Abstract
Background
Recent studies have shown that hematopoietic stem cells can undergo clonal expansion due to somatic mutations in leukemia-related genes, leading to an age-dependent accumulation of mutant leukocytes in the blood. This somatic mutation-related clonal hematopoiesis is common in the healthy elderly, but it has been associated with an increased incidence of future cardiovascular disease. The epigenetic regulator TET2 is frequently mutated in blood cells of individuals exhibiting clonal hematopoiesis.
Objectives
Here, we investigated whether Tet2 mutations within hematopoietic cells can contribute to heart failure in two models of cardiac injury.
Methods
Heart failure was induced in mice by pressure overload, achieved by transverse aortic constriction or chronic ischemia induced by the permanent ligation of the left anterior descending artery. Competitive bone marrow transplantation strategies with Tet2-deficient cells were used to mimic TET2 mutation-driven clonal hematopoiesis. Alternatively, Tet2 was specifically ablated in myeloid cells using Cre recombinase expressed from the LysM promoter.
Results
In both experimental heart failure models, hematopoietic or myeloid Tet2 deficiency worsened cardiac remodeling and function, in parallel with increased IL-1β expression. Treatment with a selective NLRP3 inflammasome inhibitor protected against the development of heart failure and eliminated the differences in cardiac parameters between Tet2-deficient and wild-type mice.
Conclusions
Tet2 deficiency in hematopoietic cells is associated with greater cardiac dysfunction in murine models of heart failure due to elevated IL-1β signaling. These data suggest that individuals with TET2-mediated clonal hematopoiesis may be at greater risk of developing heart failure and respond better to IL-1β/NLRP3 inflammasome inhibition.
Keywords: clonal hematopoiesis, heart failure, interleukin 1 beta, ten-eleven translocation 2, myocardial infarction, pressure overload hypertrophy
Introduction
The prevalence of heart failure approximately doubles with each decade of life (1,2), and due to dramatic increases in the aging population, the heart failure epidemic is anticipated to grow significantly in the coming decades (3). While aging is the major risk factor for heart failure and overall cardiovascular disease, we do not have a clear understanding of how this condition promotes disease progression.
The accumulation of somatic DNA mutations in proliferative tissues is an inevitable consequence of the aging process that leads to the generation of tissues that are a mosaic of slightly different genotypes (4). In this context, the highly proliferative hematopoietic system is known to accumulate a substantial number of somatic mutations, which leads to an intense Darwinian selection due to the competition among the different mutant clones (5). Thus, the accumulation of mutations that provide a selective proliferative advantage to hematopoietic stem/progenitor cells (HSPC) will over time lead to the clonal expansion of specific mutations that become selectively overrepresented in the blood cells genomes. This phenomenon of somatic mutation-induced clonal hematopoiesis has been found to be relatively common in non-symptomatic, cancer-free, elderly individuals (6–8). Recent longitudinal studies have reported that detectable clonal hematopoiesis in non-symptomatic individuals is associated with all-cause mortality (6,7,9). While clonal hematopoiesis increases the risk of developing a hematological malignancy, this condition is relatively rare and does not significantly contribute to overall mortality under these conditions. Instead mortality associated with clonal hematopoiesis is attributed to larger increases in the incidence of coronary heart disease and stroke (7).
The protein encoded by TET2 (ten-eleven translocation 2) is an epigenetic regulatory enzyme that modulates HSPC self-renewal (10–13). TET2 gene was the first gene reported to exhibit somatic mutations in blood cells in individuals with clonal hematopoiesis in the absence of hematological malignancies (14). More than 130 distinct mutations have been reported in the TET2 gene in cancer-free individuals (6–9,14–18), the majority being small insertions/deletions, nonsense mutations, etc., that are predicted to inactivate the enzyme. Previously, we provided experimental evidence supporting that somatic TET2 mutations in blood cells play a causal role in atherosclerosis (19). In this study, partial bone marrow reconstitution with Tet2-deficient hematopoietic cells led to their clonal expansion and markedly increased atherosclerotic plaque size in hyperlipidemic mice. Mechanistically, Tet2-deficient macrophages exhibited greater interleukin-1β secretion, and the administration of an NLRP3 inhibitor showed greater athero-protective activity in chimeric mice reconstituted with Tet2-deficient cells than in non-chimeric mice and suppressed differences in plaque size between genotypes.
Recently, the clonal expansion of leukocytes with somatic mutations in TET2 has been associated with coronary heart disease and early-onset myocardial infarction (MI) (16). However, there have been no experimental studies in model systems to test whether somatic TET2 mutations in hematopoietic cells are causally linked to myocardial infarction or heart failure. Here, we analyzed the effects of genetic inactivation of hematopoietic Tet2 in murine models of heart failure involving chronic ischemia and pressure overload hypertrophy.
Methods
C57Bl/6J Tet2-deficient mice (Tet2−/− and +/−), C57Bl/6J LysM-Cre mice, C57Bl/6J Tet2-floxed mice and C57Bl/6 Cd45.1+ PepBoy mice were used for these studies. These mice were employed to examine the consequences of hematopoietic Tet2-deficiency using competitive bone marrow transplantations (BMT, 10% CD45.2 and 90% CD45.1 bone marrow cells) or conditional myeloid-restricted inactivation of Tet2. Both of these models of genetic Tet2 deficiency were employed in two surgical models of murine heart failure: the permanent ligation of the left anterior descending artery (LAD) and transverse aortic constriction (TAC) as described previously (20–26). Surgically-treated and sham mice were characterized by echocardiographic, histological and qPCR analyses. In some experiments, mice were continuously infused with the NLRP3 inflammasome inhibitor MCC950 (27) at a dose of 5 mg per kg per day or with phosphate-buffered saline (PBS) vehicle via subcutaneous osmotic pumps. In the BMT approach, the expansion of Tet2-deficient HSPC into the different blood lineages was assessed by flow cytometry using the gating strategy shown in Online Figure 1. Leukocyte levels in heart were determined by flow cytometry using the gating strategy outlined in Online Figure 2 (28,29). For some assays, bone marrow-derived macrophages from conditional or whole-body Tet2-deficient mice were utilized for qPCR analyses. Detailed materials and methods are included in the Online Appendix.
Statistics
Data were expressed as mean + SEM, or median (minimum to maximum). Data distribution was evaluated by Shapiro-Wilk normality test. An F-test was used to evaluate homogeneity of variance. Normally distributed data with only 1 variable were analyzed by parametric analysis: unpaired (2-tailed) Student’s t test (with Welch correction when variance was unequal) for 2 groups and 1-way ANOVA with Tukey’s multiple comparison test for ≥3 groups. Non-normally distributed data with only one variable were analyzed by non-parametric analysis: Mann-Whitney U test (2-tailed) for 2 groups. Data with more than one variable were evaluated by 2-way ANOVA, with post-hoc Tukey’s multiple comparison tests. Results of echography were evaluated by 2-way repeated measure ANOVA with post-hoc Sidak’s or Tukey’s multiple comparison tests. In survival analysis, Kaplan-Meier survival curves were compared by log-rank test. All the statistical analysis was performed by GraphPad Prism 7 software (GraphPad Software, La Jolla, California).
