Abstract
Higher arterial stiffness is associated with increased risk of atherosclerotic events. However, its contribution toward risk of heart failure (HF) and its subtypes, HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF), independent of other risk factors is not well-established. In this study, we included HealthABC study participants without prevalent HF that had arterial stiffness measured as carotid-femoral pulse wave velocity (cf-PWV) at baseline (n = 2,290). Adjusted Cox-proportional hazard models were constructed to determine the association between continuous and data-derived categorical measures (tertiles) of cf-PWV and incidence of HF and its subtypes [HFpEF (ejection fraction, EF > 45%) & HFrEF (EF ≤ 45%)]. We observed 390 HF events (162 HFpEF, 145 HFrEF events) over 11.4 years of follow-up. In adjusted analysis, higher cf-PWV was associated with greater risk of HF after adjustment for age, sex, ethnicity, mean arterial pressure, and heart rate [Hazard ratio (HR, 95% CI) for cf-PWV Tertile-3 vs. Tertile-1 (ref) = 1.35 (1.05 – 1.73)]. However, this association was not significant after additional adjustment for other cardiovascular risk factors [HR (95% CI): 1.14 (0.88 – 1.47)]. cf-PWV velocity was also not associated with risk of HFpEF and HFrEF after adjustment for potential confounders [most adjusted HR (95% CI) for cf-PWV Tertile-3 vs. Tertile-1 (ref), HFpEF: 1.06 (0.72 – 1.56); HFrEF = 1.28 (0.83 – 1.97)]. In conclusion, arterial stiffness, as measured by cf-PWV, is not independently associated with risk of HF or its subtypes after adjustment for traditional cardiovascular risk factors.
Keywords: Arterial stiffness, Heart failure, Hypertension, Ejection fraction, Pulse wave velocity
Introduction
Heart failure (HF) is a significant public health problem that affects an estimated 5.7 million Americans adults and is associated with increased morbidity, mortality, and healthcare cost 1,2. Despite important advances in medical and device therapies over the past two decades, outcomes associated with HF remain poor highlighting the need for effective preventive strategies 3,4.
An important first step in HF prevention is to identify the intermediate at-risk phenotypes that play a constitutive role in pathogenesis of HF and may be targeted with novel preventive therapies. Previous studies have demonstrated that progression from at-risk to symptomatic HF stage occurs through a series of intermediate cardiac phenotypes characterized by left ventricular (LV) remodeling (i.e. concentric LV hypertrophy, eccentric LV hypertrophy), changes in systolic function (i.e. ejection fraction, strain) and/or diastolic function 5–11. Recently, there has also been an interest in characterizing the contribution of abnormal vascular remodeling with increased arterial stiffness and LV afterload toward development of HF and its two subtypes, HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF) 12–15.
Higher carotid-femoral pulse wave velocity (cf-PWV), a well-established measure of arterial stiffness, is associated with increased risk of atherosclerotic cardiovascular disease (CVD) and mortality 16–19. Furthermore, cross-sectional studies have demonstrated higher arterial stiffness among HF patients compared with hypertensive adults 12,20–24. However, the contributions of increased arterial stiffness, independent of other established risk factors, on the risk of incident HF and its subtypes are not well established. This is largely due to conflicting findings from previous cohorts and lack of adequate power to assess HFpEF and HFrEF outcomes 19,25. In this study, we evaluated the association between measures of cf- PWV and risk of HF, HFpEF, and HFrEF in a community-based cohort of well-functioning older adults.
Methods
Study Population
The Health, Aging, and Body Composition (Health ABC) study is a prospective cohort study of community-dwelling older adults that evaluated the impact of changes in weight and body composition on age-related physiological and functional changes. The details of the study design, participant eligibility criteria, and recruitment strategy have been reported previously 26. Briefly, well-functioning adults aged 70–79 years who were free of life-threatening illnesses and could perform daily activities of living without difficulty were recruited between March 1987 and July 1998 from a random sample of white and all black Medicare beneficiaries residing around Pittsburgh and Memphis. The study participants underwent detailed examination at baseline and subsequent annual follow-up visits for first 6 years. Participants also had detailed telephone interviews every 6 months. All study participants provided written informed consent. Institutional Review Boards at the University of Tennessee and University of Pittsburgh approved the study protocols. Of the 3,075 participants that were initially enrolled in the Health ABC study, baseline measures of cf- PWV were available in 2,488. For the present study, we further excluded 111 participants with prevalent HF and 87 participants with missing co-variates of interest at baseline. The final study population consisted of 2,290 participants. The baseline characteristics of study participants with vs. without cf-PWV measures have been compared previously 19.
Health ABC baseline examination and covariate definitions
Details about baseline examination, laboratory tests protocols and variable definitions for the Health ABC study have been described previously 26–28. Information on demographic characteristics including age, sex, ethnicity, education level, smoking and alcohol use was self-reported. Prevalent co-morbidities such as diabetes and hypertension were assessed based on self-reported physician diagnosis and confirmed by use of specific medications, or positive examination or laboratory tests. Exercise and physical activity levels were also self-reported and estimated in Kilocalories per week using a standardized questionnaire29. Prevalent CVD included coronary heart disease (CHD) identified based on history of myocardial infarction, angina, or coronary revascularization; cerebrovascular disease identified based on history of stroke, transient ischemic attack, or carotid endarterectomy; or peripheral vascular disease identified by history of intermittent claudication, or peripheral vascular procedures.
