Abstract
Background/Aim
Plasma trimethylamine-N-oxide (TMAO), a product of intestinal microbial metabolism of dietary phosphatidylcholine has been recently associated with atherosclerosis and increased risk of cardiovascular diseases (CVD) in rodents and humans. However, the molecular mechanisms of how TMAO induces atherosclerosis and CVD progression are still unclear. The present study tested whether TMAO induces NLRP3 inflammasome formation and activation and thereby contributes to endothelial injury initiating atherogenesis.
Methods
Inflammasome formation and activation was determined by confocal microscopy, caspase-1 activity was measured by colorimetric assay, IL-1β production was measured using ELISA, cell permeability was determined by microplate reader and ZO-1 expression was determined by western blot analysis and confocal microscopy. In in vivo experiments, TMAO was infused by osmotic pump implantation.
Results
TMAO treatment significantly increased the colocalization of NLRP3 with Asc or NLRP3 with caspase-1, caspase-1 activity, IL-1β production, cell permeability in carotid artery endothelial cells (CAECs) compared to control cells. Pretreatment with caspase-1 inhibitor, WEHD or Nlrp3 siRNA abolished the TMAO-induced inflammasome formation, activation and cell permeability in these cells. In addition, we explored the mechanisms by which TMAO activates NLRP3 inflammasomes. TMAO-induced the activation of NLRP3 inflammasomes was associated with both redox regulation and lysosomal dysfunction. In animal experiments, direct infusion of TMAO in mice with partially ligated carotid artery were found to have increased NLRP3 inflammasome formation and IL-1β production in the intima of wild type mice.
Conclusion
The formation and activation of NLRP3 inflammasomes by TMAO may be an important initiating mechanism to turn on the endothelial inflammatory response leading to endothelial dysfunction.
Keywords: TMAO, inflammasome, tight junction protein, endothelial cell permeability
INTRODUCTION
Trimethylamine-N-oxide (TMAO) has been recently highlighted as a potential diagnostic marker for cardiovascular diseases (CVD). Recent studies demonstrated that elevated TMAO levels in plasma are associated with an increased risk of CVD [1–8]. Circulating TMAO levels were found to be elevated in distinct cohorts of cardiac patients with stable heart failure and were associated with increased risk for myocardial infarction, stroke and mortality [1–5, 9–15]. Plasma TMAO concentrations were found to independently predict coronary atherosclerosis and mortality in patients with chronic kidney disease [12]. Increased TMAO concentrations have also been associated with impaired glucose tolerance [16], diabetes [17]. Recently, TMAO have been associated with greater risk of colorectal and prostate cancer. Despite the clear association of TMAO with various chronic diseases, the exact mechanism through which TMAO leads to development and progression of various diseases is still unclear.
In this regard, some mechanisms postulated to date are alteration in host sterol/lipid metabolic pathway leading to changes in cholesterol transport and excretion [2, 7, 9, 15], modulation of platelet responsiveness [8] and activation of profibrotic pathways [3]. Recent reports indicated activation of mitogen-activated protein kinase, nuclear factor-kappa B signaling cascade and promotion of leukocyte adhesion in vivo [3]. Together, these studies suggests that TMAO may trigger endothelial and vascular inflammation, injury and fibrotic processes that may contribute to atherogenesis. However, the exact mechanism through which TMAO leads to development and progression of atherosclerotic vascular diseases is currently unclear.
Nlrp3 inflammasome act as a sensor to detrimental exogenous and endogenous substances and switch on both inflammatory and non-inflammatory responses which play a vital role in the development of atherosclerosis. Recent studies have indicated that Nlrp3 inflammasome activation is critical for the development of atherosclerosis upon atherogenic stimuli such as cholesterol crystals [18, 19]. However, whether Nlrp3 acts as a sensor to the recently recognized proatherogenic metabolite, TMAO, is unknown and the role of inflammasome signaling in TMAO-induced atherogenisis has not been explored. Since TMAO is a biologically active atherogenic molecule it is important to understand the role of TMAO in eliciting inflammatory and non-inflammatory responses via inflammasome activation during atherosclerotic vascular disease. The earliest event in the development of atherosclerosis is endothelium dysfunction, which can be triggered by several insults. Hence, it is plausible to determine whether TMAO induces endothelial inflammasome activation and contribute to the endothelial dysfunction in the very early stages of atherosclerosis.
MATERIAL AND METHODS
Cell culture and treatments
The mouse carotid arterial endothelial cells were isolated and characterized as described earlier [20, 21]. For the TMAO stimulation, cells were treated with TMAO (30 μm) and then incubated for overnight. In case of inhibitors used, the cells were pretreated with 1 mmol/L Z-WEHD-FMK (WEHD; R&D Systems, Minneapolis, MN), cathepsin B inhibitor Ca-074Me (5 μM, Sigma), potassium channel blocker glibenclamide (Glib, 10 μM, Sigma) or ROS scavenger N-acetyl-L-cysteine (NAC, 10 μM, Sigma) for 30 min.
