Abstract
Objective
Investigate role of dose/duration of zoledronic acid (ZOL), a powerful anti-resorptive (pAR), on prevalence of medication-related osteonecrosis of the jaw (MRONJ) in rice rats (Oryzomys palustris), a species with natural susceptibility to food impaction-induced localized periodontitis (FILP). We hypothesize that ZOL induces MRONJ lesions in rice rats with FILP, and that the prevalence of MRONJ rises with increasing dose and duration of ZOL treatment.
Methods
We performed a toxicology experiment with clinically-relevant doses of ZOL in female rats (N = 230) fed standard (STD) rodent chow. At age 4 weeks (baseline), 12 rats were necropsied. The rest were randomized into five groups that began to receive 0, 8, 20, 50 or 125 µg/kg ZOL IV/q 4 weeks. After 12, 18, 24 and 30 weeks, subgroups (N = 9–16) from each of the dose groups were necropsied. High-resolution macroscopic photos of all jaw quadrants were given a gross quadrant grade (GQG) (0–4 or MRONJ) that classified FILP lesion severity and determined presence of gross MRONJ. Quadrants with GQG ≥ 1 were examined histopathologically. Logistic regression analysis (ZOL dose/duration) of MRONJ prevalence was completed.
Results
We found: 1) 75% of 0 µg/kg ZOL rats developed FILP lesions; 2) baseline rats and rats treated with 0 µg/kg ZOL had no MRONJ; 3) 29 gross MRONJ cases were identified; 4) all gross MRONJ cases were confirmed histopathologically by the observation of exposed necrotic bone, and 53 new cases were discovered (total = 82); 5) ZOL dose (P < 0.001), but not duration (P = 0.326), was a significant predictor of MRONJ prevalence; 6) 13% prevalence of gross MRONJ among all rats, with 22% prevalence among rats exposed to ZOL oncologic doses (20–125 µg/kg); 7) 38% prevalence of histopathologic MRONJ among all rats, with 73% prevalence among rats exposed to ZOL oncologic doses.
Conclusions
This is the first experiment to show a dose response relationship between clinically relevant doses of ZOL and MRONJ prevalence.
Keywords: Anti-resorptives, Periodontitis, Toxicology, Duration
1. Introduction
Medication-related osteonecrosis of the jaw (MRONJ) is a potentially severe adverse event in humans primarily associated with powerful anti-antiresorptive (pAR) drugs, including nitrogen-containing bisphosphonates (N-BPs) [1–5] and receptor activator of NFκB ligand (RANKL) antibodies [6–8]. It is also associated with anti-angiogenic therapies, such as tyrosine kinase and vascular endothelial growth factor inhibitors [9–11]. The American Association of Oral and Maxillofacial Surgeons (AAOMS) defines MRONJ as exposed bone, or bone that can be probed through a fistula in the maxillofacial region that has persisted for more than eight weeks in patients with no history of radiation therapy to the jaws, no obvious metastatic disease in the jaws, and a history of pARs and/or anti-angiogenic therapy [12].
The role of the medications implicated in the etiology and pathophysiology of MRONJ has not been fully elucidated. Randomized, prospective human studies indicate that oncologic doses of ZOL, and longer duration of ZOL treatment, increase MRONJ incidence. For instance, a trial of 3300 women taking oncology doses of ZOL, reported increasing cumulative MRONJ incidence in the ZOL group, with a maximum of 2.1% after six years treatment [13–15], compared to a 0.0% cumulative incidence in the non-ZOL group. In comparison, a trial of over 8000 women taking ZOL for osteoporosis (OP) reported a cumulative MRONJ incidence of only ~0.01% with a dose that was only 10-fold lower than that used in oncology patients [16]. Despite these findings, the dose relationship between ZOL and MRONJ incidence appears to be disproportionate, given the ~200-fold lower incidence in OP patients that occurs at a dose only 10-fold lower than the oncology doses. Furthermore, the relatively low prevalence of MRONJ among patients receiving even the highest doses of pARs [13–15], raises a strong likelihood that risk factors other than the medications themselves are crucial [17,18].
The localization MRONJ to the jaws suggests that pre-existing or coinciding oral risk factors play an important role in the disease. Multiple preclinical experiments have already investigated aspects of event-related MRONJ by observing tooth extraction-related healing in pAR-treated small animals [19–27]. Reducing event-related MRONJ incidence by strategically timing tooth extractions in pAR patients is a highly successful recommendation already in place [28,29]. In contrast, spontaneous MRONJ, which accounts for one third of all cases, is inherently more difficult to prevent and treat due to the absence of an identifiable precipitating event. Spontaneous MRONJ, appears to be associated with oral inflammation and infection, including periodontitis (PD) and periapical infection [27,30–34]. PD is a bacteria-driven inflammatory disease of the periodontium, and is an important risk factor for both spontaneous and event-related MRONJ [1,4,12,28,29]. However, there have been no prospective studies to formally investigate the dose and duration relationship between pARs and MRONJ in animals affected by oral infection or inflammatory conditions, such as PD.
The rice rat (Oryzomys palustris) spontaneously develops two distinctive types of PD. When fed a high sucrose casein diet (HSC), rice rats develop a well-described generalized form of PD by age 12–18 weeks [35–39]. Furthermore, when combined with intravenous (IV) oncologic doses of pARs (clodronate, alendronate, or ZOL), HSC-fed rice rats develop MRONJ-like lesions in alveolar bone [32,40]. More recently, rats fed standard rodent chow (STD) have been shown to develop food impaction-induced localized periodontitis (FILP) [41], which is distinctive from the generalized PD seen in HSC-fed rice rats. FILPs occur with 1) high prevalence of ~60–80% between ages 16–34 weeks; 2) consistent location at the palatal aspect of the interdental space between theM2M3 maxillary molars; and 3) relatively stable size and severity level in untreated rats. These features suggest that the FILP rice rat model could be used in toxicology studies that address the interaction of oral infection and inflammation with pARs, or other medications, that may cause MRONJ.
The purpose of this study was two-fold: 1) generate pre-clinical evidence that a pAR induces MRONJ in FILP-affected rats; and 2) conduct a toxicology study in FILP-affected rats that examines the relationship of MRONJ prevalence to increasing dose and duration of pAR treatment. We hypothesize that ZOL induces MRONJ lesions in rice rats with FILP and that the prevalence of MRONJ rises with increasing dose and duration of ZOL.
2. Materials and methods
2.1. Animal care
A monogamous continuous-breeding system was used to generate pups for the studies. At weaning (age 4 weeks), clinically normal females [BW ≥ 30 g and body condition score (BCS) ≥ 3.0] [42] were randomized into the experiment, with efforts made to distribute littermates among different groups. Experimental animals ate STD diet (Envigo Teklad LM-485 (irradiated 7912) Rodent Diet; Tampa, FL USA). BW was measured bi-weekly. Rats with BW loss and/or BCS deterioration were offered gel diet and supplemental fluids. Breeder animals ate rodent breeder chow (Envigo Teklad, irradiated 2919). All rats were housed (2–5 rats per cage) in static filter top cages (area: 143 in.2) with pine shavings as bedding and continuous access to food and water. The housing room was maintained at 68–79 °F with an average humidity of 30–70% and a 12:12 h light:dark cycle. Breeder pairs were housed in the same conditions as experimental rats, but with a 14:10 h light:dark cycle. The Animal Care Services resource at the University of Florida (UF) is an AAALAC-accredited animal care and use program. Both protocols were approved by the UF Institutional Animal Care and Use Committee (IACUC).
2.2. Study design
2.2.1. Study 1: effects of an oncologic dose of ZOL
The extent that MRONJ lesions could be induced in STD diet rice rats prone to FILP lesions was examined. We hypothesized that STD-fed rice rats IV-injected with oncologic ZOL (80 µg/kg) every 4 weeks (q4wk) would develop MRONJ. Female rice rats (n = 48, age 4 weeks) were fed STD diet and injected in the tail vein with either vehicle (saline, ZOL0, N = 24) or ZOL80 (N = 24) starting at age 4 weeks, and then every four weeks, thereafter. Half the rats from each group were randomly euthanized after 12 weeks (age 16 weeks) and the remainders were euthanized after 18 weeks (age 22 weeks).