Assessment of central arterial stiffness
cf-PWV, a gold-standard measure of arterial stiffness 30, was measured non-invasively using a well-established, highly reproducible technique that has been reported previously 19,31. Higher measures of cf-PWV reflect higher central arterial stiffness. Non-directional transcutaneous Doppler flow probes (model 810A, 9.0- to 10.0-Mhz Probes, Parks Medical Electronics, Inc.) were used to obtain Doppler flow signals from right carotid and femoral arteries. For each participant, the data were recorded for 3 runs, each with at least with 10 pairs of simultaneous flow waves, and then averaged. A metal tape was used to measure the distance between the ipsilateral carotid and femoral artery above the body surface. cf-PWV was calculated as the ratio of the distance between the carotid and femoral arteries and the time differential between the onset of flow at carotid and femoral (defined as foot of the pressure tracing at each site) sites. The National Institute on Aging, Laboratory of Cardiovascular Science, Gerontology Research Center (Baltimore, MD) trained and certified all study personnel before data collection, read the wave forms, and evaluated data quality. As reported previously, cf-PWV measures demonstrated high degree of reproducibility with an interclass correlation coefficient of 0.88 between sonographers and 0.84 between readers 32.
Heart failure outcomes assessment
The study participants were contacted every 6-months to obtain information about interval adverse cardiovascular events 26. Incident HF was identified by hospitalizations related to HF among participants without prevalent HF at baseline. The HF diagnosis was clinically adjudicated and confirmed based on the review of index hospitalization records using criteria similar to that from the Cardiovascular Health Study33. These criteria required a physician diagnosis of HF with documentation of heart failure symptoms and signs, supportive radiological findings, and medical therapy for HF, including use of a diuretic agent and either digitalis or vasodilator or beta-blocker 34. A subset of HF cases had ejection fraction (EF) data available, which was used to identify cases of incident HFpEF (EF > 45%) and HFrEF cases (EF ≤ 45%) 35. The EF cutoff used for HFpEF and HFrEF diagnosis in consistent with that used in other longitudinal cohort studies and large-scale randomized controlled trials on HFpEF patients36–39.
Statistical analysis
The study participants were stratified into tertiles of baseline measures of cf-PWV. Descriptive analyses were performed and data are presented as mean (standard deviation) for continuous and percentage for categorical variables. Baseline clinical and demographic characteristics were compared across the three groups using the Chi-square test for categorical variables and Kruskal-Wallis test for continuous variables. Nelson-Aalen estimates of the cumulative hazard function were plotted to assess and compare the unadjusted cumulative risk of overall HF, HFpEF, and HFrEF across the cf-PWV tertiles. Multivariable adjusted Cox-proportional hazards models were constructed to determine the association between categorical (tertiles) and continuous measures of cf-PWV and different HF outcomes with adjustment for following covariates: Model 1: age, sex; Model 2: model 1 + ethnicity, heart rate and mean arterial pressure; Model 3: model 2 + body mass index, prevalent CHD, anti-hypertensive use, education status, diabetes status, smoking, drinking status, physical activity, and renal function. Systolic blood pressure was not included as a co-variate in the adjusted models as it is often a consequence of arterial stiffness in the pathogenesis of HF. Separate models were constructed for overall HF, HFpEF and HFrEF outcomes. Proportional hazards assumption of the Cox models was assessed and satisfied for the study outcomes. Death was treated as a censoring event in these models. Owing to the skewed distribution of cf-PWV in the study population, the variable was log transformed for continuous Cox proportional hazard models.
Sensitivity analysis was performed treating death as a competing risk with additional adjustment for incident CHD on follow-up as a time varying covariate in Model 3. Sensitivity analysis were also performed excluding participants with baseline cardiovascular disease and without adjustment for heart rate and anti-hypertensive use as these characteristics may have a more mediatory role in the relationship between cf-PWV and incident HF. All statistical analyses were performed using SATA version 12 (Stata Corp, College Station, Texas).
Results
We included 2,290 participants in our study (53% women, 35% blacks). The baseline characteristics of the study participants are compared across tertiles of cf-PWV in Table 1. Higher cf-PWV was associated with black race, prevalent CVD, and prevalent CVD risk factors (hypertension and diabetes). Blood pressure, resting heart rate, and body mass index were also significantly higher among participants with higher cf-PWV. We observed 390 incident HF events over a median follow-up of 11.4 years, of which 41.5% were HFpEF, 37.2% were HFrEF, and 21.3% were unclassified HF.
Table 1.
