Abstract
IMPORTANCE
Spontaneous preterm birth is a leading cause of infant mortality. Prediction, largely based on prior pregnancy outcomes, is not possible in women pregnant for the first time.
OBJECTIVE
To assess the accuracy of universal screening to predict spontaneous preterm birth in nulliparous women using serial measurements of vaginal fetal fibronectin levels and cervical length.
DESIGN, SETTINGS, AND PARTICIPANTS
A prospective observational cohort study of nulliparous women with singleton pregnancies, from 8 clinical sites across the United States between October 2010 and May 2014. Women and clinicians were blinded to results unless cervical shortening less than 15 mm was identified.
EXPOSURES
Transvaginal cervical length and quantitative vaginal fetal fibronectin levels were reviewed at 2 study visits 4 or more weeks apart.
MAIN OUTCOMES AND MEASURES
Spontaneous preterm birth at less than 37 weeks was the primary outcome. Cervical length and quantitative fetal fibronectin were considered independently and together at each visit. Measurement distributions were compared for spontaneous preterm birth vs all other births. Spontaneous preterm birth before 32 weeks was a secondary outcome.
RESULTS
The study included 9410 women (median age, 27.0 [interquartile range, 9.0] years; 60.7% non-Hispanic white, 13.8% non-Hispanic black, 16.5% Hispanic, 4.0% Asian, and 5.1% other), of whom 474 (5.0%) had spontaneous preterm births, 335 (3.6%) had medically indicated preterm births, and 8601 (91.4%) had term births. Among women with spontaneous preterm birth, cervical length of 25 mm or less occurred in 35 of 439 (8.0%) at 16 to 22 weeks’ gestation and in 94 of 403 (23.3%) at 22 to 30 weeks’ gestation. Fetal fibronectin levels of 50 ng/mL or greater at 16 to 22 weeks identified 30 of 410 women (7.3%) with spontaneous preterm birth and 31 of 384 (8.1%) at 22 to 30 weeks. The area under the receiver operating characteristic curve for screening between 22 and 30 weeks for fetal fibronectin level alone was 0.59 (95% CI, 0.56–0.62), for transvaginal cervical length alone was 0.67 (95% CI, 0.64–0.70), and for the combination as continuous variables was 0.67 (95% CI, 0.64–0.70).
CONCLUSIONS AND RELEVANCE
Among nulliparous women with singleton pregnancies, quantitative vaginal fetal fibronectin and serial transvaginal ultrasound cervical length had low predictive accuracy for spontaneous preterm birth. These findings do not support routine use of these tests in such women.
Preterm birth, affecting approximately 12% of the deliveries in the United States, was responsible for 35% of the world’s 3.1 million annual neonatal deaths in 2006.1 Although rates have decreased over the past decade, health care costs and long-term health consequences for children born preterm are enormous, reaching more than $26.2 billion, or $51 600 for every infant born prematurely in the United States in 2006.2 Current strategies to identify women at risk are largely based on prior pregnancy outcomes, but risk assessment in women pregnant for the first time is difficult. Short cervical length has been associated with an increased risk of preterm birth in some studies.3,4 Randomized trials demonstrating reduced rates of premature birth in women with a short cervix treated with vaginal progesterone5,6 have prompted consideration of universal ultrasound screening for short cervix.7–9 Despite the fact that the American College of Obstetricians and Gynecologists does not recommend routine screening in low-risk populations, routine evaluation of cervical length in all patients is a common practice.10
A positive qualitative test for fetal fibronectin in cervicovaginal fluid is also associated with premature birth risk, but the sensitivity and positive predictive value (PPV) are low, and there are no known treatments for women with a positive test result.11,12 However, use of quantitative, rather than qualitative, fetal fibronectin measures may improve accuracy of this test.13
The combination of transvaginal cervical length and fetal fibronectin levels to identify women at risk has been studied, with conflicting results.14,15 The current study was designed to assess the accuracy of universal screening using serial transvaginal cervical length and quantitative measurement of fetal fibronectin levels to predict spontaneous preterm birth in a large, prospective cohort of nulliparous women.
Methods
Participants and Recruitment
The Eunice Kennedy Shriver National Institute of Child Health and Human Development established the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b) to study nulliparous women with an overarching goal to identify factors that predict adverse pregnancy outcomes such as spontaneous preterm birth.16 The study is a prospective cohort study with a target recruitment of 10 000 nulliparous women. Nulliparous women with singleton pregnancies were recruited from hospitals in 8 clinical centers in the United States. All local institutional review boards approved the study protocol. Participants provided written informed consent. A detailed description of study methods and procedures is available.16
Pregnant women who planned to deliver their infants at one of the clinical site hospitals were recruited before 14 weeks’ gestational age. Women were invited to participate if they had a viable singleton gestation, were between 6 weeks 0 days of gestation and 13 weeks 6 days of gestation based on a documented ultrasound crown-rump length measurement by a certified study sonographer, and were nulliparous, defined as having had no prior pregnancy lasting 20 weeks or more based on self-report.
Maternal race/ethnicity was self-reported by participants in response to prespecified options defined by the investigators. Race/ethnicity was used in this report to demonstrate that this population represents the overall racial and ethnic distribution of the general pregnant population in the United States.
Primary Outcome
Spontaneous preterm birth, defined as delivery before 37 weeks 0 days of gestation occurring subsequent to spontaneous onset of labor or premature rupture of the membranes, regardless of subsequent labor augmentation or cesarean delivery, was the primary outcome. Pregnancy losses before 20 weeks’ gestation and pregnancy terminations were excluded. Spontaneous preterm birth was documented by chart abstraction by certified research personnel. Women without spontaneous labor or premature rupture of the membranes and who were delivered by their physician before 37 weeks’ gestation (for example, for preeclampsia, intrauterine growth restriction, or maternal indications) were included in the control group. Spontaneous preterm birth occurring before 32 weeks 0 days of gestation was prospectively identified as a secondary outcome.
Study Visits
Participants completed 3 study visits: visit 1, between 6 weeks 0 days and 14 weeks 6 days of gestation (median, 12.4 weeks); visit 2, between 16 weeks 0 days and 22 weeks 6 days of gestation (median, 19.0 weeks); and visit 3, between 22 weeks 0 days and 30 weeks 6 days of gestation (median, 28.0 weeks), allowing a 1-week extension from the protocol windows because of mistimed visits. For each participant, all study visits were at least 4 weeks apart. Detailed interviews were performed at each visit to collect demographic characteristics, medical history, and other pertinent clinical data. After delivery, final chart abstraction was performed by trained research staff to document and confirm key clinical outcomes.
Fetal Fibronectin
During visit 1, visit 2, and visit 3, participants were given 3 swabs: 1 swab for a fetal fibronectin test and 2 polyethylene terephthalate (Dacron) swabs for other tests. Fibronectin test kits were provided without charge by Hologic Inc. Participants were instructed to insert the 2 Dacron swabs together about 2 inches into the vagina and rotate the swabs around for 30 seconds, making sure to touch the walls of the vagina to absorb fluid. They were instructed to repeat this procedure with the fetal fibronectin swab. The fetal fibronectin swab was then placed in a tube containing buffer solution, barcoded, and stored at −80°C for shipment to Hologic for assay.
All fetal fibronectin samples were analyzed using enzyme-linked immunosorbent assay as previously described (Hologic Inc). Individuals performing fetal fibronectin laboratory analysis were masked to pregnancy outcomes.