Immunofluorescence microscopic analysis
Cells were grown on eight-well chamber slides and then treated as indicated. After the treatment, cells were fixed with 4% paraformaldehyde for 15 minutes. The cells were then washed in phosphate-buffer saline (PBS) and were incubated for 2 hours at 4°C with rabbit and/or mouse anti-Nlrp3 (1:500, Abcam), anti-ASC (1:500, Invitrogen, Abcam), anti-caspase 1 (1:1000; Abcam) and anti-ZO-1 (1:1000; Invitrogen). Double immunofluorescent staining was performed by incubating slides with Alexa Fluor 488 or Alexa Fluor 555-labeled secondary antibody (1:100, Invitrogen) for 1 hour at room temperature. The slides were visualized through sequentially scanning on an Olympus laser scanning confocal microscope (Fluoview FV1000, Olympus, Japan). Colocalization was analyzed by Image Pro Plus software, and the co-localization coefficient was represented by Pearson’s correlation coefficient [22–25].
Caspase-1 Activity and IL-1β production Assay
Cells were harvested and homogenized to extract proteins for caspase-1 activity assay using a commercially available kit (Biovision, CA). The data was expressed as the fold change compared with control cells. In addition, the cell supernatant was collected and IL-1β production was measured by a commercially available ELISA Kit (R&D System, Minneapolis, MN) according to the protocol described by the manufacturer.
Immunoblotting
Cells were washed twice with ice-cold PBS and homogenized in ice-cold HEPES buffer containing 25 mM Na-HEPES, 255 mM sucrose, 1 mM EDTA, and 0.1 mM phenylmethylsulfony1 fluoride (pH 7.4). After centrifugation at 1000 × g for 10 min at 4°C, the supernatants containing the membrane protein and cytosolic components, termed homogenates, were frozen in liquid N2, and stored at −80°C until use. Cell homogenates were denatured with reducing Laemmli SDS-sample buffer and boiled for 5 min. Samples were run on SDS-PAGE gel, transferred into PVDF membrane and blocked. The membranes were probed with ZO-1 antibody (Life Technology, 1:1000) or β-actin overnight at 4°C followed by incubation with secondary antibody, and then conjugated to horseradish peroxidase-labeled immunoglobulin G. The immunoreactive bands were enhanced by chemiluminescence methods and imaged on Kodak Omat film. β-actin served as a loading control.
Endothelial permeability
CAECs were cultured in 24-well transwell plates and treated as indicated for 24 hr. The transwell inserts were moved into non-used wells with 200 μl fresh media. 100 μl Fluorescein isothiocyanate (FITC)–dextran (10 KDa, Invitrogen) solution was added into each insert and the plate was incubated at 37°C for 2 hours to allow fluorescein molecules flow through the endothelial cell monolayer. The inserts were then removed and fluorescent intensity in each well was determined at excitation/emission of 485/530 nm using a fluorescent microplate reader (FL × 800, BIO-TEK Instruments). The arbitrary fluorescence intensity was used to calculate the relative permeability.
Partial Carotid Ligation and Osmotic Pump Implantation
Eight-week-old male C57BL/6J wild-type mice were used. All protocols were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. Partial carotid ligation surgery was performed as previously reported [26]. In brief, animals were sedated with 2% isoflurane that was provided through a nose cone. Next, a ventral midline incision of 4 to 5 mm was made in the neck. With the use of blunt dissection, muscle layers were separated with curved forceps to expose the left carotid artery (LCA). Three of four branches of the LCA (left external carotid, internal carotid, and occipital arteries) were ligated by using a 6-0 silk suture. The superior thyroid artery was left intact, providing the sole source for blood circulation. The incision was then closed, and the animals were kept on a heating pad until they gained consciousness. In the TMAO infusion group, the osmotic pump (model 2002; Alzet, Cupertino, CA) filled with TMAO was implanted subcutaneously, and the catheter was inserted into the external jugular vein. In another group, mice were injected intraperitoneally with WEHD, a caspase-1 inhibitor, at a dosage of 1 mg/kg per day before implantation of the TMAO pump. Fourteen days after partial ligation, animals were sacrificed by cervical dislocation after the administration of anesthesia. Blood samples were collected, LCAs and right carotid arteries were then harvested for immunohistochemistry, dual fluorescence staining, and confocal analysis.
Immunohistochemistry
Formalin-fixed, paraffin-embedded carotid arterial tissue sections (4 μm) were stained with primary antibodies (1:50 dilution) overnight at 4 °C after a 20 min wash with 3% H2O2 and 30 min blocking with serum. The slides were sequentially treated with CHEMICON IHC Select HRP/DAB Kit (EMD Millipore, MA) according to the protocol described by the manufacturer. Finally, the slides were counterstained with hematoxylin. Negative controls were prepared by leaving out the primary antibodies.