Baseline characteristics of the study participants across pulse wave velocity tertiles
| Subject Characteristics | Tertile 1 (N = 758) Low cf-PWV |
Tertile 2 (N = 764) | Tertile 3 (N = 768) High cf-PWV |
P value |
|---|---|---|---|---|
| Age, years | 73.4 (2.8) | 73.8 (2.9) | 73.9 (2.9) | <0.01 |
| African Americans | 35 % | 40 % | 43 % | 0.01 |
| Body mass index, Kg/m2 | 26.5 (4.5) | 27.6 (4.6) | 27.8 (4.9) | <0.01 |
| Systolic BP, mm Hg | 131 (19) | 136 (20) | 142 (22) | <0.01 |
| Hypertension | 38 % | 49 % | 61 % | <0.01 |
| Anti-hypertensive use | 43 % | 54 % | 63 % | <0.01 |
| CVD | 23 % | 25 % | 29 % | <0.01 |
| Diabetes Mellitus | 9 % | 14 % | 21 % | 0.01 |
| Physical Activity Kcal/kg/week | 87.9 (66.5) | 84.7 (71.3) | 78.4 (70.4) | 0.03 |
| Heart Rate, beats/min | 63 (10) | 66 (11) | 67 (12) | <0.01 |
| Pulse Wave Velocity, cm/sec | 566.6 (91.4) | 812.3 (75.2) | 1329.7 (389.0) | NA |
Data presented as mean (SD) or %
Cf-PWV: Carotid femoral pulse wave velocity; BP: Blood pressure; CVD: Cardiovascular disease; NA: not applicable
Association between cf-PWV categories and risk of overall HF
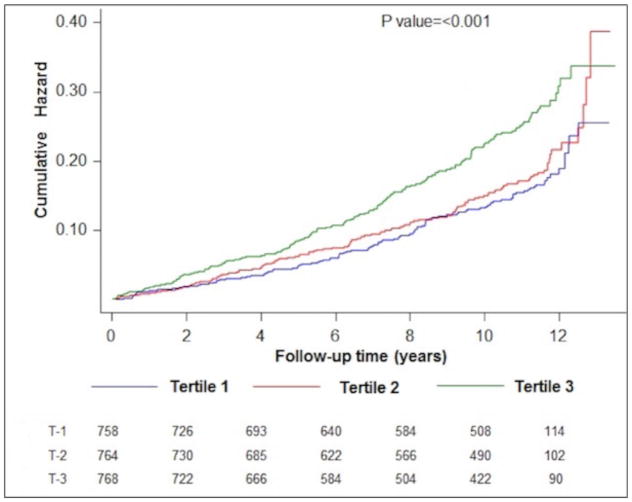
In unadjusted comparisons, higher cf-PWV was associated with increased risk of HF (log rank p value < 0.0001, Figure 1) with the highest risk among tertile 3 participants. The cumulative hazard function curves indicate a threshold effect between tertile 2 and 3 for HF risk. Table 2 shows the multivariable adjusted association between categorical measures of cf-PWV and risk of HF. After adjustment for age and sex, higher measures of cf-PWV were significantly associated with higher risk of HF (Model 1). This associated was slightly attenuated but remained significant after additional adjustment for ethnicity, heart rate, and mean arterial blood pressure (Model 2). Further adjustment for additional risk factors attenuated this relationship significantly such that cf-PWV was no longer associated with HF risk (Model 3)
Figure 1.
Cumulative risk of heart failure across different carotid-femoral pulse wave velocity-based study groups (cf-PWV T1 through T3).
Table 2.
Adjusted association between categorical measures of pulse wave velocity and risk of heart failure
| Multivariable adjusted models | Hazard Ratio (95% CI) Tertile 1 |
Hazard Ratio (95% CI) Tertile 2 |
Hazard Ratio (95% CI) Tertile 3 |
|---|---|---|---|
|
Model 1 (age & sex adjusted) |
ref | 1.09 (0.84 – 1.42) | 1.55 (1.21 – 1.98) |
|
Model 2 (age, sex, ethnicity, heart rate & MAP adjusted) |
ref | 1.00 (0.76 – 1.29) | 1.35 (1.05 – 1.73) |
|
Model 3 (age, sex, ethnicity, heart rate, MAP, BMI, CHD, education status, anti-HTN use, diabetes, smoking, drinking status, physical activity, and renal function adjusted) |
ref | 0.88 (0.68 – 1.14) | 1.14 (0.88 – 1.47) |
HR: heart rate; MAP: mean arterial pressure; BMI: Body mass index; CHD: coronary heart disease; Anti-HTN: antihypertensive medication; CI: confidence interval
Association between cf-PWV categories and risk of HF subtypes
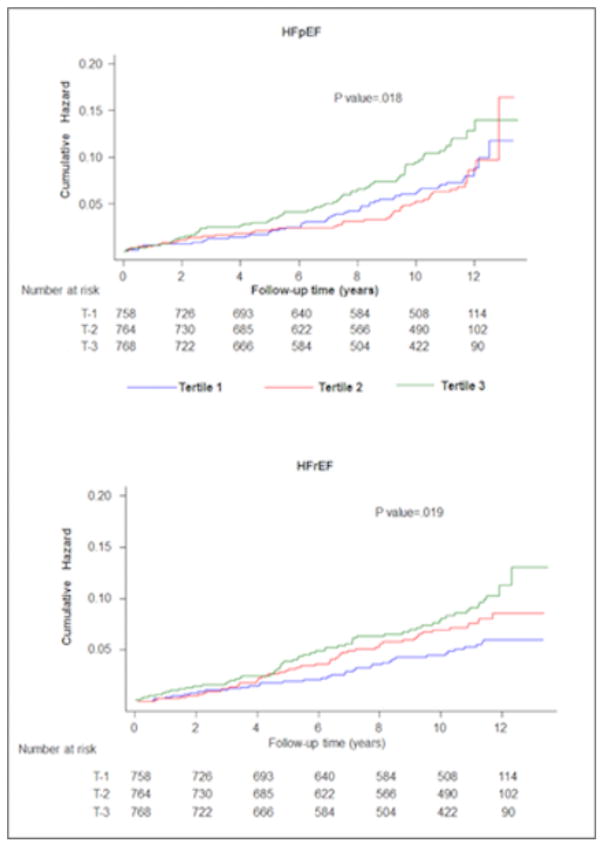
In unadjusted analysis, higher cf-PWV was associated with higher risk of both HFpEF and HFrEF (P long rank: 0.02 for both, Figure 2), with the highest risk among Tertile 3 participants. In adjusted analysis, higher cf-PWV was significantly associated with higher risk of HFrEF and HFpEF after adjustment for age and sex (Model 1, Table 3). This association attenuated significantly and was no longer significant after further adjustment for heart rate, mean arterial blood pressure (model 2, Table 3) and other risk factors (model 3, Table 3).
