Abstract
Background
Chronic pancreatitis (CP) has a profound independent effect on quality of life (QOL). Our aim was to identify factors that impact the QOL in CP patients.
Methods
We used data on 1,024 CP patients enrolled in the three NAPS2 studies. Information on demographics, risk factors, co-morbidities, disease phenotype and treatments was obtained from responses to structured questionnaires. Physical (PCS) and mental (MCS) component summary scores generated using responses to the Short Form-12 (SF-12) survey were used to assess QOL at enrollment. Multivariable linear regression models determined independent predictors of QOL.
Results
Mean PCS and MCS scores were 36.7±11.7 and 42.4±12.2, respectively. Significant (p<0.05) negative impact on PCS scores in multivariable analyses was noted due to constant mild-moderate pain with episodes of severe pain or constant severe pain (10 points), constant mild-moderate pain (5.2), pain-related disability/unemployment (5.1), current smoking (2.9 points) and medical co-morbidities. Significant (p<0.05) negative impact on MCS scores was related to constant pain irrespective of severity (6.8-6.9 points), current smoking (3.9 points) and pain-related disability/unemployment (2.4 points). In women, disability/unemployment resulted in an additional reduction 3.7 point reduction in MCS score. Final multivariable models explained 27% and 18% of the variance in PCS and MCS scores, respectively. Etiology, disease duration, pancreatic morphology, diabetes, exocrine insufficiency and prior endotherapy/pancreatic surgery had no significant independent effect on QOL.
Conclusion
Constant pain, pain-related disability/unemployment, current smoking, and concurrent co-morbidities significantly affect the QOL in CP. Further research is needed to identify factors impacting QOL not explained by our analyses.
Keywords: chronic pancreatitis, quality of life, pain, disability, NAPS2
Introduction
Chronic pancreatitis (CP), a chronic inflammatory disorder of the pancreas, is characterized by irreversible morphologic changes of the pancreas, abdominal pain – which is often chronic and debilitating, episode(s) of acute pancreatitis, impairment of endocrine and exocrine function, and in a small subset of patients, development of pancreatic cancer.(1)
In previous single center studies, quality of life (QOL) in patients with CP was noted to be significantly impaired when compared with historical population controls.(2, 3) In a large, multicenter study, we demonstrated a profound effect of CP on physical and a modest effect on mental QOL when compared with subjects without pancreatitis, independent of demographics, risk factors, etiology and common medical conditions.(4) While these data document the poor QOL in CP patients, they do not provide an explanation for the factors responsible for this observation. Understanding these factors is of importance in the clinical management of patients, especially if they can be modified by behavioral changes, treatment of disease or its complications.
Studies evaluating independent factors associated with low QOL in CP patients have consistently reported abdominal pain to be an important predictor.(3, 5–10) Treatment of pain by surgical or endoscopic therapy has documented effect not only in achieving pain relief but also in improving QOL.(11–14) Etiology of CP has not been found to affect QOL.(3, 5, 8) Younger age, female sex, smoking, alcohol consumption, low body mass index, diabetes, exocrine pancreatic insufficiency, pancreatic morphology, and disease duration lower QOL, but their association has been inconsistent across studies.(3, 5, 7, 8, 10) The reasons for this inconsistency include variability in the instruments used to determine QOL, small sample size of studies and the lack of use of uniform predictor variables. The effect of pain-related disability or unemployment on QOL has not been adequately studied.
The North American Pancreatitis Study 2 (NAPS2) studies have prospectively ascertained over 1,000 well-phenotyped patients with CP from several US centers. Systematically collected data on QOL and several potential predictor variables provided us with an opportunity to determine which factors may affect QOL in this cohort.
Methods
Study Population
We utilized data from the NAPS2 studies for this analysis. NAPS2 consists of a series of three studies (original NAPS2, NAPS2-CV, and NAPS2-AS) from 27 centers in the United States that prospectively ascertained 1195 CP, 569 RAP and 1109 controls from 2000-2014. One of the participating centers only enrolled control subjects. Detailed methodology for the NAPS2 studies has been published.(15–17) CP was defined by definitive changes on imaging studies (primarily endoscopic retrograde cholangiopancreatography or computed tomography scan, or equivalent changes on magnetic resonance cholangiopancreatography or endoscopic ultrasound) or histology. The study was approved by the Institutional Review Board of each center and all subjects provided written informed consent prior to enrollment.
The present study includes 1024/1195 (85.6%) CP patients from the three NAPS2 studies who provided complete responses for determination of QOL, disability/unemployment, and pain characteristics (pain pattern and severity).
Data collection
Study subjects completed a detailed questionnaire including information on demographics, family and personal history, exposure to smoking and alcohol, pain experience, disability/unemployment related to pain from pancreatitis, and QOL. Self-reported alcohol use during the maximum drinking period of life was used to create drinking categories (abstainers: no alcohol use or <20 drinks in a lifetime; light drinkers: ≤3 drinks per week; moderate drinkers: 4-7 drinks per week for females and 4-14 drinks per week for males; heavy drinkers: 8-34 drinks per week for females and 15-34 drinks per week for males; very heavy drinkers: ≥35 drinks per week for both sexes) and smoking (status: never, past current; amount: <1 packs per day or ≥1 packs per day; and <12, 12-35 and ≥35 packs years) as described previously.(15)
The enrolling physician investigator completed a separate questionnaire consisting of information on etiology, TIGAR-O risk factors, phenotypic characteristics (history of acute pancreatitis [AP], age at AP, age at the onset of CP symptoms, age at CP diagnosis, presence of diabetes, exocrine insufficiency, imaging features), and treatments received (medications, surgical and endoscopic interventions), and their perceived effectiveness.