2.2.2. Study 2: effects of ZOL dose and duration on MRONJ prevalence
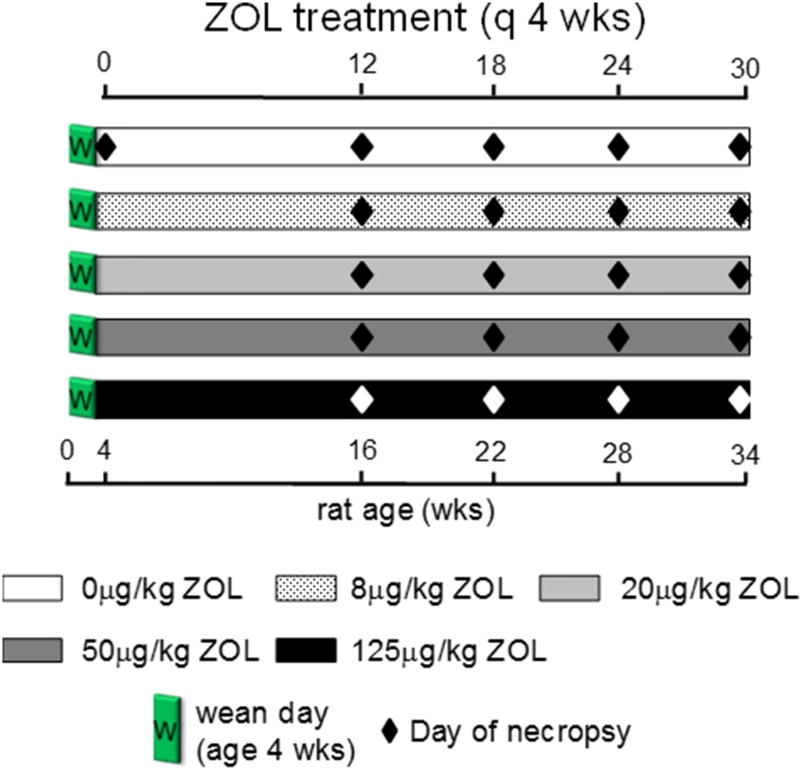
Two hundred thirty female rice rats were BW-randomized into a baseline necropsy group (N = 12, age 4 weeks), or one of five dose groups (N = 43–54) (Fig. 1). All groups were fed STD diet and received IV injections of 0, 8, 20, 50 or 125 µg/kg of ZOL q4wk, starting at age four weeks [43]. These doses encompass the osteoporosis (8 µg/kg) and oncologic doses (20–125 µg/kg) of ZOL (see below).
Fig. 1.
Study design. Two hundred thirty weanling female rice rats were BW-randomized into a baseline necropsy group (N = 12, age 4 weeks), or one of five dose groups (N = 43–54) Five doses of ZOL (0, 8, 20, 50, or 125 µg/kg) were given IV (tail vein) once every four weeks (q4wks), starting at age 4 weeks. Duration subgroups of 9–16 rice rats were necropsied (diamonds) after 12, 18, 24, and 30 weeks of treatment for each dose level.
After 12, 18, 24 and 30 weeks of treatment, duration sub-groups (N = 9–16) of rats from each of the five dose groups were necropsied. Rats were pre-assigned to dose groups at the start of the experiment, but not to duration sub-groups. Each duration subgroup was populated with rats that had lost ≥15% BW from its maximum, in order to comply with humane endpoints specified in the IACUC protocol. The rest of the group was completed by BW-randomization. No more than 25% of the rats in each subgroup were preselected as a result of reaching endpoint criteria for euthanasia due to BW lost.
2.3. ZOL dose and duration explanation
The minimum dose of ZOL that fully blocks ovariectomy-induced bone loss in adult rats is 8 µg/kg IV q4wk [44], but there are no predictive animal studies that have tested oncology outcomes of ZOL, such as prevention of bone metastases and hypercalcemia of malignancy. The ZOL oncology dose for Study 1 was 80 µg/kg BW, 10-fold higher than the OP ZOL dose. This was estimated from the 9.6:1 ratio (roughly 10-fold) of oncologic (48 mg IV/yr) to OP (5 mg IV/yr) ZOL in human medicine. The doses for Study 2 ranged from 8 to 125 µg/kg, to include both the OP dose and several doses in an oncologic range (20–125 µg/kg). ZOL was dissolved in normal saline (pH 7.2) and injected at 1 ml/kg BW IV. Zoledronic acid was kindly provided by Novartis Pharma AG (Basel, Switzerland).
Although our previous findings indicate that MRONJ lesions could be induced by 80 µg/kg ZOL in HSC diet rice rats after 18–22 weeks, the duration of this study was extended to 30 weeks since duration and cumulative exposure appear to play important roles in MRONJ. Cumulative dose, not dosing interval, is the primary determinant of N-BP efficacy [45]. Our oncology dose represents pre-clinical dose-interval combinations that yield a cumulative dose of 240 µg/kg quarterly or 40 µg/kg 2×/mo.
2.4. Euthanasia and tissue collection
Rats were euthanized by CO2 inhalation followed by cervical dislocation. Maxillae and mandibles were excised and trimmed, leaving gingiva and oral mucosa intact. When necessary, saline irrigation was used to remove excessive accumulation of bacterial plaque that obscured molars. High resolution (HR) photographs from the ventral aspect of each hemi-maxilla (Fig. 2) and medial aspect of each hemimandible (Supplemental Fig. 1) were taken with a digital camera (Canon EOS 6D; Tokyo, Japan) attached to a macro lens (Canon EF 100 mm 1:2.8; Tokyo, Japan). The right femur was disarticulated from the acetabulum and separated from the tibia. All tissue samples were fixed in freshly prepared 4% paraformaldehyde at 4 °C for 48 h then transferred to 70% ethanol for storage at 4 °C.
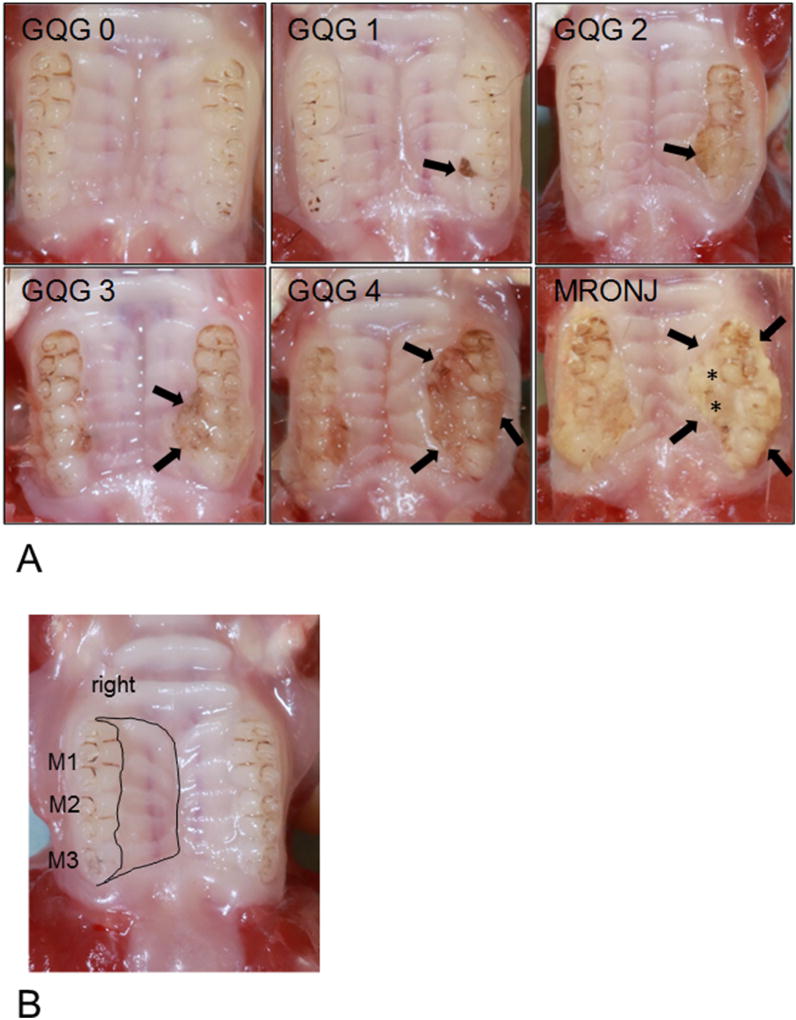
Fig. 2.
High Resolution Photographs of Maxillary gross quadrant grades [(GQG) 0–4 and MRONJ]. (A) GQG scores are at the top of each HR photograph. GQG0: Normal Maxilla. GQG1 (slight): Discrete lesion containing impacted food and hair at lingual aspect of interdental region of M2 M3 (arrow); FILP lesion occupies less than half of the lingual gingival margin of M2. GQG2 (mild): Lesion occupies two-thirds of lingual gingival margin of M2 and one-third of M3 (arrow), but does not extend toward midline. GQG3 (moderate): Lesion occupies complete lingual-gingival margin of M2 and two-thirds of M3, and extends almost halfway to the midline (arrows). GQG4 (severe): Lesion in left maxilla occupies complete lingual-gingival margin of M1–M3 and extends more than halfway to midline (arrows). MRONJ: A lesion of GQG = 3–4 (arrows) with exposed alveolar bone (*). (B) Total maxillary area (Tt.Mx.Ar) (black lines).
2.5. Gross analysis of oral lesions
2.5.1. Identification and grading of gross oral lesions
Photographs of jaws were analyzed independently in a blinded fashion by three observers (JGM, DBK, JIA). Quadrants were assigned a gross quadrant grade (GQG) (0–4, or MRONJ) (Table 1). Photographs of representative maxillae and mandibles at each GQG are shown in Fig. 2A and Supplemental Fig. 1A, respectively. Pocket depth or clinical attachment level evaluation by probing was not completed. When GQG was not consistent between investigators jaws were re-examined as a group to reach consensus. When at least one quadrant from a rat had a GQG ≥ 3 that included an area of exposed bone, the rat was considered positive for gross MRONJ.
Table 1.
