Abstract
Background and Objectives
Soft tissue sarcomas of the head and neck (STSHN) comprise a rare group of malignancies. Our objective is to determine the utility of soft tissue sarcoma staging systems within the head and neck, and to validate an individualized soft tissue sarcoma nomogram within head and neck primary sites.
Methods
Previously-untreated patients with STSHN diagnosed and treated between 1982 and 2012 were eligible (n=319, median follow-up 46 months). Clinical variables were extracted from a prospectively-maintained database. The performance of AJCC/UICC and MSK staging systems and a soft tissue sarcoma-specific nomogram were assessed.
Results
Four-year overall survival (OS), disease specific survival (DSS), and recurrence-free survival (RFS) were 72%, 76%, and 71%, respectively. AJCC/UICC and MSK staging systems accurately stratified outcomes (OS, DSS, and RFS; p<0.001 for all comparisons). The nomogram stratified outcomes by quartile (p<0.001), and predicted risk of death at 4, 8 and 12 years (p<0.001). Concordance indices for overall survival for the AJCC/UICC system, MSK system, and the nomogram were 0.71, 0.70, and 0.78, respectively.
Conclusions
Oncologic outcomes among groups of patients with STSHN can be accurately predicted using both the AJCC/UICC and MSK staging systems. A soft tissue sarcoma-specific nomogram provides reliable, individualized prognostic information for patients with STSHN.
Keywords: Soft tissue sarcoma, head and neck cancer, staging, nomograms
Introduction
Soft tissue sarcomas of the head and neck comprise a rare and heterogeneous group of malignancies1. As a result, prospective treatment protocols have not been developed, management is not standardized, and clinical outcomes research frequently relies upon analyses of diverse patient cohorts2. There are no staging schemas or nomograms specifically designed for soft tissue sarcomas of the head and neck. Established staging systems that are not site-specific are frequently employed, and a nomogram developed from all tumor sites has been described3. However relevance and utility of these tools in the head and neck, where anatomic and oncologic considerations are unique, remain unknown.
Despite this lack of data, establishing a definitive diagnosis, accurate staging, and individualized risk stratification are critical prior to contemplation of treatment options for soft tissue sarcomas. This entails a comprehensive history and physical examination, rigorous histologic review by a pathologist familiar with sarcoma, and targeted imaging studies to assess the extent of local disease and to exclude regional or distant metastases. This tumor staging workup serves to confirm the correct diagnosis, assess surgical candidacy, inform operative and reconstructive planning, and risk-stratify clinical outcomes.
There are three established staging systems for soft tissue sarcoma. The Enneking classification only applies to musculoskeletal sarcoma4. The AJCC/UICC staging system is widely cited and used, and relies upon tumor size, histology, and the presence of regional/distant metastatic disease5. The MSK staging employs similar criteria as the AJCC/UICC staging system, however is slightly more user-friendly in that it uses a collection of “unfavorable characteristics” and thus stresses the importance of each clinical variable slightly differently6.
The latter two staging systems are not site-specific, and thus may not be applicable to head and neck primary sites. Of particular concern is the importance of tumor depth, defined based upon superficial fascial invasion. This designation may be less relevant in the head and neck, where many tumors (even when small and “less-aggressive”) will frequently invade the superficial investing fascia7. In addition, critical anatomic considerations specific to the head and neck, such as resectability of tumors involving the skull base and great vessels, are not included within these staging systems.
A nomogram is a prognostic model that serves to provide individualized, patient-specific predictions of outcomes of interest. The data generated from a nomogram can be invaluable in patient counseling, consideration of adjuvant treatment, post-treatment surveillance, and/or clinical trial candidacy8. A large institutional cohort of patients with soft tissue sarcomas from all body sites was used to create a soft-tissue sarcoma nomogram designed to predict the probability of sarcoma-specific death at 4, 8 and 12 years3. This nomogram was independently validated in a different institution, also utilizing a diverse patient population9. To date, the nomogram has not been evaluated among head and neck-specific sites of disease. Because the nomogram variables overlap considerably with the AJCC/UICC and MSK staging systems, there is the potential that the nomogram may be less predictive within head and neck sites than elsewhere.
