Abstract
Segmental bone defects (SBDs) secondary to trauma invariably result in a prolonged recovery with an extended period of limited weight bearing on the affected limb. Soldiers sustaining blast injuries and civilians sustaining high energy trauma typify such a clinical scenario. These patients frequently sustain composite injuries with SBDs in concert with extensive soft tissue damage. For soft tissue injury resolution and skeletal reconstruction a patient may experience limited weight bearing for upwards of 6 months.
Many small animal investigations have evaluated interventions for SBDs. While providing foundational information regarding the treatment of bone defects, these models do not simulate limited weight bearing conditions after injury. For example, mice ambulate immediately following anesthetic recovery, and in most cases are normally ambulating within 1–3 days post-surgery. Thus, investigations that combine disuse with bone healing may better test novel bone healing strategies. To remove weight bearing, we have designed a SBD rodent healing study in microgravity (μG) on the International Space Station (ISS) for the Rodent Research-4 (RR-4) Mission, which launched February 19, 2017 on SpaceX CRS-10 (Commercial Resupply Services). In preparation for this mission, we conducted an end-to-end mission simulation consisting of surgical infliction of SBD followed by launch simulation and hindlimb unloading (HLU) studies. In brief, a 2 mm defect was created in the femur of 10 week-old C57BL/6J male mice (n=9–10/group). Three days after surgery, 6 groups of mice were treated as follows: 1) Vivarium Control (maintained continuously in standard cages); 2) Launch Negative Control (placed in the same spaceflight-like hardware as the Launch Positive Control group but were not subjected to launch simulation conditions); 3) Launch Positive Control (placed in spaceflight-like hardware and also subjected to vibration followed by centrifugation); 4) Launch Positive Experimental (identical to Launch Positive Control group, but placed in qualified spaceflight hardware); 5) Hindlimb Unloaded (HLU, were subjected to HLU immediately after launch simulation tests to simulate unloading in spaceflight); and 6) HLU Control (single housed in identical HLU cages but not suspended). Mice were euthanized 28 days after launch simulation and bone healing was examined via micro-Computed Tomography (μCT). These studies demonstrated that the mice post-surgery can tolerate launch conditions. Additionally, forces and vibrations associated with launch did not impact bone healing (p=0.3). However, HLU resulted in a 52.5% reduction in total callus volume compared to HLU Controls (p=0.0003). Taken together, these findings suggest that mice having a femoral SBD surgery tolerated the vibration and hypergravity associated with launch, and that launch simulation itself did not impact bone healing, but that the prolonged lack of weight bearing associated with HLU did impair bone healing. Based on these findings, we proceeded with testing the efficacy of FDA approved and novel SBD therapies using the unique spaceflight environment as a novel unloading model on SpaceX CRS-10.
Keywords: Fracture Healing, Spaceflight, Launch Simulation, Hindlimb Unloading
1. Introduction
Orthopaedic injuries may result from high energy trauma such as car accidents or battlefield explosions, and are a focus in orthopaedic research. Current surgical techniques, however, are not always effective for repairing damaged bone. In many cases, to allow for successful healing the injuries will require a secondary bone healing intervention. An example is traumatic blast injuries, which often require significant skeletal reconstructions to allow the patient to regain an acceptable level of function. An effective treatment could improve the outcome of military casualties, as 60–70% of modern war injuries involve the musculoskeletal system, predominantly caused by explosive devices (Covey, 2002). Unhealed fractures lead to pain, loss of function, and potential amputation. These surgeries have long recovery times with limited weight bearing on the limb. Soldiers and civilians alike undergo this type of recovery process. Additionally, patients with SBDs usually have extensive soft tissue damage and weight bearing limitations for up to 6 months (Robert Rozbruch et al., 2006). Even with these interventions, patients may not heal completely.
For many years, small animal investigations have evaluated SBD interventions. While these studies have provided the basis of much of our knowledge of SBD healing, they do not simulate the limited weight bearing clinical patients experience following high impact trauma injuries. In particular, popular human analogs (mice, rats, and pigs) ambulate immediately post anesthesia and are fully ambulatory approximately 1–3 days post operation (personal observation, MAK and (Moran et al., 2016)). In contrast, a human patient with a Gustilo-Anderson type 3 tibial injury (large SBD in the tibia of humans) will not walk for up to 6 months; therefore, animal models do not mimic human injury in terms of weight bearing.
It is well established that weight bearing has profound implications for skeletal homeostasis and healing (Kershaw et al., 1993). For this reason, there is a need to test better bone healing models in combination with non-ambulatory methods. Microgravity (μG), like that experienced aboard the ISS, may prove to be a useful model to test limited weight bearing in laboratory animals. When preparing for spaceflight operations aboard the ISS, the National Aeronautics and Space Administration (NASA) performs mission or procedure simulations as needed to improve the chances of mission success. The RR-4 (Rodent Research 4) Mission has a number of firsts, and a full mission simulation was deemed of value. Specifically, the mission simulation was designed to test the hypotheses that after a SBD surgery i) mice can tolerate the forces associated with launch, ii) male mice can be successfully housed in NASA Rodent Research hardware, and iii) our SBD model would be negatively impacted by disuse. To answer these questions we used micro-Computed Tomography or μCT to characterize the healing region, and measured animal organ and body weight, as well as food and water intake to understand the impact of experimental and housing conditions on animal health. Finding no significant differences between healing and animal health, between our groups, would validate our model.
2. Materials and Methods
Ethics Statement: All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the Indiana University School of Medicine, IN and NASA AMES Research Center, CA and were performed in facilities accredited by AAALAC.
The effects of launch simulation and HLU on bone healing were tested by inducing a 2 mm mid-diaphyseal segmental defect in the femurs of ninety, 10-week-old, male C57BL/6 mice. “Allo-reared” (cage-mated from weaning) mice were purchased from Jackson Laboratories (Bar Harbor, ME) two weeks prior to surgery and maintained in their initial cohorts of 15 mice (caged together) in large mouse cages (Ancare N40, polycarbonate, 19″ × 10.5″ × 6.125″) through surgery and until they were placed into their final hardware/group (see below). One week prior to surgery, raised wire floors (Ancare, 19″ × 10.5″ × .90″) were placed into the N40 cages, and mice began acclimation to water bottles outfitted with Lixits and NASA Nutrient-upgraded Rodent Food Bar (NuRFB) (Sun et al., 2012, Sun et al., 2010). Mice then underwent a SBD surgery as detailed below. At 2 days post-surgery, mice were selected for different groups based on evaluations of gait and activity level (see below).
As detailed in Table 1, all mice were subjected to surgery and separated into the following groups (n=10): Vivarium Control, Launch Negative Control, Launch Positive Control, Launch Positive Experimental, HLU, and HLU Control. For each group, all mice were group housed except the HLU and HLU Control mice which were housed individually. Each of the 6 groups of mice were obtained from cages originally containing 15 allo-reared males. Based on previous surgical experience, approximately 0–2 mice per cohort may experience a surgical complication (personal observation MAK). Therefore, we anticipated that cohorts of 15 mice would yield at least 10 “healthy, post-surgery” mice. Four of the experimental groups were selected from the cohorts of 15 cagemates (Launch Negative Control, Launch Positive Control, Launch Positive Experimental, and HLU). Two groups (Vivarium Control and HLU Control) were derived by combining mice from separate cohorts; this was necessitated by an unanticipated anesthetic-analgesic complication in the course of surgery, likely caused by providing the mice a pre-operative dose of buprenorphine SR (slow release).
Table 1.
Bone healing was assessed in 6 experimental groups and the impacts of caging and launch simulation was determined.
| Transporter | Launch Simulation | Spaceflight Qualified Hardware | Habitat Equivalent | HLU | Single Housed | |
|---|---|---|---|---|---|---|
| Vivarium Control | − | − | − | − | − | − |
| Launch Negative Control | + | − | − | + | − | − |
| Launch Positive Control | + | + | − | + | − | − |
| Launch Positive Experimental | + | + | + | − | − | − |
| HLU | + | + | − | − | + | + |
| HLU Control | − | − | − | − | − | − |
All mice were provided NuRFB and autoclaved deionized water ad libitum for nourishment. Water bottles for HLU cages had standard ball sipper tubes, whereas all other cage types used modified Lixits for water delivery. Custom video recording systems captured video from all groups except HLU and HLU control mice for four hours per day, 2 hours at each changeover from dark to light cycle.
