Abstract
Introduction
Dabigatran, as compared with warfarin, was associated with lower rates of stroke and systemic embolism with similar rates of major hemorrhage. But it has a significantly higher risk of gastrointestinal bleeding (GIB). There are limited data on how to prevent GIB from dabigatran and what are the risk factors.
Methods
We performed a retrospective cohort study of patients with atrial fibrillation who have ever taken dabigatran for thromboprophylaxis from October 2010 to February 2013.
Results
A total of 247 patients were identified. There were 10 (4%) patients who developed GIB (6 (6.5%) in PPI/H2RA users vs 4 (2.6%) in non‐PPI/H2RA users; P = .184). History of GIB within 1 year prior to dabigatran initiation and HAS‐BLED score ≥3 are independent risk factors for GIB, with odds ratio of 25.14 (95% CI, 2.85‐221.47; P < .01) and 5.85 (95% CI, 1.31‐26.15; P = .021), respectively.
Conclusion
In this real‐world cohort, PPI/H2RA use was not associated with reduced GIB events. HAS‐BLED score ≥3 and prior history of GIB within 1 year are independent risk factors for GIB among dabigatran users.
Keywords: atrial fibrillation, dabigatran, gastrointestinal bleeding, HAS‐BLED
1. INTRODUCTION
Dabigatran, a direct thrombin inhibitor, is a direct‐acting oral anticoagulant (DOAC) that was initially approved by the Food and Drug Administration (FDA) for stroke prevention in nonvalvular atrial fibrillation in October 2010.1 Dosage approved by FDA was 150 mg twice daily in patients with creatinine clearance (CrCl) of more than 30 mL/min/1.73 m2 and 75 mg twice daily for patients with CrCl of 15‐30 mL/min/1.73 m2. One of the advantages of dabigatran over warfarin is that there is no need for drug level or INR monitoring due to predictable pharmacokinetics and pharmacodynamics. Dabigatran, as compared with warfarin, was associated with lower rates of stroke and systemic embolism with similar rates of major hemorrhage.2 Dabigatran has less intracranial hemorrhage, but concerns have been raised regarding a significantly higher risk of gastrointestinal bleeding (GIB) with dabigatran as compared to warfarin with relative risk of 1.5.2, 3 Meta‐analysis of 5 randomized controlled trials confirmed a higher GIB in dabigatran compared to warfarin (relative risk, 1.51; 95% CI, 1.23‐1.84).3 Recent meta‐analysis of observational studies also showed 18% increased risk of GIB in dabigatran 150 mg twice daily compared to warfarin.4
There are 2 possible explanations regarding why dabigatran caused more GIB than warfarin. First, dabigatran etexilate, which is a prodrug, has limited absorption from GI tract into portal vein system with only 3‐7% bioavailability.5 This low bioavailability is partly because a P‐glycoprotein transport system actively pumps dabigatran etexilate from enterocytes back into the GI tract,6 which is later hydrolyzed by enterocyte esterase into active form and causes local anticoagulant effect. Secondly, dabigatran etexilate absorption is dependent on an acid environment; therefore, it is formulated together with tartaric acid to reduce its variability. This acidity may partly explain the increased incidence of dyspeptic symptoms and the increased risk of GIB. Rivaroxaban and edoxaban also have significantly higher GIB rates when compared to warfarin, but the mechanisms are not well understood.
Dabigatran increased risk of both upper GIB and lower GIB. From the RE‐LY trial, 53% of major GIB occurred in the lower GI tract and 47% occurred in the upper GI tract.7 There are limited data regarding effective strategy to reduce GIB among dabigatran users. Proton pump inhibitor or H2‐receptor antagonist (PPI/H2RA) has been shown to reduce upper GIB in patients on warfarin, aspirin, or NSAIDs.8 Therefore, we hypothesized that PPI/H2RA may reduce a significant portion of GIB events from dabigatran by protecting stomach and duodenal mucosa from local anticoagulation effect, irritation from tartaric acid, and concomitant use of aspirin or NSAIDs. The aim of this study was to identify risk factors associated with increased GIB in patients with atrial fibrillation taking dabigatran and whether PPI/H2RA can prevent dabigatran‐related GIB in a real‐world observational cohort.