Results
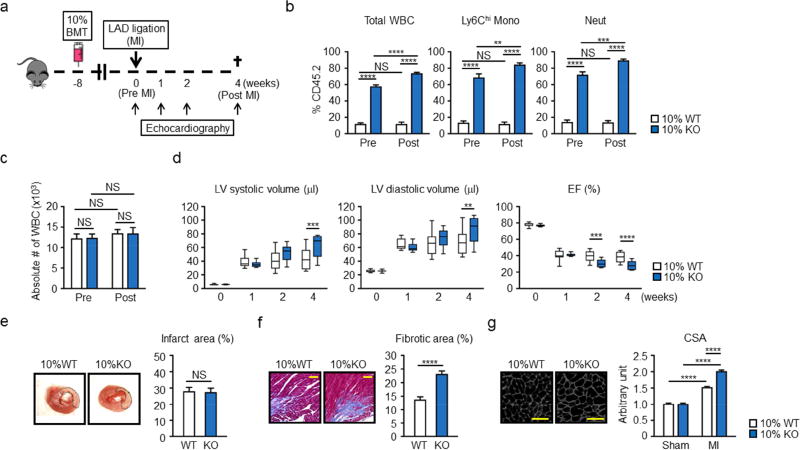
A competitive bone marrow transplantation (BMT) strategy was employed to mimic the clonal hematopoiesis that results from Tet2 inactivation and study its effects on experimental MI (Figure 1A). Lethally irradiated mice were transplanted with bone marrow cells containing 10% Tet2−/− cells and 90% Tet2+/+ cells (i.e., the 10% knock out [KO] test condition). To distinguish between these different genotypes, wild-type Tet2+/+ cells were harvested from mice carrying the CD45.1 variant of the CD45 antigen, and Tet2−/− cells were harvested from mice harboring the CD45.2 variant. Control wild-type (WT) mice received 10% CD45.2+Tet2+/+ and 90% CD45.1+Tet2+/+ cells. At 8 weeks after BMT and at 4 weeks after LAD ligation in BMT-treated animals (pre and post, respectively) flow cytometry was performed on blood leukocytes to assess the expansion of Tet2−/− cells (Figure 1B). Consistent with our prior report (19),. Tet2−/− CD45.2+ cells displayed a time dependent expansion into the white blood cells (WBC), Ly6Chi monocytes and neutrophils (Figure 1B). Although Tet2-deficient donor mice displayed a small elevation in lineage marker−, c-Kit+, Sca1+ (LSK), this difference did not appear to account for the large increase in Tet2−/− cell expansion into the difference lineages (Online Figure 3). Tet2-deficient donor mice also did not display differences in levels of GMP or MDP progenitor cells, neutrophils, or monocytes, or spleen weights at this age. These Tet2−/− cells expanded into multiple blood lineages, but a reduced expansion into the T cell lineage was observed (Online Figure 4). Total WBC levels were unaffected by genotype (Figure 1C).
Figure 1. Hematopoietic Tet2-KO mice show greater post-infarction remodeling.
a. Scheme of the experimental study. Mice underwent partial (10%) bone marrow reconstitution with Tet2-deficient cells (10% KO-BMT) or wild type cells (10% WT-BMT) following lethal irradiation. After 8 weeks of recovery, mice underwent permanent LAD ligation. Echocardiography was performed at the indicated time points. b. Tet2-KO bone marrow cells (Cd45.2+) display a competitive advantage over wild type competitor cells (Cd45.1+) in their ability to expand into multiple blood cell lineages in vivo. Peripheral blood was obtained 8 weeks and 12 weeks after BMT (before (Pre) and 4 weeks after (Post) MI, respectively) from 10% WT-BMT (n=11) mice and 10% KO-BMT mice (n=10). Statistical analysis was evaluated by evaluated by two-way ANOVA with Tukey’s multiple comparison tests. c. Absolute numbers of WBC before (Pre) and 4 weeks after (Post) LAD ligation of 10% KO-BMT mice (n=10) and 10% WT-BMT mice (n=11). Statistical analysis was evaluated by evaluated by two-way ANOVA with Tukey’s multiple comparison tests. d. Echocardiographic analysis shows that 10% KO-BMT mice (n=10) display worsening cardiac remodeling after LAD ligation compared to 10% WT-BMT mice (n=11). Statistical analysis was evaluated by two-way repeated measure ANOVA with Sidak’s multiple comparison tests. e. Representative images and analysis of infarct size in myocardial tissue sections from 10% WT-BMT (n=3) mice and 10% KO-BMT mice (n=3) stained with TTC 2 days after LAD ligation, showing there is no statistical significance between both groups. Hearts were sliced at 2 mm below from the ligation site. Statistical analysis was evaluated by two-tailed unpaired Student’s t test. f. Representative images and analysis of fibrosis in marginal zone of myocardial tissue sections from 10% WT-BMT (n=7) mice and 10% KO-BMT mice (n=7) stained with Masson’s Trichrome dye at 4 weeks after ligation, showing worsening fibrosis in 10% KO-BMT mice. The percentage of the fibrotic area was calculated with the image-J software. Statistical analysis was evaluated by two-tailed unpaired Student’s t test. Scale bars indicate 100 µm. g. Representative images and analysis of WGA staining of the heart sections from hearts 10% WT-BMT (n=7) mice and 10% KO-BMT mice (n=7) isolated at 4 weeks after LAD ligation. Staining shows that the non-infarcted, remote area of the heart displays greater hypertrophy of the cardiac myocytes. Statistical analysis was evaluated by two-way ANOVA with Tukey’s multiple comparison test. Scale bars indicate 50 µm. **p<0.01, ***p<0.001, ****p<0.0001, NS: not significant. WT: wild type, KO: knockout, BMT: bone marrow transfer, LAD: left anterior ascending artery. WBC: white blood cells, Mono: monocytes, Neut: neutrophils, LV: left ventricle, EF: ejection fraction, TTC: 2,3,5-triphenyl-tetrazolium chloride, CSA: cross-sectional area of myocytes.