2.1 Groups
All mice were maintained on a 12 hour light/dark cycle. The ambient temperature for all animals was maintained at 76–78°F. Table 1 summarizes the 6 experimental groups, including hardware/caging, launch simulation, HLU, and whether the mice were housed singly or in groups. Figure 1 summarizes the experimental timeline.
Figure 1. Experimental Timeline.
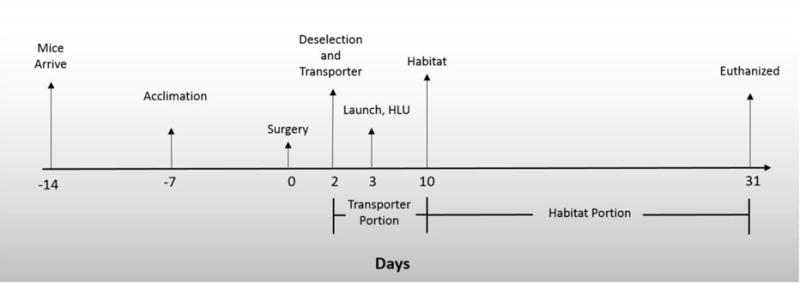
Mice were purchased from Jackson Laboratories two weeks prior to surgery and placed in large N40 mouse cages. One week prior to surgery, raised wire floors were placed into N40 cages and mice began acclimation to spaceflight water Lixit hardware and spaceflight food bars. Two weeks after arrival mice underwent a segmental bone defect surgery. Two days after surgery some mice were placed in a Transporter. One day later some mice in Transporters were subjected to launch simulation and/or were placed in HLU cages and were either HLU or served as singly housed controls. One week later mice in Transporters were moved to Habitat caging. Thirty one days post-surgery all mice were euthanized and bones/tissue collected.
2.1.1 Vivarium Control
Vivarium Control mice received surgery, but remained in standard polycarbonate small mouse cages (Ancare N10, 11.5″ × 7.5″ × 5″) with raised wire grid floors throughout and were not subjected to launch simulation. Vivarium Controls remained in the animal facility for the entire experimental period and were obtained from two surgical cohorts (5 mice/cohort/cage) as detailed above. Vivarium cages were changed twice weekly.
2.1.2 Launch Negative Control
Launch Negative Control mice were placed in the NASA Rodent Transporter (“Transporter”), which houses mice (n=10/side of Transporter, 2 sides/unit) during launch and ascent to the ISS (see Figure 2A); the Transporter was then transported to the facilities where the launch vibration and hypergravity (centrifugation) testing were performed. The hardware unit containing mice was placed on the relevant equipment for the appropriate amount of time, but not subjected to any launch stressors. After launch timeframe simulation, the Transporter housing the mice was returned to the animal facility, where it remained for the remainder of the study. After 1 week in the Transporter to mimic post-launch ascent to, and docking with, the ISS, mice were moved into a Rodent Habitat cage (”Habitat Equivalent”) where 5 mice were loaded on either side of the habitat hardware. The Habitat Equivalent hardware presents a similar animal interface as the Habitats that house mice on the ISS (food, water, cage dimensions), but with the exceptions of forced air flow and lighting conditions; the Habitat Equivalents rely on room ambient temperature and lighting conditions whereas Habitats use active air flow and lighting provided within the cage. A filter beneath the wire grid flooring collects animal waste and debris and was not changed during the course of the experiment to mimic spaceflight conditions. As with the other groups, Launch Negative mice received procedures such as replenishment of food and water following the protocols and timeline proposed for our spaceflight investigations.
Figure 2. Hardware/Cages and Launch Simulation Testing Equipment.

(A) Outside view of the Transporter, NASA developed cage for transport of mice from Earth to the ISS on the SpaceX Dragon. (B) Inside view of the Habitat, NASA developed cage used to house mice while on the ISS. (C) and (D) Hindlimb unloading (HLU) cages. Mice were suspended via tail by the clip with ambulation throughout the cage via a pulley system (Morey-Holton and Globus, 2002). (E) and (F) Vibration table for vibration testing (mimics SpaceX launch profile) and how the Transporter was secured to the vibration table. (G) and (H) 20G Centrifuge for g-force testing (mimics SpaceX launch profile) showing how the Transporter was secured on the centrifuge. Photos provided by NASA Ames Research Center.
2.1.3 Launch Positive Control
The Launch Positive Control group was treated identical to the Launch Negative group, but was subjected to vibration and hypergravity to simulate launch stressors.
2.1.4 Launch Positive Experimental
Launch Positive Experimental mice were mice treated as detailed for the Launch Positive Control group, but were placed into a flight-certified Rodent Habitat after launch and ascent simulation. Flight-certified Rodent Habitats are powered units that provide temperature, relative humidity, and power consumption data logs (1 measurement per second), forced air flow, four video angles, and filtered inlet and exhaust air (Habitat, see Figure 2B), and are built and validated to spaceflight specifications. Mice are housed in flight-certified Habitats on the ISS. This housing presents the same geometry and water/food as the Habitat Equivalent, but is noisier with higher air flow, and provides some heat. The temperatures of the rooms containing the Habitat and Habitat Equivalents were adjusted so that the temperatures experienced by the groups were within 3°F of each other.
2.1.5 HLU
HLU mice were placed into a Transporter, underwent launch simulation, and then were hindlimb unloaded and singly housed in HLU cages (Figures 2C&D). HLU was accomplished as previously described (Morey-Holton and Globus, 2002). In brief, custom built cages were equipped at the top with a bar that spanned the length of the cage. To give the animals full mobility across the bar and the cage area, a roller with a 360° swivel was placed over each rod. The animals hindlimb were then elevated using a chain and binder clip, via a custom harness which was attached to the tail. The chain length was adjusted daily to ensure the animals were not weight bearing on hindlimbs and to achieve a 30° angle of the mouse body to the floor (Morey-Holton and Globus, 2002) (Figure 2C&D). Animals were able to ambulate using only their front appendages to reach food and water (Swift et al., 2010). Unlike groups with spaceflight hardware where large food bars were attached to the walls, small pieces of food were placed across the floor in HLU cages to ensure the animals do not use the food as a resting spot. Health checks were performed twice daily and the food was changed out twice daily to provide animals with fresh food. Waste and debris fell through the grid flooring, which was cleaned and changed every other day. Of note, all mice were successfully suspended for the entire 28 day duration with no escapes.
2.1.6 HLU Control
Finally, HLU Controls were singly housed mice in similar custom built HLU cages, but were not suspended. The purpose of this group was two-fold. The first purpose was to control for the impacts of single housing vs. group housing, as mice are social animals. The second purpose was to control for differences between custom built cages with perforated flooring and standard shoebox cages. The same husbandry measurements were in place for the HLU Control animals as detailed for HLU mice. Mice in this group were obtained from various surgery cohorts as described above and did not undergo launch simulation nor housing in Transporters.
All mice were examined twice daily as well as through video recording, and body weight was measured weekly until euthanasia to ensure animal welfare. Thirty one days post-surgery (28 days after launch simulation), all mice were euthanized (150–45 mg/kg of Ketamine-Xylazine) and bones/tissue collected. Surgical femurs were analyzed via μCT as described below.
