Abstract
The known antibiotic and cytotoxic compounds griseorhodin A (1) and griseorhodin C (2) were produced in solid culture by Streptomyces puniceus AB10, which was isolated from the leaf-cutter ant Acromyrmex rugosus rugosus. Their absolute configurations were unambiguously established as 6S,6aR,7S,8S and 6R,6aR,7S,8R, respectively, using vibrational circular dichroism (VCD) and density functional theory (DFT) calculations.
Keywords: Streptomyces puniceus, Acromyrmex rugosus rugosus, Rubromycin, Griseorhodins, Vibrational circular dichroism
Graphical Abstract
1. Introduction
Griseorhodins A (1) and C (2) belong to the rubromycin family and represent an interesting group of polyketide pigments with wide spectrum of biological activities. They possess a naphthoquinone ring and an isocoumarin ring connected through a 5,6-spiroketal system.1
These compounds have been reported from actinobacteria of the genera Streptomyces, Dactylosporangium, and Actinoplanes.2–5 Griseorhodins A and C have been described with antimicrobial activity against B. subtilis, S. aureus, P. notatum and others; and with cytotoxic activity against cells derived from human carcinoma of nasopharynx (KB).6,7 Griseorhodin A also inhibits human leukocyte elastase at micromolar concentrations.8 Both griseorhodins A and C inhibit the moloney murine leukemia virus reverse transcriptase (M-MLV RT) with IC50 values of 7.38 and 9.38 μM, respectively.9 Compounds 1 and 2 show also similar levels of activity against human immunodeficiency virus type 1 reverse transcriptase (HIV-1 RT), as well as inhibition of the telomerase enzyme (IC50 = 6–12 μM).9
Despite their biological potential, the relative and absolute configurations of most of the rubromycin family members have yet to be assigned. As part of our efforts to identify biologically active natural products from bacterial symbionts of social insects,10 we have selected the actinobacterium Streptomyces puniceus AB10, isolated from leaf-cutter ants Acromyrmex rugosus rugosus for isolation of small molecules. Griseorhodin A had its absolute configuration previously determined by time-dependent density functional theory calculations (TD-DFT) of electronic circular dichroism (ECD) spectra as 6S,6aS,7S,8S.8 As unambiguous stereochemical information is very important for biological and biosynthetic studies, in this work we decided to reinvestigate the absolute stereochemistry of griseorhodin A and also assign that of griseorhodin C, for the first time, using vibrational circular dichroism (VCD) spectroscopy. VCD is a well-established and reliable method for stereochemical studies of natural molecules directly in solution-phase.11
2. Results and discussion
Fractionation of S. puniceus AB10 extract led to the isolation of griseorhodins A (1) and C (2). The planar structure of griseorhodin C was determined by comparing UV, NMR and MS data with that reported in the literature.3 The 1 and 2 D NMR analyses were carried out in both DMSO-d6 and Acetone-d6 (See Figs. S1, S4–S10). NOESY experiments in acetone-d6 showed NOE correlations between H-6 and H-7, H-7 and H-8, H-8 and H-9 as well as H-9 and H-10 using NOESY-1D and NOEDIFF experiments (See Figs. S11 and S12). The 1H NMR spectrum showed broad singlets assigned to hydrogens H-7 (δH 4.64) and H-8 (δH 4.78) (Fig. S1). So, they could be in the same plane in equatorial position in the ring of six members of spiroketal moiety. These results allowed us to determine its relative configuration as 6R*,6aR*,7S*,8R*. Griseorhodin A was identified by comparing its 1H NMR and MS data with that of observed in the 1H NMR spectra of these two compounds are the chemical shifts of H-7 and H-8 [δH 4.36 and 4.40 for 1 and δH 4.64 and 4.78 for 2, respectively]. Such differences arise from the fact that 1 presents an oxirane between C-7 and C-8 while 2 is a dihydroxy derivative. The key NOE correlations of griseorhodin A and C are showed in Fig. 1.
Fig. 1.