We hypothesized that existing soft tissue sarcoma staging systems and a soft tissue sarcoma-specific individualized nomogram would be suboptimal in predicting outcomes within head and neck sites of disease. Our objectives were to (1) determine the utility of current soft tissue sarcoma staging systems within the head and neck, and to (2) validate an individualized soft tissue sarcoma risk stratification schema within head and neck primary sites.
Materials and Methods
Patients and Data Collection
All previously-untreated patients diagnosed with soft tissue sarcoma of the head and neck that were older than 16 years, treated with primary surgical extirpation with curative intent at Memorial Sloan-Kettering Cancer Center between 1982 and 2012, and without distant metastatic disease at presentation, were eligible for inclusion. For the past three decades, our institutional practice has favored primary surgical excision of resectable soft tissue sarcomas, reserving chemotherapy and radiotherapy for selected adjuvant indications or recurrent/unresectable disease, often as part of prospective clinical trials. The Memorial Sloan-Kettering Cancer Center Institutional Review Board reviewed and approved this study.
Demographic, clinical, surgical and oncologic variables were extracted from a prospectively-maintained institutional oncologic database. These data have been longitudinally updated, and were independently verified in the medical record prior to data analysis. Over the years, descriptive manuscripts have continually updated these data10,11. The social security death index was used to confirm overall mortality. Determination of nodal status was based upon pathological data when available, and clinical or radiologic findings when not available. The MSK and AJCC/UICC 7th edition staging systems were utilized (Table 1). Histological classifications (size, depth of invasion, and grade) were defined based upon the criteria established in the AJCC/UICC soft tissue sarcoma staging system. Depth of invasion refers to whether the tumor is located exclusively above the superficial fascia without invasion of the fascia (superficial), or whether it is located either within or below the superficial fascia (deep).
Table 1.
Staging System Schemas
| AJCC / UICC | |
|---|---|
|
| |
| T1 | < 5 cm in greatest dimension |
| T2 | ≥ 5 cm in greatest dimension (a: superficial; b: deep) |
|
| |
| N0 vs. N1 | (Any nodal disease) |
|
| |
| M0 vs. M1 | (Any distant metastases) |
|
| |
| G1 | Well-differentiated |
| G2 | Moderately differentiated |
| G3 | Poorly differentiated |
| G4 | Undifferentiated |
|
| |
| I | G1-2, T1a-b or T2a, N0, M0 |
| II | G1-2, T2b, N0, M0 or G3-4, T1a-b / T2a, M0 |
| III | G3-4, T2b, N0, M0 or N1 (any G, any T) |
| IV | Any M1 |
|
| |
| MSK | |
|
| |
| Unfavorable signs | ≥ 5 cm in greatest dimension Deep invasion High grade |
|
| |
| Stage 0 | 0 Unfavorable signs |
| Stage I | 1 Unfavorable sign |
| Stage II | 2 Unfavorable signs |
| Stage III | 3 Unfavorable signs |
| Stage IV | Any metastases (nodal or distant) |
Data Analysis
Data were collected and stored in Microsoft Excel (version 12, Microsoft, Seattle, WA). Statistical analyses were performed using SPSS version 21 and R version 3.0.2. Overall survival was defined from time of diagnosis to time of death from any cause, and described with the Kaplan-Meier method. Disease-specific survival was defined from time of diagnosis to time of death in patients with confirmed disease; patients who died of other causes were censored appropriately. Recurrence-free survival was defined from time of diagnosis to either their most recently documented clinical follow-up or their first confirmed recurrence (local, regional or distant).
The performance of the AJCC/UICC and MSKCC staging systems were used to estimate prognosis using the Kaplan-Meier method. For each of the oncologic outcomes, patients were grouped by quartiles. Kaplan-Meier curves were plotted by the quartiles for 4 year, 8 year, and 12 year prediction of disease-specific death. Hazard ratios were calculated for each of the estimated probabilities as a continuous predictor in a univariate Cox regression model. Concordance indices were then calculated for the staging systems based upon observed versus expected outcomes.
