Abstract
Increasing researches suggest that inflammatory response is involved in vascular remodeling, which plays an important role in the development of aortic dissection. Glucocorticoids have been widely used in the clinical practice due to its powerful and effective anti-inflammatory property. However, the potential relationship between glucocorticoids and aortic dissection was still obscure. This study sought to elucidate the effect of glucocorticoids on the development and progression of aortic dissection, and the potential mechanism involved. Serum cortisol in aortic dissection patients was significantly higher than that in non-ruptured aortic aneurysm patients and healthy volunteers by radioimmunoassay. In modified C57BL/6 mouse model of aortic dissection, glucocorticoids reduced the incidence of aortic dissection and protected the collagen from degradation. Furthermore, glucocorticoids inhibited the TNF-α secretion of THP-1 monocytes, decreased the migration, phenotype switch from contractile type to synthetic type, and the apoptosis of human aortic smooth muscle cells induced by TNF-α. Finally, TNF-sRII was identified as an important cytokine in cellular interaction that participated in vascular remodeling by targeting the p38 MAPK-HSP27 pathway. These results indicate that glucocorticoids inhibit the incidence of aortic dissection by decreasing the TNF-α secretion and increasing the uncombined TNF-sRII, positively participating in vascular remodeling.
Keywords: Aortic dissection, Glucocorticoid, Vascular remodeling, TNF-α, Soluble TNF-RII
Highlights
-
•
Glucocorticoids participate in the vascular remodeling of aortic dissection mediated by soluble TNF-RII.
-
•
Soluble TNF-RII may be used as a potential and attractive target for the intervention of aortic dissection in the future.
In clinical study, we found the serum cortisol in aortic dissection patients was significantly higher than that in non-ruptured aortic aneurysm patients and healthy volunteers. In modified C57BL/6 mouse model, we found glucocorticoids reduced the incidence of aortic dissection, and protected the collagen from degradation. Furthermore, glucocorticoids inhibited the TNF-α secretion of macrophages, decreased the migration, the phenotype switch from contractile type to synthetic type, and the apoptosis of human aortic smooth muscle cells induced by TNF-α. In general, glucocorticoids participate the vascular remodeling of aortic dissection via the p38 MAPK-HSP27 pathway mediated by TNF-sRII.
1. Introduction
Aortic dissection is the most devastating disease of thoracic aorta (Goldfinger et al., 2014). The estimated annual incidence of aortic dissection is approximately 2–5 per 100,000 individuals (Clouse et al., 2004, Howard et al., 2013), which may underestimate the true incidence and case fatality by the incomplete inclusion of deaths before hospital admission (Nienaber and Clough, 2015). The most feared clinical consequence of aortic dissection progression is lethal rupture of aorta, which occurs in about 1–2% per hour within the initial 24 h and almost 50% by 1 week after clinical symptoms onset for acute type A dissection patients (Hagan et al., 2000). Risk factors of aortic dissection include hypertension, physical trauma, cigarette smoking, prior cardiac surgery, male sex, and genetic disorders, etc. (Goldfinger et al., 2014). In addition, increasing pathologic researches have shown that inflammatory mechanisms are involved in the process of vascular remodeling (Rodriguez et al., 2008, Rodriguez-Menocal et al., 2014), which plays an important role in the development and progression of aortic dissection (Luo et al., 2009, del Porto et al., 2010, Ait-Oufella et al., 2013).
Glucocorticoids have been widely used in clinical practice by reason of the powerful and effective anti-inflammatory properties (Rhen and Cidlowski, 2005, Owens et al., 2014). Previous studies demonstrated that glucocorticoids inhibited the immune-inflammatory responses mediated by macrophages (Usher et al., 2010), vascular smooth muscle cells (Pross et al., 2002) or mast cells (Zhou et al., 2011). To date, however, the effect of glucocorticoids on the incidence and development of aortic dissection was still unclear.
We hypothesized that the level of serum glucocorticoids in aortic dissection patients would be increased; the exogenous glucocorticoids intervention could influence the process of vascular remodeling and lower the incidence of aortic dissection, and thereby allowing us to potentially offer an attractive target for the intervention of aortic dissection.
2. Materials and Methods
The study protocol complied with the declaration of Helsinki, and was approved by the Institutional Review Board of our hospital. The human samples were collected in our hospital, and signed informed consent forms for using the samples in the experiments were obtained from all subjects. The animal experiments were performed conform the NIH guidelines on the protection of animals used for scientific purposes, and approved by the Institutional Animal Care and Use Committee of our university.