QOL evaluation
The Short Form-12 (SF-12) version 2 was used to assess QOL in the NAPS2-CV and NAPS2-AS studies. In the original NAPS2 study, QOL was assessed using the SF-12 version 1, and scores were transformed to version 2 for interpretation.(18) SF-12 is a multipurpose generic QOL questionnaire that was derived from the SF-36 Health Survey.(18, 19) The SF-36 and SF-12 have been validated in several languages and disease conditions, including CP.(2, 3, 20) The SF-12 is comprised by twelve questions that measure 8 domains (role physical, role emotional, physical function, social function, mental health, vitality, bodily pain, and general health), and can be summarized into the physical component summary (PCS) and mental component summary (MCS) scores. SF-12 questionnaire takes approximately two minutes to complete without a significant loss of information, and is based on a standard recall of 4 weeks.(18) The summary scores for each domain are then transformed to z-scores using the SF-12 scale means and standard deviations (SD) from the 1998 general US population to produce a normally distributed population score. PCS and MCS scores range from 0–100 with a score of 50±10 representing the mean ± SD for the general population. These scores provide a direct comparison of the distribution in the population of interest when compared with the general population and between groups. A difference of 3 points in the PSC or MCS scores between groups has been suggested to be clinically relevant.(4)
Pain and disability/unemployment assessment
We used patient-reported pain experience for all comparisons in this study. In the NAPS2-CV and NAPS2-AS studies, there was a leading question on the presence of pain (i.e. Have you experienced abdominal pain related to pancreatitis in the past year?). Patients who reported “yes” were asked to choose from one of the five pre-defined pain patterns (episodes of mild to moderate pain; constant mild to moderate pain; usually pain free with episodes of severe pain; constant mild pain plus episodes of severe pain; and constant severe pain that does not change). For this study, we incorporated the presence, severity and temporal nature of pain to classify patients into the following 5 categories: 1) No pain; 2) Intermittent mild to moderate episodes of pain; 3) Intermittent severe episodes of pain; 4) Constant mild to moderate pain; 5) Constant mild to moderate pain with episodes of severe pain, or constant severe pain that does not change. In the original NAPS2 study, a leading question on the presence of pain was not asked and patients were directly asked to complete the question in the five pain patterns.(15) Therefore, patients in the original NAPS2 study who did not identify with a pain pattern were excluded from the final analysis (n=126) as it is impossible to distinguish those patients without pain and those with pain who simply left their pain category blank. In addition, patients from the three NAPS2 studies were asked for the presence of pain-related disability or unemployment.
Statistics
The PCS and MCS scores were computed using the scoring method system provided in the SF-12 version 2 reference manual, and presented as mean ± standard deviation (SD).(18) Descriptive analyses are presented as proportions for categorical data and as mean ± SD for continuous data. Comparison between the PCS and MCS scores within each group variable was done using the Kruskal-Wallis test. All variables with a p-value of <0.2 on univariate analysis comparing the PCS and MCS scores were considered for potential inclusion in the multivariable models.
Multivariable linear regression models were used to determine the independent predictors of physical and mental QOL. A backwards selection technique was used to determine significant independent predictors. Age and gender were included in both final models because of clinical significance. Other variables were removed one by one in order of the magnitude of the p-value for the type III F-test, with the exception of diabetes which was removed from the PCS model due to its small effect size compared to the other variables remaining. Models were then re-run until a final model was reached containing only the variables with P <0.05. For the MCS final model, history of liver disease was borderline significant and was included in the final model. There were missing data in some variables, but in no case did they exceed ~6%. The PCS model was not examined for interactions to maintain easier interpretation and out of concern of including too many variables in the final model. Significant interactions were examined for the MCS model. For easier interpretation, the final models were centered at the age of 50 years. Since the original NAPS2 study used an earlier version of SF12, and presence of pain was not assessed by a leading question, a sensitivity analysis was performed after excluding these patients and using the same variables included in the final models. Data analysis was performed using SAS version 9.4.
Results
Demographics, risk factors and clinical characteristics
Of the 1024 CP patients who formed the final study cohort, 381 (37.2%) were enrolled in the original-NAPS2 study, 510 (49.8%) in the NAPS2-CV study and 133 (13.0%) in the NAPS2-AS study. The distribution of baseline socio-demographic and clinical characteristics is shown in table 1.
Table 1.
Predictors of physical and mental QOL for CP patients in the NAPS2 study