The performance of a soft tissue sarcoma-specific nomogram was assessed, both stratified by quartile and as a continuous variable12. This nomogram was developed at our institution in 2001 and included patients with tumors of all sites (including the head and neck)3. Because patients within the current dataset who were treated before 2001 were used in the nomogram development, they were excluded from the analysis. Data analyses were performed using the subset of patients treated from 2001 to 2012 (n=187). A concordance index was calculated for the nomogram performance with bootstrapping to mitigate bias. Because of the timeline of inclusion (which afforded a median follow-up time of 28 months), the primary endpoint was 4 year disease-specific death.
Results
Descriptive Data
A total of 319 eligible patients were identified (median follow-up 46 months). The mean age was 50 years; 59% were male. Primary sites were varied; the neck was most common (43%). Histologies were similarly heterogeneous (Table 3). Approximately half (49%) demonstrated low grade histology, and 30% exhibited superficial invasion (above deep tissue fascia, based upon AJCC staging definition). The majority (64%) had R0 resections, 29% had R1 resections, and 7% had R2 resections. Oncologic details are presented in Table 2.
Table 3.
Histologic Characteristics (n=319)
| HISTOLOGY | N | % |
|---|---|---|
| Malignant Fibrous Histiocytoma (MFH) | 44 | 14 |
| Dermatofibrosarcoma Protuberans (DFSP) | 32 | 10 |
| Angiosarcoma | 30 | 9 |
| Solitary Fibrous Tumor/Hemangiopericytoma | 25 | 8 |
| Desmoid/Fibromatosis (Deep Seated) | 23 | 7 |
| Leiomyosarcoma | 23 | 7 |
| Liposarcoma | 23 | 7 |
| Synovial | 21 | 7 |
| Fibrosarcoma | 18 | 6 |
| Malignant Peripheral Nerve Sheath Tumor | 17 | 5 |
| Sarcoma | 13 | 4 |
| Rhabdomyosarcoma | 12 | 4 |
| Myofibroblastic | 11 | 3 |
| Undifferentiated | 6 | 2 |
| Epithelioid | 4 | 1 |
| Dendritic Cell Tumor | 3 | 1 |
| Ewing’s/Primitive Neuroectodermal Tumor (PNET) | 3 | 1 |
| Hemangioendothelioma | 3 | 1 |
| Angiomatoid Fibrous Histiocytoma | 2 | <1 |
| Chondrosarcoma | 2 | <1 |
| Alveolar-Soft Part | 1 | <1 |
| Carcinosarcoma Ex-Pleomorphic Adenoma | 1 | <1 |
| Inflammatory Myofibroblastic Tumor | 1 | <1 |
| Plexiform Fibrohistiocytic | 1 | <1 |
| GRADE | ||
| Low | 156 | 49 |
| High | 163 | 51 |
| DEPTH | ||
| Superficial | 96 | 30 |
| Deep | 223 | 70 |
| COMPLETENESS OF RESECTION (R) | ||
| R0 | 204 | 64 |
| R1 | 94 | 29 |
| R2 | 21 | 7 |
Table 2.
Oncologic Characteristics (n=319)
| N | % | |
|---|---|---|
| TUMOR SIZE | ||
| < 5 cm | 220 | 69 |
| 5 – 10 cm | 85 | 27 |
| > 10 cm | 13 | 4 |
| Unknown | 1 | <1 |
| N CLASSIFICATION | ||
| cN0/pNx | 217 | 68 |
| cN0/pN0 | 93 | 29 |
| cN0/pN1 | 1 | <1 |
| cN1/pN1 | 8 | 3 |
| AJCC/UICC STAGE | ||
| Stage I | 113 | 35 |
| Stage II | 151 | 47 |
| Stage III | 55 | 17 |
| Stage IV | 0 | 0 |
| MSK STAGE | ||
| Stage 0 | 53 | 17 |
| Stage I | 95 | 30 |
| Stage II | 116 | 36 |
| Stage III | 46 | 14 |
| Stage IV | 9 | 3 |
Outcomes by Stage
Four-year overall survival (OS), disease specific survival (DSS), and recurrence-free survival (RFS) were 72%, 76%, and 71%, respectively. The AJCC/UICC and MSK staging systems accurately stratified outcomes by overall disease stage (OS, DSS, and RFS; p<0.001 for all comparisons; Figures 1 and 2). The c-index for AJCC staging was 0.71 (se=0.02). The c-index for the MSK staging was 0.70 (se=0.03).