2.1. Human Blood and Aortic Samples Collection
Between October 2012 and December 2013, 82 patients diagnosed with Stanford type B aortic dissection, 68 patients with non-ruptured aortic aneurysm and 76 healthy volunteers were prospectively registered in the study. All the aortic dissection patients were free from connective tissue disorders such as Marfan syndrome, Ehlers-Danlos syndrome and aortitis. Diagnosis of aortic dissection and aneurysm were confirmed by computed tomography angiography. The venous blood was drawn in a fasting state at 8:00 AM on the second morning of hospital admission using minimally traumatic venepuncture. To maintain the biological activity, the blood was immediately stored at 4 °C and centrifuged within 10 min, and the supernatant was transferred to several freezing tubes and stored at − 80 °C until being assayed for cortisol (Laboratory Medicine of Changhai Hospital), adrenocorticotropic hormone (ACTH, Phoenix Pharmaceuticals, RK-001-01, California, USA) using radioimmunoassay kit, and for soluble tumor necrosis factor receptor II (TNF-sRII, Raybiotech, ELH-TNFR2, Norcross, GA, USA) using a multiplex, bead based ELISA kit, according to the manufacturer's instructions.
At the same period of time, aortic specimens were obtained from eight aortic dissection patients and eight non-ruptured aortic aneurysm patients, who underwent open surgery for aortic graft. Additionally, healthy aortic specimens were obtained from eight body donation volunteers.
2.2. Entry Tear Number Counting
Entry tear was defined as the communication between true and false lumen that caused entry flow into the patent false lumen. Number of the entry tear was assessed by computed tomography angiography in transverse sections.
2.3. Definitions
Renal insufficiency was defined as the level of plasma creatinine > 130 μmol/L. Smoking history was defined as those who had smoked no < 100 cigarettes in their entire life. Coronary artery disease included stable angina, acute coronary syndrome, acute myocardial infarction, and major cardiac event.
2.4. Development of Aortic Dissection Model in Mice
Six-month old male wide-type C57BL/6 mice were purchased from the animal centre of our university and randomly allocated to one of the three treatment groups according to the computer-generated randomization sequence stratified by weight: the intervention group received Angiotensin II (AngII, Sigma-aldrich, A9525, St. Louis, MO) infusion followed by intraperionteal administration of corticosterone (Sigma-Aldrich, 27840), the aortic dissection model group received AngII infusion followed by intraperionteal administration of PBS, and the control group received saline infusion followed by intraperionteal administration of PBS. The animals were fed on a regular diet and drinking water with 12-hour dark/light circle for one-month. To eliminate the interference of endogenous glucocorticoids, bilateral adrenalectomy was performed in all mice.
After intraperitoneal anesthesia (ketamine, 5.6 × 10− 3 g/mL), the mice received subcutaneous micro-osmotic pumps (Alzet, model 1004, Durect Corp., Cupertino, CA) delivering either saline (control group) or Ang II (intervention group and model group) at 2500 ng/kg/min for 28 days. The administration of either corticosterone (intervention group, 0.015 μg/g) or PBS (model group and control group) was started on the day of micro-pump implantation and administered at 9:00 AM daily until the end of the experiment.
2.5. Aortic Specimen Harvest
Ketamine-anesthetized mice were perfused with PBS via the left ventricle to remove blood from tissue; then the entire aorta from ascending aorta to iliac artery was excised in one piece and placed in sterile PBS. After the periadventitial fat was removed, the aortic specimen was observed and photographed. The location and scope of hematoma was carefully recorded. Then the whole aorta was averagely cut into nine segments in different processing.
2.6. External Aortic Diameter Measurement of Mice
External aortic diameter was measured in three transverse sections using image pro-plus software. Ascending aorta was defined as the proximal 2 mm section from the ostium of the innominate artery, aortic arch as the section between the ostia of left common carotid artery and left subclavian artery, descending aorta as the distal 2 mm section from the ostium of the left subclavian artery (Supplemental Fig. I).
2.7. Histology and Immunohistochemistry
Sections (4 μm) were cut from the paraffin-embedded aortic specimens obtained from the aortic dissection patients, non-ruptured aneurysm patients, healthy body donation volunteers or mice. Human aortic sections were immunostained with rabbit polyclonal antibody against human glucocorticoid receptor (GCR, 1:100, Abcam, ab3580, Manassas, VA, USA), and mouse monoclonal antibody against human macrophage (CD 64, 1:150, Abcam, ab140779). The negative control slides were stained with control IgG (Supplemental Fig. II). Blind evaluation of positive staining percentages was performed in 10 different fields at 400 × magnification from two independent pathologists, with an intervariability < 5%.
