Abstract
Chemotherapy is considered “state of the art” for the treatment of poorly differentiated neuroendocrine neoplasms. Unfortunately, there is no standard effective post-first-line treatment for relapsing high-grade gastroenteropancreatic neuroendocrine neoplasms. We report the case of a patient with a gastric neuroendocrine carcinoma stage IV, with massive gastrointestinal bleeding at diagnosis. After the first line of platin-based chemotherapy a major tumoral response was documented, but the patient relapsed after 4 months. A second line of chemotherapy treatment was given, with the FOLFOX regimen, and the patient has been free of progression for almost 2 years. There is no second-line standard treatment accepted for this type of carcinoma, but 5-fluorouracil combined with oxaliplatin showed interesting antitumor activity.
Keywords: Carcinoma, Neuroendocrine; Stomach Neoplasms; Neoplasm Metastases; Medical Oncology
INTRODUCTION
Neuroendocrine neoplasms (NENs), which arise from the gastroenteropancreatic (GEP) system, define a group of heterogeneous diseases with a growing incidence.1,2 According to histopathological characteristics, NENs can be classified in neuroendocrine tumors (NETs) and neuroendocrine carcinomas (NECs). NECs usually show more aggressive behavior, and patients are usually diagnosed with metastatic disease.2,3 The European guidelines recommends that systemic chemotherapy should be the treatment of choice for metastatic poorly differentiated NECs.2,4 We present the case of a poorly differentiated gastric neuroendocrine carcinoma that had a sustained major response to a second line of chemotherapy with 5-fluorouracil (5-FU) combined with oxaliplatin.
CASE REPORT
A 63-year-old male with no previous significant medical history was admitted to our department after hemorrhagic shock. He complained of dysphagia and asthenia for 2 weeks followed by abdominal pain and melena over the past 3 days. On physical examination, he presented with pale skin, blood pressure of 99/69 mmHg and tachycardia of 110 bpm. There were no palpable masses on the abdomen. The laboratory tests showed a hemoglobin level of 6.8 g/dL (reference value [RV]: 14-17.5 g/dL), a hematocrit of 20.5% (RV: 40-54%), and a normal platelet count. The liver function tests, renal function tests, and coagulation results were normal. The electrocardiogram, and the chest and abdominal x-rays showed no abnormal findings. A gastroesophageal transit and an upper abdominal endoscopy were performed; an important ulcerated tumor was seen, which involved the esophagogastric junction and the gastric fundus with active bleeding (Figure 1A).
Figure 1. A – Gastroesophageal transit showing a huge mass involving the esophagogastric junction and the gastric fundus; B – CT scan showing an exophytic mass in the lesser gastric curvature referring to the known gastric cancer (asterisk).

A computed tomography (CT) scan (Figure 1B) was also performed and revealed extensive gastric wall thickening extending from the cardia through the minor curvature. Large masses in the gastrohepatic ligament and celiac trunk were also described, which were consistent with a conglomerate of lymph nodes. The size of the primary tumor was around 10.6 × 7.8 × 8.4 cm, and the left gastric artery was invaded. The CT also showed several enlarged lymph nodes in the retroperitoneum, a 20 mm mass in segment VII of the liver, nodules of 16 mm and 10 mm in the right adrenal gland, and 12 mm in the left adrenal gland, which were consistent with metastases.
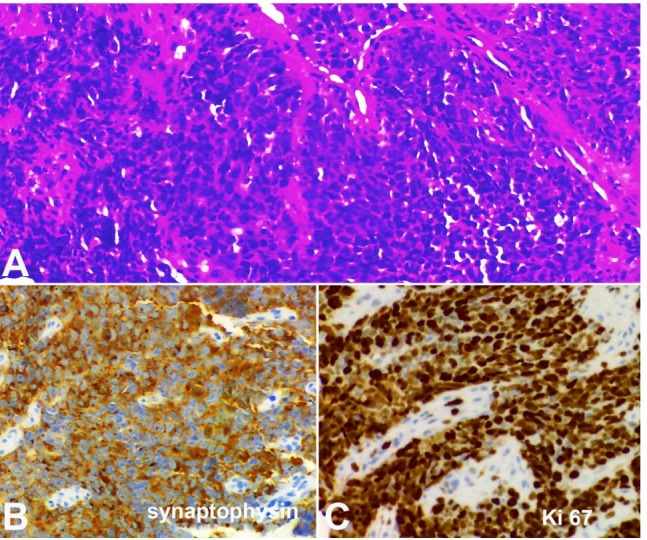
The gastric tumor was biopsied. Microscopic examination showed malignant epithelial proliferation forming nests of large cells, with no glandular differentiation. Immunohistochemistry disclosed diffuse cytokeratin AE1/AE3 and diffuse synaptophisin staining, focal chromogranin positivity and Ki-67 proliferation index of 90%. Histopathological diagnosis was gastric carcinoma with neuroendocrine differentiation, grade 3.
(Figure 2). As the biopsy was taken by endoscopy, the mitotic index could not be established in 10 high-power fields (HPF). However, in an isolated HPF, four mitotic figures were found.