| Variable (n=1024) | N (%) | Physical QOL score | Mental QOL score | ||
|---|---|---|---|---|---|
|
| |||||
| Mean ± SD | p-value | Mean ± SD | p-value | ||
|
| |||||
| Study type | |||||
| NAPS2-Original | 381 (37.2) | 37.4±11.2 | 0.07 | 43.4±11.6 | 0.06 |
| NAPS2-CV | 510 (49.8) | 36.9±12.1 | 42.2±12.7 | ||
| NAPS2-AS | 133 (13.0) | 34.5±10.9 | 40.6±11.9 | ||
|
| |||||
| Age | |||||
| <45 years | 344 (33.5) | 36.5±11.3 | < 0.001 | 41.1±12.2 | < 0.001 |
| 45-60 years | 427 (41.7) | 35.6±11.5 | 40.7±11.9 | ||
| >60 years | 253 (24.8) | 39.1±12.2 | 47.2±11.7 | ||
|
| |||||
| Gender | |||||
| Male | 559 (54.6) | 37.5±12.0 | 0.02 | 42.9±12.1 | 0.15 |
| Female | 465 (45.4) | 35.8±11.2 | 41.8±12.3 | ||
|
| |||||
| Race (n=1023) | |||||
| Black | 228 (22.3) | 35.0±11.2 | 0.06 | 40.9±12.0 | 0.03 |
| Caucasian | 765 (74.8) | 36.2±10.8 | 43.0±12.3 | ||
| Other | 30 (2.9) | 37.2±11.8 | 40.6±12.4 | ||
|
| |||||
| Body Mass Index (n=1017) | |||||
| Normal/Low | 586 (57.6) | 36.5±11.7 | 0.5 | 42.5±12.4 | 0.8 |
| Overweight | 275 (27) | 37.4±11.9 | 42.1±12.2 | ||
| Obese | 156 (15.4) | 36.1±11.1 | 42.6±11.8 | ||
|
| |||||
| Smoking (n=1022) | |||||
| Never | 251 (24.6) | 39.2±12.0 | < 0.001 | 45.1±10.7 | < 0.001 |
| Past | 259 (25.3) | 38.5±12.1 | 45.1±11.6 | ||
| Current | 512 (50.1) | 34.6±10.9 | 39.7±12.6 | ||
|
| |||||
| Current Drinking | |||||
| Yes | 201 (19.6) | 39.0±12.3 | 0.003 | 43.7±12.0 | 0.1 |
| No | 823 (80.4) | 36.2±11.4 | 42.1±12.3 | ||
|
| |||||
| Drinking category (n=998) | |||||
| Abstainer | 171 (17.1) | 35.5±11.0 | <0.001 | 43.2±12.1 | < 0.001 |
| Light | 170 (17.0) | 37.4±11.6 | 44.9±11.7 | ||
| Moderate | 159 (15.9) | 40.2±12.2 | 44.2±11.2 | ||
| Heavy | 151 (15.1) | 37.2±12.0 | 42.2±12.0 | ||
| Very Heavy | 347 (34.9) | 35.3±11.4 | 40.0±12.6 | ||
|
| |||||
| Alcohol etiology | |||||
| Yes | 510 (49.8) | 36.0±11.2 | 0.06 | 40.9±12.3 | < 0.001 |
| No | 513 (50.2) | 37.4±12.1 | 44.0±12.0 | ||
|
| |||||
| Disease duration, years (n=968) | |||||
| 1 or less | 103 (10.7) | 37.4±12.6 | 0.06 | 45.1±11.6 | 0.008 |
| 1 – < 2 | 122 (12.6) | 38.2±12.5 | 43.9±12.9 | ||
| 2 – < 3 | 117 (12.1) | 35.8±11.3 | 40.9±12.4 | ||
| 3 – < 4 | 97 (10.0) | 39.5±11.1 | 40.3±12.8 | ||
| 4 or more | 529 (54.6) | 36.2±11.4 | 42.2±11.9 | ||
|
| |||||
| Pain pattern | |||||
| No pain | 86 (8.4) | 43.7±11.9 | < 0.001 | 48.7±11.8 | < 0.001 |
| Intermittent mild to moderate episodes of pain | 131 (12.8) | 43.0±11.1 | 46.1±11.1 | ||
| Intermittent severe episodes of pain | 246 (24.0) | 40.6±11.2 | 47.0±10.8 | ||
| Constant mild to moderate pain | 64 (6.3) | 37.2±10.7 | 39.3±10.7 | ||
| Constant mild to moderate pain with episodes of severe pain, or constant severe pain | 497 (48.5) | 31.9±9.9 | 38.5±11.9 | ||
|
| |||||
| Disability/unemployment | |||||
| Yes | 277 (27.1) | 30.6±8.6 | < 0.001 | 37.1±12.1 | < 0.001 |
| No | 747 (72.9) | 39.0±11.8 | 44.4±11.7 | ||
|
| |||||
| Exocrine insufficiency | |||||
| Yes | 390 (38.1) | 36.1±12.0 | 0.11 | 41.5±12.3 | 0.05 |
| No | 634 (61.9) | 37.2±11.5 | 43.0±12.2 | ||
|
| |||||
| Endocrine Insufficiency | |||||
| Yes | 339 (33.1) | 35.3±11.9 | 0.004 | 41.9±12.1 | 0.2 |
| No | 685 (66.9) | 37.5±11.5 | 42.7±12.3 | ||
|
| |||||
| Pseudocysts | |||||
| Yes | 309 (30.2) | 36.0±11.6 | 0.12 | 41.4±12.4 | 0.06 |
| No | 715 (69.8) | 37.1±11.7 | 42.9±12.1 | ||
|
| |||||
| Pancreatic surgery | |||||
| Yes | 210 (21.5) | 35.8±11.5 | 0.12 | 41.6±11.8 | 0.19 |
| No | 814 (79.5) | 37.0±11.7 | 42.7±12.3 | ||
|
| |||||
| Endoscopic therapy | |||||
| Pancreatic endotherapy | 386 (37.7) | 36.2±11.0 | 0.58 | 41.9±11.9 | 0.31 |
| Other endotherapy | 126 (12.3) | 36.9±12.3 | 43.9±11.7 | ||
| None | 512 (50.0) | 37.1±12.0 | 42.5±12.6 | ||
|
| |||||
| History of heart disease/heart attack/stroke | |||||
| Yes | 121 (11.8) | 32.9±11.2 | < 0.001 | 42.4±13.2 | 0.9 |
| No | 903 (88.2) | 37.3±11.6 | 42.4±12.1 | ||
|
| |||||
| History of cancer | |||||
| Yes | 38 (3.7) | 32.7±10.3 | 0.03 | 41.5±13.4 | 0.7 |
| No | 986 (96.3) | 36.9±11.7 | 42.5±12.2 | ||
|
| |||||
| History of liver disease | |||||
| Yes | 92 (9.0) | 33.3±10.7 | 0.002 | 39.6±12.0 | 0.03 |
| No | 932 (91.0) | 37.1±11.7 | 42.7±12.2 | ||
|
| |||||
| History of gallstones/cholecystectomy | |||||
| Yes | 454 (44.3) | 35.4±11.1 | 0.002 | 42.3±11.8 | 0.6 |
| No | 570 (55.7) | 37.8±12.0 | 42.6±12.5 | ||
|
| |||||
| History of renal disease | |||||
| Yes | 62 (6.1) | 33.3±10.7 | 0.02 | 41.8±11.5 | 0.6 |
| No | 962 (93.9) | 37.0±11.7 | 42.5±12.3 | ||
- Numbers are provided in parenthesis for variables where the number of patients was less than the total sample size.
- Body Mass Index categories: Normal/Low – ≤25; Overweight – >25 – ≤30; Obese – >30.
- Drinking category definition- Abstainers: no alcohol use or <20 drinks in a lifetime; Light drinkers: ≤ 3 drinks/week; Moderate drinkers: 4-7 drinks/week for females, 4-14 drinks/week for males; Heavy drinkers: 8-34 drinks/week for females, 15-34 drinks/week for males; Very heavy drinkers: ≥ 35 drinks/week for both sexes.
Overall, patients had a mean age of 50.4±14.6 years, 54.6% were male, 74.8% were white, 19.6% were current drinkers, and the majority had a normal/low BMI (57.6%). Heavy or very heavy drinking during the maximum drinking period of life was reported by 15.1% and 34.9%, and past or current smoking by 25.3% and 50.1% patients respectively. Physicians considered alcohol as the main etiologic factor in about half of (50.2%) patients. In greater than 50% patients (54.6%) the duration of CP was 4 or more years.