Statistics
Data are presented as means±SEM. Significant differences between and within multiple groups were examined using ANOVA for repeated measures, followed by Duncan’s multiple-range test. P < 0.05 was considered statistically significant
RESULTS
TMAO induces formation and activation of NLRP3 inflammasome in mouse carotid artery endothelial cells (CAECs)
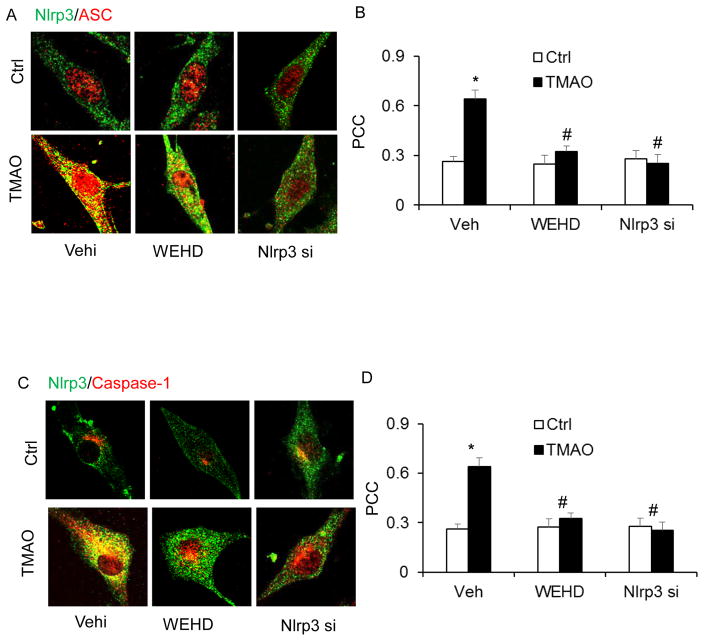
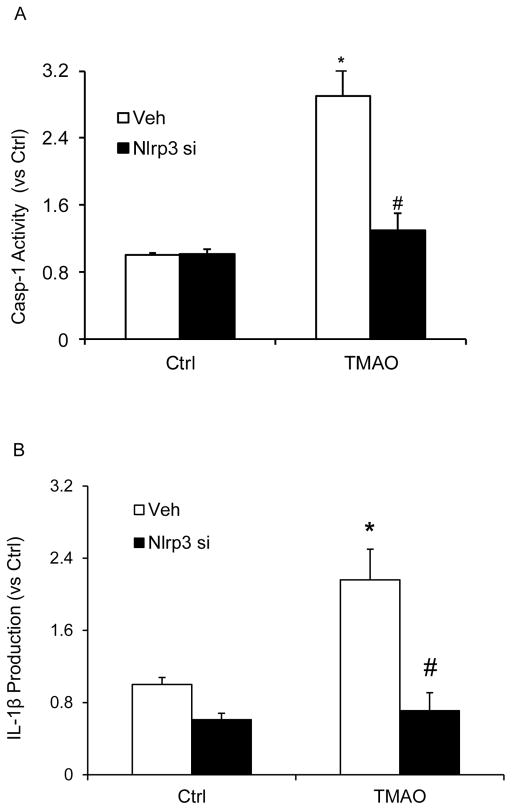
We tested the hypothesis that TMAO induces inflammasome formation and activation and thereby contributes to endothelial injury. In cultured CAECs, we examined whether TMAO could trigger the formation and activation of Nlrp3 inflammasome complexes by analyzing the co-localization of Nlrp3 inflammasome components, the cleavage of pro-caspase-1 to activate caspase-1, and the production of IL-1β. Our confocal microscopic images showed that TMAO-induced co-localization of inflammasome molecules between Nlrp3 (green) with ASC (red) or Nlrp3 (green) with Caspase-1(red) as shown by increased yellow staining (yellow spots) in CAECs, which were blocked by caspase-1 inhibitor (WEHD) or silencing Nlrp3 gene by Nlrp3 siRNA (Nlrp3si) transfection (Fig. 1). Nlrp3 inflammasome complex formation results in cleavage of pro-caspase-1 protein to their bioactive form, which in turn binds to and cleaves its substrates such as pro-interleukin 1β (IL-1β). In line with the confocal findings of inflammasome complex formation, we have shown that TMAO increased caspase 1 activity (Fig. 2A) and also enhanced IL-1β production (Fig. 2B). Caspase-1 activity and IL-1β production were abolished in CAECs with prior treatment of Nlrp3 gene silencing.
Figure 1. TMAO-induced NLRP3 inflammasome formation and activation in CAECs.
Representative confocal fluorescence images show the colocalization of NLRP3 with ASC (A) or NLRP3 with caspase-1 (C). Summarized data shows the fold changes of pearson coefficient correlation (PCC) for the colocalization of NLRP3 with ASC (B) and NLRP3 with caspase-1 (D) in CAECs of Nlrp3+/+ mice. * Significant difference (P<0.05) compared to the values from control cells, # Significant difference (P<0.05) compared to the values from TMAO treated group. Nlrp3 si, Nlrp3 siRNA; cells were transfected with Nlrp3 siRNA or WEHD and then stimulated with TMAO. N=5–6.
Figure 2. Effects of TMAO on caspase-1 activity and IL-1β production in CAECs.
Values are arithmetic means ± SEM (n=6 each group) of caspase-1 activity (A), IL-1β production (B) in CAECs of Nlrp3+/+ mice with or without stimulation of TMAO and/or Nlrp3 siRNA transfection. * Significant difference (P<0.05) compared to the values from control cells, # Significant difference (P<0.05) compared to the values from TMAO treated group. Nlrp3 si, Nlrp3 siRNA; cells were transfected with Nlrp3 siRNA or WEHD and then stimulated with TMAO. N=6.