Criteria for gross quadrant grade (GQG).
| GQG | Degree | Gross lesion description |
|---|---|---|
| 0 | Absence | None |
| 1 | Slight | Discrete lesion, area of M2M3 or M1M2; minimal gingival recession; visible impacted materials (hair, food debris); inflammation at margin of impacted materials |
| 2 | Mild | Single large lesion area of M2M3 or M1M2 with ulceration/recession of lingual gingiva along 1/2 to 2/3 the mesiodistal aspect of the lingual gingival margin of one involved molar; limited extension into the lingual mucosa; OR presence of two separate GQG = 1 lesions within the same hemimaxilla/hemimandible; inflammation/swelling/redness at margin of impacted material |
| 3 | Moderate | Continuous recession/ulceration of gingiva involving entire mesiodistal aspect of lingual gingival margin of both involved molars; ulceration extending into lingual mucosa toward midline in maxilla or onto lingual mucosa (lingual plate) in mandible; inflammation/swelling/redness at margin of impacted material; possible involvement of buccal mucosa |
| 4 | Severe | Continuous gingival recession/ulceration involving all three molars; ulceration extending into lingual mucosa toward the midline and onto the buccal mucosa; gingival inflammation/swelling/redness at margin of impacted material; possible tooth migration or loss |
| MRONJ lesion | Lesion of GQG = 3 or 4 with exposure of alveolar or palatal bone |
See Fig. 2A for corresponding photographs.
2.5.2. Quantification of gross oral lesion area
The areas (mm2) of gross oral lesions in each quadrant with GQG ≥ 1 were measured using the outline tool as previously described using AxioVision SE64 Rel software version 4.9.1 (Carl Zeiss, Germany) [41]. Total maxillary area (Tt.Mx.Ar) was demarcated mesially by a perpendicular line mesial to M1; caudally, a perpendicular line distal to M3, dorsally, a line along the gingival margins, and approximating the mesial and distal lines; and ventrally, a longitudinal line, along the sagittal palatal line, and approximating the mesial and distal lines (Fig. 2B). Total hemi-mandible area (Tt.Mb.Ar) was demarcated mesially by a perpendicular line mesial to M1; caudally, a perpendicular line distal to M3, dorsally, a line along the gingival margins, and approximating the mesial and distal lines; and ventrally a longitudinal line along the dorsal border of the incisor, and approximating the mesial and distal lines (Supplemental Fig. 1B). FILP area (FILP.Ar), lesion area (Le.Ar), MRONJ lesion area (MRONJ.Ar), Tt.Mx.Ar, and Tt.Mb.Ar were collected. FILP.Ar, Le.Ar, and MRONJ.Ar are then expressed as a percentage of Tt.MxAr or Tt.Mb.Ar (=100 * FILP.Ar or MRONJ.Ar/Tt.[Mx or Mb]Ar). Fig. 2A and Supplemental Fig. 1A show panels of photos that include representative cases of maxillary and mandibular GQG scores (0–4) and MRONJ lesions, respectively. All lesions (n = 343; Supplemental Table 1) were measured in random order by one investigator blinded to GQG (JIA).
2.6. Histopathological assessment of oral lesions
2.6.1. Specimen selection, preparation, and examination of jaw quadrant sections
All quadrants containing a gross MRONJ lesion, and any quadrant with GQG 1–4 underwent histopathological examination to identify and/or confirm exposed, necrotic bone (see below). Quadrants were decalcified in 5% formic acid under gentle agitation for four weeks, with three acid changes per week. Decalcified quadrants were trimmed to include the molar region, surrounding alveolar bone, and, for maxillae, the hard palate. Tissue was paraffin-embedded, and serially sectioned in the mesio-distal plane at 5-µm thickness at five to six different levels for quadrant. Each level was separated by about 250 µm. Multi-level examination of each quadrant was conducted on palatal or lingual tissues (one or two levels), interdental regions (M1M2, M2M3; three levels), and buccal regions (one level). Leica/Jung 2265 and Accu-Cut SRM 200 Sakura microtomes were used to section (Sakura Finetek Europe B.V, Zoeterwoude, The Netherlands). Sections were stained with H&E and coverslipped, and analyzed by one observer, blinded to the GQG.
2.6.2. Histological definition of MRONJ lesion
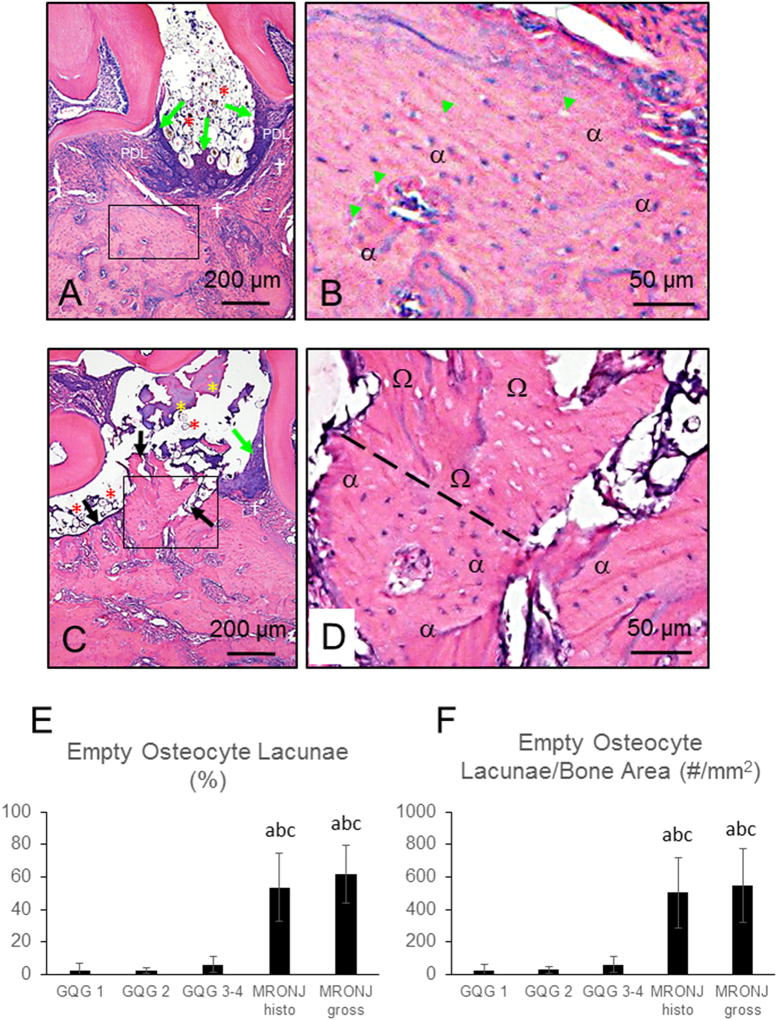
MRONJ lesions were defined histologically as bone that was both exposed and necrotic in at least one level within a quadrant. Exposed bone was identified by a lack of overlying soft tissue including gingival epithelium, lamina propria, connective tissue, and periodontal ligament (PDL) (Fig. 5). Necrotic bone was defined as a region of bone matrix containing ≥10 adjacent empty osteocyte lacunae, or lacunae with pyknotic osteocyte nuclei, as previously described [46,47]. These lacunae occasionally appeared enlarged and/or contained cellular debris. Exposed necrotic bone was almost always associated with adherent bacterial plaque. When at least one quadrant in a rat was assigned a histopathologic diagnosis of MRONJ, the rat was considered positive for histopathologic MRONJ.
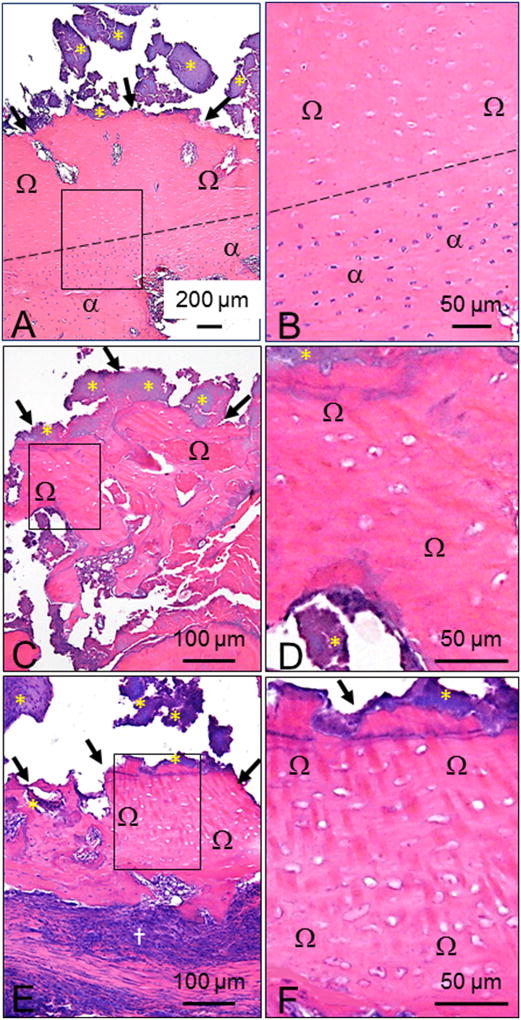
Fig. 5.