2.2 Surgical Procedure
Mice were anesthetized with Ketamine-Xylazine (125–20 mg/kg), then their right hindlimbs were shaved and scrubbed with ethanol and betadine. After ensuring the limb was sterile, a 1 cm lateral incision was made over the right hindlimb approximately at the level of the femur midshaft. After the initial incision, blunt dissection was carried out to expose the femur, where the muscle was bluntly stripped to the diaphyseal region. The knee was then flexed and a 27-gauge needle was manually inserted between the condyles of the femur and threaded retrograde into the intramedullary canal. The needle was then partially removed (anterograde, so that the tip cleared the site of the intended defect), then a sterile dremel rotary cutting tool was used to remove a 2 mm intercalary segment from the femoral diaphysis. Next, we placed a synthetic graft composed of poly(propylene fumarate)/tricalcium phosphate (2 mm in height and having an internal diameter of 1 mm, external diameter of 2 mm, and a 0.7 mm side port to allow for fluid flow) to maintain the defect size. The synthetic scaffold used is identical to that previously described except reduced in size for mice compared to that used in rats (Chu et al., 2007). The needle was advanced once again retrograde, this time through the center hole in the scaffold to the proximal end of the femur. The needle tip was used with a twisting motion to bore through the proximal femur. The exposed needle tip was bent back on itself and the needle was pulled anterograde to stabilize the femur and defect. The opposite end of the needle was cut as close to the distal femur as possible. Next, a saline-soaked collagen sponge (RCM6 Resorbable Collagen Membrane, ACE) was wrapped around the synthetic scaffold and sutured into place using 3-0 vicryl suture (Ethicon, J215H). The muscle was then closed using 2 interrupted sutures (3-0 vicryl suture). The skin was then closed using 7 mm wound clips (Braintree Scientific, RF7CS). Mice were then taken to a recovery area where they were placed on heating pads and monitored until they recovered from anesthetic. After recovery, mice were returned to their original cages and resting boards (Bio-Serve, K3392 Rest Stops) were added for the first 2 days of recovery before mice were transferred to their groups as detailed above. Resting boards are flat, plastic boards that provide a flat surface as opposed to a wire bottom cage upon which the animals could walk and rest. These boards were used to ease the transition to ambulating after surgery.
2.3 Launch Simulation Procedures
One day after loading animals into Transporters, the Transporters were driven to the launch simulation facility. The Vivarium and HLU Controls remained at the Animal Care Facility. The launch simulation entails application of 2 main mechanical factors: random vibration and hypergravity from centrifugal acceleration. The equipment at NASA Ames cannot provide vibration and acceleration simultaneously, which would be a higher fidelity simulation. Therefore, vibration is followed by acceleration. The vibration and acceleration profiles were provided by the SpaceX recommended testing envelope requirements document. To simulate launch, the mice were first subjected to approximately ten minutes of vibration by mounting each Transporter on a Ling Electronics A300-B (5000 lb–f) electrodynamic shaker vibration table (Figures 2E&F). Following vibration, the Transporter was then mounted in a Genisco 1230-2 200 g centrifuge (Figures 2G&H). The centrifugation profile also lasted approximately 10 minutes. NASA is not allowed to provide specific launch simulation conditions because they are considered defense secrets.
A custom video capture system was used throughout the launch simulation and transport to and from the animal care facility, which allowed live viewing and recording. The NASA Attending Veterinarian observed the mice via video throughout the launch simulations to ensure animal welfare and to help evaluate whether the SBD mice could withstand the rigors of launch factors. To better mimic our spaceflight mission, mice were not handled once placed into the Transporters until transfer into the Habitat or Habitat Equivalent hardware units seven days after launch simulation.
2.4 Bone Healing Analyses and Stress Assessment
At euthanasia weights for thymus, adrenal gland, spleen, and whole body were obtained to evaluate changes corresponding to stress. For bone healing, the femora were analyzed by μCT (Skyscan 1172) (Feher et al., 2010, Warden et al., 2009, Weatherholt et al., 2013). The fracture callus region of interest (ROI) was defined as a 6 mm region of the femoral midshaft (the 2mm segmental defect region + 2 mm of bone on either end). Based on our historical studies, a 2mm distance on either end of the segmental defect ensures that the entire fracture callus would be evaluated. The bones were scanned at 60kv with a 6μm pixel size and a 0.7 degree rotation step. Two images were averaged per frame (Bouxsein et al., 2010). Following scanning, the binarized images were used to calculate several three-dimensional bone healing parameters as detailed previously (Burgers et al., 2016, Komatsu et al., 2010). In brief, tissue volume (TV) generated by NRecon delineates tissue from non-tissue, which we defined as the callus volume. Mineralized tissue volume (BV) was defined as tissue within the callus volume with a pixel intensity with a threshold of 60 or more. This threshold was determined by its ability to distinguish mineralized tissue in the cortical shaft from background. These values were used to determine the percent of mineralized callus (BV/TV, %). Additionally, fracture healing was assessed using a modified Radiographic Union Score for Tibial Fractures method (RUST) (Kooistra et al., 2010, Whelan et al., 2010), adapted and applied to the mouse femora, as a semi-quantitative method for analysis. Here the medial, lateral, anterior, and posterior cortices visible on anteroposterior and lateral μCT images were scored. Specifically, each cortex was given a score of 1 (no bridging), 2 (partial bridging), or 3 (complete bridging) and the scores for all 4 cortices were added up to provide a final score ranging from 4 (not healed) to 12 (maximally healed). Images were scored by 3 blinded readers and the average score was used for each specimen.
2.5 Statistics
Unless otherwise indicated, all data were presented as the Mean ± SEM. One-way factorial analyses of variances with LSD were used to make multiple group comparisons. For analysis of animal body weights over time, a repeated measures ANOVA with a Bonferroni post-hoc test (α=0.05) was performed. All analyses were performed with the Statistical Package for Social Sciences software (IBM SPSS 24; SPSS Inc., Armonk, NY) and were two tailed with a level of significance set at 0.05.
3. Results
3.1 Adaptation to Caging
Food and water consumption and body weight were monitored for each cage as an indication of adaption of the mice to the different caging systems. Figure 3A shows the apparent food consumption (measured as food depletion, because wastage cannot be reliably quantified) by the mice in the different groups, while Figure 3B shows the apparent water consumption by the same mice. HLU and HLU Control food consumption data was not recorded because the pieces of food crumbled and dried out so much any weights would not be accurate. For the other groups, no differences in apparent food or water consumption were observed during the Transporter phase of this study. For the Habitat phase, the mice in the Launch Positive Experimental group had a higher apparent water consumption rate (4.10±0.83 mL/mouse/day consumed) than the other groups with Lixits (1.93±0.34 mL/mouse/day or 69.3% lower for the Vivarium Controls, 2.82±0.70 mL/mouse/day or 55.2% lower for the Launch Negative Controls, and 3.17±0.45 mL/mouse/day or 64.3% lower for the Launch Positive group), but more comparable to the HLU caged animals (5.17±2.39 mL/mouse/day or 26.1% lower for HLU mice and 4.78±1.63 mL/mouse/day or 16.6% lower for HLU Controls), which had sipper tubes (Figure 3B). Lixits usually provide less water per activation than sipper tubes, which may explain the difference between HLU and Habitat cages/vivarium, but does not explain why the Launch Positive Experimental group in the spaceflight-qualified Habitat had a higher water depletion rate. It may be that the increased air flow and temperature inside the Habitat dries the animals more, inducing them to drink more. Of note, only one water consumption measurement at the termination of the test is possible for the spaceflight-qualified Habitat, because the unit must be disassembled to take the measurement.
Figure 3. Apparent Food and Water Depletion and Mouse Weight.