Effect of TMAO on tight junction proteins and endothelial cell permeability in CAECs
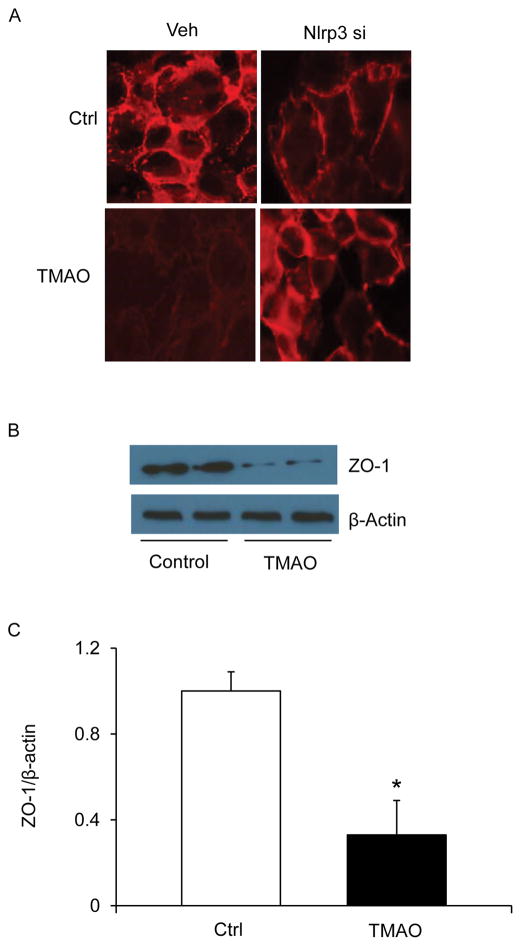
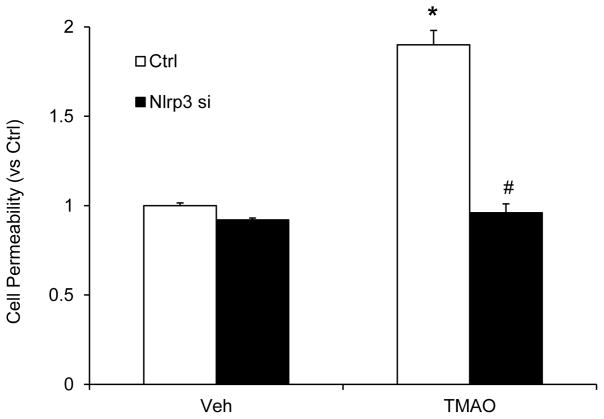
Endothelial cells are connected by tight junction proteins which maintain the integrity of the endothelium. Tight junctions function as a barrier in regulating paracellular permeability and maintaining cell polarity. ZO-1 is an essential tight junction protein which is associated with junction integrity and its down regulation leads to junctional disruption and enhnaced cellular permealibity. Hence, we investigated whether TMAO-induced Nlrp3 inflammasome activation could cause disassembly of tight junction protein ZO-1. Our confocal analysis showed that TMAO markedly decreased the expression of tight junction protein ZO-1 on endothelial cell monolayers (Fig. 3A). TMAO induced downregulation of ZO-1 which was prevented by silencing Nlrp3 in the CAECs. Down-regulation of ZO-1 by TMAO was further confirmed by western blot analyses which indicate that TMAO decreased ZO-1 protein expression (Fig. 3B and 3C). To further determine the functional significance of NLRP3 inflammasome activation, we examined its influence on TMAO-induced changes in barrier function of endothelial monolayers. As shown in Fig. 4, dextran flux significantly increased in ECs treated with TMAO compared to vehicle treated ECs. This TMAO-induced increase in EC permeability was markedly reduced in the presence of Nlrp3 siRNA transfection (Fig. 4). These results indicate that activation of Nlrp3 inflammasome by TMAO causes disruption of tight-junction proteins and alters EC permeability.
Figure 3. Effects of Nlrp3 gene silencing on TMAO-induced tight junction protein ZO-1 in CAECs.
A: Representative fluorescence images shows the ZO-1 expression in CAECs with or without stimulation of TMAO and/or Nlrp3 siRNA transfection (n=5). B: Representative Western blot gel document showing the expression of ZO-1 (n=3–5). C: Summarized data showing the expression of ZO-1 (n=3–5). * Significant difference (P<0.05) compared to the values from control cells.
Figure 4. Inhibition of inflammasome abolishes TMAO-induced cell permeability in CAECs.
Values are arithmetic means ± SEM (n=6 each group) of cell permeability in CAECs of Nlrp3+/+ mice with or without stimulation of TMAO and/or Nlrp3 siRNA transfection. * Significant difference (P<0.05) compared to the values from control cells, # Significant difference (P<0.05) compared to the values from TMAO treated group. Nlrp3 si: Nlrp3 siRNA; cells were transfected with Nlrp3 siRNA and then stimulated with TMAO.
TMAO-induced inflammasome signaling pathways
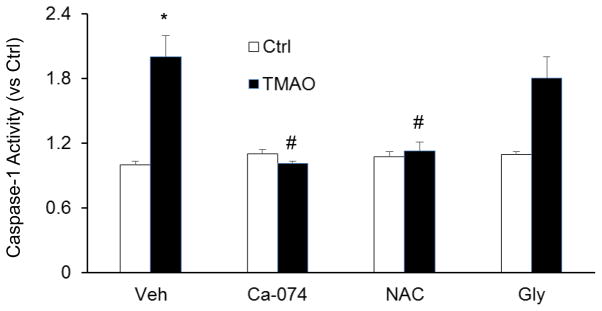
Endothelial inflammasomes are known to be activated by three major signaling pathways which include reactive oxygen species (ROS) activation, lysosome rupture and ion channel gating (K+ efflux). We examined TMAO-induced inflammasome signaling mechanisms by using inhibitors of the above mentioned pathways which lead to activation of Nlrp3 inflammasomes. It was found that inhibition of both cathepsin B activity (Ca-074Me) and ROS release (N-acetyl-L-Cysteine or Nac) in ECs markedly attenuated TMAO-induced caspase-1 activity in ECs (Fig. 5). In contrast, K Channel blocker (Glibenclamide) had a no significant effect on TMAO-induced Nlrp3 inflammasome activation. Therefore our results indicate that TMAO could act via lysosomal destabilization and blockade of ROS.