Figure 2.
Cumulative risk of heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF) across different carotid-femoral pulse wave velocity-based study groups (cf-PWV T1 through T3).
Table 3.
Adjusted association between categorical measures of pulse wave velocity and risk of HFpEF and HFrEF
| Multivariable adjusted Models | Hazard Ratio (95% CI) Tertile 1 |
Hazard Ratio (95% CI) Tertile 2 |
Hazard Ratio (95% CI) Tertile 3 |
|---|---|---|---|
| HFpEF | |||
|
Model 1 (age & sex adjusted) |
Ref. | 0.91 (0.61 – 1.36) | 1.46 (1.01 – 2.11) |
|
Model 2 (age, sex, ethnicity, HR & MAP adjusted) |
Ref. | 0.84 (0.56 – 1.26) | 1.30 (0.89 – 1.89) |
|
Model 3 (age, sex, ethnicity, HR, MAP, BMI, baseline CHD, education status, anti-HTN use, diabetes, smoking, drinking status, physical activity, and renal function adjusted) |
Ref. | 0.72 (0.48 – 1.09) | 1.06 (0.72 – 1.56) |
| HFrEF | |||
|
Model 1 (age & sex adjusted) |
Ref | 1.39 (0.91 – 2.14) | 1.78 (1.17 – 2.70) |
|
Model 2 (age, sex, ethnicity, HR & MAP adjusted) |
Ref. | 1.21 (0.78 – 1.86) | 1.49 (0.98 – 2.27) |
|
Model 3 (age, sex, ethnicity, HR, MAP, BMI, CHD, education status, anti-HTN use, diabetes, smoking, drinking status, physical activity, and renal function adjusted) |
Ref. | 1.11 (0.72 – 1.71) | 1.28 (0.83 – 1.97) |
HFpEF: Heart failure with preserved ejection fraction; HFrEF: Heart failure with reduced ejection fraction; HR: Heart rate; MAP: Mean arterial pressure; BMI: Body mass index; CHD: coronary heart disease; Anti-HTN: antihypertensive medication; CI: confidence interval
Association between continuous measures of cf-PWV and risk of HF outcomes
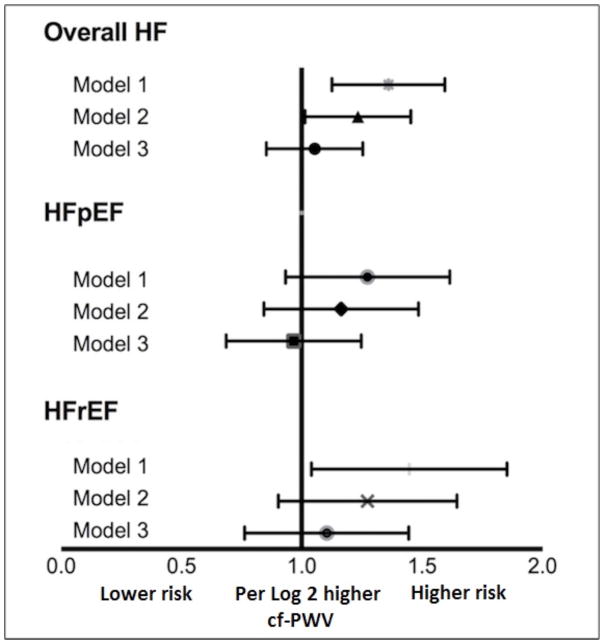
The continuous association between cf-PWV and risk of HF, HFpEF, and HFrEF were assessed in separate Cox proportional hazards models using log-transformed measures of cf-PWV. We observed a progressive attenuation in the association between cf-PWV and risk of HF, HFpEF and HFrEF with sequential adjustment for potential confounders, similar to that observed in categorical analysis. Higher cf-PWV was not associated with risk of HF outcomes in the most adjusted model (Figure 3). Furthermore, no significant interaction was observed between participant characteristics such as body mass index, physical activity levels and cf-PWV for risk of HF (P-interaction > 0.2 for all, supplemental Figure S1).
Figure 3.