Histologic features of MRONJ lesions in ZOL-treated rats. Necrotic bone in MRONJ lesions was predominantly present in maxillae at (A) palatal and/or (C and E) M2M3 interdental space. B, D, and F are magnified fields of the rectangles demarcated in A, C, and E, respectively. (A, C, E) MRONJ lesions with characteristic exposed bone (black arrows) lacking overlying gingival epithelium, lamina propria, connective tissue, and PDL. Large fields of empty osteocyte lacunae (B, D, F) that were occasionally enlarged, containing nuclear debris (Ω). Adherent bacterial colonies resembling Actinomyces sp. (yellow asterisks) were frequently found on exposed bone. Dotted black line demarcates a region with extensive necrotic bone (Ω), overlying a region of vital bone (α) (A and B). Severe inflammatory cell infiltration and fibrosis were often observed surrounding necrotic palatal and alveolar bone (†). Five-µm sections stained with H&E.
2.6.3. Histological quantification of empty osteocyte lacunae
To assess necrotic bone the number of lacunae that were empty or contained pyknotic nuclei were counted. Data collection was performed at 200× magnification in sections within a 0.15–0.25 mm2 region of interest (ROI) that contained exposed bone matrix in an MRONJ lesion or an area of bone with periodontal tissue destruction and inflammation in FILP lesions with GQG ≥ 1. Total bone area (Tt.Ar;mm2), total number of osteocyte lacunae (#OtLc), and number of empty osteocyte lacunae (#Em.OtLc) were measured. The number of lacunae per bone area (density of osteocyte lacunae; #OtLc/mm2), number of empty lacunae per bone area (#Em.OtLc/mm2), and percentage of empty lacunae (100 × #Em.OtLc / #Ot.Lc) were calculated. For analysis, means were calculated from quadrants with 1) FILP lesions with GQG 1; 2) FILP lesions with GQG 2; 3) FILP lesions with GQG 3–4; 4) MRONJ lesions identified solely by histology; or 5) gross MRONJ lesions confirmed histologically.
2.7. Peripheral quantitative computed tomography (pQCT)
pQCT analysis of the right femur was used to verify the antiresorptive efficacy of ZOL. Femurs were scanned at 1 mm-thick cross sections using a Stratec XCT Research M instrument (v 5.40, Norland Medical Systems; Fort Atkinson, WI). Sites of interest were 5 mm proximal to the distal end of the femur (total metaphysis), and at the longitudinal midpoint of the femur (mid-diaphysis). Volumetric bone mineral content (vBMD, mg), volumetric bone mineral density (vBMD, mg/cm3), and cortical area (mm2) were determined for total bone (trabecular and cortical bone at the metaphysis, and cortical bone at the mid-diaphysis), as previously described [48].
2.8. Statistical analysis
Data are expressed as mean ± SD for each group, except BW which is express as mean ± SEM. For Study 1, Fisher's Exact test was used to assess prevalence of MRONJ lesions in ZOL0 and ZOL80 groups at 12 and 18 weeks. For Study 2, data were evaluated to assess intergroup differences using one-way ANOVA with Holm-Sidak post hoc tests for BW, percent of empty osteocyte lacunae, and vBMD, vBMD and cortical bone area. When assumptions of data normality were not met, a Kruskal-Wallis ANOVA test followed by Dunn's multiple comparison was applied. Multiple logistic regression was used to test the effects of ZOL dose/duration on MRONJ prevalence. Pearson correlation test was used to test for an association between FILP GQG and the area (mm2) of oral lesions. P values <0.05 were considered statistically significant.
3. Results
3.1. Study 1
3.1.1. ZOL0
FILP lesions were generally discrete and localized at the lingual surface of the interdental area of M2M3, with minimal/moderate gingival recession (Fig. 2A; GQG = 1–3). 75% of rats had FILP lesions in at least one maxilla was after 12 and 18 weeks (Fig. 3). FILP GQGs ranged from 1 to 3, with a median of 1 (data not shown). No differences in GQG or FILP.Ar/T.Ar were observed between rats on STD diet for 12 vs. 18 weeks (data not shown). Similar to previous findings, no FILP or PD-like lesions were observed in the mandible [41] (Fig. 3), and no rats developed MRONJ.
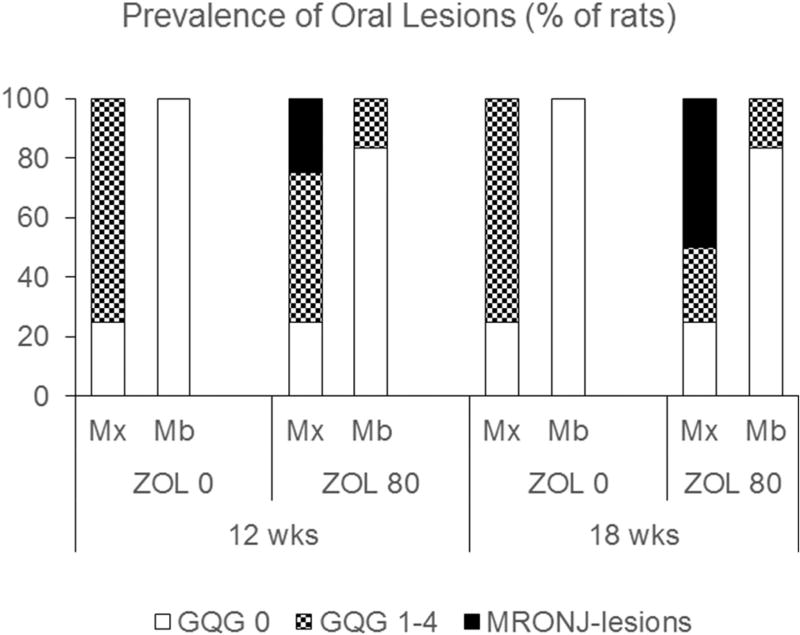
Fig. 3.
Prevalence of gross oral lesions (study 1). In ZOL0 rats, the prevalence of GQG = 1–4 oral lesions in the maxilla was 75% at both 12 and 18 weeks treatment. Mandibles had no oral lesions at either time point. In ZOL80 rats, the prevalence of GQG = 1–4 oral lesions (non-MRONJ) in the maxilla was 50% at 12 weeks and 17% at 18 weeks. The prevalence of MRONJ lesions in the maxilla was 25% at 12 weeks and 50% at 18 weeks. The prevalence of GQG = 1–4 oral lesions (non-MRONJ) in the mandible was 17% at both time points.
3.1.2. ZOL80
Like ZOL0, 75% of ZOL80 rats showed FILP lesions in at least one maxilla (Fig. 3), ranging from QGQ1–4, with median GQG2 (data not shown). FILP lesions tended to be larger and more severe in ZOL80 rats than in time-matched ZOL0 rats, with greater areas of recession/ulceration often involving the entire gingival margin of M2M3 (Fig. 2A; GQG scores 3–4). Some ZOL80 rats also had gross oral lesions in mandibles similar to PD lesions that have been previously reported in rice rats [32] (Fig. 3; Supplemental Fig. 1A).
Unlike ZOL0 rats, ZOL80 rats had gross maxillary MRONJ lesions at 12 and 18 weeks, with a prevalence of 25% and 50%, respectively (Fig. 3). In maxillae, MRONJ lesions were found at locations where FILP lesions also developed (Fig. 2A). MRONJ lesions were never observed in mandibles (Fig. 3). Histopathologic analysis confirmed the existence of exposed, necrotic bone in all gross MRONJ cases. Prevalence of MRONJ lesions was associated with jaw (maxilla) and ZOL treatment (P < 0.001).
3.2. Study 2: dose and duration effects of ZOL on MRONJ prevalence
3.2.1. Prevalence of rats with gross MRONJ
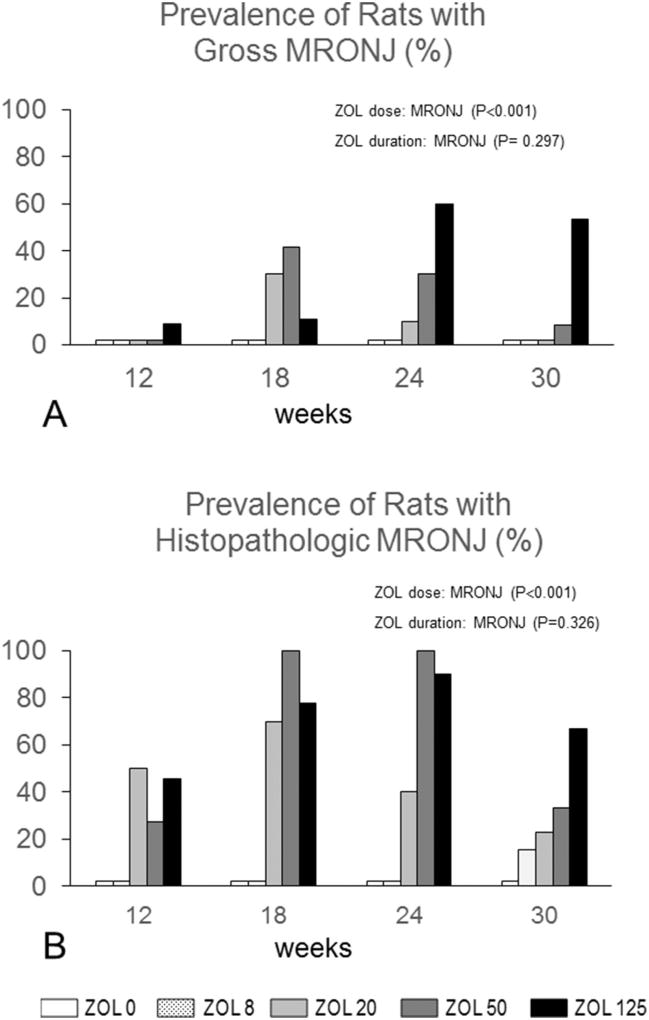
No MRONJ lesions were present at four weeks. The prevalence of rats with MRONJ by ZOL dose and treatment duration is shown (Fig. 4A). ZOL dose (P < 0.001), but not duration of ZOL treatment (P = 0.297), was a significant predictor of MRONJ. The overall prevalence of gross MRONJ in the whole study was 13%. The prevalence of rats with MRONJ was 22% in rats receiving oncologic doses of ZOL (≥20 µg/kg), which was significantly greater than 0 or 8 µg/kg (P < 0.001 and P < 0.001, respectively).