Figure 2. Photomicrography of the gastric biopsy. A – Nests of a poorly differentiated carcinoma stained with hematoxylin-eosin; B – Diffuse cytoplasmic synaptophysin immunostaining; C – High cell proliferation index, showing 90% of Ki-67 positive cells.
The extended laboratory blood work-up showed an elevated chromogranin A of 586 ng/mL (RV: <36.4 ng/mL) and enolase of 94.8 ng/mL (RV: ≤15 ng/mL).
The clinical staging was cT4N1M1, stage IV, according to the tumor, node, and metastasis (TNM) classification for gastric endocrine tumors (European Neuroendocrine Tumor Society).5-7 The case was presented at the hospital multidisciplinary tumor board and systemic treatment was initiated with carboplatin (AUC5, at day 1) and etoposide (100 mg/m2 at days 1, 2, and 3). The patient was discharged after the first cycle of chemotherapy; he was clinically steady, tolerating oral diet and without any signs of bleeding.
After the end of six cycles of chemotherapy, a control CT scan (Figures 3A and 3B) revealed a partial tumoral response, with almost complete recovery of the gastric wall; however, a lymph node of 20 mm remained in the celiac axis.
Figure 3. A and B – CT-scans revealing a major tumoral response, with almost complete recovery of the gastric wall (black arrow), but a lymph node of 20 mm remained in the celiac axis (white arrow).

On the fourth month of follow-up, a new CT scan (Figure 4A) showed a 70 mm mass in the gastrohepatic ligament.
Figure 4. A – CT scan showing local relapse (asterisk); B – The sustained major tumoral response after the 44th cycle of chemotherapy (arrow).

The patient started second-line treatment with mFOLFOX6 (oxaliplatin 85 mg/m2; folinic acid 200 mg/m2; 5-FU 400 mg/m2; and an infusion of 2 400 mg/m2 for 46 hours), every 14 days. Tree months after, on re-evaluation of the first CT scan, there was a partial tumoral response. The patient presented with grade 3 neuropathy, which lead to the discontinuation of oxaliplatin on the 16th cycle, while the CT scans showed a sustained partial response, and continued with the De Gramont scheme ((folinic acid 200 mg/m2; 5-FU 400 mg/m2; and an infusion of 2 400 mg/m2 for 46 hours, every 14 days). The patient has been free of tumor progression for 24 months, on the 44th cycle of the De Gramont scheme and the last CT scan (Figure 4B) documented a sustained major tumoral response.
The patient signed an informed consent and the case report is in accordance with the institution’s ethics committee.
DISCUSSION
The classification for GEP NENs was recently revised. The World Health Organization defines grade 3 GEP carcinomas according to a Ki-67 greater than 20% and/or mitosis greater than 20/HPF. The recommended therapeutic regimen for grade 3 neuroendocrine tumors is the systemic chemotherapy with platinum plus etoposide.2,4,8 Grade 3 carcinomas show diverse responses and outcomes to chemotherapy. Some studies have shown a correlation between Ki-67 and the response to a platinum-based chemotherapy, while others have shown longer survival rates with lower Ki-67 values.9-11 A new sub-classification, which was recently proposed by Fazio and Milione,12 uses the term “tumor” for morphological well-differentiated neoplasms, and “carcinoma” for poorly differentiated neoplasms, and classified GEP NENs according to the Ki67 index. In this setting, they suggest using an alkylating-based or oxaliplatin-based chemotherapy for poorly differentiated GEP NET G3, with a Ki-67 inferior to 55%, while the poorly-differentiated GEP NECs with a Ki-67 superior to 55% should be treated with cisplatin/carboplatin plus etoposide.12
Treatment with first-line chemotherapy usually results in response rates around 42%; the median duration of response is approximately 9.2 months.11 However, our patient responded well to the platin-etoposide chemotherapeutic regimen, but relapsed within 4 months. There is no standard second-line therapy established for GEP tumors. The European Society for Medical Oncology guidelines refer to promising results regarding a fluoropyrimidine drug associated with oxaliplatin or irinotecan.2 The response rate with irinotecan is known to be around 31%, with disease stabilization also around 31%, and a median progression free survival (PFS) of 4 months.13 Alternatively, Hadoux et al.14 evaluated the antitumor activity of 5-FU and oxaliplatin (FOLFOX) chemotherapy in poorly differentiated grade 3 neuroendocrine carcinomas that relapsed after the cisplatin-based regimen. This French retrospective study revised 17 patients—all with distant metastases—in which 11 patients (64%) had a stable disease/partial response with FOLFOX; the median PFS was 4.5 months. An Italian Group15 also analyzed the response to the oxaliplatin-based scheme (n = 78) and documented a PFS of 8 months. After receiving 16 cycles (approximately 8 months) of FOLFOX, our patient is still on the De Gramont scheme with good tolerability, showing a PFS (until now) superior to the aforementioned studies.