Continuous association between log-transformed carotid-femoral pulse wave velocity measures and risk of heart failure (HF), heart failure with preserved ejection fraction (HFpEF), and heart failure with reduced ejection fraction (HFrEF) in different multivariable adjusted Cox models. Model 1 is adjusted for age and sex. Model 2 is additionally adjusted for ethnicity, heart rate and mean arterial blood pressure, Model 3 is adjusted for Model 2 covariates + body mass index, prevalent cardiovascular disease, education status, anti-hypertensive use, diabetes, smoking, drinking status, physical activity, and renal function. CI indicates confidence interval; and HR, hazard ratio.
Sensitivity Analysis
Sensitivity analysis was performed to evaluate the association between measures of cf-PWV and risk of HF among participants without cardiovascular disease at baseline and without adjustment for potential mediator characteristics including hypertension status and heart rate in the final model. The pattern of association between cf-PWV and risk of HF in this subgroup was not different from the overall study population such that higher measures of cf-PWV were not significantly associated with HF risk after adjustment for potential confounders [Hazard Ratio (95% CI) per Log 2 higher cf-PWV = 1.12 (0.89 – 1.42)] (Supplemental Table S1).
Of the 2,290 participants included in the analysis, 1,027(44.8%) died during follow up. In sensitivity analysis treating death as a competing risk with additional adjustment for incident CHD as a time varying covariate, categorical and continuous measures of cf-PWV were not significantly associated with risk of HF outcomes (Supplemental Table S2).
Discussion
In the present study of community dwelling elderly individuals, we observed that higher cf-PWV was associated with greater risk of overall HF and its subtypes, HFpEF and HFrEF. However, these associations were attenuated substantially after adjustment for CVD and its risk factors. Taken together, our study findings suggest that the association between vascular remodeling and increased arterial stiffness, as measured by cf-PWV, and HF risk in older adults may be related to the burden of coexistent CVD and/or risk factors.
Higher measures of arterial stiffness have been associated with greater risk of major adverse cardiovascular events including MI, incident CHD, and mortality16–19. A previous analysis from the Health ABC study with 181 HF events demonstrated no association between cf-PWV and HF risk, even in unadjusted analysis 19. In contrast, in the present study with 390 HF events, we observed significant inverse association after adjustment for age, sex, blood pressure, and heart rate. This could be related to greater statistical power and higher number of HF events in the present analysis.
The association between arterial stiffness, as measured by cf-PWV, and HF risk has been explored in other cohort studies. Chirions et al. 40 demonstrated that higher measures of cf-PWV were associated with greater risk of HF in a cohort of chronic kidney disease patients. Recently, in a secondary analysis from the Framingham Heart Study (FHS), Tsao, et al. 25 demonstrated a significant association between cf-PWV and HF risk [HR: 1.29 (1.02 – 1.64), p-value = 0.04]. Findings in our study are in contrast to that from Tsao, et al. and could be related to several factors. First, there are significant differences in the population characteristics that may influence the observed associations between measures of arterial stiffness and HF risk. The FHS population is relatively younger and racially/ethnically homogenous as compared with the Health ABC study population. Second, the multivariable adjusted model by Tsao, et al. did not account for many potential confounders including heart rate, renal function, and physical activity levels. Thus, the observed association between cf-PWV and HF risk may be related to residual confounding. It is noteworthy that the association between cf-PWV and HF in the age- and sex- adjusted models were not different between the two studies. Finally, as noted previously, there were differences in the cf-PWV assessment methods between the Health ABC and FHS 41. It is unclear if this difference in cf-PWV measurements may have influenced the study findings since its association with other cardiovascular outcomes is not different between the two cohorts.
Availability of EF information from index HF hospitalization in a substantial proportion of HF patients allowed us to evaluate the association between arterial stiffness and risk of HF subtypes, HFpEF and HFrEF. In age and sex- adjusted models, cf-PWV was more strongly associated with HFrEF risk as compared with HFpEF risk. However, these associations were substantially attenuated and not significant after adjustment for blood pressure, heart rate, and other potential confounders. Tsao, et al. 25 had evaluated the association between cf-PWV and HF subtypes in the FHS and observed stronger association for HFrEF than HFpEF, similar to our study findings. However, their study was considerably limited in their statistical power with only 77 HFpEF and 61 HFrEF events. The present study with 162 HFpEF and 147 HFrEF events adds significantly to the existing literature with adequate power to evaluate the association between cf-PWV and risk of HF subtypes.
Previous cross-sectional studies have reported higher arterial stiffness among patients with HFpEF as compared with hypertensive control patients 12,20,42. However, there were significant differences in age, sex distribution, and other clinical characteristics that may have lead to the observed differences. In the present study, we observe that the contributions of arterial stiffness, as measured by cf-PWV, beyond differences in traditional risk factors toward risk of HF subtypes are not significant. Our findings are supported by the cross-sectional observations from Lam, et al. who demonstrated that after adjustment for age, sex, and body size, measures of arterial stiffness were similar among HFpEF patients and hypertensive control participants 43.