Figure 5. Effect of cathespsin B inhibition, potassium channel blockade or ROS scavenging on TMAO- induced NLRP3 inflammasomes activation in CAECs.
Summarized data showing the, caspase-1 activity in CAECs with or without stimulation of TMAO. Ca-074: Ca-074Me, cathepsin B inhibitor, Gly: Glybeclamide, potassium channel blocker, NAC: N-acetyl-L-cysteine, ROS scavenger. * P<0.05 vs. Ctrl group; # P<0.05 vs. TMAO (n=6).
TMAO-induced endothelial inflammasome formation and activation in the carotid arteries of mice
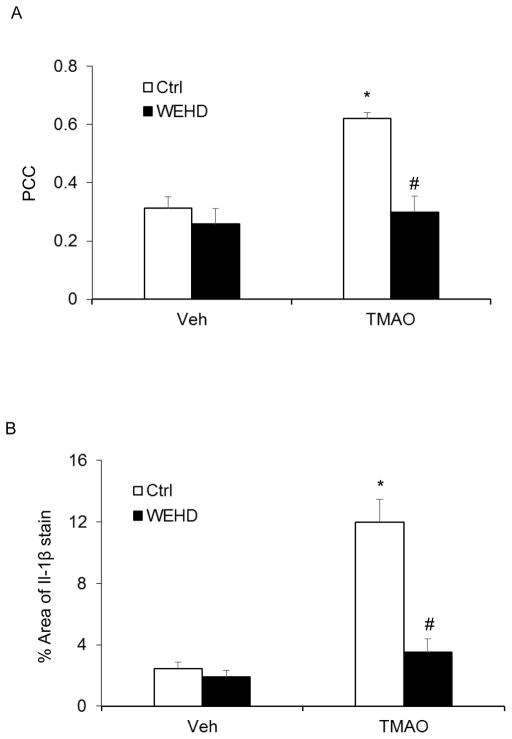
Confocal microscopic analysis demonstrated that TMAO treatment increased the co-localization of NLRP3 with ASC in carotid arteries of wild type mice (Fig. 6A). In addition, the TMAO-induced IL-1β production in the intima in wildtype mice (Fig. 6B). This data suggests the formation and activation of NLRP3 inflammasomes in the endothelium of these arteries.
Figure 6. Nlrp3 inflammasome formation and activation in wild type mice with or without stimulation of TMAO and PLCA.
A: Summarized data showing the co-localization coefficient (PCC) of Nlrp3 with Asc. B: IL-1β production in the intima of vehicle or TMAO treated wild type mice. * Significant difference (P<0.05) compared to the values from control mice. # Significant difference (P<0.05) compared to the values from mice on the TMAO.
DISCUSSION
The primary goal of the present study was to reveal whether TMAO induced NLRP3 inflammasome activation and leads to the development of endothelial dysfunction. We first confirmed that TMAO stimulation induced the formation and activation of the NLRP3 inflammasome complex in CAECs. However, such inflammasome formation and activation were abolished in ECs with prior treatment with Nlrp3 siRNA or Caspase-1 inhibitor, WEHD. Thus TMAO lead to the formation and activation of NLRP3 inflammasomes in ECs. The findings demonstrate the critical role of TMAO in the activation of NLRP3 inflammasomes which could be associated with subsequent endothelial dysfunction and atherogenesis.