At this 8 week time point, mice from both experimental groups underwent permanent LAD ligation surgery as described previously (20,25,26). Permanent LAD ligation surgery did not affect the expansion of Tet2−/− cells (Online Figure 5). Consistent with prior studies (30), all transplanted mice survived surgery following irradiation (Online Figure 6). At 4 weeks after myocardial infarction (MI) surgery, the 10% KO-BMT mice displayed statistically significant increases in left ventricle (LV) systolic volume and LV diastolic volume, indicative of greater systolic dysfunction, compared to control mice that also underwent BMT and MI surgery (Figure 1D). Trends in these echocardiographic parameters were observed at the 2 week time point but did not reach statistical significance. Ejection fraction was significantly reduced in the 10% KO-BMT group at 2 and 4 weeks post-MI. Consistent with a lack of difference in echocardiographic parameters at the 1 week time point, there was no detectable difference in infarct size between 10% KO-BMT and 10% WT-BMT mice (Figure 1E). There was a statistically significant increase in fibrosis in the marginal zone of the 10% KO-BMT mice (Figure 1F). Wheat germ agglutinin staining was employed on tissue sections, to determine cardiac myocyte cross-sectional area, revealed that myocytes from 10% KO-BMT mice displayed greater hypertrophy at the marginal zone (Figure 1G), consistent with greater post-MI cardiac remodeling. Analysis of the infarct marginal zone for F4-80-positive cells revealed greater macrophage infiltration at the 4 week post-MI time point (Online Figure 4). In separate studies, competitive BMT experiments with Tet2+/− cells revealed that Tet2 heterozygosity is sufficient to promote cardiac dysfunction in this model, albeit at a reduced level compared to homozygous Tet2-deficient cells (Online Figure 7). These findings are consistent with the slower kinetics of Tet2-heterozygous cell expansion.
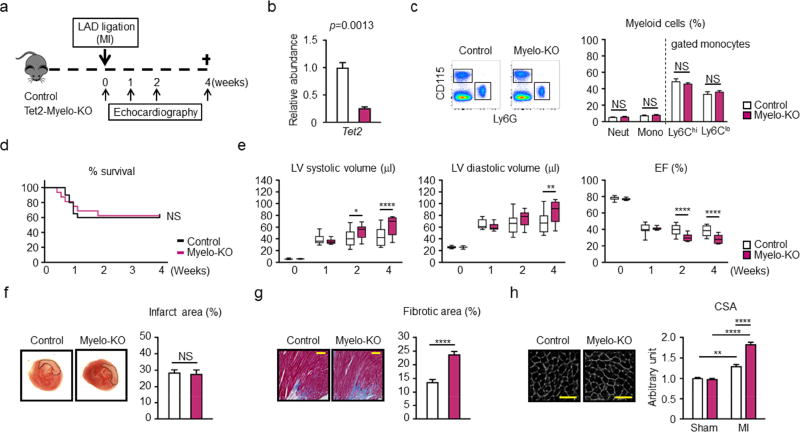
Due to the central role of macrophage activation in post-MI remodeling (31) and the preferential expansion of human TET2-mutant cells into the myeloid lineage in mice (32), we investigated whether the targeted ablation of Tet2 would be sufficient to promote post-MI remodeling. Myeloid-deficient Tet2 mice (Myelo-Tet2-KO mice) were generated using LysM-Cre/LoxP strategies and subjected to permanent LAD ligation (Figure 2A). Using this system, the ablation of Tet2 in bone marrow-derived macrophages occurred at an efficiency of 74% (Figure 2B). This condition did not lead to detectable changes in the levels of hematopoietic cells (Figure 2C; Online Figure 8). No differences in mouse survival following LAD ligation was detected between Tet2-deficient and control strains of mice over the time course of this experiment (Figure 2D). However, Myelo-Tet2-KO mice displayed a statistically significant increase in LV systolic and LV diastolic volumes at the 4 week post-MI time point, and trends toward increases in these parameters at the 2 week time point, compared with control mice (Figure 2E). Calculated ejection fraction showed statistically significant reductions at both 2 and 4 week time points in the Myelo-Tet2-KO mice. Infarct size was unaffected by the different myeloid Tet2 genotypes (Figure 2F). At the terminal 4 week time point hearts from Myelo-Tet2-KO mice displayed an increase in fibrosis in cardiac tissue sections from the marginal zone (Figure 2G), and an increase in cardiac myocyte cross sectional area (Figure 2H), consistent with greater cardiac remodeling.
Figure 2. Conditional myeloid Tet2-deficiency in mice leads worse cardiac remodeling in hearts subjected to LAD ligation.
a. Scheme of the study. Control and conditional myeloid Tet2-knockout (Myelo-KO) mice underwent LAD ligation. Mice underwent permanent LAD ligation, and echocardiography was performed at the indicated time points. b. The efficiency of Tet2 ablation was analyzed by qPCR in BMDM at 7 days after in vitro differentiation from conditional Tet2-Myelo-KO mice and control mice (3 mice per genotype). Two-tailed Student’s t test was performed for statistical analysis. c. Flow cytometry representative data and analysis of peripheral blood from Tet2-Myelo-KO mice (n=6) and control mice (n=6) to show there are no detectable changes in myeloid populations. Statistical significance of difference was evaluated by multiple t test. d. Mice survival curve after LAD ligation. The mortality of the conditional KO mice and control mice after surgery was 37.5% and 40.0%, respectively. Log-rank test was used for statistical analysis (n= 20 for control mice and n= 16 for conditional Tet2-KO mice). e. Echocardiographic evaluation shows that surviving mice with conditional Tet2 ablation in myeloid cells (n=10) display worsening cardiac remodeling after LAD ligation surgery compared to control mice (n=12). Statistical analysis was evaluated by two-way repeated measure ANOVA with Sidak’s multiple comparison tests. f. Representative images and analysis of infarct size in myocardial tissue sections from conditional KO mice (n=3) and control (n=3) mice stained with TTC 2 days after LAD ligation. Hearts were sliced at 2 mm below from the ligation site, showing that there was no difference in initial infarct size. Statistical analysis was evaluated by Mann-Whitney U test. g. Representative images and analysis of fibrosis in the marginal zone of myocardial tissue sections from conditional KO mice (n=6) and control (n=6) mice stained with Masson’s Trichrome dye at 4 weeks after ligation, showing worsening fibrosis in conditional KO mice. The percentage of the fibrotic area was calculated with the image-J software. Statistical analysis was evaluated by two-tailed unpaired Student’s t test. Scale bars indicate 100 µm. h. WGA staining of the heart sections from hearts control (n=6) mice and conditional KO mice (n=6) isolated at 4 weeks after LAD ligation. Analysis of CSA shows that the non-infarcted, remote area of the heart of conditional KO mice display greater hypertrophy of the cardiac myocytes. Statistical analysis was evaluated by two-way ANOVA followed with Tukey’s multiple comparison tests. Scale bars indicate 50 µm. *p<0.05, **p<0.01, ****p<0.0001, NS: not significant. WT: wild type, KO: knockout, LAD: left anterior ascending artery, qPCR: quantitative polymerase chain reaction, BMDM: bone marrow-derived macrophages, LAD: left anterior ascending artery, Mono: monocytes, Neut: neutrophils, LV: left ventricle, EF: ejection fraction, TTC: 2,3,5-triphenyl-tetrazolium chloride, WGA: wheat germ agglutinin, CSA: cross-sectional area of myocytes.