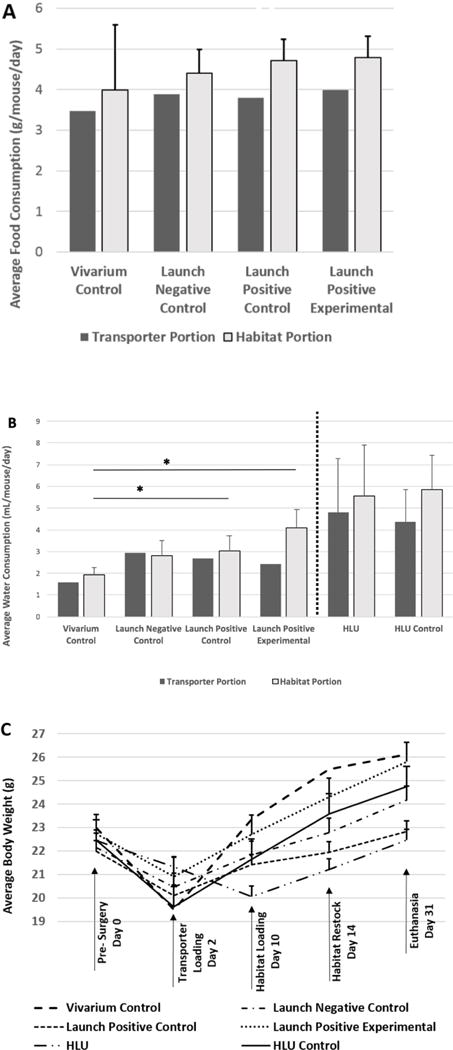
(A) Apparent food and (B) water depletion were measured while the mice were housed in the different pieces of hardware. Mice were (C) weighed prior to surgery, 2 days after surgery (just before placement into Transporters), 1 week after launch simulation when mice were moved into Habitat cages, 2 weeks later when the Habitats were restocked with food and water, and at the time of euthanasia. Food and water consumption were expressed as values per mouse per day. Bars represent the mean ± SEM. There are no error bars associated with either food or water consumption during the Transporter phase because per standard protocol, the Transporter was only accessed after 7 days. Additionally, for the Habitat phase of the Launch Positive Experimental group, there were no water consumption error bars because the unit must be disassembled to take this measurement. No significant differences were detected between groups for food consumption during the Habitat phase. Significant differences were detected for water consumption between Vivarium and Launch Positive and Launch Positive Experimental groups (* indicates p<0.05) but not between Vivarium and Launch Negative groups (p>0.05). HLU and Vivarium/Habitat groups were not compared for water consumption due to different water delivery mechanisms (denoted by dotted line). However, HLU vs. HLU Controls were not significantly different from each other for water consumption. With regard to body weight, no significant differences were observed between the groups when using a repeated measures ANOVA followed by a Bonferroni post-hoc test (p<0.05).
With respect to body weight (Figure 3C), all groups lost weight immediately following surgery, but increased their body weight between Transfer Day (2 days post-surgery) and 29 days later at the time of euthanasia. It should be noted that the HLU mice did not experience a net change in body weight over the course of the experiment. However, the Vivarium Controls gained 3.1 grams (23.0g to 26.1g), Launch Negative Controls gained 2.0 g (22.2g to 24.2g), Launch Positive Controls gained 0.8g (22.0g to 22.8g), Launch Positive Experimental mice gained 3.1g (22.7g to 25.8g), and HLU Controls gained 2.2g (22.5g to 24.7g). Next, we analyzed the change in body weight over time for all groups with a repeated measures ANOVA followed by a Bonferroni post-hoc test. Difference between the Vivarium Control and HLU groups approached significance (p=0.063). Of note, all other group comparisons were not different (p>0.1), including the HLU vs. HLU Control groups. Interestingly, a significant difference was detected when using a paired Student’s t-test to examine final body weights between HLU and HLU Control group (22.5±0.9g vs. 24.7±2.3g respectively, p<0.05). That two different statistical approaches gave different results, may explain the fact that in previous HLU studies some differences in body weight were detected, while in other studies differences were not detected (Morey-Holton and Globus, 2002).
Next, we examined whether launch simulation and/or unloading impacted adrenal gland, thymus, or spleen weights. The weights of the dissected organs were normalized to final body weight, and are shown in Figure 4. These weights are known to change as a physiological response to stress (Everds et al., 2013). Statistical analyses demonstrated that the HLU and HLU Control mice (i.e. singly housed mice) were not significantly different from each other, but were both significantly lower than Vivarium Controls, Launch Negative Controls, and Launch Positive Experimental mice. However, no significant differences were observed between the Launch Positive and HLU or HLU Control mice. These data suggest that housing mice individually might introduce an element of stress which is consistent with thymus involution (Gamallo et al., 1986, Abou-Ismail and Mahboub, 2011). Of note, no significant differences were found between groups for adrenal gland or spleen weight.
Figure 4. Thymus, Spleen, and Adrenal Gland Weight.
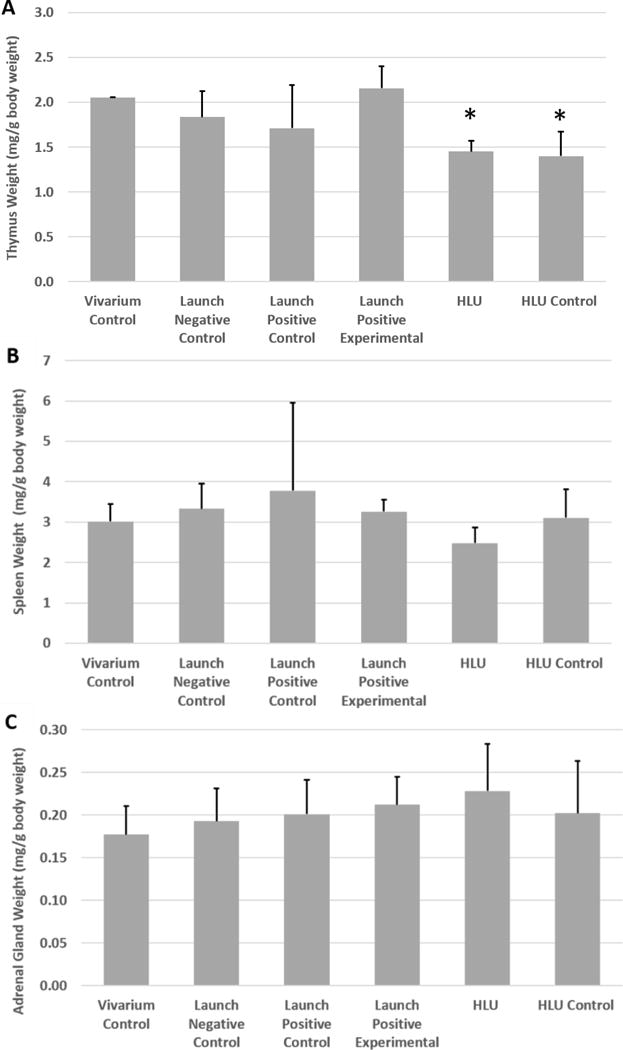
Weights of the (A) thymus, (B) spleen, and (C) adrenal gland were measured and normalized to body weight. Bars represent the mean ± SEM. No differences were detected between groups for spleen or adrenal gland weight; however, the two singly housed mouse groups (HLU and HLU Control) both experienced significantly lower thymus weights compared to all of the other groups (* indicates significant difference from all group housed cohorts, p<0.05).
Just prior to euthanasia, the research team examined the gait of each mouse for overt abnormalities such as lack of weight bearing, limps, and the inability to flex or extend the leg or foot. Aside from HLU mice, which were excluded from visual gait observations (as they remained suspended until they were euthanized), all mice were noted to have a similar gait. Additionally, at this time the Ames Attending Veterinarian performed an individual assessment of health and well-being and indicated that all mice were of suitable condition for scientific study. This health assessment by the veterinarian was done prior to loading of the mice into the spaceflight hardware, via video as the mice underwent launch simulation testing, at the time of transfer from Transporters to Habitat or Habitat Equivalents, and prior to euthanasia.
An important qualitative observation is that the mice were ambulating similarly in the vivarium, HLU control, Transporter, and Habitat cages. The standard polycarbonate vivarium cages contained 1/3″ grid raised wire flooring, the HLU control cages contain a polycarbonate 1/2″ grid floor, and the Transporter and Habitat/Habitat Equivalent both contain stainless steel 1/3″ grid floors on four surfaces of the cage (one surface is the smooth Lexan door and the other surface contains the food), so that the mice can utilize the entire volume. Thus, it appeared that irrespective of the flooring, the surgery did not impede the ability of the mice to move throughout their cages, nor did the wound clips snag on the cage grids. Non-surgery mice usually display a period of adaptation for 2–4 days where they stretch up from the ground to eat the food, which is placed about 10 cm above the cage floor; as they get acclimated to the cage, they generally switch to a typical pattern of sitting on top of the food and eating downwards. By contrast, the mice in this study climbed the cage walls to sit on top of the food bar, rather than stand up on their hindlimbs to reach the food, within hours of transfer into the different cages (personal observation, CSG).