Transvaginal Cervical Length Measurement
Participants underwent transvaginal ultrasound to measure transvaginal cervical length at visits 2 and 3 using a previously described technique.16 All persons performing transvaginal cervical length measurements were required to complete an educational module and submit 3 images from each of 5 pregnant women for review by a single reviewer (J.D.I.) using published criteria or to have been previously credentialed using the same process and reviewer for other research studies.17
Study visits were not part of clinical care. Research participants, clinicians, and those performing the chart reviews were blinded to the results of the transvaginal ultrasonography and fetal fibronectin assays. Those performing the fetal fibronectin assays were blinded to the cervical length results. Research data were communicated to the clinician only when an ultrasound revealed major fetal structural malformation, hydrops, fetal demise, estimated fetal weight less than fifth percentile, oligohydramnios, cervical length less than 15 mm before 28 weeks, fetal bradycardia or tachycardia, or placenta or vasa previa found at the third study visit. Clinical care of women found to have a short cervix was left to the discretion of the referring physician.
Statistical Analysis
Study participants with pregnancies carried 20 weeks or more were eligible for this analysis. Women with pregnancy outcome data and at least 1 visit at which either fetal fibronectin or cervical length were obtained were included. Fetal fibronectin and cervical length were considered as individual screening tools at each study visit for spontaneous preterm birth using previously described thresholds (cervical length ≤20 mm or ≤25 mm; fetal fibronectin levels ≥10, ≥50, and ≥200 ng/mL),3,4,8 and we investigated all potential thresholds using receiver operating characteristic (ROC) curves. Rates of change in the measurements relative to the weeks elapsed between visits were also assessed, and multiple logistic regression models were used to combine information on fetal fibronectin levels and cervical length in prediction of spontaneous preterm birth. Test characteristics, including sensitivity, specificity, positive and negative predictive values, and likelihood ratios, were computed for selected thresholds and Mann-Whitney U statistics provided estimates of the areas under the ROC curves (AUCs). The method of Delong, Delong, and Clarke-Pearson was used for comparing AUCs.18
The assay range for fetal fibronectin was reported by the laboratory analyst at Hologic as 0 to 500 ng/mL. Reported values greater than 500 were set to 500, and an indicator variable for outside the assay range was included with the measurement in a multiple logistic regression model to assess fetal fibronectin measurement as an individual screening tool. Before using cervical length and fetal fibronectin level together, the distributions of the measurements were reviewed for departures from normality. Fetal fibronectin measurements were highly skewed to the left, and Box-Cox log transformations19 were taken, identifying the reciprocal square root transformation best in normalizing the data. Thus, the multiple logistic regression model combining cervical length and fetal fibronectin level included cervical length on the original scale, fetal fibronectin level on the reciprocal square root scale, and an indicator for fetal fibronectin level outside the assay range. The Hosmer-Lemeshow test20 was used to evaluate goodness-of-fit in the model combining cervical length and fetal fibronectin values.
Generally, characteristics and measurements were contrasted between groups using Wilcoxon rank sum tests for distributions of measurements on a continuous scale, and χ2 tests for categorical data.
By protocol, women were informed of cervical length measurements less than 15 mm at a study visit, and their clinician may have opted to treat with progesterone for preterm birth. We performed a post hoc sensitivity analysis in which women given progesterone after 16 weeks’ gestation were included as spontaneous preterm births (regardless of actual pregnancy outcome) when calculating AUCs for cervical length in prediction of spontaneous preterm birth.
All tests in this report were performed at a nominal significance level of α = .05; all tests with 1 df were 2-sided; and no correction was made for multiple comparisons. Analyses were conducted using SAS version 9.3/9.4 (SAS Institute Inc).
Results
Between October 2010 and May 2014 (date of last delivery), the parent protocol enrolled and followed up 10 038 women.16 Of these, 459 were excluded because of missing pregnancy outcome data and 110 were excluded who had a pregnancy loss prior to 20 weeks’ gestation or a pregnancy termination, leaving 9469 women for analysis (Figure 1). Of these, 477 (5%) experienced a spontaneous preterm birth and 8992 (95%) experienced either a term birth or a nonspontaneous preterm birth. There were 3 women with spontaneous preterm births and 56 women with other births who did not have at least 1 documented transvaginal cervical length or fetal fibronectin measurement; these women also were excluded from the analysis, leaving 474 spontaneous preterm birth cases and 8936 other birth controls (335 medically indicated preterm births and 8601 term births). There were 303 women with a cervical length measurement less than 15 mm.
Figure 1. Enrollment and Inclusion in Analysis in the Nulliparous Pregnancy Outcome Study: Monitoring Mothers-to-Be (nuMoM2b).
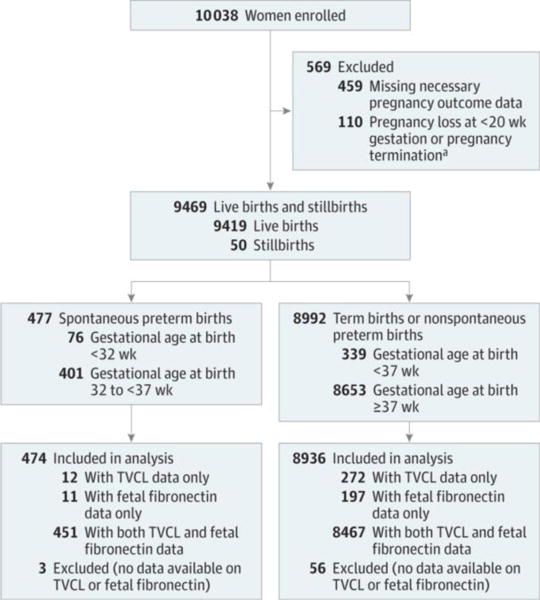
This analysis assessed serial transvaginal ultrasound cervical length and quantitative vaginal fetal fibronectin, alone and in combination, and measured at different points during pregnancy and as rates of change, to detect spontaneous preterm birth in nulliparous women with singleton pregnancies successfully carried 20 weeks or more. Women enrolled in the study who carried their pregnancy to 20 weeks or more were eligible for the analysis. To determine eligibility required collection of the necessary pregnancy outcome data to exclude pregnancy losses at less than 20 weeks. Furthermore, the analysis was only possible among the women with results from at least 1 serial transvaginal ultrasound or 1 sample assayed for fetal fibronectin level. TVCL indicates transvaginal cervical length.
aElective termination (n = 10), indicated termination (n = 23), and fetal demise at less than 20 weeks’ gestational age (n = 77).
Baseline demographic characteristics of the study population are presented in Table 1. Overall, the women had a median age of 27.0 (interquartile range, 9.0) years; 60.7% were non-Hispanic white, 13.8% non-Hispanic black, 16.5% Hispanic, 4.0% Asian, and 5.1% other race/ethnicity or multiracial. Women who experienced a spontaneous preterm birth were more likely to be at the extremes of maternal age, to have smoked in the 3 months prior to pregnancy, to have fewer years of education, to be single and never married, and to have preexisting diabetes compared with controls. The groups did not differ with respect to race and ethnicity, body mass index, or preexisting hypertension. eTable 1 in the Supplement contrasts baseline demographic characteristics for study participants with and without outcome data. Those without outcome data tended to be younger, less educated, more often of minority race/ethnicity, and more often single.
Table 1.