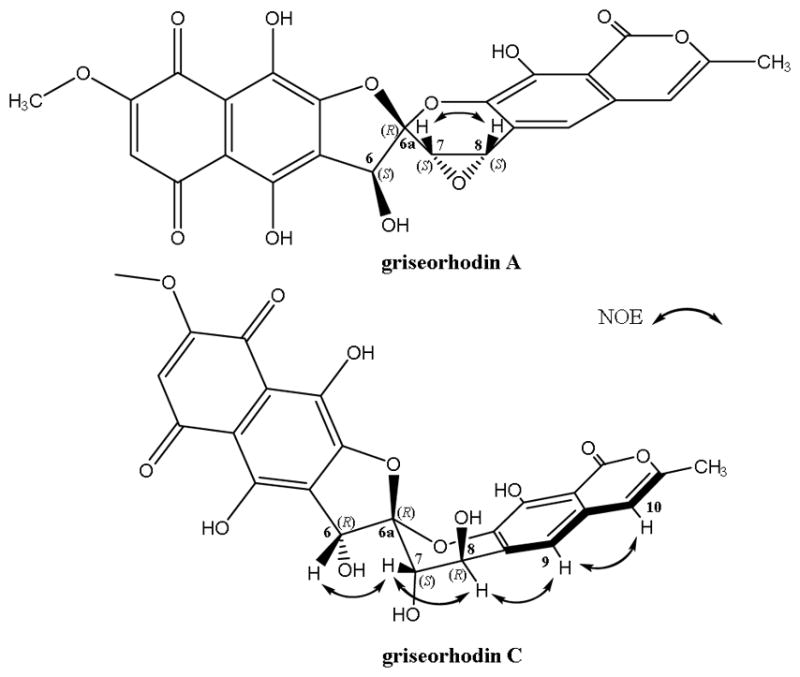
Key NOE correlations of griseorhodin A (1) and C (2)
Griseorhodin A (1) was reported previously with the configuration 6S,6aS,7S,8S 8 based on TD-DFT calculations of its ECD spectrum. Our experimental and theoretical VCD investigation on this molecule (Fig. S16) revealed exactly the same spatial arrangement of the atoms, however, we identified a misassignment of the CIP (Cahn-Ingold-Prelog sequence rules) priority of the stereogenic center at C-6a. Therefore, the stereodescriptors of griseorhodin A should be corrected to 6S,6aR,7S,8S.
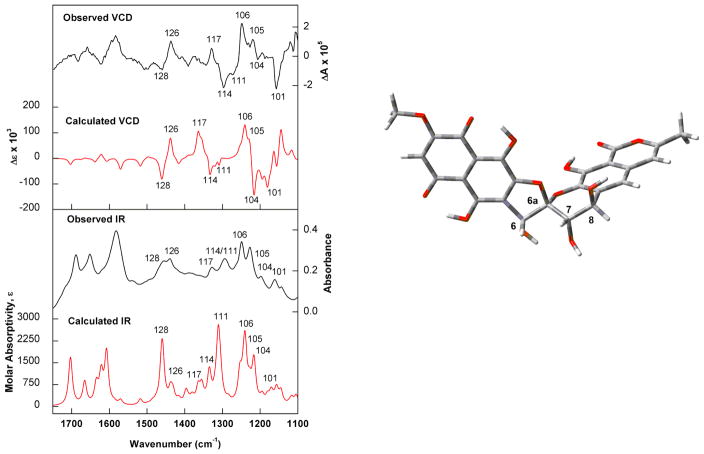
Experimental and calculated IR and VCD spectra of griseorhodin C were compared in order to assign its absolute configuration. Due to the polycyclic nature of the molecule a single conformer was identified after molecular mechanics conformational search and B3LYP/PCM(DMSO)/6-31G(d) geometry optimization steps. The good agreement between observed and DFT-predicted IR/VCD spectra led to the assignment of compound 2 as (−)-(6R,6aR,7S,8R) (Fig. 2).
Fig. 2.
(Left) Comparison of the observed IR and VCD spectra of (−)-2 with the calculated (B3LYP/PCM(DMSO)/6-31G(d)) IR and VCD spectra of the lowest-energy conformer identified for (6R,6aR,7S,8R)-griseorhodin C (right). Numbers on spectra represent selected vibrational modes.