In our final study cohort, abdominal pain in the year preceding enrollment was present in 91.6%. The most common pattern of abdominal pain identified was constant mild to moderate pain with episodes of severe pain or constant severe pain (48.5%). Less frequently patients identified intermittent episodes of severe pain (24%), intermittent episodes of mild to moderate pain (12.8%), and constant mild to moderate pain (6.3%). Pain-related disability/unemployment was reported by 27.1% of patients. About one-third of patients had exocrine insufficiency (38.1%) or diabetes (33.1%). While 40.3% received only medical management, a prior history of pancreatic endotherapy was noted in 37.7% and pancreatic surgery in 21.5% patients. Almost half of the patients (44.3%) had history of gallstones or cholecystectomy. The most commonly reported comorbidities noted were heart disease/heart attack/stroke (11.8%), liver disease (9%), renal disease (6.1%), or prior history of cancer (3.7%).
Predictors of physical quality of life
Univariable analysis
Mean PCS score for the study cohort was 36.7±11.7. Univariable analysis for PCS scores based on demographics, risk factors and clinical characteristics are shown in table 1. A significantly lower physical QOL was noted with younger age, female sex, black race, extremes of alcohol consumption, current smoking, disability/unemployment, pain and diabetes. A medical history of heart disease/heart attack/stroke, cancer, liver disease, renal disease, gallstones or cholecystectomy was also significantly associated with lower PCS scores. Interestingly, the association was only borderline with disease duration, and no association was noted with BMI, etiology, exocrine insufficiency, pancreatic morphology, prior endotherapy or pancreatic surgery.
Multivariable analysis
Results for multivariable regression analyses for physical QOL are shown in table 2. Temporal nature and severity of pain had the greatest impact on physical QOL. Compared to patients with no pain, constant mild to moderate pain with episodes of severe pain or constant severe pain reduced the PCS score by 10 points, while constant mild to moderate pain alone decreased it by 5.2 points. Presence of severe pain which was intermittent was also important but the impact of this (2.6 points) was less than constant pain. Moreover, a history of gallstones or cholecystectomy, which likely reflects an indirect impact of pain, also had a negative impact (1.6 points). Other disease related factors with a negative impact included disability/unemployment (5.1 points) and current smoking (2.9 points). The effect of self-reported drinking history was interesting in that when compared with lifetime abstainers, the PCS score was significantly higher in patients who reported moderate (4.3 points) or heavy (2.5 points) drinking during their heaviest period of drinking in life. Presence of co-morbid conditions also had a significant negative impact – a history of cancer, heart disease/heart attack/stroke and renal failure reduced the PCS score by 4.4, 3.3 and 2.8 points, respectively. Other factors associated with lower PCS scores on the univariable analysis were not significant after adjusting for other variables and were not included in the final regression model. Variables in the final multivariable model explained 27% of the variance in PCS.
Table 2.
Multivariable regression model showing significant determinants for Physical Quality of Life in Chronic Pancreatitis
| Variable | Reference category | Parameter Estimate | SE | p-value |
|---|---|---|---|---|
|
| ||||
| Intercept | – | 45.8 | 1.5 | <0.001 |
|
| ||||
| Age 50ˆ | – | −0.04 | 0.02 | 0.1 |
|
| ||||
| Female | Male | −1.2 | 0.7 | 0.09 |
|
| ||||
| Pain | ||||
| Intermittent mild to moderate episodes of pain | No pain | −0.8 | 1.5 | 0.6 |
| Intermittent severe episodes of pain | −2.6 | 1.4 | 0.05 | |
| Constant mild to moderate pain | −5.2 | 1.8 | 0.003 | |
| Constant mild to moderate pain with episodes of severe pain, or constant severe pain | −10.0 | 1.3 | < 0.001 | |
|
| ||||
| Disabled or unemployed | No disabled or unemployed | −5.1 | 0.8 | <0.001 |
|
| ||||
| Smoking status | ||||
| Past | Never smoker | −1.0 | 0.9 | 0.3 |
| Current | −2.9 | 0.9 | 0.002 | |
|
| ||||
| Drinking category | ||||
| Light | Abstainer | 2.1 | 1.1 | 0.06 |
| Moderate | 4.3 | 1.2 | < 0.001 | |
| Heavy | 2.5 | 1.2 | 0.04 | |
| Very Heavy | 1.6 | 1.1 | 0.2 | |
|
| ||||
| History of cancer | No cancer | −4.4 | 1.7 | 0.01 |
|
| ||||
| History of heart disease/heart attack/stroke | No heart disease/heart attack/stroke | −3.3 | 1.0 | 0.001 |
|
| ||||
| History of renal failure | No renal failure | −2.8 | 1.4 | 0.04 |
|
| ||||
| History of gallstones/cholecystectomy | No gallbladder disease/chole-cystectomy | −1.6 | 0.7 | 0.02 |
- R-square – 27%.
- For drinking category definition – refer to methods section.
Age variable centered at 50 years.
To illustrate the impact of individual factors and incremental effect of predictive factors, representative PCS score for a 50-year-old man with CP and progressive addition of select attributes are shown in figure 1. By adding constant mild to moderate pain with episodes of severe pain or constant severe pain, current smoking, and pain-related disability/unemployment, the PCS score would decrease from 45.8 to 27.8.
Figure 1. Incremental decrease in physical and mental QOL scores with additional attributes in a representative CP patient.