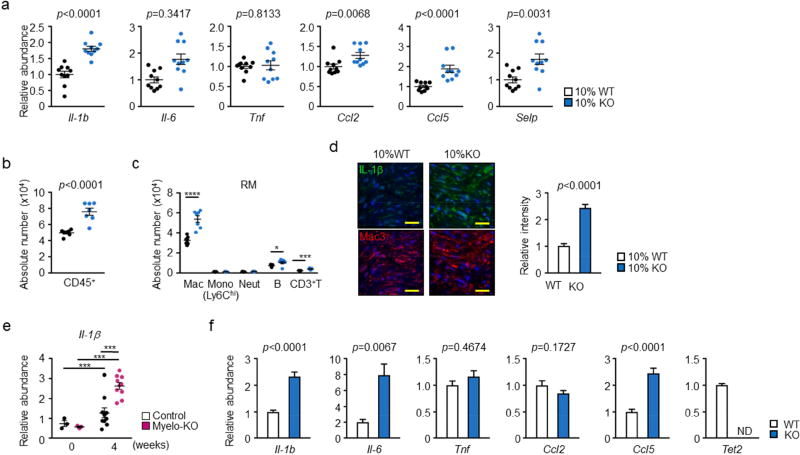
A survey of transcripts revealed that interleukin 1 beta (IL-1β), IL-18, Cxcl2, Ccl2, Ccl5 and P-selectin were upregulated in the post-MI hearts of 10% KO-BMT mice compared to 10% WT-BMT mice (Figure 3A). In contrast, little or no statistical differences were detected in the transcript levels of IL-6, TNFα. The 10% KO-BMT, post-MI hearts also displayed an increase in CD45+ leukocytes that could primarily by attributed to the abundance of macrophages (Figure 3B, C, and Online Figure 9). The increase in macrophage number was not accompanied by an increase in proliferation, as assessed by Ki67 (Online Figure 10). To document changes in IL-1β protein, confocal microscopy immunofluorescence staining of IL-1β was performed on marginal zone sections of hearts at the 4 week post-MI time point. Cardiac sections from 10% KO-BMT mice displayed significantly greater IL-1β expression than sections of 10% WT-BMT mice (Figure 3D). Consistent with these results, IL-1β transcript levels were upregulated in the myocardium of the -Tet2-KO mice compared to control mice at the 4 week post-MI time point (Figure 3E). Bone marrow-derived macrophages isolated from whole body Tet2-deficient mice also displayed elevated IL-1β, IL-6 and Ccl5 transcript expression (Figure 3F).
Figure 3. Effect of Tet2-deficient hematopoietic cells on the expression of pro-inflammatory cytokines and chemokines in the remodeling heart tissue.
a. Analysis of transcript expression in the non-infarcted marginal zone obtained from 10% KO-BMT mice (n=10) and 10% WT-BMT (n=11) mice. Gene expression was analyzed by qPCR analysis. Statistical significance was evaluated by two-tailed unpaired Student’s t tests with Welch’s Correction when variance was unequal or by Mann Whitney U tests for data which failed to pass the Shapiro-Wilk normality test. b. Flow cytometry analysis of cardiac remote area from 10% KO-BMT mice (n=7) and 10% WT-BMT (n=7) mice to show the absolute number of total CD45+ immune cells are increased in the myocardial tissue from 10% KO-BMT mice. Data is expressed as number of cells per 100 mg wet weight. Statistical analysis was evaluated by two-tailed unpaired Student’s t test. c. Flow cytometry analysis of cardiac remote area from 10% KO-BMT mice (n=7) and 10% WT-BMT (n=7) mice to show the absolute number of each immune cell populations. Statistical significance of difference was evaluated by multiple t tests. d. IL-1β immunofluorescence staining in Mac3-positive macrophage-enriched marginal zone of 10% KO-BMT (n=5) mice and 10% WT-BMT mice (n=5) showing IL-1β signal is higher in 10% KO-BMT mice. Scale bars = 20 µm. Images were quantified for integrated fluorescence intensity with Image J software. Statistical analysis was performed by two-tailed unpaired Student’s t tests. e. Remote area samples were obtained from conditional myeloid-specific KO mice and control mice, and gene expression was analyzed by qPCR at the indicated time points (n=3 for sham and n = 10 at 4 weeks after LAD ligation, per genotype). Statistical significance was evaluated by two-way ANOVA with Tukey’s multiple comparison tests. f. Bone marrow-derived macrophages 2 days after in vitro differentiation obtained from Tet2-null mice (n=6) and wild type (n=7) were obtained in vitro and gene expression was analyzed by qPCR analysis. Statistical significance of difference was evaluated by two-tailed unpaired Student’s t tests with Welch’s Correction when variance was unequal or by Mann Whitney U tests for data which failed to pass the Shapiro-Wilk normality test. *p<0.05, **p<0.01, ****p<0.0001. WT: wild type, KO: knockout, BMT: bone marrow transfer, LAD: left anterior ascending artery, qPCR: quantitative polymerase chain reaction, RM: remote area, Mac: macrophages, Mono: monocytes, Neut: neutrophils, B: B cells, T: T cells, ND: not detected.
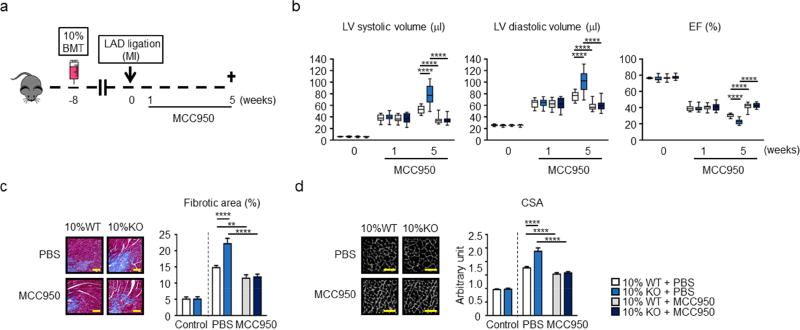
To assess the functional significance of NLRP3-mediated IL-1β production in cardiac remodeling, 10% KO-BMT and 10% WT-BMT mice were infused with the specific NLRP3 inhibitor MCC950 starting at one week post-MI and continued until sacrifice at 5 weeks (Figure 4A). At the one week time point (initiation of MCC950 infusion), there were no detectable differences in echocardiographic parameters in the different experimental groups of mice (Figure 4B). However, by week 5 post-MI, MCC950 treatment led to significant protection against cardiac remodeling in both 10% KO-BMT and 10% WT-BMT mice. Notably, the MCC950 treatment completely eliminated echocardiographic differences in LV systolic volume, LV diastolic volume or ejection fraction, between the Tet2-deficient and WT experimental conditions (Figure 4B). Treatment with MCC950 infusion also diminished marginal zone fibrosis at the 5-week time point in both 10% KO-BMT and 10% WT-BMT mice and eliminated the difference in this parameter between these experimental groups (Figure 4C). Similarly, cardiac myocyte hypertrophy was markedly reduced by MCC950 treatment in both Tet2-deficient and WT mice and the drug eliminated the difference in this parameter between experimental groups (Figure 4D). In a separate experiment, treatment with MCC950 had no effect on the expansion of Tet2−/− HSPC into the different blood lineages (Online Figure 11), consistent with our previous study (19).