Demographic Characteristics and Baseline Risk Factors
| Baseline Characteristicsa | Pregnancy Outcome | P Valueb | |
|---|---|---|---|
| SPTB (n = 474) | Other Births (n = 8936) | ||
| Maternal age, y | |||
| Median (IQR) | 27.0 (9.0) | 27.0 (9.0) | .31 |
| Category, No. (%) | |||
| 13–21 | 118 (24.9) | 1849 (20.7) | .01 |
| 22–35 | 315 (66.5) | 6500 (72.8) | |
| >35 | 41 (8.6) | 584 (6.5) | |
| Maternal race/ethnicity, No. (%) | |||
| White non-Hispanic | 273 (57.6) | 5435 (60.8) | .13 |
| Black non-Hispanic | 82 (17.3) | 1212 (13.6) | |
| Hispanic | 74 (15.6) | 1477 (16.5) | |
| Asian | 16 (3.4) | 362 (4.1) | |
| Other | 29 (6.1) | 447 (5.0) | |
| BMIc | |||
| Median (IQR) | 24.5 (7.2) | 24.6 (7.2) | .47 |
| Category, No. (%) | |||
| <25 | 252 (54.4) | 4658 (53.1) | .83 |
| 25 to <30 | 110 (23.8) | 2174 (24.8) | |
| ≥30 | 101 (21.8) | 1947 (22.2) | |
| Gravidity, No. (%) | |||
| 1 | 352 (74.3) | 6656 (74.5) | .84 |
| 2 | 88 (18.6) | 1695 (19.0) | |
| ≥3 | 34 (7.2) | 582 (6.5) | |
| Smoked during 3 mo prior to pregnancy, No. (%) | 113 (23.9) | 1547 (17.3) | <.001 |
| Maternal education obtained, No. (%) | |||
| Less than high school | 54 (11.4) | 702 (7.9) | <.001 |
| Completed high school or GED | 75 (15.8) | 1010 (11.3) | |
| Some college | 94 (19.8) | 1706 (19.1) | |
| Associate or technical degree | 55 (11.6) | 899 (10.1) | |
| Completed college | 104 (21.9) | 2516 (28.2) | |
| Degree work beyond college | 92 (19.4) | 2098 (23.5) | |
| Marital status, No. (%) | |||
| Single, never married | 214 (45.1) | 3429 (38.4) | .01 |
| Married | 253 (53.4) | 5401 (60.5) | |
| Widowed | 8 (0.1) | 0 | |
| Divorced | 5 (1.1) | 76 (0.9) | |
| Separated | 2 (0.4) | 13 (0.1) | |
| Chronic hypertension, No. (%) | 9 (1.9) | 231 (2.6) | .35 |
| Diabetes, No. (%) | 17 (3.6) | 134 (1.5) | <.001 |
Abbreviations: BMI, body mass index; GED, General Educational Development; IQR, interquartile range; SPTB, spontaneous preterm births.
Sample sizes vary slightly by baseline characteristic: maternal age (n = 9407); maternal race/ethnicity (n = 9407); BMI (n = 9242); gravidity (n = 9407); smoking status (n = 9403); maternal education (n = 9405); marital status (n = 9401); chronic hypertension (n = 9391); diabetes (n = 9399).
P values shown are from χ2 tests for spontaneous preterm birth and the categorical baseline characteristics and from Wilcoxon rank sum tests for spontaneous preterm birth and continuous baseline characteristics.
Calculated as weight in kilograms divided by height in meters squared.
The relationship between transvaginal cervical length and spontaneous preterm birth is presented in Table 2. Women with spontaneous preterm births (cases) before 37 weeks had shorter transvaginal cervical length measurements than those with other births (controls) at visit 2 (median cervical length, 36 mm [25th–75th percentiles, 31 to 41] for cases vs 39 mm [25th–75th percentiles, 35 to 44] for controls; P < .001) and visit 3 (median cervical length, 32 mm [25th–75th percentiles, 26 to 38] for cases vs 37 mm [25th–75th percentiles, 32 to 42] for controls; P < .001). Cervical length was significantly shorter for those with spontaneous preterm birth before 32 weeks compared with controls at both visit 2 (median cervical length, 32 mm [25th–75th percentiles, 26 to 37] for cases vs 39 mm [25th–75th percentiles, 35 to 44] for controls; P < .001) and visit 3 (median cervical length, 20 mm [25th–75th percentiles, 4 to 33] for cases vs 37 mm [25th–75th percentiles, 32 to 42] for controls; P < .001). Also, transvaginal cervical length decreased to a greater degree between visit 2 and visit 3 for those with spontaneous preterm birth (median rate of change, −0.55 mm/wk [25th–75th percentiles, −1.36 to 0.01] for cases vs −0.23 mm/wk [25th–75th percentiles, −0.83 to 0.28] for controls; P < .001). A transvaginal cervical length of 25 mm or less identified 35 of 439 women (8.0%) with spontaneous preterm birth before 37 weeks at visit 2 and 94 of 403 (23.3%) at visit 3.
Table 2.
Transvaginal Cervical Length and Quantitative Vaginal Fetal Fibronectin Level at Study Visits by Stratifications on Births
| Stratification 1, No. (%) | P Valuee | Stratification 2, No. (%) | P Valuee | |||
|---|---|---|---|---|---|---|
| SPTB <37 wk Gestation | Other Births | SPTB <32 wk Gestation | Other Births | |||
| Transvaginal Cervical Length, mm | ||||||
| Visit 2 (16–22 wk gestational age), No.a | 439 | 8332 | 67 | 8704 | ||
| Median (25th–75th percentile) | 36 (31 to 41) | 39 (35 to 44) | <.001 | 32 (26 to 37) | 39 (35 to 44) | <.001 |
| ≤25 | 35 (8) | 181 (2) | <.001 | 16 (24) | 200 (2) | <.001 |
| ≤20 | 18 (4) | 98 (1) | <.001 | 10 (15) | 106 (1) | <.001 |
| Visit 3 (22–30 wk gestational age), No.a | 403 | 8304 | 25 | 8682 | ||
| Median (25th–75th percentile) | 32 (26 to 38) | 37 (32 to 42) | <.001 | 20 (4 to 33) | 37 (32 to 42) | <.001 |
| ≤25 | 94 (23) | 530 (6) | <.001 | 13 (52) | 611 (7) | <.001 |
| ≤20 | 70 (17) | 266 (3) | <.001 | 13 (52) | 323 (4) | <.001 |
| Rate of change from visit 2 to visit 3, mm/wk, No.a | 378 | 7865 | 23 | 8220 | ||
| Median (25th–75th percentile) | −0.55 (−1.36 to 0.01) | −0.23 (−0.83 to 0.28) | <.001 | −1.47 (−3.11 to −0.12) | −0.24 (−0.84 to 0.26) | .002 |
| ≤25th percentileb | 159 (42) | 1905 (24) | <.001 | 14 (61) | 2050 (25) | <.001 |
| Fetal Fibronectin, ng/mL | ||||||
| Visit 1 (6–14 wk gestational age), No.c | 411 | 7691 | 66 | 8036 | ||
| Median (25th–75th percentile) | 5 (3 to 32) | 4 (2 to 11) | <.001 | 9 (3 to 70) | 4 (2 to 11) | <.001 |
| ≥10 | 142 (35) | 1989 (26) | <.001 | 32 (48) | 2099 (26) | <.001 |
| ≥50 | 87 (21) | 953 (12) | <.001 | 19 (29) | 1021 (13) | <.001 |
| ≥200 | 39 (9) | 417 (5) | <.001 | 11 (17) | 445 (6) | <.001 |
| Visit 2 (16–22 wk gestational age), No.c | 410 | 7873 | 64 | 8219 | ||
| Median (25th–75th percentile) | 3 (2 to 6) | 3 (2 to 5) | .18 | 3 (2 to 15) | 3 (2 to 5) | .02 |
| ≥10 | 62 (15) | 902 (11) | .02 | 19 (30) | 945 (11) | <.001 |
| ≥50 | 30 (7) | 312 (4) | <.001 | 10 (16) | 332 (4) | <.001 |
| ≥200 | 12 (3) | 133 (2) | .06 | 5 (8) | 140 (2) | <.001 |
| Visit 3 (22–30 wk gestational age), No.c | 384 | 8092 | 28 | 8448 | ||
| Median (25th–75th percentile) | 3 (2 to 8) | 3 (2 to 5) | <.001 | 9 (3 to 103) | 3 (2 to 5) | <.001 |
| ≥10 | 84 (22) | 665 (8) | <.001 | 14 (50) | 735 (9) | <.001 |
| ≥50 | 31 (8) | 258 (3) | <.001 | 9 (32) | 280 (3) | <.001 |
| ≥200 | 15 (4) | 92 (1) | <.001 | 6 (21) | 101 (1) | <.001 |
| Rate of change from visit 1 to visit 2, ng/mL per wk, No.c | 378 | 7238 | 57 | 7559 | ||
| Median (25th–75th percentile) | −0.16 (−2.43 to 0.14) | −0.10 (−0.86 to 0.15) | .02 | −0.45 (−5.58 to 0.12) | −0.10 (−0.88 to 0.15) | .06 |
| ≥75th percentiled | 90 (24) | 1814 (25) | .58 | 14 (25) | 1890 (25) | .94 |
| Rate of change from visit 2 to visit 3, ng/mL per wk, No.c | 345 | 7475 | 25 | 7795 | ||
| Median (25th–75th percentile) | 0.06 (−0.11 to 0.36) | −0.00 (−0.20 to 0.17) | <.001 | 0.50 (−0.05 to 8.40) | 0.00 (−0.20 to 0.17) | .002 |
| ≥75th percentiled | 126 (37) | 1829 (24) | <.001 | 15 (60) | 1940 (25) | <.001 |
Abbreviation: SPTB, spontaneous preterm birth.