Fig. 4.
Prevalence of MRONJ lesions (study 2). (A) Prevalence of rats with gross MRONJ by ZOL dose and duration. Prevalence increased with increasing ZOL dose (P < 0.001), but not increasing duration of ZOL exposure (P=0.297). Gross MRONJ lesions were not found in ZOL0 or osteoporosis (OP) ZOL (8 µg/kg) groups. (B) Prevalence of rats with histopathologic MRONJ by time and ZOL dose in quadrants with GQG ≥ 1. Prevalence of histopathologic MRONJ increased with increasing ZOL dose (P < 0.001) but not increasing duration of ZOL exposure (P = 0.326). Histopathologic MRONJ was never found in ZOL0 rats; two cases were present in the OP ZOL (8 µg/kg) group at 30 weeks.
Four quadrants were missing, broken, or the HR photo was of insufficient quality for analysis. Supplemental Table 1 shows Le.Ar, expressed as Le.Ar/Tt.Ar, and number of GQG (0–4) and MRONJ lesions by arch and ZOL dose. At age 4 weeks (baseline), rats had no MRONJ or FILP lesions. A total of 916 jaw quadrants were given a GQG. At ages 16–34 weeks, 573 quadrants were lesion-free (63%; 41% of maxillae and 84% of mandibles). More maxillary quadrants than mandibular quadrants had lesions (P < 0.001). A positive correlation was found between lesion area and GQG (Pearson r = 0.867), independent of dose and duration of ZOL (Supplemental Table 1).
56 MRONJ lesions (Supplemental Table 1) were distributed among 29 rats. There were no MRONJ lesions in control or OP ZOL (8 µg/kg) rats. All 29 rats with MRONJ lesions had at least one maxilla with an MRONJ lesion. 79% of MRONJ lesions were in the maxilla (P < 0.001 vs mandible). Mandibular MRONJ lesions were never found without accompanying maxillary MRONJ lesions.
3.2.2. Histopathological analysis
FILP lesions were located primarily at the maxillary M2M3 interdental region (Fig. 6A and B). ZOL0 rats had FILP lesions with GQG ≤ 3, but never had MRONJ. FILP lesions displayed substantial hyperplasia of the gingival epithelium; inflammatory cell infiltration of the lamina propria (Fig. 6A and B); PDL disruption; apical migration of the junctional epithelium; and alveolar bone crest resorption. Isolated, empty osteocyte lacunae were occasionally observed, but most lacunae were occupied by round basophilic osteocyte nuclei (Figs. 6B).
Fig. 6.
Histologic Features of FILP and MRONJ lesions and osteocyte lacunae data in ZOL-treated rats. Five-µm sections stained with H&E. B and D are magnified fields of the rectangles demarcated in A and C, respectively. (A & B) FILP lesion with GQG3 with vital alveolar bone characterized by basophilic osteocyte nuclei within lacunae (α) and only a few scattered, empty lacunae (green arrowheads [B]); substantial hyperplasia of the gingival epithelium (green arrows [A]), inflammatory cell infiltration of the lamina propria (†), disruption of the periodontal ligament (PDL), apical migration of the junctional epithelium, and alveolar bone crest resorption. (C and D) MRONJ lesion with bone exposed to the oral cavity (black arrows) with adherent bacteria (yellow asterisks); confluent area of necrotic bone with empty osteocyte lacunae (Ω) is shown above an area of vital bone, demarcated by dashed line [D]. Note presence of impacted hair and food debris (red asterisks) in both FILP and MRONJ lesions. (E and F) Quantification of empty osteocyte lacunae as (E) a percent of total lacunae (#Em.Lc/Tot.#.Lc) and as (F) number per bone tissue area (#Em.Lc/Tt.Ar). Both gross and histopathologic-only MRONJ lesions had significantly higher percentage of empty lacunae compared to GQG 1–4 lesions without MRONJ. Data are Mean ± SD. a- different from GLG 1 (P < 0.05). b- different from GLG 2 (P < 0.05); c- different from GLG3–4 (P < 0.05).
We found that 96% of quadrants with gross MRONJ lesions (54/56) contained exposed necrotic bone, and that all 29 rats with gross MRONJ lesions fulfilled the histopathologic criteria for an MRONJ positive case (Figs. 5, 6C, and D). We identified an additional 53 rats, not diagnosed with gross MRONJ, that had at least one quadrant with histopathologic features of MRONJ [GQG 1 (n = 0/100), GQG 2 (n = 15/96), 3 (n = 28/71), or 4 (n = 10/20)].
There was a significantly higher percentage of empty osteocyte lacunae (P < 0.001) (Fig. 6E) and a greater number of empty osteocyte lacunae/mm2 (P < 0.001) (Fig. 6F) in MRONJ lesions than in GQG 1–4 quadrants not diagnosed with MRONJ. No differences in these endpoints were found between gross and histopathologic MRONJ lesions (Fig. 6E and F).
3.2.3. Prevalence of rats with histopathologic MRONJ
Fig. 4B shows the prevalence of rats with histopathological MRONJ by dose and duration. The prevalence of rats with histopathologic MRONJ in the whole study populations was 38%. The prevalence of histopathologic MRONJ in rats exposed to oncologic doses of ZOL (≥20 µg/kg) was 73%, with most cases occurring at ≥18 weeks. Several duration groups had 100% prevalence of MRONJ (Fig. 4B). Only 5% of MRONJ cases occurred in rats given 8 µg/kg ZOL (OP dose), all at 30 weeks (Fig. 4B). ZOL dose (P < 0.001), but not duration (P=0.326), was a predictor of histopathologic MRONJ prevalence (Fig. 4B). Prevalence in ZOL-treated rats was 31% (13/42); 62% (26/42); 57% (23/40), and 36% (20/55) at 12, 18, 24 and 30 weeks, respectively. To examine the effect of pre-selecting rats for necropsy due to BW loss on the effect of ZOL duration on MRONJ prevalence, ZOL-treated rats with BW loss were removed from the analysis at the 12 (n = 6), 18 (n = 16), and 24 week (n= 10) time points, and data were re-analyzed. When BW loss cases were removed, MRONJ prevalence was 19, 38, 43, and 36% at 12, 18, 24, and 30 weeks, respectively, and both ZOL dose (P < 0.001) and duration (P = 0.017) were significant predictors of MRONJ prevalence (Supplemental Fig. 4). The proportion of rats with histopathologic MRONJ within each GQG was also determined (Supplemental Fig. 5). There were no cases of histopathologic MRONJ in animals with GQG 0 or 1. In rats with GQG 2, 3, and 4 the proportion of animals with MRONJ was about 40, 80, and 100%, respectively, at each time point.
3.2.4. Body weight
BW of rats in all experimental groups increased with age throughout the study, with no significant effects of ZOL (Supplemental Fig. 2A). However, when this study population was grouped into rats that had either gross MRONJ, histopathologic-only MRONJ, or PD 0, we found significantly lower BW in rats with gross MRONJ compared to GQG 0 rats at 12–24 weeks of ZOL treatment (Supplemental Fig. 2B). In addition, BW were lower in rats with histopathologic-only MRONJ, but only at week 24 of ZOL treatment compared with GQG0 rats (Supplemental Fig. 2B).
3.2.5. Anti-resorptive efficacy of ZOL in the femur
Total metaphyseal vBMD and vBMD, and cortical area were significantly greater in all ZOL groups compared to the ZOL 0 group (P < 0.001) at all time points (Supplemental Fig. 3A–C). Cortical BMC was higher in groups treated with ZOL only after 18 weeks compared to ZOL 0 (Supplemental Fig. 3D). Total metaphyseal vBMD and vBMD; and cortical bone area and BMC were significantly greater at ZOL doses ≥20 µg/kg compared to ZOL dose 8 µg/kg, particularly at 24 and 30 weeks (P < 0.05) (Supplemental Fig. 3A–D).
4. Discussion
These studies demonstrate that MRONJ can be induced in rice rats fed a STD diet when simultaneously given clinically relevant doses of ZOL at both the osteoporosis and oncology range. The MRONJ lesions in this model resemble those seen in human MRONJ, and were localized primarily to the same location where FILP lesions occurred. Additionally, higher doses of ZOL were associated with higher prevalence of MRONJ. To our knowledge, this is the first experiment to show a dose response relationship between clinically relevant doses of ZOL and MRONJ prevalence.