Figure 4. Inflammasome inhibition reverses post-infarction remodeling associated with hematopoietic Tet2-deficiency.
a. Scheme of the experimental study. Mice underwent partial (10%) bone marrow reconstitution with Tet2-deficient cells (10% KO-BMT mice) or wild type cells (10% WT-BMT mice) following lethal irradiation. After 8 weeks of recovery, mice underwent permanent LAD ligation. 1 week after LAD ligation, MCC950 and PBS was continuously infused with osmotic pumps for 4 weeks. Echocardiography was performed at the indicated time points. b. Echocardiographic analysis reveals that treatment with the NLRP3 inflammasome inhibitor MCC950 protects against adverse cardiac remodeling in 10% KO-BMT and 10% WT-BMT mice, and eliminates the differences in cardiac parameters between Tet2-deficient and WT conditions at the post-LAD ligation time point of 5 weeks. Sample sizes were n = 12 for 10% WT-BMT with PBS, n=12 for 10% KO-BMT with PBS, n = 14 for 10% WT-BMT with MCC950, n = 14 for 10% KO-BMT with MCC950. Statistical significance was evaluated by 2-way repeated measure ANOVA with Tukey’s multiple comparison tests. c. Representative images and analysis of fibrosis in marginal zone of myocardial tissue sections stained with Masson’s Trichrome dye at 5 weeks after ligation. MCC950 inhibits the development of cardiac fibrosis after LAD ligation in mice reconstituted with Tet2-KO or WT bone marrow, and eliminates the differences in cell size between Tet2-deficient and WT genotypes. Statistical significances of differences among groups of 10% WT/10%KO with PBS or MCC950 were evaluated by two-way ANOVA with Tukey’s multiple comparison tests. Scale bars indicate 100 µm. d. Representative images and analysis of WGA staining of the heart sections of hearts at 5 weeks after LAD ligation. MCC950 inhibits the development of cardiac myocyte hypertrophy after LAD ligation in mice reconstituted with Tet2-KO or WT bone marrow, and eliminates the differences in cell size between Tet2-deficient and WT genotypes. For c and d, sham-operated mice without any pump infusion were used as control (n=3 per genotype). Sample sizes were n=6 for 10% WT-BMT with PBS, n=6 for 10% KO-BMT with PBS, n=8 for 10% WT-BMT with MCC950, n=8 for 10% KO-BMT with MCC950. Statistical significances of differences among groups of 10% WT/10%KO with PBS or MCC950 were evaluated by two-way ANOVA with Tukey’s multiple comparison tests. Scale bars indicate 50 µm. **p<0.01, ***p<0.001, ****p<0.0001. WT: wild type, KO: knockout, BMT: bone marrow transfer, LAD: left anterior ascending artery, PBS: phosphate-buffered saline, WGA: wheat germ agglutinin, CSA: cross-sectional area of myocytes.
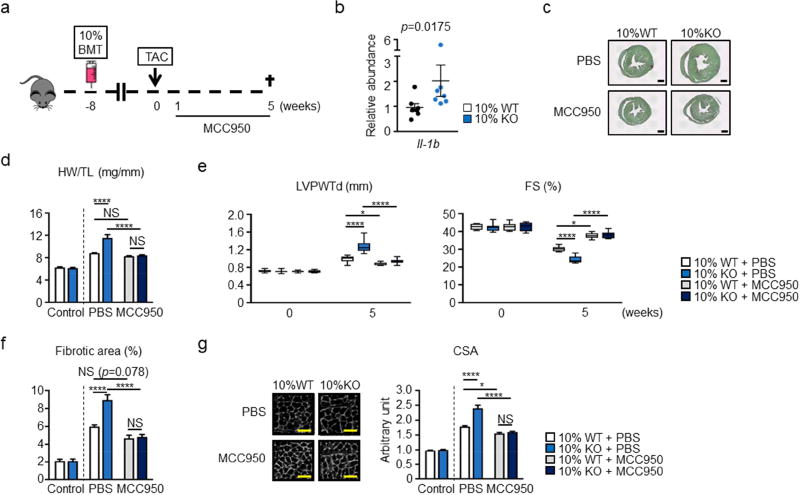
To corroborate these findings in a different model of heart failure, experimental groups of mice were also subjected to pressure overload hypertrophy by performing transverse aortic constriction (TAC) (Figure 5A). In this model, the heart initially undergoes compensatory hypertrophy, but transitions to decompensated hypertrophy due to capillary rarefaction and other chronic pathological alterations (33,34). Because 10% KO-BMT mice exhibited increased IL-1β expression in this model (Figure 5B), similar to our observation in the LAD model, TAC experiments were performed in mice treated with the NLRP3 inflammasome inhibitor MCC950 or vehicle control as described above to assess the effects of blockade of NLRP3 inflammasome-mediated IL-1β secretion. In this setting, MCC950 treatment was started at the time of surgery and maintained until termination of the experiment. Vehicle-infused 10% KO-BMT mice exhibited marked cardiac hypertrophy after TAC, as reflected by a greater increase in relative heart size and heart weight to tibia length ratio compared to 10% WT-BMT mice (Figure 5C,D). 10% KO-BMT mice also displayed greater LV posterior wall thickness and a greater impairment in fractional shortening in the hearts of the 10% KO-BMT mice (Figure 5E). Correspondingly, 10% KO-BMT mice displayed more fibrosis (Figure 5F) and cardiac myocyte hypertrophy (Figure 5G) following LAD ligation. As with the partial BMT, myeloid cell-specific ablation of Tet2 led to greater cardiac hypertrophy (Online Figure 7) and diminished cardiac function (Online Figure 8) compared to control mice. Mye-Tet2-KO mice also displayed greater expression of IL-1β after TAC than the control strain (Online Figure 9).surgery. Treatment with MCC950 had a marked inhibitory effect on the development of pressure overload-induced cardiac hypertrophy and it suppressed the differences in heart size, cardiac function, myocyte hypertrophy and interstitial fibrosis observed between the 10% KO-BMT and 10% WT-BMT mice in this model (Figure 5C–G).