2. METHODS
This is a single‐center retrospective cohort study of patients with atrial fibrillation who have ever taken dabigatran for thromboprophylaxis. We identified atrial fibrillation patients using ICD‐9 coding at University Medical Center, Texas Tech University Health Sciences Center, Lubbock, Texas, from October 1, 2010, to February 1, 2013. We included patients with atrial fibrillation, aged between 21 and 89 years, who were ever prescribed dabigatran. Exclusion criteria included history of thrombocytopenia or coagulopathy. Dabigatran dosage was 150 mg twice a day if CrCl > 30 mL/min/1.73 m2 and 75 mg twice a day if CrCl = 15‐30 mL/min/1.73 m2.
We collected baseline characteristics, GIB events, length of stay, amount of blood transfusion, serious complications from GIB (shock, acute renal failure, death), ischemic stroke, hemorrhagic stroke, and HAS‐BLED score. The HAS‐BLED score is an acronym for hypertension (uncontrolled, >160 mm Hg systolic), abnormal renal/liver function (1 point each for presence of renal or liver impairment, maximum 2 points), stroke (previous history), bleeding history or predisposition (anemia), labile INR (ie, therapeutic time in range <60%), elderly (older than 65 years), and use of drugs/alcohol (antiplatelet agents, nonsteroidal anti‐inflammatory drugs; 1 point for drugs plus 1 point for alcohol excess).9 Abnormal liver function was defined as presence of cirrhosis or bilirubin >2 times normal or AST/ALT/ALP >3 times normal. Abnormal renal function in HAS‐BLED score was defined as presence of chronic dialysis, renal transplantation, or serum creatinine >200 mmol/L (>2.26 mg/dL).
The primary outcome was GIB defined as bleeding that occurs in any part of the gastrointestinal tract which includes esophagus, stomach, small intestine, large intestine, rectum, and anus.
We used simple descriptive statistics to describe baseline characteristics, incidence of GIB, and ischemic stroke. We used Pearson chi‐square test, Fisher's exact test, or t test to compare differences in baseline characteristics, GIB, and ischemic stroke rate between patients with PPI/H2RA and without PPI/H2RA. Fisher's exact test was used over chi‐square test when more than 20% cells had expected count <5. We used multivariate binary logistic regression to identify independent risk factors for GIB. All analyses were performed using SPSS Windows version 15.0 (SPSS Inc., Chicago, IL, USA). The Texas Tech University Health Sciences Center Lubbock/Odessa Institutional Review Board approved the study protocol.
3. RESULTS
A total of 247 patients were identified between October 2010 and February 2013. Mean age was 72 years, and 48.2% were female. Baseline characteristics between groups were similar, except for history of GU/gastritis/GERD, history of stroke, alcohol abuse, and HAS‐BLED score which were higher in the PPI/H2RA group (Table 1). Mean HAS‐BLED score in PPI/H2RA users was 2.1, which was significantly higher than that of non‐PPI/H2RA users (1.6).
Table 1.