The combination of experimental and DFT-predicted IR and VCD spectra led to assignment of the absolute configurations of griseorhodins A (1) and C (2) as 6S,6aR,7S,8S and 6R,6aR,7S,8R, respectively. Even though the spatial arrangement of the atoms of griseorhodin A (1) was the same as that reported previously,8 in this work we identified a misassignment of the CIP priority of the stereogenic center at 5,6-spiroketal system. Interestingly, the griseorhodins A and C showed a different configuration at C-6. Other related compounds, such as heliquinomycin and γ-rubromycin, have been reported12 from Streptomyces sp. with opposite configuration at their spiro centers (C-6a) (R and S configuration respectively), showing the complex biosynthesis of this family of secondary metabolites.
Highlights.
Streptomyces puniceus AB10 is associated with Acromyrmex rugosus rugosus ants
Actinobacteria produced complex antibiotic polyketides
Absolute configurations were determined using VCD and DFT calculations
Acknowledgments
The authors acknowledge the financial support from São Paulo Research Foundation (FAPESP) grants 2013/50954-0, 2014/14095-6, 2015/07089-2, 2014/25222-9, and 2015/01001-6; Fogarty International Center, National Institutes of Health (FIC-NIH) grant U19TW009872; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). This research was also supported by resources supplied by the Centre for Scientific Computing (NCC/GridUNESP) of the São Paulo State University (UNESP).
Footnotes
Supplementary data
Supplementary data associated with article can be found in the online version at http://.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Atkinson DJ, Brimble MA. Nat Prod Rep. 2015;32:811–840. doi: 10.1039/c4np00153b. [DOI] [PubMed] [Google Scholar]
- 2.Panzone G, Trani A, Ferrari P, Gastaldo L, Colombo L. J Antibiot. 1997;50:665–670. doi: 10.7164/antibiotics.50.665. [DOI] [PubMed] [Google Scholar]
- 3.Eckardt K, Tresselt D, Ihn W. J Antibiot. 1978;31:970–973. doi: 10.7164/antibiotics.31.970. [DOI] [PubMed] [Google Scholar]
- 4.Coronell C, Pagani H, Bardone MR, Lancini GC. J Antibiot. 1974;27:161–168. doi: 10.7164/antibiotics.27.161. [DOI] [PubMed] [Google Scholar]
- 5.JP57032286-A, JP88023996-B, JP1474218-C. 1982.
- 6.Stroshane RM, Chan JA, Rubalcaba EA, Garretson AL, Aszalos AA, Roller PP. J Antibiot. 1979;32:197–204. doi: 10.7164/antibiotics.32.197. [DOI] [PubMed] [Google Scholar]
- 7.Suetsuna K, Seino A, Kudo T, Osajima Y. Agric Biol Chem. 1989;53:581–583. [Google Scholar]
- 8.Yunt Z, Reinhardt K, Li A, Engeser M, Dahse HM, Gutschow M, Bruhn T, Bringmann G, Piel J. J Am Chem Soc. 2009;131:2297–2305. doi: 10.1021/ja807827k. [DOI] [PubMed] [Google Scholar]
- 9.Ueno T, Takahashi H, Oda M, Mizunuma M, Yokoyama A, Goto Y, Mizushina Y, Sakaguchi K, Hayashi H. Biochemistry. 2000;39:5995–6002. doi: 10.1021/bi992661i. [DOI] [PubMed] [Google Scholar]
- 10.Pupo MT, Currie CR, Clardy J. J Braz Chem Soc. 2017;28:393–401. [Google Scholar]
- 11.Batista JM, Blanch EW, Bolzani VD. Nat Prod Rep. 2015;32:1280–1302. doi: 10.1039/c5np00027k. [DOI] [PubMed] [Google Scholar]
- 12.Bringmann G, Kraus J, Schmitt U, Puder C, Zeeck A. Eur J Org Chem. 2000:2729–2734. [Google Scholar]