Figure 5. Inflammasome inhibition reverses pressure overload-induced cardiac remodeling associated with hematopoietic Tet2-deficiency.
a. Scheme of the experimental study. Mice underwent partial (10%) bone marrow reconstitution with Tet2-deficient cells (10% KO-BMT mice) or WT cells (10% WT-BMT) following lethal irradiation. After 8 weeks of recovery, mice underwent permanent TAC surgery to produce pressure overload on the heart. MCC950 or PBS were infused from 1 week after TAC. b. IL-1β transcripts were determined in heart samples from 10% WT-BMT (n=7) mice and 10% KO-BMT mice (n=7) after pressure overload were by qPCR analysis. Statistical significance was evaluated by Mann-Whitney U test. c. Representative images of Picrosirius red staining to show the heart from 10% KO-BMT mice is larger compared to the heart from 10% WT-BMT mice 5 weeks after TAC. Scale bar indicates 1 mm. d. HW adjusted by TL, showing that MCC950 ameliorates the increase of cardiac mass after pressure overload in both strains of mice and eliminates the differences in these parameters between the Tet2-deficient and WT conditions (n=7 for TAC with PBS and n=8 for TAC with MCC950 per genotype). Sham operated mice without any infusion were used as control (n=3 per genotype). Statistical significances of differences among groups of 10% WT/10%KO with PBS or MCC950 were evaluated by two-way ANOVA with Tukey’s multiple comparison tests. e. Echocardiographic analysis shows that infusion with MCC950 protects against adverse cardiac remodeling in mice reconstituted with Tet2-KO and wild-type bone marrow, and eliminates the differences in cardiac parameters between Tet2-deficient and WT genotypes at the 5 weeks after TAC surgery. Echocardiography was performed at the indicated time points. Sample sizes were n=7 for 10% WT-BMT with PBS, n=7 for 10% KO-BMT with PBS, n=8 for 10% WT-BMT with MCC950, n=8 for 10% KO-BMT with MCC950. Statistical significance of difference was evaluated by two-way repeated measure ANOVA with Tukey’s multiple comparison tests. f. Quantitative analysis of cardiac sections stained with Picro sirius red as presented in Fig. 5c. shows that mice reconstituted with Tet2-knockout bone marrow exhibit greater cardiac fibrosis after pressure overload that can be reversed by treatment with MCC950. The MCC950 treatment eliminates the difference in this parameter between the Tet2-deficient and WT conditions. Sample sizes were n=7 for 10% WT-BMT with PBS, n=7 for 10% KO-BMT with PBS, n=8 for 10% WT-BMT with MCC950, n=8 for 10% KO-BMT with MCC950. Sham mice without any infusion were used as control (n=5 per genotype). Statistical significances of differences among groups of 10% WT/10%KO with PBS or MCC950 were evaluated by two-way ANOVA with Tukey’s multiple comparison tests. g. Representative images and measurement of CSA stained with WGA shows that MCC950 inhibits hypertrophy after pressure overload both in wild type and hematopoietic Tet2-KO mice and eliminates the difference in parameters between the Tet2-deficient and WT conditions. Sample sizes were n=7 for 10% WT-BMT with PBS, n=7 for 10% KO-BMT with PBS, n=8 for 10% WT-BMT with MCC950, n=8 for 10% KO-BMT with MCC950. Sham mice without any infusion were used as control (n=5 per genotype). Statistical significances of differences among groups of 10% WT/10%KO with PBS or MCC950 were evaluated by two-way ANOVA with Tukey’s multiple comparison tests. *p<0.05, ****p<0.0001; NS: not significant. WT: wild type, KO: knockout, BMT: bone marrow transfer, TAC: transverse aortic constriction, PBS: phosphate-buffered saline, qPCR: quantitative polymerase chain reaction, HW: heart weight, TL: tibia length, WGA: wheat germ agglutinin, WGA: wheat germ agglutinin, CSA: cross-sectional area of myocytes.
In separate experiments employing the TAC model of heart failure, the targeted ablation of Tet2 in myeloid cells also led to greater cardiac hypertrophy and diminished function compared to control mice (Online Figure 12). Myeloid Tet2-deficiency also led to greater lung congestion, myocardial fibrosis and myocyte hypertrophy. Hearts from these mice displayed greater expression of IL-1β transcript and more macrophages in the myocardium.
Discussion
A number of recent studies have shown that it is common to find non-symptomatic individuals that exhibit clonal hematopoiesis associated with the expansion of hematopoietic cells that contain a mutation in 1 of a number of cancer-related genes (Central Illustration). The most common mutations occur in epigenetic regulatory genes, such as TET2, that are frequently mutated in hematological cancers (6–8). Deep sequencing of mutational hotspots within candidate genes or whole exome sequencing of a well characterized cohort revealed that clonal hematopoiesis is common in the elderly (6–9,16–18,35). Remarkably, a study employing highly sensitive targeted error-corrected sequencing revealed that 95% of individuals in their 50’s possesses low levels of these mutations (predominantly DNMT3A and TET2), suggesting that the seeding of bone marrow with mutated hematopoietic cells is essentially ubiquitous by middle age (36). These findings have led to the compelling notion that age-associated chronic diseases—both cardiovascular disease (CVD) and non-CVD—may be influenced by the occurrence of somatic mutations that lead to clonal hematopoiesis. While previous studies in our laboratory demonstrated that clonal hematopoiesis associated with Tet2 deficiency accelerates atherosclerosis in mice (19), our experimental findings presented herein suggest that this phenomenon may have broader implications in the context of CVD, as it may also promote heart failure in post-MI patients.
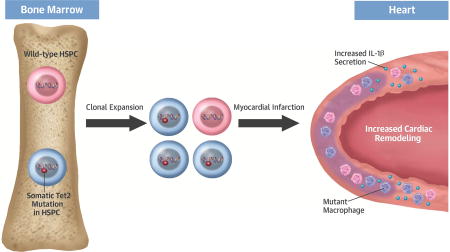
Central Illustration. Clonal Hematopoiesis Promotes Heart Failure.
Somatic Tet2 mutations within hematopoietic stem and progenitor cells (HSPC) will lead to their clonal amplification. These HSPC give rise to myeloid cell progeny that promote cardiac remodeling through excessive production of IL-1β.
