lobular carcinoma. Am J Surg Pathol. 2007;31:417–26. doi: 10.1097/01.pas.0000213368.41251.b9. [DOI] [PubMed] [Google Scholar]
- 27.Gupta SK, Douglas-Jones AG, Fenn N, et al. The clinical behavior of breast carcinoma is probably determined at the preinvasive stage (ductal carcinoma in situ) Cancer. 1997;80:1740–5. [PubMed] [Google Scholar]
- 28.Buerger H, Otterbach F, Simon R, et al. Different genetic pathways in the evolution of invasive breast cancer are associated with distinct morphological subtypes. J Pathol. 1999;189:521–6. doi: 10.1002/(SICI)1096-9896(199912)189:4<521::AID-PATH472>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 29.Burkhardt L, Grob TJ, Hermann I, et al. Gene amplification in ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2010;123:757–65. doi: 10.1007/s10549-009-0675-8. [DOI] [PubMed] [Google Scholar]
- 30.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 32.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25:2127–32. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida T, Takei H, Kurosumi M, et al. True recurrences and new primary tumors have different clinical features in invasive breast cancer patients with ipsilateral breast tumor relapse after breast-conserving treatment. Breast J. 2010;16:127–33. doi: 10.1111/j.1524-4741.2009.00884.x. [DOI] [PubMed] [Google Scholar]
- 34.Alexandrova E, Sergieva S, Kostova P, et al. Ipsilateral in-breast tumor recurrence after breast conserving therapy: true recurrence versus new primary tumor. J BUON. 2015;20:1001–8. [PubMed] [Google Scholar]
- 35.Sarsenov D, Ilgun S, Ordu C, et al. True Local Recurrences after Breast Conserving Surgery have Poor Prognosis in Patients with Early Breast Cancer. Cureus. 2016;8:e541. doi: 10.7759/cureus.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bright CJ, Rea DW, Francis A, et al. Comparison of quadrant-specific breast cancer incidence trends in the United States and England between 1975 and 2013. Cancer Epidemiol. 2016;44:186–194. doi: 10.1016/j.canep.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Abner AL, Recht A, Eberlein T, et al. Prognosis following salvage mastectomy for recurrence in the breast after conservative surgery and radiation therapy for early-stage breast cancer. J Clin Oncol. 1993;11:44–8. doi: 10.1200/JCO.1993.11.1.44. [DOI] [PubMed] [Google Scholar]
- 38.Gentilini O, Botteri E, Veronesi P, et al. Repeating conservative surgery after ipsilateral breast tumor reappearance: criteria for selecting the best candidates. Ann Surg Oncol. 2012;19:3771–6. doi: 10.1245/s10434-012-2404-5. [DOI] [PubMed] [Google Scholar]
- 39.Arthur DW, Winter KA, Kuerer HM, et al. NRG Oncology-Radiation Therapy Oncology Group Study 1014: 1-Year Toxicity Report From a Phase 2 Study of Repeat Breast-Preserving Surgery and 3-Dimensional Conformal Partial-Breast Reirradiation for In-Breast Recurrence. Int J Radiat Oncol Biol Phys. 2017;98:1028–1035. doi: 10.1016/j.ijrobp.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vicini FA, Antonucci JV, Goldstein N, et al. The use of molecular assays to establish definitively the clonality of ipsilateral breast tumor recurrences and patterns of in-breast failure in patients with early-stage breast cancer treated with breast-conserving therapy. Cancer. 2007;109:1264–72. doi: 10.1002/cncr.22529. [DOI] [PubMed] [Google Scholar]
- 41.Bollet MA, Servant N, Neuvial P, et al. High-resolution mapping of DNA breakpoints to define true recurrences among ipsilateral breast cancers. J Natl Cancer Inst. 2008;100:48–58. doi: 10.1093/jnci/djm266. [DOI] [PubMed] [Google Scholar]