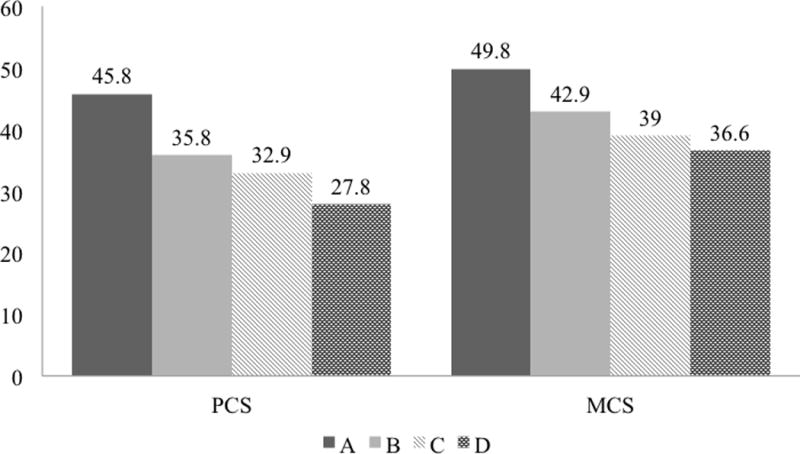
Each column represents a clinical scenario with additive attributes: (A) A 50-year-old man with CP who is nonsmoker, has no pain, disability/unemployment, or comorbidities. (B) Subject in A with constant mild to moderate pain with episodes of severe pain, or constant severe pain that does not change. (C) Subject in B who is also a current smoker. (D) Subject in C with disability/unemployment.
Predictors of mental quality of life
Univariable analysis
The mean MCS score for the study cohort was 42.4±12.2. Univariable analysis for MCS scores based on demographics, risk factors and clinical characteristics are shown in table 1. A significantly lower mental QOL was noted with younger age, very heavy drinking, current smoking, disability/unemployment and pain. In contrast to physical QOL, mental QOL was significantly lower in patients with physician-defined alcohol etiology and the duration of disease. Race, presence of pseudocysts and exocrine insufficiency showed borderline association with MCS score, while sex, BMI, diabetes, other features on pancreatic morphology, and prior endotherapy or pancreatic surgery showed no association.
Multivariable analysis
The results of multivariable regression analyses for mental QOL are shown in table 3. Similar to physical QOL, presence of pain had the most impact on mental QOL. Interestingly, compared to those patients with no pain, a negative impact was seen only for constant pain irrespective of its severity. The impact of constant mild to moderate pain (6.8 points) was almost similar to constant mild to moderate pain with episodes of severe pain or constant severe pain (6.9 points). Intermittent pain irrespective of severity did not significantly impact MCS score compared to those patients with no pain. Current smoking (3.9 points) and pain-related disability/unemployment (2.4 points) also significantly reduced the MCS score. An interaction was noted between gender and disability/unemployment –the effect of disability/unemployment on MCS was an additional 3.7 points lower for women than for men. Other factors associated with lower MCS scores on the univariable analysis were not significant after adjusting for other variables and were not included in the final regression model. Variables in the final multivariable models explained 17.9% of the variance in MCS score.
Table 3.
Multivariable regression model showing significant determinants for Mental Quality of Life in Chronic Pancreatitis
| Variable | Reference category | Parameter Estimate | SE | p-value |
|---|---|---|---|---|
|
| ||||
| Intercept | – | 49.81 | 1.4 | <0.001 |
|
| ||||
| Age 50ˆ | – | 0.04 | 0.03 | 0.16 |
|
| ||||
| Female | Male | −0.3 | 0.8 | 0.70 |
|
| ||||
| Pain | ||||
| Intermittent mild to moderate episodes of pain | No pain | −1.3 | 1.6 | 0.41 |
| Intermittent severe episodes of pain | −0.2 | 1.4 | 0.87 | |
| Constant mild to moderate pain | −6.8 | 1.9 | < 0.001 | |
| Constant mild to moderate pain with episodes of severe pain, or constant severe pain | −6.9 | 1.4 | < 0.001 | |
|
| ||||
| Disabled or unemployed | No disabled or unemployed | −2.4 | 1.1 | 0.03 |
|
| ||||
| Smoking status | ||||
| Past | Never smoker | −0.3 | 1.0 | 0.79 |
| Current | −3.9 | 0.9 | < 0.001 | |
|
| ||||
| Liver disease | No liver disease | −2.4 | 1.2 | 0.052 |
|
| ||||
| Female * Disability/unemployment | – | −3.7 | 1.6 | 0.02 |
- R-square = 17.9%.
Age variable centered at 50 years.
A significant interaction was found for female gender and disability/unemployment.
To illustrate the impact of individual factors and incremental effect of predictive factors, representative MCS score for a 50-year old CP man are shown in figure 1. Progressive addition of select attributes, such as constant mild to moderate pain with episodes of severe pain or constant severe pain, current smoking, and disability/unemployment, would decrease the MCS score from 49.8 to 36.6.
Sensitivity analysis
Sensitivity analysis after excluding patients from the original NAPS2 study showed slight differences in the estimated effect sizes, but were overall consistent with the main analyses (data not shown).
Discussion
In this largest prospective study to date, we found that pain – especially constant, pain-related disability/unemployment, current smoking, and concurrent co-morbidities to have a significant negative impact on the QOL of CP patients. Interestingly, physician-defined alcohol etiology, disease duration, pancreatic morphology, and prior endoscopic or surgical treatments did not independently affect QOL. These data underscore the need to better understand factors driving pain and to develop effective strategies to address them. Facilitating behavioral modification will also have an impact on QOL in these patients. Since only a fraction of variance in PCS and MCS scores was explained by our analyses, future studies should focus on identifying other factors affecting the QOL of CP patients.
Pain occurs in up to 90% of CP patients, and about half of them have constant pain.(9) While the association of pain with poor QOL is consistent across studies(3, 5–10), whether the effect is greater with severity or temporal nature of pain is debatable. A small single center Polish study of 69 patients revealed that pain intensity correlates with QOL scores more often than frequency.(8) More recently, Olesen et al assessed the association between the intensity and temporal pattern of pain with the QOL of 106 CP patients from two tertiary medical centers in Netherlands and Denmark.(10) In addition to measuring pain severity by the modified brief pain inventory short form (m-BPI-sf),(21) the authors used this scale to indirectly classify pain frequency into 3 patterns: intermittent, constant, and constant with acute exacerbations. After adjustment for confounders, they found that pain severity but not pain pattern was significantly correlated with global health status, all functional subscales, and most symptom subscales.