3.2 Launch Simulation Tolerance
A critically important aspect of this study was to demonstrate that our surgical model could withstand the random vibration and hypergravity associated with launch. Thirty mice in this study were subjected to launch simulation conditions three days after surgery (Launch Positive Control, Launch Positive Experimental, and HLU groups, n=10/group). No surgical hardware failures were observed during, immediately after, or 28 days after launch testing was completed. A surgical failure was defined as failure to recover from anesthetic, an open wound (e.g. wound clip loss), inability of an animal to walk, appearing excessively moribund, lack of activity or normal behaviors (e.g. grooming), excessive bleeding, swelling, visible infection, obvious fracture or malalignment of the leg (including inability to flex or extend the leg or foot), or other untoward observations upon visual inspection by the research team and/or attending veterinarian. One mouse in the launch positive group died 4 days after launch simulation while in the Transporter. While not conclusive, the attending veterinarian speculated this could be due to stress resulting from a combination of the analgesic-anesthetic dose reaction and the hypergravity stress.
Mice subjected to vibration initially displayed low activity, mostly resting with some eating and grooming. As the vibration test began, they stopped eating and grooming and then began moving around the cage. Mice on top of the food moved to the floor. Immediately after vibration testing was completed the mice behaved and ambulated normally. Launch Negative Control mice also explored the cage while it was on the vibration table, but with more controlled movements than mice exposed to vibration.
After transfer into the centrifuge, the mice continued an average activity level including exploring/ambulating with some eating and grooming. At the beginning of centrifugation as acceleration was slowly rising, the mice continued to explore the cage, but their movements slowed. As the centrifugation continued, the movement of the mice essentially stopped. They formed a group on one side of the cage and they remained in the same position for the remainder of the centrifugation. Slight movement was seen in several individuals during this approximate 10 minute period. Upon termination of the centrifugation, mice remained in position before activity slowly began increasing as mice began moving by rolling, standing, and lifting their heads. Some mice began ambulating as early as 20–30 minutes after centrifugation ended; however, the majority displayed their first movements after 60 minutes, with all mice moving 90 minutes post-centrifugation. By that time, ambulation matched that prior to launch simulation testing. This behavior is consistent with what has been observed in other launch simulation tests with female and male rodents that had not been subjected to surgeries prior to the simulation of launch (personal observation CSG and NASA Attending Veterinarian). Importantly, no overt surgical failures (as described above) were observed.
3.3 Bone Healing
No gross differences were observed in the experimental hindlimbs. RUST scores demonstrated that all groups had virtually identical bone bridging or fracture healing scores (average of 8.1 ± 0.5, range 5–11, Figure 4A). Higher scores indicate more complete healing, with 12 as maximum. Of interest, other studies using a different model, without a scaffold, have shown that a 2mm segmental defect (same size as in this study) in a mouse femur does not reliably heal (5 of 8 animals healed) (Zwingenberger et al., 2013). Since the surgical bone defect in our model, with a scaffold, is a SBD, not a critical sized defect (which by definition require surgical and/or pharmacological intervention to heal), bones will heal with time even with saline treatment, and a score of ~8 is what we typically see for 31 days post-surgery (personal observation, MAK). Based on our previous studies, we would anticipate healing to occur 8+ weeks post-surgery without further treatment (personal observation, MAK).
Total callus volume was calculated from the μCT images, and the results for each group of femurs is reported in Figure 4B. A higher total callus volume indicates greater tissue regeneration. As shown in Figure 4B, the callus volumes for all but the HLU were similar. The callus volume was significantly reduced (52.5%) in HLU compared to HLU Control mice (p=0.0003). The callus volume was also significantly reduced in the HLU group compared to all other groups (p<0.05).
Figure 4C reports the percentage of mineral contained within the callus from each group. No significant differences were detected among the different groups in terms of the percentage of mineralized callus.
4. Discussion
Microgravity is the ultimate disuse environment which can be exploited for biological testing. Because this environment mimics some aspects of the unloading observed in traumatic skeletal reconstruction patients, we can use it to better assess the efficacy of bone healing agents in the context of unloading (Milstead et al., 2004). Indeed, this type of approach has been suggested for development of novel drugs for bone loss diseases such as osteoporosis (Milstead et al., 2004). Unfortunately, in many cases promising animal data does not translate into the clinic. With regard to fracture healing, this may be due to the animal models bearing weight much sooner than human patients do, as weight bearing improves fracture healing.
The gold standard for surgical techniques to treat human SBDs is to use an autograft to assist with bone healing, which is not feasible in some cases because of insufficient availability (due to defect size) or quality of autograft tissue (due to patient health, e.g. smoking, diabetes etc.). One of the most widely utilized alternatives to autografts is the use of bone morphogenetic proteins (BMPs) to assist with bone healing. BMP treatment results in robust bone regeneration in laboratory animals, but human clinical data show mixed outcomes (Einhorn and Gerstenfeld, 2015, Gautschi et al., 2007). In recent years, BMP use has become limited due to side effects such as heterotopic bone formation and an association with cancer risk (Boraiah et al., 2009, Carragee et al., 2011, Comer et al., 2012, Heggeness, 2011, Helgeson et al., 2011, Mindea et al., 2009, Woo, 2012). Thus, there is a need for both novel bone healing treatment development and animal models that better mimic the clinical scenario. Our planned spaceflight investigation will provide the unique opportunity to test both FDA approved bone healing treatments along with a novel bone healing drug with the important disuse component, better mimicking the treatment course in human patients. However, before spaceflight investigations can be conducted, a number of important preflight ground control experiments were required to increase our chances for a successful mission.
As detailed above, we had three primary issues which were addressed by conducting our spaceflight simulation studies. First, we demonstrated that mice that have undergone our surgical procedure can withstand the forces associated with launch. This was determined qualitatively during and after launch simulation by observing the gait and apparent health of the mice. Just as has been observed with mice which have not undergone surgery, surgical mice remained immobile for 30–90 minutes immediately post-centrifugation but ambulated normally by about the 120 minute mark. Additionally, μCT imaging of the femurs 28 days after launch simulation revealed no surgical failures or alterations in the surgical hardware, and no difference between mice that had undergone launch simulation vs. mice that had not.
Our second goal was to demonstrate that the mice having undergone surgery could successfully ambulate on the wire flooring and successfully be housed in the spaceflight hardware, including being able to access food and water. Indeed, Figures 3A&B showed the average water and food consumption by the mice housed in the different hardware were very similar, indicating that as a group, the mice were able to access food. That said, individual consumption of food was not monitored; therefore, it is possible that some individual mice did experience problems. Our daily in person and video recording checks demonstrated that the mice had full access to the NASA spaceflight hardware and could successful walk on and climb the wire grid cage with no apparent problems in both the Transporter and the Habitat. Also, mice in the Habitat demonstrated robust activity such as chasing and allo-grooming. The mice quickly learned to climb up to the top of the food bar to sit and eat downward, demonstrating this activity within the first day of placement into the hardware as contrasted with a typical 2 – 4 day period in similar tests where mice that have not undergone surgery stretch up to eat the foodbar from below before switching to the top down eating method (personal observation, CSG). Importantly, all mice housed in the spaceflight hardware gained weight over the 28 day post-launch simulation time frame (Figure 3C), confirming that they were able to access the food and water.
Our third objective was to understand the impact of launch conditions, HLU, and different housing/hardware options on bone healing. As shown in Figures 4A–C, the only difference observed in the bone healing parameters measured was a significant 52.5% reduction in total callus volume in HLU mice compared to HLU Controls. This suggests that while HLU does negatively impact the overall callus volume, the callus that is formed is mineralizing to a level consistent with the callus in all of the other mice examined. These observations are consistent with previous HLU studies which examined the impact of HLU on fracture healing, and showed reductions in callus volume and expressions of genes associated with bone healing (Kirchen et al., 1995, Midura et al., 2006, Androjna et al., 2012). While not formally tested here, a smaller callus is expected to be weaker than a larger callus of equivalent BMD.