Recently, NLRP3 inflammasome has been implicated in different auto inflammatory diseases such as gout, myocardial infarction, and type II diabetes, obesity, glomerular injury [18, 24, 27–36] and also to a number of other diseases including silicosis, liver toxicity, Alzheimer’s disease, cystic fibrosis and acute lung injury [23, 24, 37–42]. However, little is known about inflammasome contribution to the initiation or development of atherosclerosis. Among different types of inflammasomes, the NLRP3 inflammasome has been well characterized, which consists of a proteolytic complex formed by Nlrp3, the adaptor protein ASC, and caspase-1. Caspase-1 is activated when the inflammasome complex is formed to produce active IL-1β and IL-18 by cleavage of their precursors [22, 29, 43]. NLRP3 acts as the sensory component to recognize both endogenous and exogenous danger signals [44–46], when ASC and caspase-1 are recruited to form a protein complex, where caspase-1 is activated [47–49]. The active caspase-1 not only proteolytically cleaves IL-1β and/or IL-18 into their biologically active form [22, 29, 43]. In macrophages, NLRP3 inflammasome activation is critical for the foam cell formation and other atherosclerotic lesions upon proatherogenic stimuli such as cholesterol crystals (ChC) [18, 19]. More interestingly, some non-atherogenic endanger factors also activate NLRP3 inflammasomes including adenosine triphosphate (ATP), uric acid, visfatin and DAMPs [26, 28, 30, 34, 50–53], which may enhance the susceptibility to atherosclerosis or other vascular diseases, cell pyroptosis and alterations of cell membrane permeability, turning on the inflammatory response and directly inducing cell dysfunction or injury. Moreover, recent study shows that TMAO activates the expression of inflammasomes in human umbilical vein endothelial cells [54]. However, it remains unknown whether TMAO induces NLRP3 inflammasomes activation in both in vitro and in vivo and how activated NLRP3 inflammasomes lead to endothelial dysfunction. In the present study, we first confirmed that TMAO-induced the formation and activation of the NLRP3 inflammasome complex in CAECs, as shown by colocalization of NLRP3 with ASC or NLRP3 with caspase-1 using confocal microscopy and by biochemical analysis of caspase-1 activity and production of IL-1β. However, such inflammasome formation and activation were abolished in CAECs with prior treatment with Nlrp3 siRNA or caspase-1 inhibitor, WEHD (Fig. 1 and 2). In addition in in vivo studies, mice infused with TMAO for 2 weeks had increased the NLRP3 inflammasome formation (colocalization of NLRP3 with ASC) and activation (IL-1β production) in carotid arteries. Taken together, these results clearly suggest that TMAO-induced NLRP3 inflammasome activation in endothelial cells, which may contribute to the development of endothelial dysfunction or atherogenic pathology. To our knowledge, the results from the present study provide the first experimental evidence demonstrated that TMAO-induced endothelial inflammasome activation in both in in vitro and in vivo models.
Vascular endothelium serves as a semi-selective interface between the vessel lumen and surrounding tissue and acts as a barrier, controlling the passage of materials and contributes to vascular homeostasis [55, 56]. Alterations of endothelial cells play a central role in the pathogenesis of a many human diseases, as endothelial cells have the key function in the maintenance of vascular homeostasis. Vascular endothelial damage is involved in peripheral vascular disease, stroke, heart disease, diabetes, insulin resistance, venous thrombosis, chronic kidney failure, metastasis, tumor growth and some viral infectious diseases [55, 56]. Endothelium is altered by various molecules including proteins, lipid-transporting particles, metabolites, and hormones, as well as through specific junctional proteins and receptors [55, 56]. The present study demonstrated that stimulation of endothelial cells with TMAO decreased the expression of tight junction protein ZO-1 in endothelial cell monolayers, where as Nlrp3 gene silencing prevented such TMAO-induced down regulation of tight junction protein. These findings demonstrate for the first time that TMAO-induced endothelial hyperpermeability which is associated with inflammasome-dependent tight junction disruption.
Many physiologic and pathophysiologic stimuli can induce changes in endothelial permeability. For example bacterial endotoxin LPS, environmental toxins, high fat diet can contribute to endothelial dysfunction by increasing endothelial permeability and subsequently arterial lipid accumulation in the subendothelial space, thereby initiating atherosclerotic plaque development. Other injurious stimuli like thrombin, histamine and other acute inflammatory mediators can act on endothelium to stimulate opening of their intercellular junctions at the level of adherens and tight junctional complexes [57, 58]. It has been well established that loss of the integrity of inter-endothelial tight junctions contributes to enhanced paracellular endothelial permeability and plasma proteins including albumin and visfatin can impair renal tubular or endothelial tight junctions via activation of Nlrp3 inflammasomes [23, 24, 57, 58]. Consistent with these studies, the present study demonstrates that TMAO treatment induces increases in permeability to dextrans in CAECs, via activation of Nlrp3 inflammasomes (Fig. 4) which is prevented by inhibition of Nlrp3 by Nlrp3 siRNA.
Next we examined how TMAO-induced NLRP3 inflammasome activation in endothelial cells. Several mechanisms underlying inflammasome activation have been reported, including lysosome rupture, K+ channel gating, and reactive oxygen species (ROS) activation [25]. We first tested which of these pathways are involved in TMAO-induced NLRP3 inflammasome activation. Using blockers or inhibitors of individual pathway, we found that TMAO-induced NLRP3 inflammasome formation and activation in endothelial cells were significantly attenuated or abolished by ROS scavenger, N-acetyl-L-cysteine (NAC) and cathepsin B inhibitor, Ca-074Me, but not by potassium channel blocker, glibenclamide (Glib). These results suggest that TMAO is able to activate NLRP3 inflammasomes in ECs at least via two reported pathways involving increased ROS and frustrated lysosomes and enhanced cathepsin B activity.
In summary, this work has studied the formation and activation of NLRP3 inflammasomes by TMAO which may be an important initiating mechanism to turn on the endothelial inflammatory response leading to endothelial dysfunction. Our data suggest that TMAO induces inflammasome-dependent endothelial hyperpermeability via activation of the Nlrp3 inflammasome in endothelial cells. Thus, our findings provide novel insights that TMAO-induced endothelial hyperpermeability via inflammasome activation may facilitate endothelial barrier dysfunction thereby contributing to endothelial dysfunction and atherogenesis.
Acknowledgments
This work was supported by by grants DK104031 to (K.B.) from National Institutes of Health and new faculty research grant to (S.K) from University of Houston.
Footnotes
CONFLICTS OF INTERESTS
The authors of this manuscript declare that they have no conflicts of interests.