Gestational age at the study visit was computed using the project-estimated date of delivery and the ultrasound date. Rate of change was restricted to ultrasounds at least 4 weeks apart.
A cutoff of −0.85 for change in cervical length per week of gestation was the lower 25th percentile for all births.
Gestational age at the study visit was computed using the project-estimated date of delivery and the date of the fetal fibronectin collection, if valid; otherwise, the date of the visit interview was used.
A cutoff of 0.15 for change in fetal fibronectin level from visit 1 to visit 2 per week gestation was the largest 25th percentile for all births. A cutoff of 0.17 for change in fetal fibronectin level from visit 2 to visit 3 per week gestation was the largest 25th percentile for all births.
P values shown are from Wilcoxon rank sum tests comparing distributions (selected percentiles are shown), and from χ2 tests comparing percentages.
The relationship between fetal fibronectin level and spontaneous preterm birth is presented in Table 2. Higher percentages of samples with fetal fibronectin values of 10 ng/mL or greater, 50 ng/mL or greater, and 200 ng/mL or greater were found in the spontaneous preterm birth group compared with the control group. Use of the most commonly accepted threshold of 50 ng/mL or greater identified 87 of 411 women (21.2%) with spontaneous preterm birth at visit 1, 30 of 410 (7.3%) at visit 2, and 31 of 384 (8.1%) at visit 3. The changes from visit 1 to visit 2 and visit 2 to visit 3 were different between women with spontaneous preterm births and those with other births but in opposite directions for visit 1 to visit 2 vs visit 2 to visit 3.
The predictive capabilities of commonly reported thresholds of quantitative fetal fibronectin level and transvaginal cervical length at each study visit are summarized in Table 3 and Table 4. All thresholds had low PPV, with a high of 20.8% for transvaginal cervical length and a high of 14.0% for fetal fibronectin level in prediction of spontaneous preterm birth at less than 37 weeks; and highs of 8.6% and 5.6%, respectively, in prediction of preterm birth at less than 32 weeks.
Table 3.
Predictive Accuracy for Spontaneous Preterm Birth at Less Than 37 Weeks’ Gestation Based on Thresholds on Transvaginal Cervical Length and Quantitative Vaginal Fetal Fibronectin Level at Study Visits
| Thresholda | Measure (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | LR+ | LR− | AUC | |
| Transvaginal Cervical Length, mm | |||||||
| Visit 2 (16–22 wk gestational age), No. | 439 | 8332 | |||||
| ≤25 | 8.0 (5.4–10.5) | 97.8 (97.5–98.1) | 16.2 (11.3–21.1) | 95.3 (94.8–95.7) | 3.67 (2.39–4.95) | 0.94 (0.91–0.97) | 0.53 (0.52–0.54) |
| ≤20 | 4.1 (2.4–6.4) | 98.8 (98.6–99.1) | 15.5 (8.9–22.1) | 95.1 (94.7–95.6) | 3.49 (1.77–5.21) | 0.97 (0.95–0.99) | 0.51 (0.51–0.52) |
| Visit 3 (22–30 wk gestational age), No. | 403 | 8304 | |||||
| ≤25 | 23.3 (19.2–27.5) | 93.6 (93.1–94.1) | 15.1 (12.3–17.9) | 96.2 (95.8–96.6) | 3.65 (2.94–4.37) | 0.82 (0.77–0.86) | 0.58 (0.56–0.61) |
| ≤20 | 17.4 (13.7–21.1) | 96.8 (96.4–97.2) | 20.8 (16.5–25.2) | 96.0 (95.6–96.4) | 5.42 (4.10–6.74) | 0.85 (0.82–0.89) | 0.57 (0.55–0.59) |
| Fetal Fibronectin, ng/mL | |||||||
| Visit 1 (6–14 wk gestational age), No. | 411 | 7691 | |||||
| ≥10 | 34.5 (30.0–39.1) | 74.1 (73.2–75.1) | 6.7 (5.6–7.7) | 95.5 (95.0–96.0) | 1.34 (1.15–1.52) | 0.88 (0.82–0.95) | 0.54 (0.52–0.57) |
| ≥50 | 21.2 (17.2–25.1) | 87.6 (86.9–88.3) | 8.4 (6.7–10.0) | 95.4 (94.9–95.9) | 1.71 (1.37–2.04) | 0.90 (0.85–0.95) | 0.54 (0.52–0.56) |
| ≥200 | 9.5 (6.7–12.3) | 94.6 (94.1–95.1) | 8.6 (6.0–11.1) | 95.1 (94.7–95.6) | 1.75 (1.20–2.30) | 0.96 (0.93–0.99) | 0.52 (0.51–0.53) |
| Visit 2 (16–22 wk gestational age), No. | 410 | 7873 | |||||
| ≥10 | 15.1 (11.7–18.6) | 88.5 (87.8–89.2) | 6.4 (4.9–8.0) | 95.2 (94.8–95.7) | 1.32 (1.01–1.63) | 0.96 (0.92–1.00) | 0.52 (0.50–0.54) |
| ≥50 | 7.3 (4.8–9.8) | 96.0 (95.6–96.5) | 8.8 (5.8–11.8) | 95.2 (94.7–95.7) | 1.85 (1.18–2.51) | 0.97 (0.94–0.99) | 0.52 (0.50–0.53) |
| ≥200 | 2.9 (1.5–5.1) | 98.3 (98.0–98.6) | 8.3 (3.8–12.8) | 95.1 (94.6–95.6) | 1.73 (0.72–2.74) | 0.99 (0.97–1.00) | 0.51 (0.50–0.51) |
| Visit 3 (22–30 wk gestational age), No. | 384 | 8092 | |||||
| ≥10 | 21.9 (17.7–26.0) | 91.8 (91.2–92.4) | 11.2 (9.0–13.5) | 96.1 (95.7–96.5) | 2.66 (2.12–3.20) | 0.85 (0.81–0.90) | 0.57 (0.55–0.59) |
| ≥50 | 8.1 (5.3–10.8) | 96.8 (96.4–97.2) | 10.7 (7.2–14.3) | 95.7 (95.2–96.1) | 2.53 (1.62–3.44) | 0.95 (0.92–0.98) | 0.52 (0.51–0.54) |
| ≥200 | 3.9 (2.2–6.4) | 98.9 (98.6–99.1) | 14.0 (7.4–20.6) | 95.6 (95.2–96.0) | 3.44 (1.59–5.28) | 0.97 (0.95–0.99) | 0.51 (0.50–0.52) |
Abbreviations: AUC, area under receiver operating characteristic curve using single threshold cutpoint; LR−, negative likelihood ratio; LR+, positive likelihood ratio; NPV, negative predictive value; PPV, positive predictive value.