These HLU results are also consistent with two previous studies examining closed fracture healing in spaceflight (Morey-Holton et al., 2005, Zhao et al., 2014). Specifically, in a composite tissue (muscle, bone & soft tissue) crush injury approximating a closed fracture, rats showed defects in callus formation including impaired cartilage deposition over a 14 day period in space (Kaplansky et al., 1991). In a shorter experiment, rats with a tibial osteotomy injury experienced delayed callus formation in microgravity. Additionally, vascular supply to the callus tissue was diminished (Kirchen et al., 1995). Thus, we predict that our spaceflight bone healing studies will show reduced bone healing as detailed above, but how bone healing therapies behave remains to be determined.
Regarding housing, no differences were observed between HLU Controls and Vivarium Controls, suggesting that singly housing mice does not impact bone healing. That being said, it is known that single housing does impact bone loss observed with spaceflight. Indeed, Wronski et al (Wronski et al., 1998) showed that in spaceflight, group housed rats lost little bone, whereas Jee et al (Jee et al., 1983) showed significant bone loss when rats were singly housed in space. Thus, while single housing appears to impact spaceflight-induced bone loss, our data suggests that single housing does not impact the bone healing parameters evaluated here. However, as shown in Figure 4A we did observe a thymus stress response in singly housed animals, which further supports the importance of evaluating housing effects. Indeed, studies by Bartos and Brain (Bartos and Brain, 1993) showed that individually housing male mice resulted in decreased thymus weights, an indicator of stress. Of importance, although the HLU and HLU Controls both exhibited reduced thymus weights (a possible indication of stress), we still noted a marked difference in the callus volume between these groups.
Finally, comparison of the Launch Negative Control mice with the Launch Positive Control and Launch Positive Experimental mice demonstrated that launch conditions do not impact bone healing or cause any other gross damage to the surgical limb. This finding gave confidence to the RR-4 mission, where our mice underwent similar launch stressors prior to reaching the microgravity environment. Additionally, as this study demonstrated that launch does not impact bone healing parameters measured 28 days post launch simulation, differences observed between spaceflight mice and Earth-based control mice during RR-4 will likely be due to the spaceflight environment and not due to launch effects.
Taken together, we demonstrated that mice with a SBD surgery can withstand the forces and vibrations associated with launch, can be successfully housed in spaceflight hardware, and exhibit effects of unloading on bone healing. These positive findings enabled us to take the next steps toward better modeling the clinical scenario by utilizing the novel microgravity laboratory to unload the mice during bone healing. While much valuable data can be gleaned from the HLU model and many aspects are similar to spaceflight (Kirchen et al., 1995, Sung et al., 2013), the spaceflight model may be a better option, as bone loss and muscle loss differ between spaceflight, HLU, and even nerve injury models (another method of unloading the hindlimb on Earth) (Aguirre et al., 2006, Blaber et al., 2013, Franquinho et al., 2010, Globus et al., 1986, Globus and Morey-Holton, 2016, Morey and Baylink, 1978, Ohara et al., 1994, Vico et al., 1991, Halloran et al., 1985). As an example, higher reductions in bone volume were observed in the tibial metaphysis of spaceflown mice as compared to that observed in HLU mice (Vico et al., 1991). Showing that our SBD model is able to withstand the launch stressors of vibration and hypergravity paved the way for our spaceflight study, and can serve to inform future investigators. We hope the RR-4 mission, which will test the efficacy of current and novel osteoinductive agents on bone healing in the disuse environment of spaceflight vs. on Earth, will lend critical insight into what therapies will truly aid healing for human patients.
5. Conclusions
In conclusion, it was found that neither launch simulation nor caging provided by the NASA Rodent Research Project impacted bone healing. Removal of weight bearing, as accomplished by hindlimb suspension, resulted in a significant reduction in total bone callus volume, whereas singly housing mice did not impact bone healing in our model. Therefore, since limited weight bearing is obligatory in microgravity (simulating injured patients with restricted weight bearing), the RR-4 mission should be a higher fidelity model for human bone healing, improving translation into the clinic.
Figure 5. Bone Healing. (A) Fracture Healing Score.
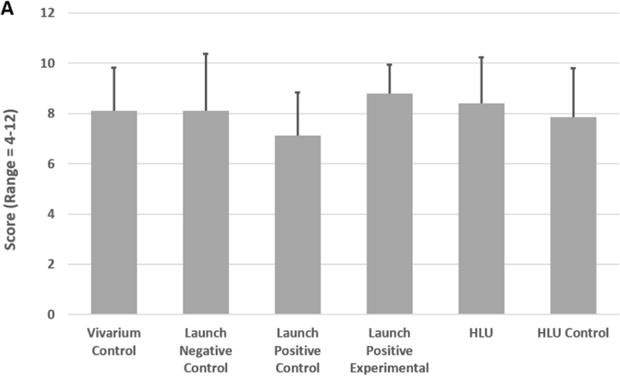
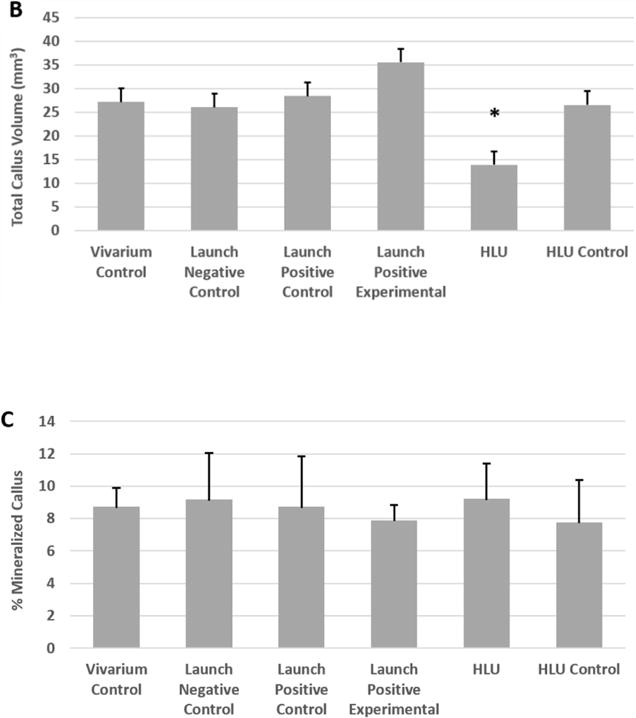
Femurs were subjected to μCT imaging and then analyzed to assess bone healing. Fracture healing was assessed using the Radiographic Union Score for Tibial Fractures (RUST) method (but applied to femurs). This method assigns a numerical value ranging from 4 (not healed) to 12 (maximally healed) based on the assessment of union in the medial, lateral, anterior, and posterior cortices visible on anteroposterior and lateral μCT images. Bars represent the mean ± SEM. No significant differences were observed between the groups. (B) Callus Volume. Femurs were imaged by μCT and analyzed to assess the total callus volume in the separate test groups. Bars represent the mean ± SEM. *HLU resulted in a significant reduction in callus volume (p<0.05, compared to all other groups). (C) Mineralized Callus. Femurs were evaluated by μCT and the percentage of mineralized callus was measured. Bars represent the mean ± SEM. No significant differences were observed between the groups.
Acknowledgments
We would like to thank the following people for their assistance in conducting the surgeries associated with the work: Kishan Shah, Aamir Tucker, Faisal Khan, Jane Han, and Yinghua Cheng. We would also like to thank all of the personnel at NASA Ames that made this study possible: America Reyes, Michelle Hing, Nicolas Cole, and Marie Dinh for supporting the HLU experiments; Daniel Rozewicz, Nicholas Mason, Duncan Espinoza, and Peter Eikemeyer for hardware preparation and test support; Allison Brown, R.V.T. and Dr. Stephanie Solis, D.V.M., for animal assessment and care; and Richard Rowan, Frank Pichay, and Lynn Hoflund of the launch simulation test facility. This work was also supported in part by a postdoctoral NIH T32 Training Grant in Hematopoiesis, T32 DK007519 (PC, DJO), a Center for Research and Learning RISE Scholarship, Indiana University Purdue University Indianapolis (AB), and the Department of Orthopaedic Surgery, Indiana University School of Medicine (MAK). This work was supported in part by a grant from the Orthopaedic Trauma Association (MAK, TOM) and the Ralph W. and Grace M. Showalter Research Trust Fund (MAK). In addition, research reported in this publication was supported in part by the following grants: NIH NIAMS R01 AR060863 (MAK) and GA-2015-217 from the Center for the Advancement of Sciences in Space (CASIS, MAK). This work was also supported by the US Army Medical Research and Materiel Command (NC, RH). Additionally, this work supported by the NASA Ames Research Center (CSG, SYC, YS-F).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
The views, opinions, and/or findings contained in this report are those of the author(s) and should not be construed as official Department of the Army, NIH, NASA, or CASIS position, policy, or decision, unless so designated by other official documentation. Citations of commercial organizations or trade names in this report do not constitute an official Department of the Army, NIH, NASA, or CASIS endorsement or approval of the products or services of these organizations.