Baseline characteristics of the study participants, according to PPI/H2RA use
| Characteristics | All patients N = 247(%) | PPI/H2RA use | P value | |
|---|---|---|---|---|
| Yes, n = 93 (%) | No, n = 154 (%) | |||
| Mean age (years) | 72.2 | 72.0 | 72.3 | .884 |
| Female | 119 (48.2) | 45 (48.4) | 74 (48.1) | .959 |
| Past medical history | ||||
| Diabetes mellitus | 83 (33.7) | 31 (33.7) | 52 (33.8) | .991 |
| Hypertension | 216 (87.4) | 84 (90.3) | 132 (85.7) | .290 |
| History of GIB prior to dabigatran initiation | 11 (4.4) | 6 (6.5) | 5 (3.2) | .330 |
| Within 1 year | 5 (2.0) | 2 (2.2) | 3 (1.9) | |
| >1 year | 6 (2.4) | 4 (4.3) | 2 (1.3) | |
| History of GU/gastritis/GERD | 117 (47.4) | 53 (57.0) | 64 (41.6) | .019b |
| History of stroke | 41 (16.6) | 22 (23.7) | 19 (12.3) | .021b |
| Coronary artery disease | 123 (49.8) | 50 (53.8) | 73 (47.4) | .333 |
| At the time of dabigatran initiation | ||||
| Smoking | 38 (15.6) | 16 (17.4) | 22 (14.5) | .542 |
| Alcohol use | 18 (7.4) | 11 (12.0) | 7 (4.6) | .035b |
| Corticosteroid use | 23 (9.3) | 11 (11.8) | 12 (7.8) | .290 |
| Antiplatelet or NSAIDs use | 137 (55.5) | 58 (62.4) | 79 (51.3) | .090 |
| Cr (mg/dL), CrCl (mL/min/1.73 m2) | 1.1, 67.8 | 1.07, 70.9 | 1.12, 65.9 | .500, .153 |
| Abnormal liver functiona | 6 (2.4) | 3 (3.3) | 3 (2.0) | .675 |
| Mean HAS‐BLED score | 1.8 | 2.1 | 1.6 | <.01b |
GIB, gastrointestinal bleeding; GU, gastric ulcer; EGD, esophagogastroduodenoscopy; GERD, gastroesophageal reflux disease; NSAIDs, nonsteroidal anti‐inflammatory drugs; Cr, creatinine; CrCl, creatinine clearance.
Cirrhosis or bilirubin >2× normal or AST/ALT/ALP >3× normal.
P value <.05.
There were 10 of 247 (4.0%) patients who developed GIB, and all of them were hospitalized. Among 10 GIB cases, 4 were upper GIB, 5 were lower GIB, and 1 was unknown due to refused endoscopy/colonoscopy. Median time to first GIB event was 204 days after the start of dabigatran use (minimum 39 days, maximum 769 days). Five patients required transfusion of 2 or more units of PRBCs, and 3 patients developed serious complications (shock, acute kidney injury, endotracheal intubation, or death).
Rate of GIB was 6.5% in PPI/H2RA users, as compared with 2.6% with non‐PPI/H2RA users (P = .184) (Table 2). After adjustment for baseline differences (history of GU/gastritis/GERD, history of stroke, alcohol use, and mean HAS‐BLED score), PPI/H2RA use was still not associated with reduction in the odds of GIB (adjusted odds ratio, 1.34; 95% CI, 0.30‐6.10; P = .70). Severity and complications from GIB comparing between the 2 groups were not different. There was no difference in prevalence of hemorrhagic or ischemic stroke between the 2 groups.
Table 2.
GIB and stroke while on dabigatran, according to PPI/H2RA use
| Event | All patients N = 247 (%) | PPI/H2RA use | P value | |
|---|---|---|---|---|
| Yes, n = 93 (%) | No, n = 154 (%) | |||
| GIB | 10 (4.0) | 6 (6.5) | 4 (2.6) | .184 |
| Mean hospital LOS (days) | 3.2 | 3.0 | 3.5 | .804 |
| Required ≥2 units of PRBCs | 5 | 2 | 3 | .483 |
| Serious complicationsa | 3 | 2 | 1 | 1.000 |
| Hemorrhagic stroke | 2 (0.8) | 2 (2.2) | 0 (0.0) | .141 |
| Ischemic stroke | 6 (2.4) | 0 (0.0) | 6 (3.9) | .086 |
GIB, gastrointestinal bleeding; PRBCs, packed red blood cells; LOS, length of stay.