For cervical length, gestational age at the study visit used the project-estimated date of delivery and the ultrasound date. For fetal fibronectin level, gestational age at the study visit used the project-estimated date of delivery and the date of the fetal fibronectin collection, if valid; otherwise, the date of the visit interview was used.
Table 4.
Predictive Accuracy for Spontaneous Preterm Birth at Less Than 32 Weeks’ Gestation Based on Thresholds on Transvaginal Cervical Length and Quantitative Vaginal Fetal Fibronectin Level at Study Visits
| Thresholda | Measure (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | LR+ | LR− | AUC | |
| Transvaginal Cervical Length, mm | |||||||
| Visit 2 (16–22 wk gestational age), No. | 67 | 8704 | |||||
| ≤25 | 23.9 (13.7–34.1) | 97.7 (97.4–98.0) | 7.4 (3.9–10.9) | 99.4 (99.2–99.6) | 10.39 (5.73–15.06) | 0.78 (0.67–0.88) | 0.61 (0.56–0.66) |
| ≤20 | 14.9 (6.4–23.5) | 98.8 (98.6–99.0) | 8.6 (3.5–13.7) | 99.3 (99.2–99.5) | 12.26 (4.87–19.64) | 0.86 (0.77–0.95) | 0.57 (0.53–0.61) |
| Visit 3 (22–30 wk gestational age), No. | 25 | 8682 | |||||
| ≤25 | 52.0 (32.4–71.6) | 93.0 (92.4–93.5) | 2.1 (1.1–3.5) | 99.9 (99.8–99.9) | 7.39 (4.55–10.23) | 0.52 (0.31–0.73) | 0.72 (0.62–0.82) |
| ≤20 | 52.0 (32.4–71.6) | 96.3 (95.9–96.7) | 3.9 (2.1–6.5) | 99.9 (99.8–99.9) | 13.98 (8.50–19.45) | 0.50 (0.30–0.70) | 0.74 (0.64–0.84) |
| Fetal Fibronectin, ng/mL | |||||||
| Visit 1 (6–14 wk gestational age), No. | 66 | 8036 | |||||
| ≥10 | 48.5 (36.4–60.5) | 73.9 (72.9–74.8) | 1.5 (1.0–2.1) | 99.4 (99.2–99.6) | 1.86 (1.39–2.32) | 0.70 (0.53–0.86) | 0.61 (0.55–0.67) |
| ≥50 | 28.8 (17.9–39.7) | 87.3 (86.6–88.0) | 1.8 (1.1–2.8) | 99.3 (99.1–99.5) | 2.27 (1.40–3.14) | 0.82 (0.69–0.94) | 0.58 (0.53–0.64) |
| ≥200 | 16.7 (7.7–25.7) | 94.5 (94.0–95.0) | 2.4 (1.2–4.3) | 99.3 (99.1–99.5) | 3.01 (1.36–4.66) | 0.88 (0.79–0.98) | 0.56 (0.51–0.60) |
| Visit 2 (16–22 wk gestational age), No. | 64 | 8219 | |||||
| ≥10 | 29.7 (18.5–40.9) | 88.5 (87.8–89.2) | 2.0 (1.2–3.1) | 99.4 (99.2–99.6) | 2.58 (1.60–3.57) | 0.79 (0.67–0.92) | 0.59 (0.53–0.65) |
| ≥50 | 15.6 (6.7–24.5) | 96.0 (95.5–96.4) | 2.9 (1.4–5.3) | 99.3 (99.1–99.5) | 3.87 (1.63–6.11) | 0.88 (0.79–0.97) | 0.56 (0.51–0.60) |
| ≥200 | 7.8 (1.2–14.4) | 98.3 (98.0–98.6) | 3.4 (1.1–7.9) | 99.3 (99.1–99.5) | 4.59 (0.65–8.52) | 0.94 (0.87–1.00) | 0.53 (0.50–0.56) |
| Visit 3 (22–30 wk gestational age), No. | 28 | 8448 | |||||
| ≥10 | 50.0 (31.5–68.5) | 91.3 (90.7–91.9) | 1.9 (1.0–3.1) | 99.8 (99.7–99.9) | 5.75 (3.58–7.91) | 0.55 (0.34–0.75) | 0.71 (0.61–0.80) |
| ≥50 | 32.1 (14.8–49.4) | 96.7 (96.3–97.1) | 3.1 (1.4–5.8) | 99.8 (99.7–99.9) | 9.70 (4.36–15.04) | 0.70 (0.52–0.88) | 0.64 (0.56–0.73) |
| ≥200 | 21.4 (6.2–36.6) | 98.8 (98.6–99.0) | 5.6 (1.2–10.0) | 99.7 (99.6–99.8) | 17.92 (4.74–31.10) | 0.80 (0.64–0.95) | 0.60 (0.52–0.68) |
Abbreviations: AUC, area under receiver operating characteristic curve using single threshold cutpoint; LR−, negative likelihood ratio; LR+, positive likelihood ratio; NPV, negative predictive value; PPV, positive predictive value.
For cervical length, gestational age at the study visit used the project-estimated date of delivery and the ultrasound date. For fetal fibronectin level, gestational age at the study visit used the project-estimated date of delivery and the date of the fetal fibronectin collection, if valid; otherwise, the date of the visit interview was used.
ROC curves are shown in Figure 2 for fetal fibronectin and transvaginal cervical length individually and combined at visit 3 for prediction of spontaneous preterm birth before 37 weeks of gestation. The AUC was highest for transvaginal cervical length (0.67 [95% CI, 0.64–0.70]), compared with fetal fibronectin level (0.59 [95% CI, 0.56–0.62]) (P < .001). Combining fetal fibronectin level with transvaginal cervical length resulted in no additional benefit, with an AUC of 0.67 (95% CI, 0.64–0.70), and the model combining these measures was assessed to have adequate fit (Hosmer-Lemeshow , P = .30). Overlaid plots of sensitivity and specificity over a range of cutoff values for cervical length and quantitative fetal fibronectin at visit 3 are provided as eFigures 1 and 2 in the Supplement. Sensitivity plus specificity was maximized for cervical length at a cutoff of 31.7 mm and for quantitative fetal fibronectin at a cutoff of 7.077 ng/mL.
Figure 2. Receiver Operating Characteristic Curves for Visit-3 Measures Predicting Spontaneous Preterm Birth at Less Than 37 Weeks’ Gestation.