Opinions, interpretations, conclusions, and recommendations are those of the author(s) and are not necessarily endorsed by the US Army, NIH, NASA, or CASIS. This research complied with the Animal Welfare Act and implementing Animal Welfare Regulations, the Public Health Service Policy on Humane Care and Use of Laboratory Animals, and adhered to the principles noted in The Guide for the Care and Use of Laboratory Animals (NRC, 2011).
References
- ABOU-ISMAIL UA, MAHBOUB HD. The effects of enriching laboratory cages using various physical structures on multiple measures of welfare in singly-housed rats. Lab Anim. 2011;45:145–53. doi: 10.1258/la.2011.010149. [DOI] [PubMed] [Google Scholar]
- AGUIRRE JI, PLOTKIN LI, STEWART SA, WEINSTEIN RS, PARFITT AM, MANOLAGAS SC, BELLIDO T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res. 2006;21:605–15. doi: 10.1359/jbmr.060107. [DOI] [PubMed] [Google Scholar]
- ANDROJNA C, MCCABE NP, CAVANAGH PR, MIDURA RJ. Effects of Spaceflight and Skeletal Unloading on Bone Fracture Healing. Clinical Reviews in Bone and Mineral Metabolism. 2012;10:61–70. [Google Scholar]
- BARTOS L, BRAIN PF. Physiological responses to social status and housing conditions in male mice subject to food competition tests. Bolletino di Zoologia. 1993;60:293–296. [Google Scholar]
- BLABER EA, DVOROCHKIN N, LEE C, ALWOOD JS, YOUSUF R, PIANETTA P, GLOBUS RK, BURNS BP, ALMEIDA EA. Microgravity induces pelvic bone loss through osteoclastic activity, osteocytic osteolysis, and osteoblastic cell cycle inhibition by CDKN1a/p21. PLoS One. 2013;8:e61372. doi: 10.1371/journal.pone.0061372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORAIAH S, PAUL O, HAWKES D, WICKHAM M, LORICH DG. Complications of recombinant human BMP-2 for treating complex tibial plateau fractures: a preliminary report. Clin Orthop Relat Res. 2009;467:3257–62. doi: 10.1007/s11999-009-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUXSEIN ML, BOYD SK, CHRISTIANSEN BA, GULDBERG RE, JEPSEN KJ, MULLER R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- BURGERS TA, VIVANCO JF, ZAHATNANSKY J, MOREN AJV, MASON JJ, WILLIAMS BO. Mice with a heterozygous Lrp6 deletion have impaired fracture healing. 2016;4:16025. doi: 10.1038/boneres.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARRAGEE EJ, HURWITZ EL, WEINER BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–91. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- CHU TMG, WARDEN SJ, TURNER CH, STEWART RL. Segmental Bone Regeneration Using a Load Bearing Biodegradable Carrier of Bone Morphogenetic Protein-2. Biomaterials. 2007;28:459–467. doi: 10.1016/j.biomaterials.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMER GC, SMITH MW, HURWITZ EL, MITSUNAGA KA, KESSLER R, CARRAGEE EJ. Retrograde ejaculation after anterior lumbar interbody fusion with and without bone morphogenetic protein-2 augmentation: a 10-year cohort controlled study. Spine J. 2012;12:881–90. doi: 10.1016/j.spinee.2012.09.040. [DOI] [PubMed] [Google Scholar]
- COVEY DC. Blast and fragment injuries of the musculoskeletal system. J Bone Joint Surg Am. 2002;84-a:1221–34. doi: 10.2106/00004623-200207000-00022. [DOI] [PubMed] [Google Scholar]
- EINHORN TA, GERSTENFELD LC. Fracture healing: mechanisms and interventions. Nature reviews. Rheumatology. 2015;11:45–54. doi: 10.1038/nrrheum.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVERDS NE, SNYDER PW, BAILEY KL, BOLON B, CREASY DM, FOLEY GL, ROSOL TJ, SELLERS T. Interpreting Stress Responses during Routine Toxicity Studies. Toxicologic Pathology. 2013;41:560–614. doi: 10.1177/0192623312466452. %! Interpreting Stress Responses during Routine Toxicity Studies. [DOI] [PubMed] [Google Scholar]
- FEHER A, KOIVUNEMI A, KOIVUNEMI M, FUCHS RK, BURR DB, PHIPPS RJ, REINWALD S, ALLEN MR. Bisphosphonates do not inhibit periosteal bone formation in estrogen deficient animals and allow enhanced bone modeling in response to mechanical loading. Bone. 2010;46:203–7. doi: 10.1016/j.bone.2009.10.023. [DOI] [PubMed] [Google Scholar]
- FRANQUINHO F, LIZ MA, NUNES AF, NETO E, LAMGHARI M, SOUSA MM. Neuropeptide Y and osteoblast differentiation–the balance between the neuro-osteogenic network and local control. Febs j. 2010;277:3664–74. doi: 10.1111/j.1742-4658.2010.07774.x. [DOI] [PubMed] [Google Scholar]
- GAMALLO A, VILLANUA A, TRANCHO G, FRAILE A. Stress adaptation and adrenal activity in isolated and crowded rats. Physiology & Behavior. 1986;36:217–221. doi: 10.1016/0031-9384(86)90006-5. [DOI] [PubMed] [Google Scholar]
- GAUTSCHI OP, FREY SP, ZELLWEGER R. Bone morphogenetic proteins in clinical applications. ANZ J Surg. 2007;77:626–31. doi: 10.1111/j.1445-2197.2007.04175.x. [DOI] [PubMed] [Google Scholar]
- GLOBUS RK, BIKLE DD, MOREY-HOLTON E. The temporal response of bone to unloading. Endocrinology. 1986;118:733–42. doi: 10.1210/endo-118-2-733. [DOI] [PubMed] [Google Scholar]
- GLOBUS RK, MOREY-HOLTON E. Hindlimb unloading: rodent analog for microgravity. J Appl Physiol (1985) 2016;120:1196–206. doi: 10.1152/japplphysiol.00997.2015. [DOI] [PubMed] [Google Scholar]
- HALLORAN BP, BIKLE DD, WRONSKI TJ, GLOBUS RK, LEVENS MJ, MOREY-HOLTON E. Effect of simulated weightlessness and chronic 1,25-dihydroxyvitamin D administration on bone metabolism. Physiologist. 1985;28:S127–8. [PubMed] [Google Scholar]
- HEGGENESS MH. Important considerations on bone morphogenetic protein-2 and neuroinflammation. Spine J. 2011;11:506. doi: 10.1016/j.spinee.2011.05.010. [DOI] [PubMed] [Google Scholar]
- HELGESON MD, LEHMAN RA, JR, PATZKOWSKI JC, DMITRIEV AE, ROSNER MK, MACK AW. Adjacent vertebral body osteolysis with bone morphogenetic protein use in transforaminal lumbar interbody fusion. Spine J. 2011;11:507–10. doi: 10.1016/j.spinee.2011.01.017. [DOI] [PubMed] [Google Scholar]
- JEE WS, WRONSKI TJ, MOREY ER, KIMMEL DB. Effects of spaceflight on trabecular bone in rats. Am J Physiol. 1983;244:R310–4. doi: 10.1152/ajpregu.1983.244.3.R310. [DOI] [PubMed] [Google Scholar]
- KAPLANSKY AS, DURNOVA GN, BURKOVSKAYA TE, VOROTNIKOVA EV. The effect of microgravity on bone fracture healing in rats flown on Cosmos-2044. Physiologist. 1991;34:S196–9. [PubMed] [Google Scholar]