Serious complications include shock, acute kidney injury, endotracheal intubation, or death.
Univariate analysis demonstrated that history of GIB within 1 year prior to initiation of dabigatran, corticosteroid use, and a high HAS‐BLED score were risk factors for GIB (Table 3). Rate of GIB was 60% in patients with prior history of GIB within 1 year, as compared with 2.8% in the group without history of GIB within 1 year (odds ratio, 50.36; 95% CI, 7.23‐350.67; P < .01). Rate of GIB was 10.3% in corticosteroid users, as compared with 3.1% in patients who did not use corticosteroids (odds ratio, 4.65; 95% CI, 1.12‐19.39; P = .035). Higher HAS‐BLED score was also associated with higher risk of GIB. According to the ROC curves from our data, a HAS‐BLED score cutoff of 3 or more was an optimal cutoff value for predicting risk of GIB with 70% sensitivity and 80% specificity (AUC = 0.722, P = .018) (Figure 1). So, we used HAS‐BLED score of ≥3 in univariate analysis which showed GIB rate of 12.7%, as compared to 1.6% in the lower HAS‐BLED score group (odds ratio, 9.19; 95% CI, 2.29‐36.85; P < .01).
Table 3.
Factors associated with increased GIB events (univariate analysis)
| Factors | All patients N = 247 (%) | GIB | Odds ratio (95% CI) | P value | |
|---|---|---|---|---|---|
| Yes, n = 10 (%) | No, n = 237 (%) | ||||
| Mean age | 72.2 | 69.8 | 72.3 | 0.98 (0.94‐1.03) | .519 |
| Female | 119 (48.2) | 5 (50.0) | 114 (48.1) | 1.08 (0.30‐3.82) | 1.000 |
| Past medical history | |||||
| Diabetes mellitus | 83 (33.7) | 4 (40.0) | 79 (33.5) | 0.76 (0.21‐2.75) | .737 |
| Hypertension | 216 (87.4) | 9 (90.0) | 207 (87.3) | 0.77 (0.09‐6.27) | 1.000 |
| History of GIB prior to dabigatran initiation | 11 (4.4) | 3 (30.0) | 8 (3.4) | 12.27 (2.67‐56.38) | <.01a |
| Within 1 year | 5 (2.0) | 3 (30.0) | 2 (0.8) | 50.36 (7.23‐350.67) | <.01a |
| >1 year | 6 (2.4) | 0 (0.0) | 6 (2.6) | — | 1.000 |
| History of GU/gastritis/GERD | 117 (47.4) | 5 (50.0) | 112 (47.3) | 1.12 (0.32‐3.96) | 1.000 |
| History of stroke | 41 (16.6) | 3 (30.0) | 38 (16.0) | 2.24 (0.56‐9.07) | .220 |
| Coronary artery disease | 123 (49.8) | 5 (50.0) | 118 (49.8) | 0.99 (0.28‐3.52) | 1.000 |
| At the time of dabigatran initiation | |||||
| Smoking | 38 (15.6) | 2 (20.0) | 36 (15.4) | 0.73 (0.15‐3.56) | .657 |
| Alcohol abuse | 18 (7.4) | 2 (20.0) | 16 (6.9) | 3.39 (0.66‐17.32) | .164 |
| Corticosteroid use | 23 (9.3) | 3 (30.0) | 20 (8.4) | 4.65 (1.12‐19.39) | .035a |
| Antiplatelet or NSAIDs use | 137 (55.5) | 9 (90.0) | 128 (54.0) | 7.66 (0.96‐61.45) | .055 |
| CrCl (mL/min/1.73 m2) | 67.8 | 79.6 | 67.3 | 1.01 (0.99‐1.03) | .149 |
| Abnormal liver function | 6 (2.4) | 0 (0.0) | 6 (2.6) | — | 1.000 |
| HAS‐BLED score | 1.8 | 2.70 | 1.76 | 2.21 (1.24‐3.95) | <.01a |
| HAS‐BLED ≥ 3 | 55 (22.3) | 7 (70.0) | 48 (20.3) | 9.19 (2.29‐36.85) | <.01a |
GIB, gastrointestinal bleeding; GU, gastric ulcer; EGD, esophagogastroduodenoscopy; GERD, gastroesophageal reflux disease; NSAIDs, nonsteroidal anti‐inflammatory drugs; Cr, creatinine; CrCl, creatinine clearance.