In 414 CP patients enrolled in the original NAPS2 study, we reported that constant pain was associated with lower physical and mental QOL scores when compared with those who had intermittent pain.(9) The present study extends those findings using multivariable analyses in a larger cohort, and provides a numeric estimate of the effect of each pain type on QOL. We found that compared to those patients with no pain, constant mild to moderate pain with episodes of severe pain or constant severe pain to be the strongest predictor of poor physical (10 points reduction) and mental QOL (6.9 points reduction), followed by constant mild to moderate pain (5.2 and 6.8 points reduction respectively). The effect of constant pain on QOL was statistically significant, clinically relevant, and independent of other covariates such as demographics, risk factors and comorbidities. Intermittent severe pain had no effect on the mental QOL, but had a borderline significant effect on physical QOL. These data suggest that both temporal nature and severity of pain are important to consider when designing pain management strategies and clinical trials in CP patients. The relevance of constant nature of symptoms was aptly demonstrated in the classic study of the natural history of alcoholic CP by Ammann et al, where the temporal nature of pain was the primary determinant for the need for surgical intervention.(22) Interestingly, constant pain has also been shown to have a significant impact on QOL in many other health conditions.(23–25)
The effect of smoking on QOL in CP differs between studies. In two Italian studies, current smoking,(3) and the amount and duration of smoking did not affect QOL.(7) In contrast, we previously had shown and this study confirmed that current smoking had a significant impact on both physical and mental QOL.(4) Similarly, Han et al reported smokers to have a significantly worse physical QOL when compared with non-smokers.(26) A Polish study found the duration of smoking to be associated with lower physical functioning and insomnia, and the amount of smoking with worse fear of future health.(8) The mechanism by which smoking has an effect on QOL is unclear. One possible explanation may be the effect of smoking on disease-related manifestations such as worsening of morphology (e.g. calcifications, exocrine and or endocrine function) – however, it is interesting to note that morphological or functional derangements by themselves did not impact QOL. Other possible explanations would be indirect, similar to the effect of current smoking on general QOL in conditions related or unrelated to smoking.(27–31) Prevalence of affective spectrum disorders in CP patients is high and patients may smoke to relieve anxiety, depression or stress that is pre-existing or develops after diagnosis of CP, or to cope with the need to abstain from drinking.(32–35) Smoking cessation by counseling and/or pharmacologic measures should be an important component of the management of CP, not only due to its association with QOL, but also for its impact on disease progression.(36, 37) While smoking cessation strategies show good results in many conditions, its results in CP can be challenging and fail in the majority of patients.(26)
The independent effect of disability/unemployment on QOL has not been adequately studied. We found disability/unemployment related to CP to have a significant negative impact on physical as well as mental QOL. Wehler et al reported an independent reduction of PCS score caused by CP-related unemployment or early retirement.(5) Disability/unemployment can lead to heavy psychological burden, financial distress, and disruption in social and family relationships, which can limit the ability to cope with the disease.(38, 39) Future strategies should focus to prevent work disability and to help disabled patients to get back into the labor force.(40)
Strengths of our study include a large, well-phenotyped cohort of patients and a comprehensive analysis of multiple relevant variables. This approach overcomes important limitations of prior work in this area. Our study also has limitations. First, this was a one-time assessment of QOL in patients with a chronic condition. Therefore, we are unable to evaluate for temporal changes in QOL, as well as the direct impact of interventions, such as pancreatic endotherapy or surgery, pain management, medical management of exocrine insufficiency or diabetes, etc. on QOL. We did not find a significant difference in QOL based on whether a patient had prior pancreatic endotherapy or surgery. This observation merely represents a cross-sectional assessment, and does not address the question of whether performance of endotherapy or surgery improves QOL. Such a question can best be answered by studies specifically designed to evaluate changes in QOL before and after an intervention – in fact, several such studies have concluded the benefit of these interventions on QOL.(11–14) A previous study of 83 CP patients (87% alcoholic) found the QOL to be stable over a 2-year period(41) – however, larger studies in patients with diverse etiologies with a longer follow-up period are needed to better assess temporal trends and determinants of QOL. Second, we excluded 126 patients in the original NAPS2 study who did not identify a pain pattern, and given the large sample size in the final cohort, we feel the impact of excluding these patients is likely small and would not affect the conclusions. Third, recruitment of patients mainly from referral centers, and, under-representation of racial groups (e.g. Hispanics, Asians, etc.) despite the multicenter effort may limit the generalizability of our findings.
Finally, even after an exhaustive evaluation of multiple factors, we only explain 27% variance in physical and 17.9% variance in mental QOL, leaving several unmeasured variables. Future studies should assess the role of factors not included in our analyses, examples of which include bowel habits (e.g. diarrhea, steatorrhea, opioid-induced constipation), malnutrition (e.g. weight loss, micronutrient deficiencies, metabolic bone disease), psychological factors (e.g. depression, anxiety, stress, sleep disturbance, substance abuse), social factors (e.g. work absenteeism, financial status, social support), healthcare utilization (e.g. frequency and duration of hospitalizations), adherence to medical care, and genetics. The impact of behavioral (e.g. smoking or alcohol cessation strategies, relaxation techniques), medical (e.g. narcotics, neuromodulating agents, antidepressants, pancreatic enzyme replacement, micronutrient supplementation), endoscopic (e.g. pancreatic or biliary stenting, endoscopic shock wave lithotripsy, celiac plexus block) and surgical (e.g. drainage and resection techniques, total pancreatectomy and islet auto transplantation) interventions, and physical therapy needs further assessment. Management of CP by an interdisciplinary team with expertise in primary care, behavioral health, gastroenterology, therapeutic endoscopy, pancreatic surgery, endocrinology and radiology has been recommended. The impact of such an approach on QOL should be empirically evaluated.
In summary, this large multicenter study demonstrated that constant pain, pain-related disability/unemployment, current smoking, and concurrent co-morbidities significantly impair the QOL of CP. These data expands our understanding that both temporal nature and severity of pain are important to consider when designing clinical trials in CP. Developing treatment strategies aimed to target modifiable factors may help in improving the QOL of CP patients. Future research is needed to identify factors that were not included in this study.
Study highlights.
What is current knowledge?
Chronic pancreatitis has a profound effect on quality of life
Factors that determine quality of life in chronic pancreatitis patients are not well established
What is new?