It is noteworthy that the lack of association between cf-PWV and HF risk may not be generalizable to other measures of pulsatile hemodynamic afterload such as reflected wave magnitude, aortic impedance, and total arterial compliance. Reflected waves and late systolic load have been associated with diastolic dysfunction and adverse myocardial remodeling in animal studies44,45. Similarly, several cross-sectional studies in humans have demonstrated the significant contribution of reflected waves and aortic characteristic impedance to left ventricular systolic and diastolic dysfunction46–48. Furthermore, in a recent longitudinal cohort study among participants of the MESA study, Chirinos, et al 49 demonstrated an independent association between reflection wave magnitude and incident HF risk. Future studies are needed to better assess the effects of afterload (reflected waves, aortic impedance, total arterial compliance) measured directly via pressure-flow relations, on the risk of incident HF among older individuals.
Our study findings have important clinical implications. The higher incidence of HF outcomes among patients with elevated cf-PWV appears to be related to greater burden of CVD and its risk factors in these patients. Thus, higher arterial stiffness is an intermediate phenotype observed in at-risk individuals but it may not contribute to development of HF independent of other risk factors. Higher cf-PWV may be useful to identify individuals that are at increased risk of HF development and may benefit from aggressive risk factor modification. This is consistent with the hypothesis recently proposed by Paulus et al. 50 in which risk factors and comorbidity were identified as the primary determinants of HFpEF pathophysiology through their inflammatory effects.
Several important issues must be considered in regards to the overall null findings in our study. These include possibility of type-II error, measurement of the exposure variable and outcomes of interest, and other potential biases. The large number of events (390 HF events) observed in our study ensures adequate statistical power to evaluate the association between cf-PWV and HF risk. Thus, a type II error is less likely in our study. The measures of cf-PWV in the Health ABC study are well established and previously validated 19,32. Furthermore, cf-PWV has been shown to be strongly associated with cardiovascular mortality and CHD outcomes in the Health ABC cohort, similar to other population based cohorts 17–19. It is possible that, the observed lack of association is related to the older age and higher burden of CVD and its risk factors in our study population. The strong associations of prevalent CVD and risk factors with HF risk in this older study cohort could mask our ability to observe an independent association for cf-PWV. Finally, there is a potential for survival bias in our study of older adults such that individuals who were more susceptible to higher arterial stiffness related HF risk or other adverse cardiovascular events may not have survived or met the eligibility criteria to be included in this study. Thus, the associations between arterial stiffness and HF risk may stronger in a middle-age population.
Several other limitations to our study are noteworthy. The study population included older community dwelling adults of black and white race and our study findings may not be generalizable to younger population and other race/ethnicities. HF adjudication was based on inpatient hospitalization and thus, we may have missed a proportion of HF events that were diagnosed in outpatient visits. Furthermore, we do not have baseline echocardiographic assessment in our study participants, and therefore, individuals with asymptomatic left ventricular dysfunction and/or other cardiac structural abnormalities may have been included in the analysis.
Perspectives
We have demonstrated in a community-based sample of well-functioning older adults that higher arterial stiffness, as measured by cf-PWV, is not independently associated with increased risk of HF or its subtypes after adjustment for prevalent CVD and risk factors. These findings suggest that higher cf-PWV associated risk of HF is largely mediated by coexistent disease and/or traditional risk factors. Future studies are needed to determine if these findings are generalizable to other measures of left ventricular afterload.
Supplementary Material
Novelty and Significance.
What is new?
Higher measures of arterial stiffness, as measured by carotid-femoral pulse wave velocity, identify individuals at greater risk of heart failure. However, this is largely related to the higher burden of established cardiovascular disease and its risk factors.
What is relevant?
Higher carotid-femoral pulse wave velocity may be an intermediate phenotype observed in at-risk individuals but it does not contribute to development of HF independent of other risk factors. Individuals with higher carotid-femoral pulse wave velocity may benefit from aggressive risk factor modification to lower heart failure risk.
Summary
Higher carotid-femoral pulse wave velocity is not independently associated with increased risk of heart failure in older individuals after adjusting for traditional risk factors. This observation is consistent in individuals with as well as without prevalent coronary artery disease. Future studies are needed to determine the contribution of other measures of left ventricular afterload toward HF risk in older individuals.
Acknowledgments
“This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.”
Funding Source
This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459.
Footnotes
Dr. Berry receives funding from (1) the Dedman Family Scholar in Clinical Care endowment at University of Texas Southwestern Medical Center, and (2) 14SFRN20740000 from the American Heart Association prevention network. All other authors report no relevant conflicts of interest or financial disclosures. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication. All authors have read and agree to the manuscript as written.