P value <.05.
Figure 1.
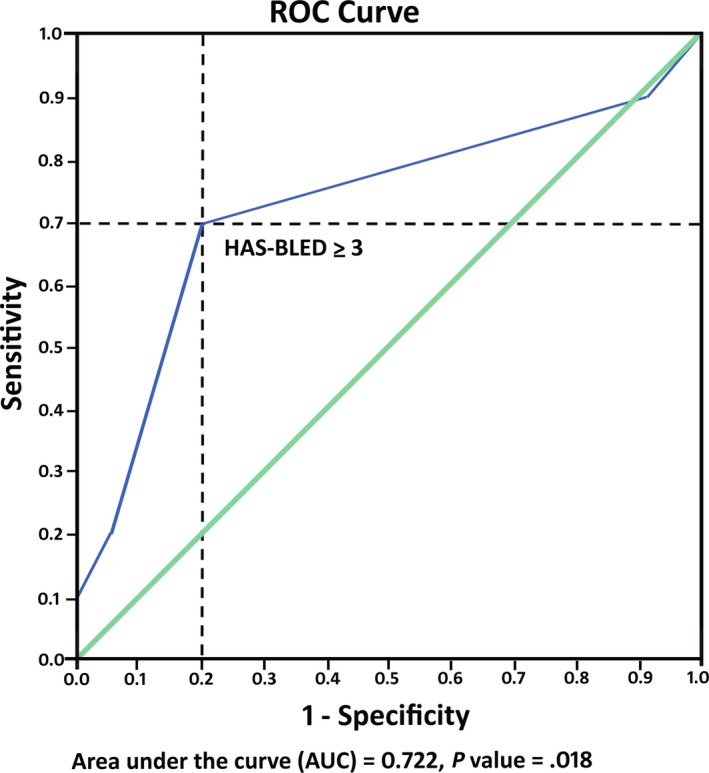
Receiver operating characteristic (ROC) curves for HAS‐BLED score predicting increased risk of gastrointestinal bleeding with dabigatran
After adjusted with multivariate analysis, corticosteroid use was not associated with increased GIB (odds ratio, 4.30; 95% CI, 0.81‐22.79; P = .087). Only history of GIB within 1 year prior to dabigatran initiation and HAS‐BLED score ≥3 were still associated with increased GIB, with odds ratio of 25.14 (95% CI, 2.85‐221.47; P < .01) and 5.85 (95% CI, 1.31‐26.15; P = .021), respectively (Table 4).
Table 4.
Multivariate analysis of factors associated with increased gastrointestinal bleeding (GIB)
| Factors | Odds ratio (95% CI) | P value |
|---|---|---|
| History of GIB within 1 year prior to dabigatran initiation | 25.14 (2.85‐221.47) | <.01a |
| Corticosteroid use | 4.30 (0.81‐22.79) | .087 |
| HAS‐BLED ≥ 3 | 5.85 (1.31‐26.15) | .021a |
P value <.05.