Constant pain had the most impact on quality of life of chronic pancreatitis patients
Other independent factors were disability/unemployment related to pain, current smoking and co-morbid conditions
Prior endoscopic or surgical pancreatic interventions had no impact on the quality of life
Alcohol etiology, disease duration, and pancreatic morphology had no effect on the quality of life
Acknowledgments
Financial support: The study was supported by R01DK061451 (DCW), R01 DK077906 (DY), and U01 DK108306-01 (DC, CEF, DCW, DY), and UL1 RR024153 and UL1TR000005 (PI—Steven E Reis, MD).
Footnotes
Data from this analysis was presented as an oral presentation at the Digestive Disorders Week 2016 in San Diego, CA and published as an abstract in Gastroenterology 2016;150(4):S191-S192
Specific author contributions:
Study design: Jorge D. Machicado, Judah N. Abberbock, and Dhiraj Yadav
Data acquisition: C. Mel Wilcox, Bimaljit S. Sandhu, Vikesh K. Singh, Andres Gelrud, Stuart Sherman, Gregory A. Cote, Samer Al-Kaade, Michelle A. Anderson, Timothy B. Gardner, Michele D. Lewis, Christopher E. Forsmark, Nalini M. Guda, Joseph Romagnuolo, John Baillie, Stephen T. Amann, Thiruvengadam Muniraj, Darwin L. Conwell, Peter A. Banks, Randall E. Brand, Adam Slivka, and Dhiraj Yadav
Statistical analysis: Judah N. Abberbock and Gong Tang
Drafting of the manuscript: Jorge D. Machicado and Dhiraj Yadav
Data interpretation, review of manuscript for important intellectual content, final approval of the manuscript: all authors.
Potential competing interests: Dr. Whitcomb is an inventor of intellectual property that is licensed to Ambry Genetics, which has been evaluated in this study. He also has an ownership interest in Ambry Genetics.
Guarantor of the article: Dhiraj Yadav, MD MPH
References
- 1.Forsmark CE. Management of chronic pancreatitis. Gastroenterology. 2013;144:1282–91 e3. doi: 10.1053/j.gastro.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Wehler M, Reulbach U, Nichterlein R, et al. Health-related quality of life in chronic pancreatitis: a psychometric assessment. Scand J Gastroenterol. 2003;38:1083–9. doi: 10.1080/00365520310005956. [DOI] [PubMed] [Google Scholar]
- 3.Pezzilli R, Morselli-Labate AM, Frulloni L, et al. The quality of life in patients with chronic pancreatitis evaluated using the SF-12 questionnaire: a comparative study with the SF-36 questionnaire. Dig Liver Dis. 2006;38:109–15. doi: 10.1016/j.dld.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Amann ST, Yadav D, Barmada MM, et al. Physical and mental quality of life in chronic pancreatitis: a case-control study from the North American Pancreatitis Study 2 cohort. Pancreas. 2013;42:293–300. doi: 10.1097/MPA.0b013e31826532e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wehler M, Nichterlein R, Fischer B, et al. Factors associated with health-related quality of life in chronic pancreatitis. Am J Gastroenterol. 2004;99:138–46. doi: 10.1111/j.1572-0241.2004.04005.x. [DOI] [PubMed] [Google Scholar]
- 6.Fitzsimmons D, Kahl S, Butturini G, et al. Symptoms and quality of life in chronic pancreatitis assessed by structured interview and the EORTC QLQ-C30 and QLQ-PAN26. Am J Gastroenterol. 2005;100:918–26. doi: 10.1111/j.1572-0241.2005.40859.x. [DOI] [PubMed] [Google Scholar]
- 7.Pezzilli R, Morselli-Labate AM, Fantini L, et al. Assessment of the quality of life in chronic pancreatitis using Sf-12 and EORTC Qlq-C30 questionnaires. Dig Liver Dis. 2007;39:1077–86. doi: 10.1016/j.dld.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Mokrowiecka A, Pinkowski D, Malecka-Panas E, et al. Clinical, emotional and social factors associated with quality of life in chronic pancreatitis. Pancreatology. 2010;10:39–46. doi: 10.1159/000225920. [DOI] [PubMed] [Google Scholar]
- 9.Mullady DK, Yadav D, Amann ST, et al. Type of pain, pain-associated complications, quality of life, disability and resource utilisation in chronic pancreatitis: a prospective cohort study. Gut. 2011;60:77–84. doi: 10.1136/gut.2010.213835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olesen SS, Juel J, Nielsen AK, et al. Pain severity reduces life quality in chronic pancreatitis: Implications for design of future outcome trials. Pancreatology. 2014;14:497–502. doi: 10.1016/j.pan.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Cahen DL, Gouma DJ, Nio Y, et al. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med. 2007;356:676–84. doi: 10.1056/NEJMoa060610. [DOI] [PubMed] [Google Scholar]
- 12.Cahen DL, Gouma DJ, Laramee P, et al. Long-term outcomes of endoscopic vs surgical drainage of the pancreatic duct in patients with chronic pancreatitis. Gastroenterology. 2011;141:1690–5. doi: 10.1053/j.gastro.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 13.Strate T, Bachmann K, Busch P, et al. Resection vs drainage in treatment of chronic pancreatitis: long-term results of a randomized trial. Gastroenterology. 2008;134:1406–11. doi: 10.1053/j.gastro.2008.02.056. [DOI] [PubMed] [Google Scholar]
- 14.Izbicki JR, Bloechle C, Broering DC, et al. Extended drainage versus resection in surgery for chronic pancreatitis: a prospective randomized trial comparing the longitudinal pancreaticojejunostomy combined with local pancreatic head excision with the pylorus-preserving pancreatoduodenectomy. Ann Surg. 1998;228:771–9. doi: 10.1097/00000658-199812000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitcomb DC, Yadav D, Adam S, et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2) Pancreatology. 2008;8:520–31. doi: 10.1159/000152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilcox CM, Yadav D, Ye T, et al. Chronic pancreatitis pain pattern and severity are independent of abdominal imaging findings. Clin Gastroenterol Hepatol. 2015;13:552–60. doi: 10.1016/j.cgh.2014.10.015. quiz e28-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilcox CM, Sandhu BS, Singh V, et al. Racial Differences in the Clinical Profile, Causes, and Outcome of Chronic Pancreatitis. Am J Gastroenterol. 2016 doi: 10.1038/ajg.2016.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware JEKM, Turner-Bowker DM, Gandek B. How to score version 2 of the SF 12 Health Survey with a supplement documenting version 1. QualityMetric Incorporated; Rhode Island: Health Assessment Lab; Boston, Massachusetts: 2002. [Google Scholar]