The present study shows for the first time that the partial inactivation of Tet2 in hematopoietic cells, a condition that contributes to clonal hematopoiesis in humans, will promote cardiac dysfunction in two murine models of heart failure. The permanent ligation of the LAD artery is a model of severe ischemia that leads to myocardial necrosis and the remodeling of the heart in response to scar formation. The TAC model initially induces a cardiac hypertrophic response to pressure overload, and this is followed by systolic dysfunction in response to capillary rarefaction (33,34). In both of these models, Tet2-deficiency in hematopoietic cells, either by specific ablation in myeloid cells or by partial reconstitution of the bone marrow with Tet2-deficient HSPC, was associated with worse late-stage cardiac remodeling and reduced cardiac function.
The current work shows that Tet2-mediated clonal hematopoiesis leads to the up-regulation of IL-1β in two models of heart failure. IL-1β is processed to an active form and secreted following cleavage in the cytosol of innate immune cells by the NLRP3 inflammasome. Treatment with the NLRP3 inflammasome inhibitor MCC950 protected against the development of heart failure in both the LAD ligation and TAC models and eliminated the differences in cardiac parameters between Tet2-deficient and wild-type (WT) mice. These findings strongly suggest a central role for exacerbated IL-1β production in the maladaptive cardiac remodeling observed in conditions of hematopoietic Tet2 loss of function. Consistent with this possibility, previous studies have demonstrated an important role of IL-1β in CVD. A number of compelling studies in animal models have shown that the neutralization of IL-1β or inhibition of the inflammasome will protect the ischemic heart (37–41). IL-1β levels are elevated after ST-Elevation Myocardial Infarction (STEMI) and associated with maladaptive remodeling (42), and an IL-1β neutralizing antibody has recently shown efficacy in high risk post-MI patients (43). IL-1β has been shown to have local effects on the heart (44) as well as early systemic actions that includes the enhancement of HSPC proliferation within 1 day of the infarct (45). However, neither inflammasome inhibition nor LAD ligation had a detectable impact on Tet2-deficient leukocyte expansion in the current study.
Tet2 is a multifaceted epigenetic regulator that is able to facilitate both transcription activation and repression depending on context. Previous studies have shown that Tet2 acts as a negative regulator of pro-inflammatory macrophage activation and that it can function to repress LPS-induced IL-6 expression (46,47). A mechanistic link between Tet2 inactivation and NLRP3-mediated IL-1β production has also been documented (19). Tet2-deficient macrophages express higher levels of IL-1β transcript and pro-IL-1β protein, its inactive precursor. Tet2-deficiency also upregulates components of the NLRP3 inflammasome, and there are increases in the levels of cleaved and secreted IL-1β that exceed the level of its transcriptional activation suggesting that Tet2 modulates multiple steps in the IL-1β pathway. Tet2-mediated IL-1β regulation is independent of its catalytic activity, i.e. the oxidation of 5-methylcytosine, but involves changes in the recruitment of histone deacetylase to the IL-1β promoter (19,46).
The Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) has shown that the IL-1β neutralizing antibody canakinumab can reduce major adverse cardiovascular events in patients with stable coronary artery disease and elevated levels of C-reactive protein, predominantly due to reductions in the incidence of repeat myocardial infarction (43). Based upon the findings of the current study and our previous atherosclerosis studies (19), it is tempting to speculate that subjects with somatic mutations in TET2 would exhibit a superior response to this treatment. Thus, subgroup analysis based upon genetic screening for somatic TET2 mutations, and perhaps related somatic mutations in WBC, should be considered in this patient cohort. In view of the infection side effect of canakinumab therapy, the identification of patients who would most benefit from this drug is warranted.
Conclusions
These experimental studies show that mice with Tet2-deficiency in hematopoietic cells display greater maladaptive cardiac remodeling and dysfunction in models of pressure overload hypertrophy and permanent LAD ligation. NLRP3 inflammasome inhibition had a disproportionately greater protective effect in these models when mice were engineered to have inactivating mutations in the Tet2 gene of hematopoietic cells. Given that somatic mutation in TET2 are relatively common in the elderly population, these data suggest that IL-1β blockade or NLRP3 inflammasome inhibition may be particularly effective for the treatment of CVD in individuals who carry these mutations.
Supplementary Material
Clinical Perspectives.
Competency in Medical Knowledge
In elderly individuals, clonal hematopoiesis arising from pre-leukemic genetic mutations in hematopoietic stem cells may include the epigenetic regulator TET2. In epidemiological studies, somatic TET2 mutations are associated with an increased risk of developing heart failure.
Translational Outlook
Clinical studies are needed to determine whether patients with somatic TET2 mutations who sustain myocardial infarction benefit from therapies that target IL-1β or the NLRP3 inflammasome.
Acknowledgments
Funding: Dr. Walsh was funded by NIH grants HL131006, HL138014 and Dr. Sano was supported by an American Heart Association post-doctoral fellowship.