4. DISCUSSION
A large trial and a meta‐analysis of randomized controlled trials have consistently shown that dabigatran increased the risk of GIB compared to warfarin.2, 3 One may ask, given this inherited problem of higher GIB risk, why dabigatran is still being used. There are a few differences between dabigatran and other DOACs: (i) Dabigatran is the only DOAC that has FDA‐approved reversal agent, idarucizumab; (ii) it is dialyzable, while apixaban, edoxaban, and rivaroxaban are not; (iii) it may be used in liver disease because 80% of total clearance is from kidney, while apixaban, edoxaban, and rivaroxaban should be avoided in patients with Child‐Pugh B or C; and (iv) only apixaban and dabigatran met superiority in preventing stroke or systemic embolism compared to warfarin, notwithstanding that all trials were designed as noninferiority studies.
In our study, GIB rate in dabigatran users was 4.0%, which was higher compared to the 1.51% in the RE‐LY trail2 and 1.54% in an additional follow‐up study,10 but similar to the 3.1% in other studies.11, 12, 13 Our study consisted of high‐risk populations that would have been excluded from the RE‐LY study,2 hence reflecting a “real‐world” practice. These high‐risk patients had GIB within 1 year prior to dabigatran initiation (2.0%), alcohol use (7.4%), CrCl <30 mL/min/1.73 m2 (4.0%), and abnormal liver function (2.4%).
PPI/H2RA did not reduce GIB events in our study. This is in contrast to the Chan et al14 study which showed that use of PPI/H2RA was associated with reduced risk of GIB, with the caveat that risk reduction was significant only for upper GIB and in patients with prior history of peptic ulcers or GIB. The differences in the results are possibly due to (i) smaller sample size in our study or (ii) because our populations have significantly higher risk of GIB. Prior history of GIB or peptic ulcers was 51.8% in our study as compared to 13.9% in Chan's study. We had 7.4% alcohol use and 2.4% abnormal liver function patients as compared to none mentioned in Chan's study. Renal disease in Chan's study was 5.1%—however, GFR was not specified—while we had 4.0% of patients with CrCl < 30 mL/min/1.73 m2; and (iii) our primary outcome was upper and lower GIB combined, with the high proportion of lower GIB of 50%. To elucidate this question, a large randomized controlled trial is needed. In the absence of strong data, PPI/H2RA use in high–bleeding risk patients should be considered on a case‐by‐case basis. Concomitant use of PPI/H2RA and dabigatran in our study was not associated with increased risk of ischemic stroke. This finding is consistent with the prior study demonstrating that although PPI can decrease bioavailability of dabigatran by 15‐28%, it did not significantly reduce the overall clinical efficacy; hence, dose adjustment is not warranted.6, 15
Multivariate analysis showed that HAS‐BLED score ≥3 and prior history of GIB within 1 year significantly increased the risk of GIB with odds ratio of 5.85 and 25.14, respectively. HAS‐BLED has been widely validated for risk of major bleeding in AF and non‐AF patients receiving warfarin as well as aspirin therapy.9, 16, 17 However, data regarding HAS‐BLED score in predicting GIB in patients taking DOACs are limited. The Danish National Patient Registry used HAS‐BLED score to evaluate intracranial hemorrhage, not GIB, in patients taking DOACs.18 One study showed that HAS‐BLED score offers predictive capacity for overall bleeding in mixed population of warfarin and fixed‐dose ximelagatran users.19 Another retrospective study found a modified HAS‐BLED score was not predictive of risk of major bleeding in patients taking dabigatran or rivaroxaban.20 Most of the studies that specifically evaluate risks for GIB from dabigatran did not collect or elaborate on HAS‐BLED score data.2, 14 There is 1 dabigatran study that described HAS‐BLED score in baseline characteristics with HAS‐BLED score, but it did not compare or analyze HAS‐BLED score between the GIB group and the no‐GIB group.21 We are the first to use HAS‐BLED score to specifically look at GIB among dabigatran users, and to demonstrate it as an independent risk factor for GIB. Prior history of GIB within 1 year is also demonstrated here as an independent risk factor for GIB, which is consistent with other dabigatran14 and rivaroxaban studies.22
NSAIDs or aspirin use has been shown to increase the risk of GIB in a few studies predating the dabigatran era.23, 24, 25 Although NSAIDs or aspirin use alone did not increase the risk of GIB in our study, it is one of the factors incorporated in HAS‐BLED score and thus should be taken into account when starting dabigatran. One study showed corticosteroids as a risk factor for GIB or perforation.26 In our study, concurrent steroid use demonstrated a trend toward increased risk of GIB, although not statistically significant.