Our results show that the prevalence of histopathological MRONJ was 0% in the ZOL0 group, 5% in rats given the OP dose (8 µg/kg), and 73% in rats given oncology doses of ZOL (20–125 µg/kg), indicating that higher doses of ZOL are associated with more prevalent MRONJ. MRONJ lesions were found after 12 weeks, reaching peak prevalence at 18 weeks after 5 monthly injections of ZOL oncology doses. This treatment regimen produced cumulative doses of 100, 250, and 625 µg/kg for the 20, 50, and 125 µg/kg doses, respectively at peak prevalence. MRONJ prevalence in groups of rice rats given oncologic doses of ZOL was 40–100% within 18–24 weeks of treatment, and appeared to plateau around the 50 µg/kg dose. Therefore, MRONJ in the rice rat FILP model occurs with sufficient prevalence, and in a reasonable amount of time, to allow future studies that can effectively address strategies to prevent or treat MRONJ.
MRONJ prevalence did not appear to be associated with duration in this study. The lower prevalence of MRONJ in rice rats at 30 weeks may be due to the necropsy of rats with greater than 15% BW loss at the earlier time points. Shorter durations may have been disproportionately populated with rats that required earlier necropsy, leading to an artificially high proportion of healthy rats at later time points, reducing MRONJ prevalence at 30 weeks. Moreover, analysis of MRONJ prevalence at each time point after removing animals with BW loss resulted in a significant duration effect on MRONJ prevalence, supporting this idea. These findings appear to contrast with, human studies where cumulative MRONJ incidence increased steadily at 39, 59, and 74 months at 0.7% [13], 1.1% [14], and 2.1% [15], respectively. However, these clinical findings could also reflect new cases of event-related MRONJ related to discrete dental events (e.g., tooth extractions, etc.) that occurred during the follow-up period. Cases of human MRONJ have also been known to spontaneously heal. Therefore, the 30 week time point may have also included rats resistant to FILPs or with MRONJ lesions that had spontaneously healed.
Histopathological analysis revealed that MRONJ in rice rats was similar to human MRONJ [4,29,49–52], in that both show exposed necrotic bone with confluent areas of empty osteocyte lacunae that is frequently colonized by bacteria. Observation of tissues in serial sections were especially valuable because we were able to fully characterize the appearance of the lesions, identify multiple areas where necrotic bone may have existed, and therefore confidently determine positive MRONJ cases. Importantly, empty osteocyte lacunae were never present beneath fully intact gingiva. If necrotic bone had an epithelial covering in one level, then necrotic bone without an epithelial covering was always found in an adjacent level of the same quadrant. This finding may suggest that MRONJ could be present in persons taking pARs, under gingiva that is recessed or ulcerated that lacks overtly exposed bone.
Although there is no direct human equivalent to the GQG scoring system, this analysis was valuable as a preclinical tool to quickly identify lesion-free quadrants, and quadrants with gross MRONJ. Gross analysis was particularly important because visibly exposed necrotic bone is one of the primary criteria for the diagnosis of MRONJ in humans. Subsequent histopathologic examination of gross MRONJ lesions confirmed the presence of exposed necrotic bone in 95% of these cases, indicating that we were able to accurately identify MRONJ cases when animals had severe lesions with frank bone exposure. The GQG system was also a practical laboratory tool that enabled efficient prioritization of quadrants for sectioning and subsequent histopathological examination. In fact, the histopathological examination of less severe gross lesions (GQG 1–4) revealed nearly three-fold as many cases of MRONJ as gross analysis alone. GQG1 never had histopathological MRONJ, but GQG 2–4 had increased prevalence of histopathological MRONJ (Supplemental Fig. 5). One of the primary features distinguishing the levels of severity was the extent of gingival ulceration, and larger lesions tended to be more likely to have exposed bone (Supplemental Table 1). GQG2 was marked by recession/ulceration of gingival tissue around impacted materials, while GQG1 was characterized only by the presence of impacted materials between the molars without ulceration or marked recession. This finding suggests that GQG2 may represent a grossly identifiable initial stage of a MRONJ-lesion that may have histopathological MRONJ in this model.
Our findings support the idea that gingival/mucosal lesions may be an identifiable non-surgical dental event in ZOL patients that triggers what has been previously termed “spontaneous” MRONJ. Our data raise the possibility that ZOL negatively affects the healing of oral lesions, thereby contributing to higher risk of MRONJ [22,26]. Previous animal studies have also shown a link between “spontaneous” MRONJ and oral risk factors other than tooth extraction [30–34]. These studies induced MRONJ by combining pARs with infection and inflammatory conditions in rodents, including experimental PD, and exposure of the dental pulp to induce periapical infection [27,30–34]. One other study has taken advantage of spontaneous oral events that require no mechanical manipulation or dentoalveolar surgical procedures, by using C57BL/6J mice that is prone to maxillofacial abscesses. Like FILPs in rice rats, those lesions are characterized by impacted hair and food debris. Similar to our study, these lesions also progressed to MRONJ when mice were given ZOL [53].
The MRONJ lesions in this study were localized to the same quadrants, and the same anatomical location as FILPs. Therefore, this model of MRONJ appears to primarily affect the maxilla, rather than then mandible, where human MRONJ is more commonly observed in humans. The majority of untreated rice rats developed at least one FILP lesion by 12 weeks, and the highest FILP lesion prevalence preceded the time point of the highest ZOL-associated MRONJ prevalence. Furthermore, 73% of ZOL-treated rats that had at least one quadrant with GQG ≥ 1 had MRONJ. These data suggest that FILP lesions in rice rats initiate MRONJ in ZOL-treated rats. Further studies are required to establish that ZOL-induced MRONJ in STD diet-fed rice rats is linked directly to the prior or concurrent development of a FILP lesion. Specifically, these experiments would require oral exams in live animals which would track the initiation and clinical progression of both FILPs and MRONJ. The visibility and predictable location of these lesions would also allow intervention studies that reduce the frequency of impaction by removing impacted materials, or preventing impactions by dietary alterations.
A subpopulation of rice rats (45%) treated with oncologic ZOL doses for 30 weeks never developed any FILP or MRONJ lesions. An additional 28% of such rats had minor lesions that were never more severe than GQG=1. This may indicate that some rice rats are resistant to the development of ZOL-induced MRONJ, even with a cumulative ZOL dose up to 1000 µg/kg over 30 weeks. Differential susceptibility in rice rats may be, at least partly, due to individual tooth/gingival morphology that facilitates the retention of impacted materials in some rats, or some rats may have an immune response that permits progression of FILP lesions into MRONJ lesions. It is possible that dental cofactors are integral to the development of MRONJ in ZOL-treated rats and that “resistant” rats never develop those dental cofactors. A MRONJ-resistant subpopulation of rice rats also has clinical implications because the majority of pAR-treated patients also never develop MRONJ.
FILP lesions appear to be a localized reaction to the hair and food debris impacted in the interdental space. In humans, food impaction lesions have been reported in follow-ups of implant prostheses [54], inducing peri-implantitis [17], and in embrasures between implant-supported fixed dental prostheses and adjacent teeth [18]. The rice rat FILP may also be comparable to oral clinical conditions that result from debris (e.g. food or fibers) retained between teeth, and in periodontal pockets, that can cause gingivitis, periodontitis, halitosis, pain, gingival abscess, alveolar bone loss, and root caries [55]. FILPs may therefore replicate the discrete injury, and subsequent inflammatory response, seen in humans with such problems. Compared to humans, however, FILPs with GQG 2–4 with gingival ulceration along the gingival margin, are more severe than food impaction lesions typically found in humans. The lack of progression of food impaction lesions in pAR treated humans could be due to the daily oral hygiene practices employed by most humans.
Although this study included a range of osteoporosis and oncology doses of ZOL in a large number of rice rats, the study design has several limitations. MRONJ due to pAR treatment is an adverse event that occurs in patients first exposed to pARs in adulthood. Though the majority of ZOL injections in these experiments were given when the rat skeleton had reached young adulthood, each rat received two ZOL injections when the rat skeleton is considered juvenile (ages 4 and 8 weeks). The initial skeletal distribution and subsequent redistribution of N-BPs differs significantly between the juvenile and adult skeletons, due to the rapid rate of bone modeling-associated bone resorption and formation that is found only in the juvenile skeleton [56]. Therefore, bone modeling in the juvenile skeleton may have played a role in MRONJ pathophysiology in this experiment that could not exist in adult humans, since adults display mostly bone remodeling activity. Future rice rat MRONJ studies in which pARs and anti-angiogenic agents are given only after age 12 weeks, when the rat skeleton is no longer rapidly growing, would assist in resolving this limitation.
In humans, MRONJ diagnosis is made only after bone exposure of eight weeks duration [12,28,29]. Reliance on photographs taken at necropsy for the gross analysis did not allow verification of the length of bone exposure before necropsy, and perhaps contributed to underestimation of MRONJ because diagnostic criteria that are available in the clinical setting (e.g., periodontal pocket probing, patient-reported pain, dental radiography, cone beam CT, etc.) cannot be achieved in a rodent study. The inability to determine the duration of bone exposure, raises the possibility that some MRONJ lesions may have been present for less than eight weeks at necropsy. Therefore, lesions may have been identified at a higher prevalence than if we had strictly used criteria applied to humans. Longitudinal observation would also allow observation of spontaneous healing of MRONJ. Spontaneous healing of MRONJ, or gradual healing during daily use of antiseptic mouth rinses, is known to occur in humans [12,28,29].