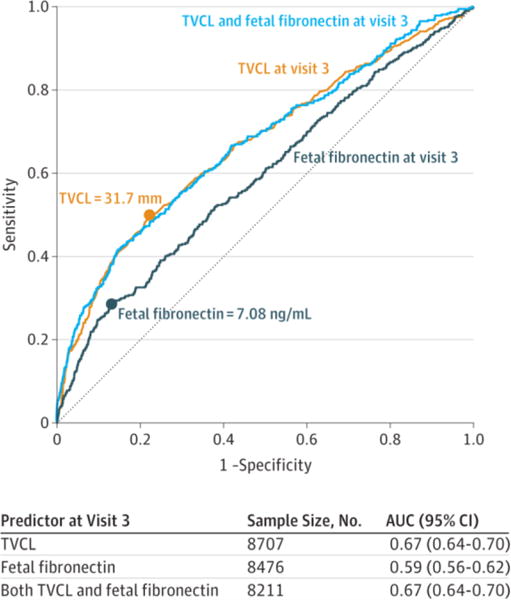
Receiver operating characteristic (ROC) curves are shown for serial transvaginal ultrasound cervical length (TVCL), quantitative vaginal fetal fibronectin, and their combination through a logistic regression model in predicting spontaneous preterm birth at less than 37 weeks’ gestation. The ultrasound used for the cervical length measurement and the sample required for the fetal fibronectin assay were taken at Nulliparous Pregnancy Outcome Study: Monitoring Mothers-to-Be (nuMoM2b) visit 3, scheduled for 22 to 30 weeks’ gestation. The graph includes all women with the cervical length measurement for the cervical length curve; all women with fetal fibronectin data for the fetal fibronectin curve; and all women with both for the combined curve. Statistics below the graph correspond to the graph (all available data). Area under the ROC curve (AUC) statistics, restricted to the women with both measures (n = 8211), are 0.67 (95% CI, 0.64–0.71) for TVCL at visit 3; 0.58 (95% CI, 0.55–0.62) for vaginal fetal fibronectin level at visit 3; and 0.67 (95% CI, 0.64–0.70) for both TVCL and fetal fibronectin level at visit 3. The AUCs are significantly different for TVCL vs fetal fibronectin level, P < .001. The AUC combining TVCL and fetal fibronectin level is not significantly different from that for TVCL alone (P = .54). Sensitivity plus specificity is maximized at 31.7 mm for TVCL and 7.08 ng/mL for fetal fibronectin.
For both transvaginal cervical length and fetal fibronectin level, AUC measures for visit 3 alone vs the rate of change from visit 2 to visit 3 were compared. AUCs for the change between visit 2 and visit 3 were lower for transvaginal cervical length (rate of change vs visit 3, 0.61 [95% CI, 0.58–0.64] vs 0.67 [95% CI, 0.64–0.70], respectively; P < .001) and not significantly different for fetal fibronectin (rate of change vs visit 3, 0.57 [95% CI, 0.53–0.60] vs 0.59 [95% CI, 0.56–0.62], respectively; P = .10).
Seven hundred forty-two women (8.0%) had a transvaginal cervical length of 25 mm or less at visit 2 or visit 3, and 66 of these women (8.9%) received progesterone therapy after 16 weeks’ gestation. In a post hoc sensitivity analysis, when women given progesterone treatment (eg, because of the finding of a short cervix) were considered as if they would have experienced a spontaneous preterm birth had they not received it, the AUC for the use of transvaginal cervical length at visit 3 to predict transvaginal cervical length was essentially unchanged (0.70 [95% CI, 0.67–0.73] for the sensitivity analysis compared with 0.67 [95% CI, 0.64–0.70] for the original analysis).
Discussion
Quantitative fetal fibronectin and transvaginal cervical length had poor predictive performance as screening tests for spontaneous preterm birth before 37 weeks in nulliparous women. All screening modalities had relatively low sensitivity and PPV. The sensitivity of transvaginal cervical length for spontaneous preterm birth before 32 weeks was higher than for 37 weeks but also had low PPV. Understanding these limitations, transvaginal cervical length at 22 to 30 weeks’ gestation was the single most accurate predictor of spontaneous preterm birth before 37 weeks, having an AUC across all potential thresholds that was higher than fetal fibronectin assessment alone. However, the most commonly used clinical cutoff (threshold of 25 mm or less) identified a minority (23.3%) of spontaneous preterm births before 37 weeks. Screening with transvaginal cervical length using a similar cutoff of 25 mm or less between 16 and 22 weeks, the most common time for screening in current clinical practice, in the epoch required for treatment with progesterone, identified only 8.0% of subsequent spontaneous preterm births. The addition of quantitative fetal fibronectin to transvaginal cervical length measurement did not increase the predictive performance of transvaginal cervical length alone.
Despite low sensitivity and predictive value, cervical length is the only predictor for which an effective intervention is potentially available. Universal cervical length screening has been proposed based on studies in which vaginal progesterone was shown to reduce the incidence of spontaneous preterm birth in women with a cervical length of 20 mm or less.21 The utility of this approach depends on the frequency of short cervix in the population being screened, the number of cases of spontaneous preterm birth that can be identified, and the efficacy of the treatment. In the large nulliparous population reported here, short cervix was uncommon; only 1.0% had a cervical length of 15 mm or less between 16 and 22 weeks. This is slightly less than rates found in 2 of 3 prospective interventional trials that screened singleton pregnancies but is similar to recent observational reports.9 To et al22 found that 1.0% of women had a cervical length 15 mm or less at 22 to 24 weeks, while Fonseca et al6 reported a rate of 1.7% and Hassan et al5 noted that 2.3% of women had similar cervical lengths. The low incidence of cervical shortening limits the utility of this screening test. Using the most conservative threshold of 25 mm or less in the most common time for clinical screening (16–22 weeks’ gestation), 247 women would need to be screened to identify 1 case of spontaneous preterm birth before 37 weeks’ gestation. Using a threshold of transvaginal cervical length of 15 mm or less at 16 to 22 weeks’ gestation, 680 women would need to be screened to identify 1 case of spontaneous preterm birth before 37 weeks in this population. The same statistic (680 to screen) applied for a threshold of transvaginal cervical length of 20 mm or less at 16 to 22 weeks’ gestation in this population.
In studies of cervicovaginal fetal fibronectin measurement to predict premature births before 36 to 37 weeks, sensitivity (10%)11,23 and PPV have been low.24–26 Several retrospective studies using enzyme-linked immunosorbent assay–based measurements of fetal fibronectin level indicate that risk of preterm birth is proportional to cervicovaginal fluid fetal fibronectin concentration.27,28 This study considered multiple thresholds and gestational ages for testing. The findings of the present study, with a maximum PPV of only 14.0% and a negative predictive value of 96.1%, indicate that fetal fibronectin level, regardless of the threshold, is not useful as a screening test in nulliparous patients.
Previous attempts to use a combination of fetal fibronectin level and transvaginal cervical length to predict spontaneous preterm birth have focused primarily on women at high risk for this outcome.14 These studies suggest that use of quantitative fetal fibronectin followed by cervical length can enhance the predictive capabilities of quantitative fetal fibronectin alone. Although the use of sequential screening was not evaluated, assessment of the contemporaneous combination of fetal fibronectin level and transvaginal cervical length as continuous measures found that quantitative fetal fibronectin did not improve the predictive capabilities of transvaginal cervical length alone in low-risk nulliparous women. Tests with relatively poor test characteristics may sometimes be useful if they are inexpensive, lack serious adverse effects of testing and treatment, and address a serious condition for which an effective intervention exists. Neither of these tests, alone or in combination, meets all of these criteria.
The strengths of the study include the large sample size, simultaneous measurement of both fetal fibronectin level and cervical length, and close attention to the accuracy of cervical length measurements and clinical outcomes. All transvaginal cervical length measurements were performed by centrally certified sonographers, reducing the potential for error due to inaccurate measurement of cervical length.