References
- 1.Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ, Shih DM. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-kappaB. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. 2014;124:4204–4211. doi: 10.1172/JCI72331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40. doi: 10.1016/j.ab.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warrier M, Shih DM, Burrows AC, Ferguson D, Gromovsky AD, Brown AL, Marshall S, McDaniel A, Schugar RC, Wang Z, Sacks J, Rong X, Vallim TA, Chou J, Ivanova PT, Myers DS, Brown HA, Lee RG, Crooke RM, Graham MJ, Liu X, Parini P, Tontonoz P, Lusis AJ, Hazen SL, Temel RE, Brown JM. The TMAO-Generating Enzyme Flavin Monooxygenase 3 Is a Central Regulator of Cholesterol Balance. Cell Rep. 2015 doi: 10.1016/j.celrep.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WH, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S, Qi H, Wu J, Pan C, Brown JM, Vallim T, Bennett BJ, Graham M, Hazen SL, Lusis AJ. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56:22–37. doi: 10.1194/jlr.M051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang WH, Hazen SL. Microbiome, trimethylamine N-oxide, and cardiometabolic disease. Transl Res. 2017;179:108–115. doi: 10.1016/j.trsl.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y, Hazen SL. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang WH, Wang Z, Shrestha K, Borowski AG, Wu Y, Troughton RW, Klein AL, Hazen SL. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2015;21:91–96. doi: 10.1016/j.cardfail.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinje S, Stroes E, Nieuwdorp M, Hazen SL. The gut microbiome as novel cardio-metabolic target: the time has come! Eur Heart J. 2014;35:883–887. doi: 10.1093/eurheartj/eht467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118:476–481. doi: 10.1016/j.jbiosc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Dambrova M, Latkovskis G, Kuka J, Strele I, Konrade I, Grinberga S, Hartmane D, Pugovics O, Erglis A, Liepinsh E. Diabetes is Associated with Higher Trimethylamine N-oxide Plasma Levels. Exp Clin Endocrinol Diabetes. 2016;124:251–256. doi: 10.1055/s-0035-1569330. [DOI] [PubMed] [Google Scholar]
- 18.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Han WQ, Boini KM, Xia M, Zhang Y, Li PL. TRAIL death receptor 4 signaling via lysosome fusion and membrane raft clustering in coronary arterial endothelial cells: evidence from ASM knockout mice. J Mol Med (Berl) 2013;91:25–36. doi: 10.1007/s00109-012-0968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koka S, Xia M, Chen Y, Bhat OM, Yuan X, Boini KM, Li PL. Endothelial NLRP3 inflammasome activation and arterial neointima formation associated with acid sphingomyelinase during hypercholesterolemia. Redox Biol. 2017;13:336–344. doi: 10.1016/j.redox.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boini KM, Xia M, Abais JM, Li G, Pitzer AL, Gehr TW, Zhang Y, Li PL. Activation of inflammasomes in podocyte injury of mice on the high fat diet: Effects of ASC gene deletion and silencing. Biochim Biophys Acta. 2014;1843:836–845. doi: 10.1016/j.bbamcr.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Li X, Boini KM, Pitzer AL, Gulbins E, Zhang Y, Li PL. Endothelial Nlrp3 inflammasome activation associated with lysosomal destabilization during coronary arteritis. Biochim Biophys Acta. 2015;1853:396–408. doi: 10.1016/j.bbamcr.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Zhang Y, Xia M, Gulbins E, Boini KM, Li PL. Activation of Nlrp3 inflammasomes enhances macrophage lipid-deposition and migration: implication of a novel role of inflammasome in atherogenesis. PLoS One. 2014;9:e87552. doi: 10.1371/journal.pone.0087552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng N, Xia M, Lu YQ, Wang M, Boini KM, Li PL, Tang WX. Activation of NLRP3 inflammasomes in mouse hepatic stellate cells during Schistosoma. J infection Oncotarget. 2016;7:39316–39331. doi: 10.18632/oncotarget.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia M, Boini KM, Abais JM, Xu M, Zhang Y, Li PL. Endothelial NLRP3 inflammasome activation and enhanced neointima formation in mice by adipokine visfatin. Am J Pathol. 2014;184:1617–1628. doi: 10.1016/j.ajpath.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abais JM, Zhang C, Xia M, Liu Q, Gehr TW, Boini KM, Li PL. NADPH oxidase-mediated triggering of inflammasome activation in mouse podocytes and glomeruli during hyperhomocysteinemia. Antioxid Redox Signal. 2013;18:1537–1548. doi: 10.1089/ars.2012.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boini KM, Xia M, Koka S, Gehr TW, Li PL. Instigation of NLRP3 inflammasome activation and glomerular injury in mice on the high fat diet: role of acid sphingomyelinase gene. Oncotarget. 2016;7:19031–19044. doi: 10.18632/oncotarget.8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitzgerald KA. NLR-containing inflammasomes: central mediators of host defense and inflammation. Eur J Immunol. 2010;40:595–598. doi: 10.1002/eji.201040331. [DOI] [PubMed] [Google Scholar]