Abbreviations list
- ACS
acute coronary syndrome
- BMT
bone marrow transplantation
- CD45
cluster of differentiation 45 antigen
- CRP
C-reactive protein
- CSA
cross-sectional area
- DNMT3A
DNA (cytosine-5)-methyltransferase 3A
- EF
ejection fraction
- FS
fractional shortening
- HSPC
hematopoietic stem/progenitor cells
- HW
heart weight
- IL-1β
interleukin 1 beta
- LAD
left anterior descending
- LVPWTd
left ventricle posterior wall thickness at diastole
- LW
lung weight
- MI
myocardial infarction
- NLRP3
NACHT, LRR and PYD domains-containing protein 3
- PBS
phosphate buffered saline
- qPCR
quantitative polymerase chain reaction
- STEMI
ST-Elevation Myocardial Infarction
- TAC
transverse aortic constriction
- TET2
ten-eleven translocation 2
- TL
tibia length
- WBC
white blood cells
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Prof. Cooper is also a shareholder in Inflazome, Ltd. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.de Freitas EV, Batlouni M, Gamarsky R. Heart failure in the elderly. J Geriatr Cardiol. 2012;9:101–7. doi: 10.3724/SP.J.1263.2011.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belsky DW, Caspi A, Houts R, et al. Quantification of biological aging in young adults. Proc Natl Acad Sci U S A. 2015;112:E4104–10. doi: 10.1073/pnas.1506264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vigen R, Maddox TM, Allen LA. Aging of the United States population: impact on heart failure. Curr Heart Fail Rep. 2012;9:369–74. doi: 10.1007/s11897-012-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vijg J. Somatic mutations, genome mosaicism, cancer and aging. Curr Opin Genet Dev. 2014;26:141–9. doi: 10.1016/j.gde.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shlush LI, Zandi S, Itzkovitz S, Schuh AC. Aging, clonal hematopoiesis and preleukemia: not just bad luck? Int J Hematol. 2015;102:513–22. doi: 10.1007/s12185-015-1870-5. [DOI] [PubMed] [Google Scholar]
- 6.Genovese G, Kahler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–87. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–98. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie M, Lu C, Wang J, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20:1472–8. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zink F, Stacey SN, Norddahl GL, et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 2017;02:769869. doi: 10.1182/blood-2017-02-769869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko M, Bandukwala HS, An J, et al. Ten-Eleven-Translocation 2 (TET2) negatively regulates homeostasis and differentiation of hematopoietic stem cells in mice. Proc Natl Acad Sci U S A. 2011;108:14566–71. doi: 10.1073/pnas.1112317108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moran-Crusio K, Reavie L, Shih A, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20:11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quivoron C, Couronne L, Della Valle V, et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell. 2011;20:25–38. doi: 10.1016/j.ccr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Cai X, Cai CL, et al. Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood. 2011;118:4509–18. doi: 10.1182/blood-2010-12-325241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busque L, Patel JP, Figueroa ME, et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat Genet. 2012;44:1179–81. doi: 10.1038/ng.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko M, Huang Y, Jankowska AM, et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature. 2010;468:839–43. doi: 10.1038/nature09586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017 doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buscarlet M, Provost S, Zada YF, et al. DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood. 2017;130:753–762. doi: 10.1182/blood-2017-04-777029. [DOI] [PubMed] [Google Scholar]
- 18.Acuna-Hidalgo R, Sengul H, Steehouwer M, et al. Ultra-sensitive Sequencing Identifies High Prevalence of Clonal Hematopoiesis-Associated Mutations throughout Adult Life. Am J Hum Genet. 2017;101:50–64. doi: 10.1016/j.ajhg.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuster JJ, MacLauchlan S, Zuriaga MA, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maruyama S, Nakamura K, Papanicolaou KN, et al. Follistatin-like 1 promotes cardiac fibroblast activation and protects the heart from rupture. EMBO Mol Med. 2016;8:949–66. doi: 10.15252/emmm.201506151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibata R, Izumiya Y, Sato K, et al. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol. 2007;42:1065–74. doi: 10.1016/j.yjmcc.2007.03.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimano M, Ouchi N, Nakamura K, et al. Cardiac myocyte-specific ablation of follistatin-like 3 attenuates stress-induced myocardial hypertrophy. J Biol Chem. 2011;286:9840–8. doi: 10.1074/jbc.M110.197079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimano M, Ouchi N, Nakamura K, et al. Cardiac myocyte follistatin-like 1 functions to attenuate hypertrophy following pressure overload. Proc Natl Acad Sci U S A. 2011;108:E899–906. doi: 10.1073/pnas.1108559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibata R, Ouchi N, Ito M, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–9. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibata R, Sato K, Kumada M, et al. Adiponectin accumulates in myocardial tissue that has been damaged by ischemia-reperfusion injury via leakage from the vascular compartment. Cardiovasc Res. 2007;74:471–9. doi: 10.1016/j.cardiores.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Wei K, Serpooshan V, Hurtado C, et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525:479–85. doi: 10.1038/nature15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coll RC, Robertson AA, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–55. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anzai A, Choi JL, He S, et al. The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes. J Exp Med. 2017;214:3293–3310. doi: 10.1084/jem.20170689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hilgendorf I, Gerhardt LM, Tan TC, et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014;114:1611–22. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Protti A, Mongue-Din H, Mylonas KJ, et al. Bone marrow transplantation modulates tissue macrophage phenotype and enhances cardiac recovery after subsequent acute myocardial infarction. J Mol Cell Cardiol. 2016;90:120–8. doi: 10.1016/j.yjmcc.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–6. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delhommeau F, Dupont S, Della Valle V, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;360:2289–301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- 33.Shiojima I, Sato K, Izumiya Y, et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–18. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Izumiya Y, Shiojima I, Sato K, Sawyer DB, Colucci WS, Walsh K. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension. 2006;47:887–93. doi: 10.1161/01.HYP.0000215207.54689.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKerrell T, Park N, Moreno T, et al. Leukemia-associated somatic mutations drive distinct patterns of age-related clonal hemopoiesis. Cell Rep. 2015;10:1239–45. doi: 10.1016/j.celrep.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young AL, Challen GA, Birmann BM, Druley TE. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Commun. 2016;7:12484. doi: 10.1038/ncomms12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toldo S, Schatz AM, Mezzaroma E, et al. Recombinant human interleukin-1 receptor antagonist provides cardioprotection during myocardial ischemia reperfusion in the mouse. Cardiovasc Drugs Ther. 2012;26:273–6. doi: 10.1007/s10557-012-6389-x. [DOI] [PubMed] [Google Scholar]
- 38.Toldo S, Mezzaroma E, Bressi E, et al. Interleukin-1beta blockade improves left ventricular systolic/diastolic function and restores contractility reserve in severe ischemic cardiomyopathy in the mouse. J Cardiovasc Pharmacol. 2014;64:1–6. doi: 10.1097/FJC.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 39.Abbate A, Salloum FN, Van Tassell BW, et al. Alterations in the interleukin-1/interleukin-1 receptor antagonist balance modulate cardiac remodeling following myocardial infarction in the mouse. PLoS One. 2011;6:e27923. doi: 10.1371/journal.pone.0027923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Tassell BW, Arena R, Biondi-Zoccai G, et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study) Am J Cardiol. 2014;113:321–7. doi: 10.1016/j.amjcard.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Hout GP, Bosch L, Ellenbroek GH, et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur Heart J. 2017;38(11):828–836. doi: 10.1093/eurheartj/ehw247. [DOI] [PubMed] [Google Scholar]
- 42.Orn S, Ueland T, Manhenke C, et al. Increased interleukin-1beta levels are associated with left ventricular hypertrophy and remodelling following acute ST segment elevation myocardial infarction treated by primary percutaneous coronary intervention. J Intern Med. 2012;272:267–76. doi: 10.1111/j.1365-2796.2012.02517.x. [DOI] [PubMed] [Google Scholar]
- 43.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 44.Saxena A, Chen W, Su Y, et al. IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. J Immunol. 2013;191:4838–48. doi: 10.4049/jimmunol.1300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sager HB, Heidt T, Hulsmans M, et al. Targeting Interleukin-1beta Reduces Leukocyte Production After Acute Myocardial Infarction. Circulation. 2015;132:1880–90. doi: 10.1161/CIRCULATIONAHA.115.016160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q, Zhao K, Shen Q, et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature. 2015;525:389–93. doi: 10.1038/nature15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cull AH, Snetsinger B, Buckstein R, Wells RA, Rauh MJ. Tet2 restrains inflammatory gene expression in macrophages. Exp Hematol. 2017;55:56–70. e13. doi: 10.1016/j.exphem.2017.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.