5. Conclusion
Powerful anti-resorptives provide unparalleled benefits to patients with both cancer and osteoporosis by reducing mortality and comorbidities associated with both diseases [57–60]. Despite the existence of MRONJ, the risk-benefit ratio for pARs is strongly positive [12,28,29,61, 62]. Therefore, the use of pARs in patients with cancer and osteoporosis will continue. Our findings raise the possibility that dental cofactors are an important element that can lead to the development of MRONJ when they are present prior to, or develop during, ZOL treatment. Our findings support the use of STD-diet fed rice rats as an animal model prone to a local periodontal event that can be used to clarify the pathophysiology of MRONJ. Prevention and intervention strategies for MRONJ are needed to manage the concerns of both patients and the dental and medical communities over this potentially severe adverse event.
Supplementary Material
Acknowledgments
CVP's Institution receives research support from Bayer. CVP receives a small amount of salary support from the research support and royalties from UpToDate for writing for Bayer.
Funding
This research was supported by the National Institute of Dental and Craniofacial Research (NIDCR); R01DE023783-01A.
This research was supported by NIH grant R01DE023783-01A from the National Institute of Dental and Craniofacial Research (NIDCR). We are thankful to Dr. Juerg Gasser and Novartis Pharma AG (Basel, Switzerland) to provide us with zoledronic acid to conduct the experiments.
Abbreviations
- FILP
food impaction-induced localized periodontitis
- GQG
gross quadrant grade
- M
molar
- MRONJ
medication-related osteonecrosis of the jaw
- PDL
periodontal ligament
- STD
standard rodent chow
- ZOL
zoledronic acid
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bone.2017.12.025.
Conflict of interest
The authors have no conflicts of interest. The authors have no conflicts of interest.
Authors' roles
Study design: JIA, DBK. Study conduct: JGM, JIA, JLMC, JMJ, EJC. Data collection: JIA, JGM, DBK, JLMC, JMJ, JMJ; EJC. Data analysis: JIA, JGM, DBK. Data interpretation: JIA, JGM, DBK, NB, CVP. Drafting manuscript: JIA, DBK, JGM. Revising manuscript content: JIA, JGM, JLMC, JMJ, EJC, CVP, NB, CVP, DBK. Approving final version of manuscript: JIA, JGM, JLMC, JMJ, EJC, CVP, NB, CVP, DBK. JIA takes responsibility for the integrity of the data analysis.
References
- 1.Bilezikian JP. Osteonecrosis of the jaw—do bisphosphonates pose a risk? N. Engl. J. Med. 2006;355:2278–2281. doi: 10.1056/NEJMp068157. [DOI] [PubMed] [Google Scholar]
- 2.Hellstein JW, Marek CL. Bis-phossy jaw, phossy jaw, and the 21st century: bisphosphonate-associated complications of the jaws. J. Oral Maxillofac. Surg. 2004;62:1563–1565. doi: 10.1016/j.joms.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J. Oral Maxillofac. Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 4.Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J. Oral Maxillofac. Surg. 2005;63:1567–1575. doi: 10.1016/j.joms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann. Intern. Med. 2006;144:753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 6.Van den Wyngaert T, Wouters K, Huizing MT, Vermorken JB. RANK ligand inhibition in bone metastatic cancer and risk of osteonecrosis of the jaw (ONJ): non bis in idem? Support Care Cancer. 2011;19:2035–2040. doi: 10.1007/s00520-010-1061-0. [DOI] [PubMed] [Google Scholar]
- 7.Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J. Clin. Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 9.Fusco V, Santini D, Armento G, Tonini G, Campisi G. Osteonecrosis of jaw beyond antiresorptive (bone-targeted) agents: new horizons in oncology. Expert Opin. Drug Saf. 2016;15:925–935. doi: 10.1080/14740338.2016.1177021. [DOI] [PubMed] [Google Scholar]
- 10.Christodoulou C, Pervena A, Klouvas G, Galani E, Falagas ME, Tsakalos G, et al. Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology. 2009;76:209–211. doi: 10.1159/000201931. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez L, Lopez-Pintor RM, Casanas E, Arriba L, Hernandez G. New non-bisphosphonate drugs that produce osteonecrosis of the jaws. Oral Health Prev. Dent. 2015;13:385–393. doi: 10.3290/j.ohpd.a34055. [DOI] [PubMed] [Google Scholar]
- 12.Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014;72:1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 13.Coleman R, Woodward E, Brown J, Cameron D, Bell R, Dodwell D, et al. Safety of zoledronic acid and incidence of osteonecrosis of the jaw (ONJ) during adjuvant therapy in a randomised phase III trial (AZURE: BIG 01-04) for women with stage II/III breast cancer. Breast Cancer Res. Treat. 2011;127:429–438. doi: 10.1007/s10549-011-1429-y. [DOI] [PubMed] [Google Scholar]
- 14.Coleman RE, Marshall H, Cameron D, Dodwell D, Burkinshaw R, Keane M, et al. Breast-cancer adjuvant therapy with zoledronic acid. N. Engl. J. Med. 2011;365:1396–1405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 15.Rathbone EJ, Brown JE, Marshall HC, Collinson M, Liversedge V, Murden GA, et al. Osteonecrosis of the jaw and oral health-related quality of life after adjuvant zoledronic acid: an adjuvant zoledronic acid to reduce recurrence trial subprotocol (BIG01/04) J. Clin. Oncol. 2013;31:2685–2691. doi: 10.1200/JCO.2012.46.4792. [DOI] [PubMed] [Google Scholar]
- 16.Grbic JT, Black DM, Lyles KW, Reid DM, Orwoll E, McClung M, et al. The incidence of osteonecrosis of the jaw in patients receiving 5 milligrams of zoledronic acid: data from the health outcomes and reduced incidence with zoledronic acid once yearly clinical trials program. J. Am. Dent. Assoc. 2010;141:1365–1370. doi: 10.14219/jada.archive.2010.0082. [DOI] [PubMed] [Google Scholar]
- 17.Bidra AS. Nonsurgical management of inflammatory periimplant disease caused by food impaction: a clinical report. J. Prosthet. Dent. 2014;111:96–100. doi: 10.1016/j.prosdent.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Jeong JS, Chang M. Food impaction and periodontal/peri-implant tissue conditions in relation to the embrasure dimensions between implant-supported fixed dental prostheses and adjacent teeth: a cross-sectional study. J. Periodontol. 2015;86:1314–1320. doi: 10.1902/jop.2015.150322. [DOI] [PubMed] [Google Scholar]
- 19.Barba-Recreo P, Del Castillo Pardo de Vera JL, Garcia-Arranz M, Yebenes L, Burgueno M. Zoledronic acid - related osteonecrosis of the jaws. Experimental model with dental extractions in rats. J. Craniomaxillofac. Surg. 2014;42:744–750. doi: 10.1016/j.jcms.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Bi Y, Gao Y, Ehirchiou D, Cao C, Kikuiri T, Le A, et al. Bisphosphonates cause osteonecrosis of the jaw-like disease in mice. Am. J. Pathol. 2010;177:280–290. doi: 10.2353/ajpath.2010.090592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jabbour Z, El-Hakim M, Henderson JE, de Albuquerque RFJ. Bisphosphonates inhibit bone remodeling in the jawbones of rats and delay healing following tooth extractions. Oral Oncol. 2014;50:485–490. doi: 10.1016/j.oraloncology.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi Y, Hiraga T, Ueda A, Nishimura R, Yatani H, Yoneda T. Zoledronic acid delayed the wound healing of tooth extraction socket but failed to cause osteonecrosis of the jaw in mice. J. Bone Miner. Res. 2007;22(Suppl. 1):S291. [Google Scholar]
- 23.Maahs MP, Azambuja AA, Campos MM, Salum FG, Cherubini K. Association between bisphosphonates and jaw osteonecrosis: a study in Wistar rats. Head Neck. 2011;33:199–207. doi: 10.1002/hed.21422. [DOI] [PubMed] [Google Scholar]