- 19.Ware JEKM, Keller SD. SF-36 physical and mental summary scales: a user’s manual. Boston, MA: The Health Institute; 1994. [Google Scholar]
- 20.Pezzilli R, Morselli Labate AM, Ceciliato R, et al. Quality of life in patients with chronic pancreatitis. Dig Liver Dis. 2005;37:181–9. doi: 10.1016/j.dld.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Mendoza T, Mayne T, Rublee D, et al. Reliability and validity of a modified Brief Pain Inventory short form in patients with osteoarthritis. Eur J Pain. 2006;10:353–61. doi: 10.1016/j.ejpain.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Ammann RW, Muellhaupt B. The natural history of pain in alcoholic chronic pancreatitis. Gastroenterology. 1999;116:1132–40. doi: 10.1016/s0016-5085(99)70016-8. [DOI] [PubMed] [Google Scholar]
- 23.Jonsdottir T, Aspelund T, Jonsdottir H, et al. The relationship between chronic pain pattern, interference with life and health-related quality of life in a nationwide community sample. Pain Manag Nurs. 2014;15:641–51. doi: 10.1016/j.pmn.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Hopwood MB, Abram SE. Factors associated with failure of trigger point injections. Clin J Pain. 1994;10:227–34. doi: 10.1097/00002508-199409000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Miller JP, Magill ST, Acar F, et al. Predictors of long-term success after microvascular decompression for trigeminal neuralgia. J Neurosurg. 2009;110:620–6. doi: 10.3171/2008.9.17660. [DOI] [PubMed] [Google Scholar]
- 26.Han S, Kheder J, Bocelli L, et al. Smoking Cessation in a Chronic Pancreatitis Population. Pancreas. 2016 doi: 10.1097/MPA.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldenberg M, Danovitch I, IsHak WW. Quality of life and smoking. Am J Addict. 2014;23:540–62. doi: 10.1111/j.1521-0391.2014.12148.x. [DOI] [PubMed] [Google Scholar]
- 28.Strandberg AY, Strandberg TE, Pitkala K, et al. The effect of smoking in midlife on health-related quality of life in old age: a 26-year prospective study. Arch Intern Med. 2008;168:1968–74. doi: 10.1001/archinte.168.18.1968. [DOI] [PubMed] [Google Scholar]
- 29.Wilson D, Parsons J, Wakefield M. The health-related quality-of-life of never smokers, ex-smokers, and light, moderate, and heavy smokers. Prev Med. 1999;29:139–44. doi: 10.1006/pmed.1999.0523. [DOI] [PubMed] [Google Scholar]
- 30.Sippel JM, Pedula KL, Vollmer WM, et al. Associations of smoking with hospital-based care and quality of life in patients with obstructive airway disease. Chest. 1999;115:691–6. doi: 10.1378/chest.115.3.691. [DOI] [PubMed] [Google Scholar]
- 31.Russel MG, Nieman FH, Bergers JM, et al. Cigarette smoking and quality of life in patients with inflammatory bowel disease. South Limburg IBD Study Group Eur J Gastroenterol Hepatol. 1996;8:1075–81. doi: 10.1097/00042737-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Swendsen J, Conway KP, Degenhardt L, et al. Mental disorders as risk factors for substance use, abuse and dependence: results from the 10-year follow-up of the National Comorbidity Survey. Addiction. 2010;105:1117–28. doi: 10.1111/j.1360-0443.2010.02902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balliet WE, Edwards-Hampton S, Borckardt JJ, et al. Depressive Symptoms, Pain, and Quality of Life among Patients with Nonalcohol-Related Chronic Pancreatitis. Pain Res Treat. 2012;2012:978646. doi: 10.1155/2012/978646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nusrat S, Yadav D, Bielefeldt K. Pain and opioid use in chronic pancreatitis. Pancreas. 2012;41:264–70. doi: 10.1097/MPA.0b013e318224056f. [DOI] [PubMed] [Google Scholar]
- 35.Palfai TP, Monti PM, Ostafin B, et al. Effects of nicotine deprivation on alcohol-related information processing and drinking behavior. J Abnorm Psychol. 2000;109:96–105. doi: 10.1037//0021-843x.109.1.96. [DOI] [PubMed] [Google Scholar]
- 36.Maisonneuve P, Lowenfels AB, Mullhaupt B, et al. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut. 2005;54:510–4. doi: 10.1136/gut.2004.039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luaces-Regueira M, Iglesias-Garcia J, Lindkvist B, et al. Smoking as a risk factor for complications in chronic pancreatitis. Pancreas. 2014;43:275–80. doi: 10.1097/01.mpa.0000437324.52598.ee. [DOI] [PubMed] [Google Scholar]
- 38.Milner A, LaMontagne AD, Aitken Z, et al. Employment status and mental health among persons with and without a disability: evidence from an Australian cohort study. J Epidemiol Community Health. 2014;68:1064–71. doi: 10.1136/jech-2014-204147. [DOI] [PubMed] [Google Scholar]
- 39.Baanders AN, Heijmans MJ. The impact of chronic diseases: the partner’s perspective. Fam Community Health. 2007;30:305–17. doi: 10.1097/01.FCH.0000290543.48576.cf. [DOI] [PubMed] [Google Scholar]
- 40.Clayton S, Bambra C, Gosling R, et al. Assembling the evidence jigsaw: insights from a systematic review of UK studies of individual-focused return to work initiatives for disabled and long-term ill people. BMC Public Health. 2011;11:170. doi: 10.1186/1471-2458-11-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pezzilli R, Morselli Labate AM, Fantini L, et al. Quality of life and clinical indicators for chronic pancreatitis patients in a 2-year follow-up study. Pancreas. 2007;34:191–6. doi: 10.1097/mpa.0b013e31802e0301. [DOI] [PubMed] [Google Scholar]