Modifiable risk factors should be addressed prior to initiation of dabigatran, such as blood pressure control, aspirin, NSAIDs, corticosteroids, or alcohol use. While dabigatran, edoxaban, and rivaroxaban carry a higher GIB risk when compared to warfarin,27 apixaban and lower dosage of dabigatran (110 mg twice daily) do not.28 A reduced dabigatran dosage of 110 mg twice daily has been shown to be as effective as warfarin in preventing stroke or systemic embolism with similar GIB and less major bleeding compared to warfarin.10 If risk factors for GIB persist after the modifiable factors have been addressed, one may consider to switch anticoagulation options to apixaban, lower dosage of dabigatran (110 mg twice daily), or warfarin. Unfortunately, dosage of dabigatran 110 mg twice daily was not approved in the United States of America.
Limitations of this study include selection bias, which is the nature of retrospective review studies. We had a small sample size, therefore possibly leading to inadequate statistical power. Although our study is not a randomized controlled trial, it provided useful information regarding how to choose the right patient for the right medicine.
5. CONCLUSIONS
In this real‐world practice observation, PPI/H2RA use was not associated with reduced GIB events. HAS‐BLED score ≥3 and prior history of GIB within 1 year are independent risk factors for GIB in dabigatran users. Possible strategies to reduce GIB rate in these high‐risk population include correcting modifiable HAS‐BLED risk factors, reducing dabigatran dose, and using other agents with less GIB risk.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest. The protocol for this research project has been approved by a suitably constituted ethics committee of the institution, and it conforms to the provisions of the Declaration of Helsinki. This project meets the criteria for exemption from formal IRB review in accordance with 45 CFR 46: 101(4), Committee of TTUHSC Lubbock/Odessa Institutional Review Board, Approval No. L13‐081.
Nantsupawat T, Soontrapa S, Nantsupawat N, et al. Risk factors and prevention of dabigatran‐related gastrointestinal bleeding in patients with atrial fibrillation. J Arrhythmia. 2018;34:30–35. https://doi.org/10.1002/joa3.12015
REFERENCES
- 1. FDA . Dabigatran (Pradaxa) full prescribing information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2013. [Google Scholar]
- 2. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 3. Bloom BJ, Filion KB, Atallah R, Eisenberg MJ. Meta‐analysis of randomized controlled trials on the risk of bleeding with dabigatran. Am J Cardiol. 2014;113:1066–74. [DOI] [PubMed] [Google Scholar]
- 4. Carmo J, Moscoso Costa F, Ferreira J, Mendes M. Dabigatran in real‐world atrial fibrillation. Meta‐analysis of observational comparison studies with vitamin K antagonists. Thromb Haemost. 2016;116:754–63. [DOI] [PubMed] [Google Scholar]
- 5. Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non‐vitamin K antagonist anticoagulants in patients with non‐valvular atrial fibrillation. Europace. 2015;17:1467–507. [DOI] [PubMed] [Google Scholar]
- 6. Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet. 2013;52:69–82. [DOI] [PubMed] [Google Scholar]
- 7. Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long‐term anticoagulant therapy (RE‐LY) trial. Circulation. 2011;123:2363–72. [DOI] [PubMed] [Google Scholar]
- 8. Lin KJ, Hernandez‐Diaz S, Garcia Rodriguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti‐inflammatory therapy. Gastroenterology. 2011;141:71–79. [DOI] [PubMed] [Google Scholar]