The study has several limitations, including use of self-collection of fibronectin swabs. Although the manufacturer’s instructions support self-collection, it may not be as reliable as collection during speculum examination. Enrollees were also not asked about symptoms at the time of collection. That women with cervix lengths less than 15 mm might have been treated with progesterone could have decreased the rate of subsequent premature birth, but the sensitivity analysis does not support this.
Although the study population reflected the demographic characteristics of the United States in general, the rate of spontaneous preterm birth before 37 weeks (5%) was less than expected (7.7%). This may have occurred because women who participate in research studies differ from the total population or because they have enhanced access to medical and social interventions that affect their outcomes. This difference requires further investigation.
Conclusions
Among nulliparous women with singleton pregnancies, quantitative vaginal fetal fibronectin and serial transvaginal ultrasound cervical length had low predictive accuracy for spontaneous preterm birth. These findings do not support routine use of these tests in such women.
Supplementary Material
Key Points.
Question
What is the accuracy of universal screening to predict spontaneous preterm birth before 37 weeks in nulliparous women using transvaginal cervical length and quantitative self-collected vaginal fetal fibronectin assessments?
Findings
In this observational study of 9410 nulliparous women with singleton pregnancies carried to 20 weeks or more, quantitative fetal fibronectin and transvaginal cervical length, alone and in combination, had poor predictive capabilities as screening tests for spontaneous preterm birth. Screening with transvaginal cervical length (threshold 25 mm or less) identified only a minority (23.3%) of cases of spontaneous preterm birth.
Meaning
Routine universal screening using transvaginal cervical length, quantitative fetal fibronectin, or both did not accurately predict subsequent spontaneous preterm birth and should not be used in routine clinical care in nulliparous women.
Acknowledgments
Funding/Support: This study is supported by grant funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) to Research Triangle Institute (U10 HD063036); Case Western Reserve University (U10 HD063072); Columbia University (U10 HD063047); Indiana University (U10 HD063037); Magee-Women’s Hospital (U10 HD063041); Northwestern University (U10 HD063020); University of California at Irvine (U10 HD063046); University of Pennsylvania (U10 HD063048); and University of Utah (U10 HD063053). Hologic USA (Marlborough, Massachusetts) provided fetal fibronectin collection kits and prepaid shipping materials and performed the fetal fibronectin assays in a blinded fashion and without charge.
Role of the Funders/Sponsors: The NICHD and the universities listed here had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication, except through the individual contributions of the authors. Hologic had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Parker and Ms Hunter had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Esplin, Elovitz, Iams, Parker, Wapner, Grobman, Simhan, Wing, Haas, Hoffman, Peaceman, Caritis, Parry, Wadhwa, Mercer, Saade, Reddy.
Acquisition, analysis, or interpretation of data: Esplin, Iams, Parker, Wapner, Grobman, Simhan, Wing, Haas, Silver, Hoffman, Caritis, Parry, Foroud, Mercer, Hunter, Saade, Reddy.
Drafting of the manuscript: Esplin, Elovitz, Iams, Parker, Wapner, Wing, Silver, Hoffman, Caritis, Parry, Foroud, Mercer, Hunter, Saade, Reddy.
Critical revision of the manuscript for important intellectual content: Esplin, Elovitz, Iams, Parker, Wapner, Grobman, Simhan, Wing, Haas, Silver, Hoffman, Peaceman, Caritis, Parry, Wadhwa, Foroud, Mercer, Hunter, Saade, Reddy.
Statistical analysis: Esplin, Parker, Hunter, Reddy.
Obtained funding: Wapner, Grobman, Simhan, Wing, Haas, Silver, Parry, Mercer.
Administrative, technical, or material support: Esplin, Parker, Simhan, Wing, Haas, Silver, Caritis, Parry, Wadhwa, Mercer, Reddy.
Supervision: Esplin, Elovitz, Iams, Parker, Wapner, Haas, Silver, Hoffman, Peaceman, Caritis, Parry, Mercer, Saade, Reddy.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Esplin reported serving as a member of the scientific advisory board for Sera Prognostics and holding stock in the company; serving as a member of the scientific advisory board for Clinical Innovations; and holding a patent issued for serum markers of preterm birth. Dr Wapner reported receiving grant support to his university from Natera, Sequenom, Illumina, March of Dimes, Ariosa Diagnostics/Roche, and KellBenx; receiving speaking honoraria from Natera, Sequenom, Illumina, and Ariosa Diagnostics/Roche; and receiving fees for serving as an expert witness for LabCorp. No other authors reported disclosures.
Group Information: The following institutions and researchers compose the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-be (nuMoM2b) Network: Case Western Reserve University/Ohio State University: Brian M. Mercer, MD, Jay Iams, MD, Wendy Dalton, RN, Cheryl Latimer, RN, LuAnn Polito, RN, JD; Columbia University/Christiana Care: Matthew K. Hoffman, MD, MPH, Ronald Wapner, MD, Karin Fuchs, MD, Caroline Torres, MD, Stephanie Lynch, RN, BSN, CCRC, Ameneh Onativia, MD, Michelle DiVito, MSN, CCRC; Indiana University: David M. Haas, MD, MS, Tatiana Foroud, PhD, Emily Perkins, BS, MA, CCRP, Shannon Barnes, RN, MSN, Alicia Winters, BS, Catherine L. McCormick, RN; University of Pittsburgh: Hyagriv N. Simhan, MD, MSCR, Steve N. Caritis, MD, Melissa Bickus, RN, BS, Paul D. Speer, MD, Stephen P. Emery, MD, Ashi R. Daftary, MD; Northwestern University: William A. Grobman, MD, MBA, Alan M. Peaceman, MD, Peggy Campbell, RN, BSN, CCRC, Jessica S. Shepard, MPH, Crystal N. Williams, BA; University of California at Irvine: Deborah A. Wing, MD, Pathik D. Wadhwa, MD, PhD, Michael P. Nageotte, MD, Pamela J. Rumney, RNC, CCRC, Manuel Porto, MD, Valerie Pham, RDMS; University of Pennsylvania: Samuel Parry, MD, Jack Ludmir, MD, Michal Elovitz, MD, Mary Peters, BA, MPH, Brittany Araujo, BS; University of Utah: Robert M. Silver, MD, M. Sean Esplin, MD, Kelly Vorwaller, RN, Julie Postma, RN, Valerie Morby, RN, Melanie Williams, RN, Linda Meadows, RN; RTI International: Corette B. Parker, DrPH, Matthew A. Koch, MD, PhD, Deborah W. McFadden, MBA, Barbara V. Alexander, MSPH, Venkat Yetukuri, MS, Shannon Hunter, MS, Tommy E. Holder Jr, BS, Holly L. Franklin, MPH, Martha J. DeCain, BS, Christopher Griggs, BS; Eunice Kennedy Shriver National Institute of Child Health and Human Development: Uma M. Reddy, MD, MPH, Marian Willinger, PhD, Maurice Davis, DHA, MPA, MHSA; University of Texas Medical Branch at Galveston: George R. Saade, MD.
Disclaimer: The comments and views in this article represent those of the author(s) and do not necessarily represent the views of the NICHD.
Previous Presentation: Presented at the annual meeting of the Society for Maternal-Fetal Medicine; February 1–6, 2016; Atlanta, Georgia.
Contributor Information
M. Sean Esplin, Intermountain Healthcare, Salt Lake City, Utah; University of Utah Health Sciences Center, Salt Lake City.
Michal A. Elovitz, University of Pennsylvania, Philadelphia.
Jay D. Iams, Ohio State University, Columbus.
Corette B. Parker, RTI International, Research Triangle Park, North Carolina.
Ronald J. Wapner, Columbia University, New York, New York.
William A. Grobman, Northwestern University, Evanston, Illinois.