- 24.Soundia A, Hadaya D, Esfandi N, de Molon RS, Bezouglaia O, Dry SM, et al. Osteonecrosis of the jaws (ONJ) in mice after extraction of teeth with periradicular disease. Bone. 2016;90:133–141. doi: 10.1016/j.bone.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zandi M, Dehghan A, Malekzadeh H, Janbaz P, Ghadermazi K, Amini P. Introducing a protocol to create bisphosphonate-related osteonecrosis of the jaw in rat animal model. J. Craniomaxillofac. Surg. 2016;44:271–278. doi: 10.1016/j.jcms.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Park S, Kanayama K, Kaur K, Tseng HC, Banankhah S, Quje DT, et al. Osteonecrosis of the jaw developed in mice: disease variants regulated by gammadelta T cells in oral mucosa barrier immunity. J. Biol. Chem. 2015;290:17349–17366. doi: 10.1074/jbc.M115.652305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song M, Alshaikh A, Kim T, Kim S, Dang M, Mehrazarin S, et al. Preexisting periapical inflammatory condition exacerbates tooth extraction-induced bisphosphonate-related osteonecrosis of the jaw lesions in mice. J. Endod. 2016;42:1641–1646. doi: 10.1016/j.joen.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 29.Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B. American Association of Oral and maxillofacial surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J. Oral Maxillofac. Surg. 2009;67:2–12. doi: 10.1016/j.joms.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Aghaloo TL, Kang B, Sung EC, Shoff M, Ronconi M, Gotcher JE, et al. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J. Bone Miner. Res. 2011;26:1871–1882. doi: 10.1002/jbmr.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aghaloo TL, Cheong S, Bezouglaia O, Kostenuik P, Atti E, Dry SM, et al. RANKL inhibitors induce osteonecrosis of the jaw in mice with periapical disease. J. Bone Miner. Res. 2014;29:843–854. doi: 10.1002/jbmr.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aguirre JI, Akhter MP, Kimmel DB, Pingel JE, Williams A, Jorgensen M, et al. Oncologic doses of zoledronic acid induce osteonecrosis of the jaw-like lesions in rice rats (Oryzomys palustris) with periodontitis. J. Bone Miner. Res. 2012;27:2130–2143. doi: 10.1002/jbmr.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li CL, WW Lu, Seneviratne CJ, Leung WK, Zwahlen RA, Zheng LW. Role of periodontal disease in bisphosphonate-related osteonecrosis of the jaws in ovariectomized rats. Clin. Oral Implants Res. 2016;27:1–6. doi: 10.1111/clr.12502. [DOI] [PubMed] [Google Scholar]
- 34.Xiong H, Peng B, Wei L, Zhang X, Wang L. Effect of an estrogen-deficient state and alendronate therapy on bone loss resulting from experimental periapical lesions in rats. J. Endod. 2007;33:1304–1308. doi: 10.1016/j.joen.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Auskaps A, Gupta O, Shaw J. Periodontal disease in the rice rat. III. Survey of dietary influences. J. Nutr. 1957;63:325–343. doi: 10.1093/jn/63.3.325. [DOI] [PubMed] [Google Scholar]
- 36.Gotcher JE, Jee WS. The progress of the periodontal syndrome in the rice rat. I. Morphometric and autoradiographic studies. J. Periodontal Res. 1981;16:275–291. doi: 10.1111/j.1600-0765.1981.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 37.Gupta O, Shaw J. Periodontal disease in the rice rat. I. Anatomic and histopathologic findings. Oral Surg. Oral Med. Oral Pathol. 1956;9:592–603. doi: 10.1016/0030-4220(56)90319-x. [DOI] [PubMed] [Google Scholar]
- 38.Hattler AB, Snyder DE, Listgarten MA, Kemp W. The lack of pulpal pathosis in rice rats with the periodontal syndrome. Oral Surg. Oral Med. Oral Pathol. 1977;44:939–948. doi: 10.1016/0030-4220(77)90038-x. [DOI] [PubMed] [Google Scholar]
- 39.Ryder MI. Histological and ultrastructural characteristics of the periodontal syndrome in the rice rat. I. General light microscopic observations and ultrastructural observations of initial inflammatory changes. J. Periodontal Res. 1980;15:502–515. doi: 10.1111/j.1600-0765.1980.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 40.Gotcher JE, Jee WS. The progress of the periodontal syndrome in the rice rat. II. The effects of a diphosphonate on the periodontium. J. Periodontal Res. 1981;16:441–455. doi: 10.1111/j.1600-0765.1981.tb00995.x. [DOI] [PubMed] [Google Scholar]
- 41.Messer JG, Jiron JM, Chen HY, Castillo EJ, Mendieta Calle JL, Reinhard MK, et al. Prevalence of food impaction-induced periodontitis in conventionally housed marsh rice rats (Oryzomys palustris) Comp. Med. 2017;67:43–50. [PMC free article] [PubMed] [Google Scholar]
- 42.Ullman-Cullere MH, Foltz CJ. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab. Anim. Sci. 1999;49:319–323. [PubMed] [Google Scholar]
- 43.Klaassen CD, Casarett LJ, Doull J. Casarett and Doull's Toxicology: The Basic Science of Poisons. McGraw-Hill Education; New York: 2013. [Google Scholar]
- 44.Gasser JA, Ingold P, Venturiere A, Shen V, Green JR. Long-term protective effects of zoledronic acid on cancellous and cortical bone in the ovariectomized rat. J. Bone Miner. Res. 2008;23:544–551. doi: 10.1359/jbmr.071207. [DOI] [PubMed] [Google Scholar]
- 45.Seedor JG, Quartuccio HA, Thompson DD. The bisphosphonate alendronate (MK-217 inhibits bone loss due to ovariectomy in rats. J. Bone Miner. Res. 1991;6:339–346. doi: 10.1002/jbmr.5650060405. [DOI] [PubMed] [Google Scholar]
- 46.Yamashita J, Koi K, Yang DY, McCauley LK. Effect of zoledronate on oral wound healing in rats. Clin. Cancer Res. 2011;17:1405–1414. doi: 10.1158/1078-0432.CCR-10-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuroshima S, Yamashita J. Chemotherapeutic and antiresorptive combination therapy suppressed lymphangiogenesis and induced osteonecrosis of the jaw-like lesions in mice. Bone. 2013;56:101–109. doi: 10.1016/j.bone.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 48.Ke HZ, Qi H, Chidsey-Frink KL, Crawford DT, Thompson DD. Lasofoxifene (CP-336,156) protects against the age-related changes in bone mass, bone strength, and total serum cholesterol in intact aged male rats. J. Bone Miner. Res. 2001;16:765–773. doi: 10.1359/jbmr.2001.16.4.765. [DOI] [PubMed] [Google Scholar]
- 49.Hansen T, Kunkel M, Weber A, James KC. Osteonecrosis of the jaws in patients treated with bisphosphonates - histomorphologic analysis in comparison with infected osteoradionecrosis. J. Oral Pathol. Med. 2006;35:155–160. doi: 10.1111/j.1600-0714.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 50.Reid IR, Cornish J. Epidemiology and pathogenesis of osteonecrosis of the jaw. Nat. Rev. Rheumatol. 2011;29:90–96. doi: 10.1038/nrrheum.2011.181. [DOI] [PubMed] [Google Scholar]
- 51.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J. Oral Maxillofac. Surg. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Ruggiero SL. Bisphosphonate-related osteonecrosis of the jaw: an overview. Ann. N. Y. Acad. Sci. 2011;1218:38–46. doi: 10.1111/j.1749-6632.2010.05768.x. [DOI] [PubMed] [Google Scholar]
- 53.de Molon RS, Cheong S, Bezouglaia O, Dry SM, Pirih F, Cirelli JA, et al. Spontaneous osteonecrosis of the jaws in the maxilla of mice on antiresorptive treatment: a novel ONJ mouse model. Bone. 2014;68:11–19. doi: 10.1016/j.bone.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song YL. The causes and treatment strategies of molar food impaction after implant restoration. Zhonghua Kou Qiang Yi Xue Za Zhi. 2016;51:7–9. doi: 10.3760/cma.j.issn.1002-0098.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Du H, Gao M, Qi C, Liu S, Lin Y. Drug-induced gingival hyperplasia and scaffolds: they may be valuable for horizontal food impaction. Med. Hypotheses. 2010;74:984–985. doi: 10.1016/j.mehy.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 56.Masarachia P, Weinreb M, Balena R, Rodan GA. Comparison of the distribution of 3H-alendronate and 3H-etidronate in rat and mouse bones. Bone. 1996;19:281–290. doi: 10.1016/8756-3282(96)00182-2. [DOI] [PubMed] [Google Scholar]
- 57.Ibrahim A, Scher N, Williams G, Sridhara R, Li N, Chen G, et al. Approval summary for zoledronic acid for treatment of multiple myeloma and cancer bone metastases. Clin. Cancer Res. 2003;9:2394–2399. [PubMed] [Google Scholar]
- 58.Wardley A, Davidson N, Barrett-Lee P, Hong A, Mansi J, Dodwell D, et al. Zoledronic acid significantly improves pain scores and quality of life in breast cancer patients with bone metastases: a randomised, crossover study of community vs hospital bisphosphonate administration. Br. J. Cancer. 2005;92:1869–1876. doi: 10.1038/sj.bjc.6602551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Facchini G, Caraglia M, Santini D, Nasti G, Ottaiano A, Striano S, et al. The clinical response on bone metastasis from breast and lung cancer during treatment with zoledronic acid is inversely correlated to skeletal related events (SRE) J. Exp. Clin. Cancer Res. 2007;26:307–312. [PubMed] [Google Scholar]
- 60.Zarogoulidis K, Boutsikou E, Zarogoulidis P, Eleftheriadou E, Kontakiotis T, Lithoxopoulou H, et al. The impact of zoledronic acid therapy in survival of lung cancer patients with bone metastasis. Int. J. Cancer. 2009;125:1705–1709. doi: 10.1002/ijc.24470. [DOI] [PubMed] [Google Scholar]
- 61.Compston J. Pathophysiology of atypical femoral fractures and osteonecrosis of the jaw. Osteoporos. Int. 2011;22:2951–2961. doi: 10.1007/s00198-011-1804-x. [DOI] [PubMed] [Google Scholar]
- 62.Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2010;25:2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.