The activity of other chemotherapy regimens has been evaluated only in small retrospective studies for poorly differentiated tumors. Welin et al.16 tested temozolomide alone or in combination with bevacizumab and capecitabine, in patients who had a platinum failure, achieving a median overall survival of 22 months. Other approaches, such as monotherapy docetaxel, or FOLFIRI, showed similar results.13,17
CONCLUSION
The prognosis for grade 3 NECs is dismal.6,10 Evidence on what should be the second line of chemotherapy is still lacking. Our patient presents an excellent response to the FOLFOX regimen as a second line of chemotherapy; nevertheless, further larger, prospective studies and guidelines are needed to establish an optimal second-line treatment.
Footnotes
How to cite: Ribeiro MJM, Alonso T, Gajate P, et al. Huge recurrent gastric neuroendocrine tumor: a second-line chemotherapeutic dilemma. Autops Case Rep [Internet]. 2018;8(1):e2018005. http://dx.doi.org/10.4322/acr.2018.005
Financial support: None
REFERENCES
- 1.Yao JC, Hassan M, Phan A, et al. . One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063-72. http://dx.doi.org/10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Öberg K, Knigge U, Kwekkeboom D, Perren A. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii124-30. [DOI] [PubMed] [Google Scholar]
- 3.Frilling A, Modlin IM, Kidd M, et al. . Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014;15(1):e8-21. http://dx.doi.org/10.1016/S1470-2045(13)70362-0. [DOI] [PubMed] [Google Scholar]
- 4.Delle Fave G, O′'Toole D, Sundin A, et al. . ENETS Consensus Conference participants: ENETS consensus guidelines update for gastroduodenal neuroendocrine neoplasms. Neuroendocrinology. 2016;103(2):119-24. http://dx.doi.org/10.1159/000443168. [DOI] [PubMed] [Google Scholar]
- 5.Rindi G, Arnold R, Bosman FT, et al. . Nomenclature and classification of neuroendocrine neoplasms of the digestive system In: Bosman FT, Carneiro F, Hruban RH, et al., editors. WHO classification of tumors of the digestive system. Lyon: IARC; 2010. p. 13-145. [Google Scholar]
- 6.Pape UF, Jann H, Muller-Nordhorn J, et al. . Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer. 2008;113(2):256-65. http://dx.doi.org/10.1002/cncr.23549. [DOI] [PubMed] [Google Scholar]
- 7.Rockall AG, Reznek RH. Imaging of neuroendocrine tumours (CT/MR/US). Best Pract Res Clin Endocrinol Metab. 2007;21(1):43-68. http://dx.doi.org/10.1016/j.beem.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Bongiovanni A, Riva N, Ricci M, et al. . First-line chemotherapy in patients with metastatic gastroenteropancreatic neuroendocrine carcinoma. Onco Targets Ther. 2015;8:3613-9. http://dx.doi.org/10.2147/OTT.S91971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HK, Ha SY, Lee J, et al. . The impact of pathologic differentiation (well/poorly) and the degree of Ki-67 index in patients with metastatic WHO grade 3 GEP-NECs. Oncotarget. 2017;8(43):73974-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorbye H, Welin S, Langer SW, et al. . Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO grade 3): the NORDIC NEC study. Ann Oncol. 2013;24(1):152-60. http://dx.doi.org/10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]
- 11.Mitry E, Baudin E, Ducreux M, et al. . Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer. 1999;81(8):1351-5. http://dx.doi.org/10.1038/sj.bjc.6690325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fazio N, Milione M. Heterogeneity of grade 3 gastroenteropancreatic neuroendocrine carcinomas: new insights and treatment implications. Cancer Treat Rev. 2016;50:61-7. http://dx.doi.org/10.1016/j.ctrv.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Hentic O, Hammel P, Couvelard A, et al. . FOLFIRI regimen: an effective second-line chemotherapy after failure of etoposide-platinum combination in patients with neuroendocrine carcinomas grade 3. Endocr Relat Cancer. 2012;19(6):751-7. http://dx.doi.org/10.1530/ERC-12-0002. [DOI] [PubMed] [Google Scholar]
- 14.Hadoux J, Malka D, Planchard D, et al. . Post-first line FOLFOX chemotherapy in Grade 3 neuroendocrine carcinoma. Endocr Relat Cancer. 2015;22(3):289-98. http://dx.doi.org/10.1530/ERC-15-0075. [DOI] [PubMed] [Google Scholar]
- 15.Spada F, Antonuzzo L, Marconcini R, et al. . Oxaliplatin-based chemotherapy in advanced neuroendocrine tumors: clinical outcomes and preliminary correlation with biological factors. Neuroendocrinology. 2016;103(6):806-14. http://dx.doi.org/10.1159/000444087. [DOI] [PubMed] [Google Scholar]
- 16.Welin S, Sorbye H, Sebjornsen S, Knappskog S, Busch C, Oberg K. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer. 2011;117(20):4617-22. http://dx.doi.org/10.1002/cncr.26124. [DOI] [PubMed] [Google Scholar]
- 17.Sorbye H, Strosberg J, Baudin E, Klimstra DS, Yao JC. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer. 2014;120(18):2814-23. http://dx.doi.org/10.1002/cncr.28721. [DOI] [PubMed] [Google Scholar]