Disclosures
Dr. Berry receives funding from (1) the Dedman Family Scholar in Clinical Care endowment at University of Texas Southwestern Medical Center, and (2) 14SFRN20740000 from the American Heart Association prevention network. All other authors report no relevant conflicts of interest or financial disclosures
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Executive Summary: Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA. 2011;306:1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng RK, Cox M, Neely ML, Heidenreich PA, Bhatt DL, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J. 2014;168:721–730. doi: 10.1016/j.ahj.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Lam CS, Lyass A, Kraigher-Krainer E, Massaro JM, Lee DS, Ho JE, Levy D, Redfield MM, Pieske BM, Benjamin EJ, Vasan RS. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation. 2011;124:24–30. doi: 10.1161/CIRCULATIONAHA.110.979203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi EY, Rosen BD, Fernandes VR, Yan RT, Yoneyama K, Donekal S, Opdahl A, Almeida AL, Wu CO, Gomes AS, Bluemke DA, Lima JA. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2013;34:2354–2361. doi: 10.1093/eurheartj/eht133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, Gardin JM, Manolio TA, Dries DL, Siscovick DS. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: The Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207–2215. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 8.Echouffo-Tcheugui JB, Erqou S, Butler J, Yancy CW, Fonarow GC. Assessing the Risk of Progression From Asymptomatic Left Ventricular Dysfunction to Overt Heart Failure: A Systematic Overview and Meta-Analysis. JACC Heart Fail. 2016;4:237–248. doi: 10.1016/j.jchf.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Yeboah J, Bluemke DA, Hundley WG, Rodriguez CJ, Lima JA, Herrington DM. Left ventricular dilation and incident congestive heart failure in asymptomatic adults without cardiovascular disease: multi-ethnic study of atherosclerosis (MESA) J Card Fail. 2014;20:905–911. doi: 10.1016/j.cardfail.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zile MR, Gaasch WH, Patel K, Aban IB, Ahmed A. Adverse left ventricular remodeling in community-dwelling older adults predicts incident heart failure and mortality. JACC Heart Fail. 2014;2:512–522. doi: 10.1016/j.jchf.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Garg S, de Lemos JA, Ayers C, Khouri MG, Pandey A, Berry JD, Peshock RM, Drazner MH. Association of a 4-Tiered Classification of LV Hypertrophy With Adverse CV Outcomes in the General Population. JACC Cardiovasc Imaging. 2015;8:1034–1041. doi: 10.1016/j.jcmg.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitzman DW, Herrington DM, Brubaker PH, Moore JB, Eggebeen J, Haykowsky MJ. Carotid arterial stiffness and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Hypertension. 2013;61:112–119. doi: 10.1161/HYPERTENSIONAHA.111.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol. 2012;60:1455–1469. doi: 10.1016/j.jacc.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 14.Bell V, Sigurdsson S, Westenberg JJ, Gotal JD, Torjesen AA, Aspelund T, Launer LJ, Harris TB, Gudnason V, de Roos A, Mitchell GF. Relations between aortic stiffness and left ventricular structure and function in older participants in the Age, Gene/Environment Susceptibility--Reykjavik Study. Circ Cardiovasc Imaging. 2015;8:e003039. doi: 10.1161/CIRCIMAGING.114.003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam CS, Gona P, Larson MG, Aragam J, Lee DS, Mitchell GF, Levy D, Cheng S, Benjamin EJ, Vasan RS. Aortic root remodeling and risk of heart failure in the Framingham Heart study. JACC Heart Fail. 2013;1:79–83. doi: 10.1016/j.jchf.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maroules CD, Khera A, Ayers C, Goel A, Peshock RM, Abbara S, King KS. Cardiovascular outcome associations among cardiovascular magnetic resonance measures of arterial stiffness: the Dallas heart study. J Cardiovasc Magn Reson. 2014;16:33. doi: 10.1186/1532-429X-16-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A, Health ABCS. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 20.Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM, Little WC. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. doi: 10.1016/s0735-1097(01)01447-4. [DOI] [PubMed] [Google Scholar]
- 21.Bonapace S, Rossi A, Cicoira M, Franceschini L, Golia G, Zanolla L, Marino P, Zardini P. Aortic distensibility independently affects exercise tolerance in patients with dilated cardiomyopathy. Circulation. 2003;107:1603–1608. doi: 10.1161/01.CIR.0000051458.39176.43. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes VR, Polak JF, Cheng S, Rosen BD, Carvalho B, Nasir K, McClelland R, Hundley G, Pearson G, O’Leary DH, Bluemke DA, Lima JA. Arterial stiffness is associated with regional ventricular systolic and diastolic dysfunction: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:194–201. doi: 10.1161/ATVBAHA.107.156950. [DOI] [PubMed] [Google Scholar]
- 23.Mottram PM, Haluska BA, Leano R, Carlier S, Case C, Marwick TH. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart. 2005;91:1551–1556. doi: 10.1136/hrt.2004.046805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rerkpattanapipat P, Hundley WG, Link KM, Brubaker PH, Hamilton CA, Darty SN, Morgan TM, Kitzman DW. Relation of aortic distensibility determined by magnetic resonance imaging in patients > or =60 years of age to systolic heart failure and exercise capacity. Am J Cardiol. 2002;90:1221–1225. doi: 10.1016/s0002-9149(02)02838-2. [DOI] [PubMed] [Google Scholar]