- 9. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 10. Connolly SJ, Wallentin L, Ezekowitz MD, et al. The long‐term multicenter observational study of dabigatran treatment in patients with atrial fibrillation (RELY‐ABLE) study. Circulation. 2013;128:237–43. [DOI] [PubMed] [Google Scholar]
- 11. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709–18. [DOI] [PubMed] [Google Scholar]
- 12. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–52. [DOI] [PubMed] [Google Scholar]
- 13. Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129:764–72. [DOI] [PubMed] [Google Scholar]
- 14. Chan EW, Lau WC, Leung WK, et al. Prevention of dabigatran‐related gastrointestinal bleeding with gastroprotective agents: a population‐based study. Gastroenterology. 2015;149:586–95. [DOI] [PubMed] [Google Scholar]
- 15. Agewall S, Cattaneo M, Collet JP, et al. Expert position paper on the use of proton pump inhibitors in patients with cardiovascular disease and antithrombotic therapy. Eur Heart J. 2013;34:1708–13. [DOI] [PubMed] [Google Scholar]
- 16. Lip GY, Banerjee A, Lagrenade I, Lane DA, Taillandier S, Fauchier L. Assessing the risk of bleeding in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation project. Circ Arrhythm Electrophysiol. 2012;5:941–8. [DOI] [PubMed] [Google Scholar]
- 17. Apostolakis S, Lane DA, Buller H, Lip GY. Comparison of the CHADS2, CHA2DS2‐VASc and HAS‐BLED scores for the prediction of clinically relevant bleeding in anticoagulated patients with atrial fibrillation: the AMADEUS trial. Thromb Haemost. 2013;110:1074–9. [DOI] [PubMed] [Google Scholar]
- 18. Banerjee A, Lane DA, Torp‐Pedersen C, Lip GY. Net clinical benefit of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus no treatment in a ‘real world’ atrial fibrillation population: a modelling analysis based on a nationwide cohort study. Thromb Haemost. 2012;107:584–9. [DOI] [PubMed] [Google Scholar]
- 19. Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS‐BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57:173–80. [DOI] [PubMed] [Google Scholar]
- 20. Tsu LV, Berry A, Wald E, Ehrlich C. Modified HAS‐BLED score and risk of major bleeding in patients receiving dabigatran and rivaroxaban: a retrospective, case‐control study. Consult Pharm. 2015;30:395–402. [DOI] [PubMed] [Google Scholar]
- 21. Lauffenburger JC, Rhoney DH, Farley JF, Gehi AK, Fang G. Predictors of gastrointestinal bleeding among patients with atrial fibrillation after initiating dabigatran therapy. Pharmacotherapy. 2015;35:560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sherwood MW, Nessel CC, Hellkamp AS, et al. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF trial. J Am Coll Cardiol. 2015;66:2271–81. [DOI] [PubMed] [Google Scholar]
- 23. Hernandez‐Diaz S, Rodriguez LA. Association between nonsteroidal anti‐inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Arch Intern Med. 2000;160:2093–99. [DOI] [PubMed] [Google Scholar]
- 24. Lanza FL, Chan FK, Quigley EM. Practice Parameters Committee of the American College of G. Guidelines for prevention of NSAID‐related ulcer complications. Am J Gastroenterol. 2009;104:728–38. [DOI] [PubMed] [Google Scholar]
- 25. Valkhoff VE, Sturkenboom MC, Kuipers EJ. Risk factors for gastrointestinal bleeding associated with low‐dose aspirin. Best Pract Res Clin Gastroenterol. 2012;26:125–40. [DOI] [PubMed] [Google Scholar]
- 26. Narum S, Westergren T, Klemp M. Corticosteroids and risk of gastrointestinal bleeding: a systematic review and meta‐analysis. BMJ Open. 2014;4:e004587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–62. [DOI] [PubMed] [Google Scholar]
- 28. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. [DOI] [PubMed] [Google Scholar]