Figure 1. Outcomes by AJCC/UICC Staging.
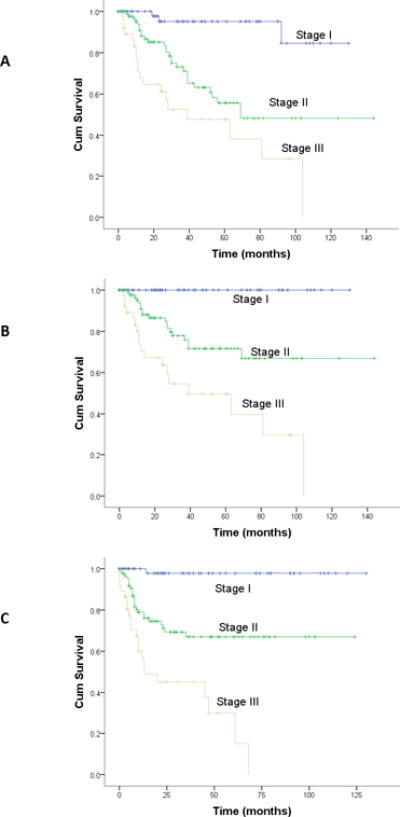
A: Overall survival; B: Disease-specific survival; C: Recurrence-free survival
p<.001 for all comparisons
Figure 2. Outcomes by MSK Staging.
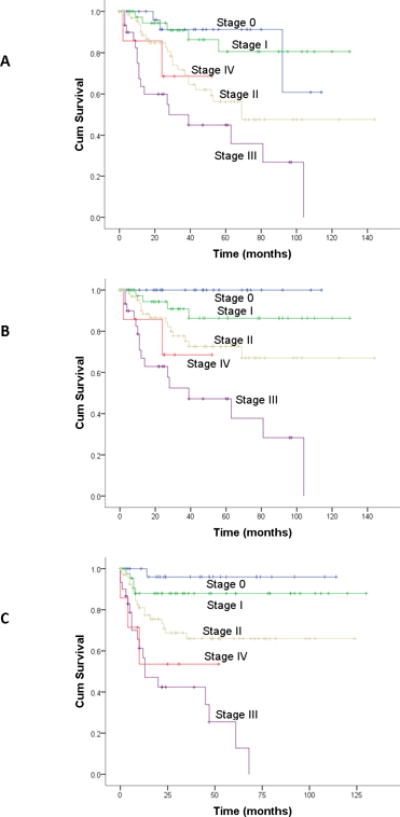
A: Overall survival; B: Disease-specific survival; C: Recurrence-free survival
p<.001 for all comparisons
Nomogram Performance
The nomogram similarly stratified outcomes by quartile (OS, DSS, and RFS; p<0.001 for all comparisons; Figure 3). In a univariate Cox regression model testing the nomogram as a continuous variable, the nomogram performed well in predicting risk of death at 4, 8 and 12 years (p<0.001). Hazard ratios for the 4 and 8 year probability of disease-specific deaths were 1.044 (95% CI 1.028-1.06; p< 0.001) and 1.043 (95% CI 1.027-1.059; p< 0.001), respectively. The concordance index for the nomogram was 0.78.
Figure 3. Nomogram Outcomes.