- 31.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 34.Xia M, Abais JM, Koka S, Meng N, Gehr TW, Boini KM, Li PL. Characterization and Activation of NLRP3 Inflammasomes in the Renal Medulla in Mice. Kidney Blood Press Res. 2016;41:208–221. doi: 10.1159/000443424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Z, Zhuang X, Xie C, Hu X, Dong X, Guo Y, Li S, Liao X. Exogenous Hydrogen Sulfide Attenuates High Glucose-Induced Cardiotoxicity by Inhibiting NLRP3 Inflammasome Activation by Suppressing TLR4/NF-kappaB Pathway in H9c2 Cells. Cell Physiol Biochem. 2016;40:1578–1590. doi: 10.1159/000453208. [DOI] [PubMed] [Google Scholar]
- 36.Xia M, Conley SM, Li G, Li PL, Boini KM. Inhibition of hyperhomocysteinemia-induced inflammasome activation and glomerular sclerosis by NLRP3 gene deletion. Cell Physiol Biochem. 2014;34:829–841. doi: 10.1159/000363046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salminen A, Ojala J, Suuronen T, Kaarniranta K, Kauppinen A. Amyloid-beta oligomers set fire to inflammasomes and induce Alzheimer’s pathology. J Cell Mol Med. 2008;12:2255–2262. doi: 10.1111/j.1582-4934.2008.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galam L, Rajan A, Failla A, Soundararajan R, Lockey RF, Kolliputi N. Deletion of P2X7 attenuates hyperoxia-induced acute lung injury via inflammasome suppression. Am J Physiol Lung Cell Mol Physiol. 2016;310:L572–581. doi: 10.1152/ajplung.00417.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajanbabu V, Galam L, Fukumoto J, Enciso J, Tadikonda P, Lane TN, Bandyopadhyay S, Parthasarathy PT, Cho Y, Cho SH, Lee YC, Lockey RF, Kolliputi N. Genipin suppresses NLRP3 inflammasome activation through uncoupling protein-2. Cell Immunol. 2015;297:40–45. doi: 10.1016/j.cellimm.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venugopal R, Galam L, Cox R, Fukumoto J, Cho Y, Parthasarathy PT, Lockey RF, Kolliputi N. Inflammasome Inhibition Suppresses Alveolar Cell Permeability Through Retention of Neuregulin-1 (NRG-1) Cell Physiol Biochem. 2015;36:2012–2024. doi: 10.1159/000430169. [DOI] [PubMed] [Google Scholar]
- 41.Hosseinian N, Cho Y, Lockey RF, Kolliputi N. The role of the NLRP3 inflammasome in pulmonary diseases. Ther Adv Respir Dis. 2015;9:188–197. doi: 10.1177/1753465815586335. [DOI] [PubMed] [Google Scholar]
- 42.Saco T, Parthasarathy PT, Cho Y, Lockey RF, Kolliputi N. Inflammasome: a new trigger of Alzheimer’s disease. Front Aging Neurosci. 2014;6:80. doi: 10.3389/fnagi.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Busso N, So A. Mechanisms of inflammation in gout. Arthritis Res Ther. 2010;12:206. doi: 10.1186/ar2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 45.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13:333–342. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, Rensen PC, Voshol PJ, Fantuzzi G, Hijmans A, Kersten S, Muller M, van den Berg WB, van Rooijen N, Wabitsch M, Kullberg BJ, van der Meer JW, Kanneganti T, Tack CJ, Netea MG. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, Hersberger M, Yamamoto M, Bachmann MF, Kopf M. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol. 2011;41:2040–2051. doi: 10.1002/eji.201041316. [DOI] [PubMed] [Google Scholar]
- 48.Poeck H, Bscheider M, Gross O, Finger K, Roth S, Rebsamen M, Hannesschlager N, Schlee M, Rothenfusser S, Barchet W, Kato H, Akira S, Inoue S, Endres S, Peschel C, Hartmann G, Hornung V, Ruland J. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat Immunol. 2010;11:63–69. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- 49.Srinivasula SM, Poyet JL, Razmara M, Datta P, Zhang Z, Alnemri ES. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem. 2002;277:21119–21122. doi: 10.1074/jbc.C200179200. [DOI] [PubMed] [Google Scholar]
- 50.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 51.Abdul-Sater AA, Koo E, Hacker G, Ojcius DM. Inflammasome-dependent caspase-1 activation in cervical epithelial cells stimulates growth of the intracellular pathogen Chlamydia trachomatis. J Biol Chem. 2009;284:26789–26796. doi: 10.1074/jbc.M109.026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang C, Boini KM, Xia M, Abais JM, Li X, Liu Q, Li PL. Activation of Nod-like receptor protein 3 inflammasomes turns on podocyte injury and glomerular sclerosis in hyperhomocysteinemia. Hypertension. 2012;60:154–162. doi: 10.1161/HYPERTENSIONAHA.111.189688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 54.Sun X, Jiao X, Ma Y, Liu Y, Zhang L, He Y, Chen Y. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res Commun. 2016;481:63–70. doi: 10.1016/j.bbrc.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 55.Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, Nishigaki I. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9:1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 57.Chen Y, Wang L, Pitzer AL, Li X, Li PL, Zhang Y. Contribution of redox-dependent activation of endothelial Nlrp3 inflammasomes to hyperglycemia-induced endothelial dysfunction. J Mol Med (Berl) 2016;94:1335–1347. doi: 10.1007/s00109-016-1481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang L, Chen Y, Li X, Zhang Y, Gulbins E, Zhang Y. Enhancement of endothelial permeability by free fatty acid through lysosomal cathepsin B-mediated Nlrp3 inflammasome activation. Oncotarget. 2016;7:73229–73241. doi: 10.18632/oncotarget.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]