- KERSHAW CJ, CUNNINGHAM JL, KENWRIGHT J. Tibial external fixation, weight bearing, and fracture movement. Clin Orthop Relat Res. 1993:28–36. [PubMed] [Google Scholar]
- KIRCHEN ME, O’CONNOR KM, GRUBER HE, SWEENEY JR, FRAS IA, STOVER SJ, SARMIENTO A, MARSHALL GJ. Effects of microgravity on bone healing in a rat fibular osteotomy model. Clin Orthop Relat Res. 1995:231–42. [PubMed] [Google Scholar]
- KOMATSU DE, MARY MN, SCHROEDER RJ, ROBLING AG, TURNER CH, WARDEN SJ. Modulation of Wnt signaling influences fracture repair. Journal of Orthopaedic Research. 2010;28:928–936. doi: 10.1002/jor.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOOISTRA BW, DIJKMAN BG, BUSSE JW, SPRAGUE S, SCHEMITSCH EH, BHANDARI M. The radiographic union scale in tibial fractures: reliability and validity. J Orthop Trauma. 2010;24(Suppl 1):S81–6. doi: 10.1097/BOT.0b013e3181ca3fd1. [DOI] [PubMed] [Google Scholar]
- MIDURA RJ, SU X, ANDROJNA C. A simulated weightlessness state diminishes cortical bone healing responses. J Musculoskelet Neuronal Interact. 2006;6:327–8. [PubMed] [Google Scholar]
- MILSTEAD JR, SIMSKE SJ, BATEMAN TA. Spaceflight and hindlimb suspension disuse models in mice. Biomed Sci Instrum. 2004;40:105–10. [PubMed] [Google Scholar]
- MINDEA SA, SHIH P, SONG JK. Recombinant human bone morphogenetic protein-2-induced radiculitis in elective minimally invasive transforaminal lumbar interbody fusions: a series review. Spine (Phila Pa 1976) 2009;34:1480–4. doi: 10.1097/BRS.0b013e3181a396a1. discussion 1485. [DOI] [PubMed] [Google Scholar]
- MORAN CJ, RAMESH A, BRAMA PA, O’BYRNE JM, O’BRIEN FJ, LEVINGSTONE TJ. The benefits and limitations of animal models for translational research in cartilage repair. J Exp Orthop. 2016;3:1. doi: 10.1186/s40634-015-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOREY-HOLTON E, GLOBUS RK, KAPLANSKY A, DURNOVA G. The hindlimb unloading rat model: literature overview, technique update and comparison with space flight data. Adv Space Biol Med. 2005;10:7–40. doi: 10.1016/s1569-2574(05)10002-1. [DOI] [PubMed] [Google Scholar]
- MOREY-HOLTON ER, GLOBUS RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol (1985) 2002;92:1367–77. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
- MOREY ER, BAYLINK DJ. Inhibition of bone formation during space flight. Science. 1978;201:1138–41. doi: 10.1126/science.150643. [DOI] [PubMed] [Google Scholar]
- OHARA S, ROTH KA, BEAUDET LN, SCHMIDT RE. Transganglionic neuropeptide Y response to sciatic nerve injury in young and aged rats. J Neuropathol Exp Neurol. 1994;53:646–62. doi: 10.1097/00005072-199411000-00012. [DOI] [PubMed] [Google Scholar]
- ROBERT ROZBRUCH S, WEITZMAN AM, TRACEY WATSON J, FREUDIGMAN P, KATZ HV, ILIZAROV S. Simultaneous treatment of tibial bone and soft-tissue defects with the Ilizarov method. J Orthop Trauma. 2006;20:197–205. doi: 10.1097/00005131-200603000-00006. [DOI] [PubMed] [Google Scholar]
- SUN GS, TOU JC, REISS-BUBENHEIM DA, HILL EL, LIITTSCHWAGER KW, GIRTEN BE, PENA-YEWKUKHIW E. Oxidative and nutrient stability of a standard rodent spaceflight diet during long-term storage. Lab Anim (NY) 2012;41:252–259. doi: 10.1038/laban0912-252. [DOI] [PubMed] [Google Scholar]
- SUN GS, TOU JC, LIITTSCHWAGER K, HERRERA AM, HILL EL, GIRTEN B, REISS-BUBENHEIM D, VASQUES M. Evaluation of the nutrient-upgraded rodent food bar for rodent spaceflight experiments. Nutrition. 2010;26:1163–9. doi: 10.1016/j.nut.2009.09.018. [DOI] [PubMed] [Google Scholar]
- SUNG M, LI J, SPIEKER AJ, SPATZ J, ELLMAN R, FERGUSON VL, BATEMAN TA, ROSEN GD, BOUXSEIN M, RUTKOVE SB. Spaceflight and hind limb unloading induce similar changes in electrical impedance characteristics of mouse gastrocnemius muscle. J Musculoskelet Neuronal Interact. 2013;13:405–11. [PMC free article] [PubMed] [Google Scholar]
- SWIFT JM, NILSSON MI, HOGAN HA, SUMNER LR, BLOOMFIELD SA. Simulated resistance training during hindlimb unloading abolishes disuse bone loss and maintains muscle strength. J Bone Miner Res. 2010;25:564–74. doi: 10.1359/jbmr.090811. [DOI] [PubMed] [Google Scholar]
- VICO L, NOVIKOV VE, VERY JM, ALEXANDRE C. Bone histomorphometric comparison of rat tibial metaphysis after 7-day tail suspension vs. 7-day spaceflight. Aviat Space Environ Med. 1991;62:26–31. [PubMed] [Google Scholar]
- WARDEN SJ, KOMATSU DE, RYDBERG J, BOND JL, HASSETT SM. Recombinant human parathyroid hormone (PTH 1-34) and low-intensity pulsed ultrasound have contrasting additive effects during fracture healing. Bone. 2009;44:485–94. doi: 10.1016/j.bone.2008.11.007. [DOI] [PubMed] [Google Scholar]
- WEATHERHOLT AM, FUCHS RK, WARDEN SJ. Cortical and trabecular bone adaptation to incremental load magnitudes using the mouse tibial axial compression loading model. Bone. 2013;52:372–9. doi: 10.1016/j.bone.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHELAN DB, BHANDARI M, STEPHEN D, KREDER H, MCKEE MD, ZDERO R, SCHEMITSCH EH. Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J Trauma. 2010;68:629–32. doi: 10.1097/TA.0b013e3181a7c16d. [DOI] [PubMed] [Google Scholar]
- WOO EJ. Recombinant human bone morphogenetic protein-2: adverse events reported to the Manufacturer and User Facility Device Experience database. Spine J. 2012;12:894–9. doi: 10.1016/j.spinee.2012.09.052. [DOI] [PubMed] [Google Scholar]
- WRONSKI TJ, LI M, SHEN Y, MILLER SC, BOWMAN BM, KOSTENUIK P, HALLORAN BP. Lack of effect of spaceflight on bone mass and bone formation in group-housed rats. J Appl Physiol (1985) 1998;85:279–85. doi: 10.1152/jappl.1998.85.1.279. [DOI] [PubMed] [Google Scholar]
- ZHAO F, LI D, ARFAT Y, CHEN Z, LIU Z, LIN Y, DING C, SUN Y, HU L, SHANG P, QIAN A. Reloading partly recovers bone mineral density and mechanical properties in hind limb unloaded rats. Acta Astronautica. 2014;105:57–65. [Google Scholar]
- ZWINGENBERGER S, NIEDERLOHMANN E, VATER C, RAMMELT S, MATTHYS R, BERNHARDT R, VALLADARES RD, GOODMAN SB, STIEHLER M. Establishment of a femoral critical-size bone defect model in immunodeficient mice. J Surg Res. 2013;181:e7–e14. doi: 10.1016/j.jss.2012.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]