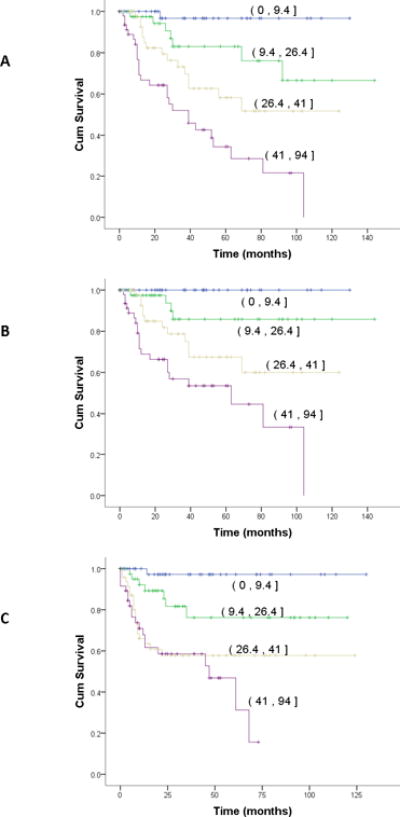
Stratified by quartile (95% CI)
A: Overall survival; B: Disease-specific survival; C: Recurrence-free survival
p<.001 for all comparisons
Discussion
Our data indicate that both the AJCC/UICC and MSK staging systems for soft tissue sarcoma are good predictors of clinically relevant outcomes within head and neck sites of disease, refuting our initial hypothesis. It is remarkable that staging systems that lack anatomic precision remain quite predictive, particularly given the strong reliance upon these very details within staging schemas for primary head and neck carcinomas. Our data demonstrate a similar concordance index for the MSK system in comparison with the AJCC/UICC system. As would be expected given the similar and overlapping criteria within these systems, they were quite comparable. In a comparison of staging systems for localized extremity soft tissue sarcoma, the MSK staging system was a slightly better predictor of systemic relapse13.
Given the excellent performance of these staging systems, what is the role or utility of a nomogram? Staging systems ably classify outcomes for groups of patients, which is critical when interpreting and reporting clinical trials and identifying other outcomes of interest among patient cohorts. However, a nomogram can provide individualized patient information in a manner that is impossible with a staging system alone (Figure 4). Thus, the concordance index of the soft tissue sarcoma nomogram confirms that this tool is a powerful way to risk-stratify a select patient who has undergone surgery, thereby providing an opportunity to tailor counseling, adjuvant treatment, clinical trial candidacy, and surveillance in a way that cannot be accomplished simply based upon his or her disease stage. It is notable that the concordance index (0.78) is comparable to its counterpart in the original nomogram development (0.69)3 and in its subsequent external validation (0.76)9, both involving patients with varied sites of disease.
Figure 4.
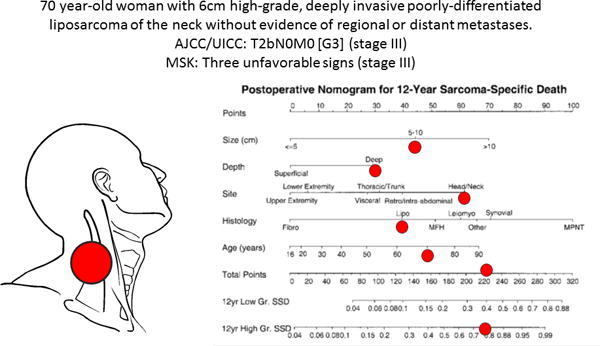
Hypothetical illustration of the advantage of a nomogram. This patient has stage III disease according to both the AJCC/UICC and MSK staging systems. However the nomogram provides patient-specific information. She has an approximately 77% chance of dying of her disease in the next 12 years.
There are notable limitations within this study. Despite relying upon a prospective database, the study is fundamentally retrospective, and suffers from its inherent limitations. Soft tissue sarcoma is a heterogeneous disease with widely variant pathology, and the somewhat arbitrary permutation within a single descriptive construct is flawed. All patients in the cohort were treated surgically with curative intent, and this selection bias may mitigate the absence of head and neck-specific anatomic details within the staging system (a patient with imaging demonstrating carotid encasement would not have undergone surgery with curative intent, for example). Similarly, the study excluded patients with distant metastatic disease, thereby eliminating our ability to assess the prognostic implications of this finding within the staging schemas. Adjuvant treatment (radiation and/or chemotherapy) were not used according to a uniform protocol, and there is a risk that such treatment may confound outcomes within our cohort. Furthermore, concordance indices demonstrated good predictive value, however they illustrate the inherent imperfections of any prognostic tool with regard to an individual patient. Finally, data derived from the nomogram requires information that can only be known postoperatively, and thus the nomogram is inappropriate for risk-stratifying newly-diagnosed patients who have not yet undergone surgery.