- 25.Tsao CW, Lyass A, Larson MG, Levy D, Hamburg NM, Vita JA, Benjamin EJ, Mitchell GF, Vasan RS. Relation of Central Arterial Stiffness to Incident Heart Failure in the Community. J Am Heart Assoc. 2015:4. doi: 10.1161/JAHA.115.002189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 27.Auer R, Bauer DC, Marques-Vidal P, Butler J, Min LJ, Cornuz J, Satterfield S, Newman AB, Vittinghoff E, Rodondi N, Health ABCS. Association of major and minor ECG abnormalities with coronary heart disease events. JAMA. 2012;307:1497–1505. doi: 10.1001/jama.2012.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Tracy RP, Rubin SM, Harris TB, Pahor M. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study) Am J Cardiol. 2003;92:522–528. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- 29.Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB, Health A Body Composition Study Research G. The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J Am Geriatr Soc. 2004;52:502–509. doi: 10.1111/j.1532-5415.2004.52154.x. [DOI] [PubMed] [Google Scholar]
- 30.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H European Network for Non-invasive Investigation of Large A. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 31.Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 32.Sutton-Tyrrell K, Mackey RH, Holubkov R, Vaitkevicius PV, Spurgeon HA, Lakatta EG. Measurement variation of aortic pulse wave velocity in the elderly. Am J Hypertens. 2001;14:463–468. doi: 10.1016/s0895-7061(00)01289-9. [DOI] [PubMed] [Google Scholar]
- 33.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 34.Rodondi N, Newman AB, Vittinghoff E, de Rekeneire N, Satterfield S, Harris TB, Bauer DC. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med. 2005;165:2460–2466. doi: 10.1001/archinte.165.21.2460. [DOI] [PubMed] [Google Scholar]
- 35.Khan H, Kalogeropoulos AP, Zannad F, Marti CN, Wilson PW, Georgiopoulou VV, Kanaya AM, Newman AB, Schelbert E, Harris TB, Kritchevsky S, Yancy C, Gheorghiade M, Fonarow GC, Butler J, Health ABCS. Incident heart failure in relation to vascular disease: insights from the Health, Aging, and Body Composition Study. Eur J Heart Fail. 2014;16:526–534. doi: 10.1002/ejhf.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seliger SL, de Lemos J, Neeland IJ, Christenson R, Gottdiener J, Drazner MH, Berry J, Sorkin J, deFilippi C. Older Adults, “Malignant” Left Ventricular Hypertrophy, and Associated Cardiac-Specific Biomarker Phenotypes to Identify the Differential Risk of New-Onset Reduced Versus Preserved Ejection Fraction Heart Failure: CHS (Cardiovascular Health Study) JACC Heart Fail. 2015;3:445–455. doi: 10.1016/j.jchf.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silverman MG, Patel B, Blankstein R, Lima JA, Blumenthal RS, Nasir K, Blaha MJ. Impact of Race, Ethnicity, and Multimodality Biomarkers on the Incidence of New-Onset Heart Failure With Preserved Ejection Fraction (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2016;117:1474–1481. doi: 10.1016/j.amjcard.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quddus AKL, Rossouw J, et al. Racial and Ethnic Differences in Preserved Ejection Fraction and Reduced Ejection Fraction Incident Heart Failure in a Multiracial Cohort of Post-Menopausal Women. Circulation. 2014;130:A16059. [Google Scholar]
- 39.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A Investigators IP. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 40.Chirinos JA, Khan A, Bansal N, Dries DL, Feldman HI, Ford V, Anderson AH, Kallem R, Lash JP, Ojo A, Schreiber M, Sheridan A, Strelsin J, Teal V, Roy J, Pan Q, Go AS, Townsend RR Investigators CS, Investigators CS. Arterial stiffness, central pressures, and incident hospitalized heart failure in the chronic renal insufficiency cohort study. Circ Heart Fail. 2014;7:709–716. doi: 10.1161/CIRCHEARTFAILURE.113.001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Canepa M, AlGhatrif M. From Arterial Stiffness to Heart Failure: Still a Long Way to Go. J Am Heart Assoc. 2015:4. doi: 10.1161/JAHA.115.002807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desai AS, Mitchell GF, Fang JC, Creager MA. Central aortic stiffness is increased in patients with heart failure and preserved ejection fraction. J Card Fail. 2009;15:658–664. doi: 10.1016/j.cardfail.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gillebert TC, Lew WY. Influence of systolic pressure profile on rate of left ventricular pressure fall. Am J Physiol. 1991;261:H805–813. doi: 10.1152/ajpheart.1991.261.3.H805. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi S, Yano M, Kohno M, Obayashi M, Hisamatsu Y, Ryoke T, Ohkusa T, Yamakawa K, Matsuzaki M. Influence of aortic impedance on the development of pressure-overload left ventricular hypertrophy in rats. Circulation. 1996;94:3362–3368. doi: 10.1161/01.cir.94.12.3362. [DOI] [PubMed] [Google Scholar]
- 46.Borlaug BA, Melenovsky V, Redfield MM, Kessler K, Chang HJ, Abraham TP, Kass DA. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol. 2007;50:1570–1577. doi: 10.1016/j.jacc.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 47.Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular-arterial interactions. J Am Coll Cardiol. 2013;61:96–103. doi: 10.1016/j.jacc.2012.08.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Canepa M, Alghatrif M, Strait JB, Cheng HM, Chuang SY, Chen CH, Brunelli C, Ferrucci L, Lakatta EG. Early contribution of arterial wave reflection to left ventricular relaxation abnormalities in a community-dwelling population of normotensive and untreated hypertensive men and women. J Hum Hypertens. 2014;28:85–91. doi: 10.1038/jhh.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chirinos JA, Kips JG, Jacobs DR, Jr, Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis) J Am Coll Cardiol. 2012;60:2170–2177. doi: 10.1016/j.jacc.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.