Hyagriv N. Simhan, University of Pittsburgh, Pittsburgh, Pennsylvania.
Deborah A. Wing, University of California at Irvine.
David M. Haas, Indiana University, Bloomington.
Robert M. Silver, Intermountain Healthcare, Salt Lake City, Utah; University of Utah Health Sciences Center, Salt Lake City.
Matthew K. Hoffman, Christiana Care, Newark, Delaware.
Alan M. Peaceman, Northwestern University, Evanston, Illinois.
Steve N. Caritis, University of Pittsburgh, Pittsburgh, Pennsylvania.
Samuel Parry, University of Pennsylvania, Philadelphia.
Pathik Wadhwa, University of California at Irvine.
Tatiana Foroud, Indiana University, Bloomington.
Brian M. Mercer, Case Western Reserve University, Cleveland, Ohio.
Shannon M. Hunter, RTI International, Research Triangle Park, North Carolina.
George R. Saade, University of Texas Medical Branch, Galveston.
Uma M. Reddy, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), Bethesda, Maryland.
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- 3.Iams JD, Goldenberg RL, Meis PJ, et al. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334(9):567–572. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 4.Crane JM, Hutchens D. Transvaginal sonographic measurement of cervical length to predict preterm birth in asymptomatic women at increased risk: a systematic review. Ultrasound Obstet Gynecol. 2008;31(5):579–587. doi: 10.1002/uog.5323. [DOI] [PubMed] [Google Scholar]
- 5.Hassan SS, Romero R, Vidyadhari D, et al. PREGNANT Trial Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH, Fetal Medicine Foundation Second Trimester Screening Group Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357(5):462–469. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 7.Parry S, Elovitz MA. Pros and cons of maternal cervical length screening to identify women at risk of spontaneous preterm delivery. Clin Obstet Gynecol. 2014;57(3):537–546. doi: 10.1097/GRF.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 8.Miller ES, Tita AT, Grobman WA. Second-trimester cervical length screening among asymptomatic women: an evaluation of risk-based strategies. Obstet Gynecol. 2015;126(1):61–66. doi: 10.1097/AOG.0000000000000864. [DOI] [PubMed] [Google Scholar]
- 9.Orzechowski KM, Boelig RC, Baxter JK, Berghella V. A universal transvaginal cervical length screening program for preterm birth prevention. Obstet Gynecol. 2014;124(3):520–525. doi: 10.1097/AOG.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 10.Khalifeh A, Quist-Nelson J, Berghella V. Universal cervical length screening for preterm birth prevention in the United States [published online September 7, 2016] J Matern Fetal Neonatal Med. doi: 10.1080/14767058.2016.1220521. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg RL, Mercer BM, Meis PJ, Copper RL, Das A, McNellis D, NICHD Maternal Fetal Medicine Units Network The preterm prediction study: fetal fibronectin testing and spontaneous preterm birth. Obstet Gynecol. 1996;87(5, pt 1):643–648. doi: 10.1016/0029-7844(96)00035-x. [DOI] [PubMed] [Google Scholar]
- 12.Foster C, Shennan AH. Fetal fibronectin as a biomarker of preterm labor: a review of the literature and advances in its clinical use. Biomark Med. 2014;8(4):471–484. doi: 10.2217/bmm.14.28. [DOI] [PubMed] [Google Scholar]
- 13.Abbott DS, Hezelgrave NL, Seed PT, et al. Quantitative fetal fibronectin to predict preterm birth in asymptomatic women at high risk. Obstet Gynecol. 2015;125(5):1168–1176. doi: 10.1097/AOG.0000000000000754. [DOI] [PubMed] [Google Scholar]
- 14.Bolt LA, Chandiramani M, De Greeff A, Seed PT, Kurtzman J, Shennan AH. The value of combined cervical length measurement and fetal fibronectin testing to predict spontaneous preterm birth in asymptomatic high-risk women. J Matern Fetal Neonatal Med. 2011;24(7):928–932. doi: 10.3109/14767058.2010.535872. [DOI] [PubMed] [Google Scholar]
- 15.Kuhrt K, Smout E, Hezelgrave N, Seed PT, Carter J, Shennan AH. Development and validation of a tool incorporating cervical length and quantitative fetal fibronectin to predict spontaneous preterm birth in asymptomatic high-risk women. Ultrasound Obstet Gynecol. 2016;47(1):104–109. doi: 10.1002/uog.14865. [DOI] [PubMed] [Google Scholar]
- 16.Haas DM, Parker CB, Wing DA, et al. NuMoM2b Study A description of the methods of the Nulliparous Pregnancy Outcomes Study: monitoring mothers-to-be (nuMoM2b) Am J Obstet Gynecol. 2015;212(4):539.e1–539.e24. doi: 10.1016/j.ajog.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iams JD, Grobman WA, Lozitska A, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network Adherence to criteria for transvaginal ultrasound imaging and measurement of cervical length. Am J Obstet Gynecol. 2013;209(4):365.e1–365.e5. doi: 10.1016/j.ajog.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 19.Box GEP, Cox DR. An analysis of transformations. J R Stat Soc Series B Stat Methodol. 1964;26(2):211–252. [Google Scholar]
- 20.Hosmer DW, Jr, Lemeshow S. Applied Logistic Regression. 2nd. New York, NY: John Wiley & Sons; 2000. [Google Scholar]
- 21.Berghella V. Universal cervical length screening for prediction and prevention of preterm birth. Obstet Gynecol Surv. 2012;67(10):653–658. doi: 10.1097/OGX.0b013e318270d5b2. [DOI] [PubMed] [Google Scholar]
- 22.To MS, Alfirevic Z, Heath VC, et al. Fetal Medicine Foundation Second Trimester Screening Group Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363(9424):1849–1853. doi: 10.1016/S0140-6736(04)16351-4. [DOI] [PubMed] [Google Scholar]
- 23.Deshpande SN, van Asselt AD, Tomini F, et al. Rapid fetal fibronectin testing to predict preterm birth in women with symptoms of premature labour: a systematic review and cost analysis. Health Technol Assess. 2013;17(40):1–138. doi: 10.3310/hta17400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hellemans P, Gerris J, Verdonk P. Fetal fibronectin detection for prediction of preterm birth in low risk women. Br J Obstet Gynaecol. 1995;102(3):207–212. doi: 10.1111/j.1471-0528.1995.tb09095.x. [DOI] [PubMed] [Google Scholar]
- 25.Faron G, Boulvain M, Lescrainier JP, Vokaer A. A single cervical fetal fibronectin screening test in a population at low risk for preterm delivery: an improvement on clinical indicators? Br J Obstet Gynaecol. 1997;104(6):697–701. doi: 10.1111/j.1471-0528.1997.tb11980.x. [DOI] [PubMed] [Google Scholar]
- 26.Greenhagen JB, Van Wagoner J, Dudley D, et al. Value of fetal fibronectin as a predictor of preterm delivery for a low-risk population. Am J Obstet Gynecol. 1996;175(4, pt 1):1054–1056. doi: 10.1016/s0002-9378(96)80052-4. [DOI] [PubMed] [Google Scholar]
- 27.Goldenberg RL, Andrews WW, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth Project: placental histology in recurrent spontaneous and indicated preterm birth. Am J Obstet Gynecol. 2006;195(3):792–796. doi: 10.1016/j.ajog.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 28.Kurtzman J, Chandiramani M, Briley A, Poston L, Das A, Shennan A. Quantitative fetal fibronectin screening in asymptomatic high-risk patients and the spectrum of risk for recurrent preterm delivery. Am J Obstet Gynecol. 2009;200(3):263.e1–263.e6. doi: 10.1016/j.ajog.2009.01.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