Conclusion
Oncologic outcomes among groups of patients with soft tissue sarcoma of the head and neck can be accurately predicted using both the AJCC/UICC and MSK staging systems. A soft tissue sarcoma-specific nomogram provides reliable, individualized prognostic information for patients with soft tissue sarcoma involving head and neck primary sites.
Synopsis.
This study was designed to determine the utility of current soft tissue sarcoma staging systems within the head and neck, and to validate an individualized soft tissue sarcoma risk stratification schema within head and neck primary sites. Oncologic outcomes among groups of patients with soft tissue sarcoma of the head and neck can be accurately predicted using both the AJCC/UICC and MSK staging systems. A soft tissue sarcoma-specific nomogram provides reliable, individualized prognostic information for patients with soft tissue sarcoma involving head and neck primary sites.
Acknowledgments
The grant is P30 CA008748
Footnotes
The authors have no financial interests, disclosures, conflicts of interest or funding sources regarding the content of this original manuscript.
This research was presented at the International Federation of Head and Neck Oncologic Societies (IFHNOS) 5th World Congress, New York, NY, July 26-30, 2014.
References
- 1.Cormier JN, Pollock RE. Soft tissue sarcomas. CA: a cancer journal for clinicians. 2004 Mar-Apr;54(2):94–109. doi: 10.3322/canjclin.54.2.94. [DOI] [PubMed] [Google Scholar]
- 2.Patel SG, Shaha AR, Shah JP. Soft tissue sarcomas of the head and neck: an update. American journal of otolaryngology. 2001 Jan-Feb;22(1):2–18. doi: 10.1053/ajot.2001.20699. [DOI] [PubMed] [Google Scholar]
- 3.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002 Feb 1;20(3):791–796. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 4.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clinical orthopaedics and related research. 1980 Nov-Dec;(153):106–120. [PubMed] [Google Scholar]
- 5.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. 7th. New York, NY: Springer; 2010. pp. 291–6. [Google Scholar]
- 6.Hajdu SI, Shiu MH, Brennan MF. The role of the pathologist in the management of soft tissue sarcomas. World journal of surgery. 1988 Jun;12(3):326–331. doi: 10.1007/BF01655665. [DOI] [PubMed] [Google Scholar]
- 7.Shellenberger TD, Sturgis EM. Sarcomas of the head and neck region. Current oncology reports. 2009 Mar;11(2):135–142. doi: 10.1007/s11912-009-0020-8. [DOI] [PubMed] [Google Scholar]
- 8.Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Annals of internal medicine. 1999 Mar 16;130(6):515–524. doi: 10.7326/0003-4819-130-6-199903160-00016. [DOI] [PubMed] [Google Scholar]
- 9.Eilber FC, Brennan MF, Eilber FR, Dry SM, Singer S, Kattan MW. Validation of the postoperative nomogram for 12-year sarcoma-specific mortality. Cancer. 2004 Nov 15;101(10):2270–2275. doi: 10.1002/cncr.20570. [DOI] [PubMed] [Google Scholar]
- 10.Kraus DH, Dubner S, Harrison LB, et al. Prognostic factors for recurrence and survival in head and neck soft tissue sarcomas. Cancer. 1994 Jul 15;74(2):697–702. doi: 10.1002/1097-0142(19940715)74:2<697::aid-cncr2820740224>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 11.Bentz BG, Singh B, Woodruff J, Brennan M, Shah JP, Kraus D. Head and neck soft tissue sarcomas: a multivariate analysis of outcomes. Annals of surgical oncology. 2004 Jun;11(6):619–628. doi: 10.1245/ASO.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 12.http://nomograms.mskcc.org/Sarcoma/
- 13.Wunder JS, Healey JH, Davis AM, Brennan MF. A comparison of staging systems for localized extremity soft tissue sarcoma. Cancer. 2000 Jun 15;88(12):2721–2730. doi: 10.1002/1097-0142(20000615)88:12<2721::aid-cncr10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]