Mouse aortic tissues were stained with hematoxylin and eosin (HE) and Masson's trichrome staining. Photomicrographs were analyzed by investigators blinded to the experimental protocol using image pro-plus software to assess aortic media thickness and collagen volume fraction. Aortic dissection was confirmed by HE photograph, which was defined as the coexistence of true lumen and false lumen, even the thrombus in false lumen. They were also immunostained with rabbit polyclonal antibody against GCR (1:100, Abcam, ab3580), rat monoclonal antibody against macrophage (F4/80, 1:20, Abcam, ab6640) or control IgG.
2.8. Cell culture
Human aortic Smooth muscle cell line (HA-SMC, ScienCell, 6110, San Diego, CA) was cultured in dedicated SMC conditional medium (ScienCell, 1101) supplemented with 2% of heat-inactivated fetal bovine serum (FBS, ScienCell, 0010), growth supplement (ScienCell, 1152), penicillin (100 U/ml) and streptomycin (100 μg/ml) (ScienCell, 0503) at 37 °C in a humidified 5% CO2 incubator. All cells used in this study were between passages 4 and 6. Human mononuclear macrophage cell line (THP-1, Chinese Academy of Science, TCHu 57, Shanghai, China) was cultured in 1640 medium (Gibco, RPMI 1640, 11875-093) supplemented with 10% FBS, penicillin (100 U/ml) and streptomycin (100 μg/ml, Gibco, 10378-016) at the same condition of culture.
2.9. Supernatant Cytokine Analysis of THP-1 Cells
THP-1 cells were seeded at 5 × 104 cells per well in 24-well culture plates for 24 h. After incubation with starvation medium (0.5% FBS) for 24 h, cells were intervened with either human cortisol (H-CORT., 10− 9 to 10− 5 mol/L, Sigma-Aldrich, HA-8880) or PBS for 48 h. Positive control group was intervened with lipopolysaccharides (LPS, 100 ng/ml, Sigma-Aldrich, L6529). Supernatant was frozen at − 80 °C until being used for matrix metalloprotein-2 (MMP-2, Raybiotech, ELH-MMP2), tissue inhibitor of metalloproteinase-2 (TIMP-2, Raybiotech, ELH-TIMP2) and tumor necrosis factor-α (TNF-α, Raybiotech, ELH-TNFα) detection.
2.10. HA-SMC Scratch Wound Assay
HA-SMC migration in vitro was determined by scratch wound assay. Briefly, HA-SMCs were seeded in 6-well plates at a concentration of 2.5 × 105 cells per well and cultured for 24 h. After incubation with starvation medium (0.5% FBS) for 24 h, a linear wound was gently introduced in the centre of the cell monolayer using 1 ml tip, followed by washing with PBS to remove the cellular debris. Cells were then intervened with H-CORT. at a final concentration of 10− 6 mol/L monitored for additional 24 h. Positive group was stimulated with human platelet derived growth factor-BB (PDGF-BB, PEPROTECH, AF-100-14B, NJ) at a final concentration of 20 ng/ml. Photos were captured using an Olympus imaging system.
2.11. HA-SMC Phenotype Switch Assay
HA-SMCs were seeded in 24-well plates at a concentration of 5 × 104 cells per well and cultured for 24 h. After incubation with starvation medium (0.5% FBS) for 24 h, cells were then intervened with H-CORT. at a final concentration of 10− 6 mol/L monitored for additional 48 h. Positive group was stimulated with PDGF-BB at a final concentration of 20 ng/ml. HA-SMCs were fixed in 4% paraformaldehyde and then permeabilized with 0.3% triton in PBS. Cells were blocked with 3% BSA and then the anti-SM22-α (Abcam, ab14106) primary antibody was used at 1:50 dilution. Goat polyclonal secondary antibody to rabbit IgG labelled with Alexa Fluor 488 was used at 1:200 dilution, and nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) at 1:100 dilution.
2.12. HA-SMC and THP-1 Co-culture System
Co-culture experiments were performed in 24-well transwell plate. HA-SMCs (5 × 104 cells/well) were grown on the bottom well, and THP-1 cells (1.5 × 104 cells/well) were added to the top of polycarbonate inserts with 0.4 μm pores. For cytokine analysis, the medium was removed and kept at − 80 °C. For flow cytometry, HA-SMCs were digested with 0.25% tyrisin free from EDTA. For neutralizing of IL-6 or TNF-sRII, 10 μg anti-IL-6 or anti-TNF-RII antibody was added daily beginning at day 0 of co-culture.
2.13. ImageStreamχ Imaging Flow Cytometry
In vitro assessment of apoptosis in HA-SMCs, ImageStreamχ MK II imaging cytometer (Amnis Corp., Seattle, WA, USA) via annexin V-FITC/PI (eBioscience, BMS500FI, San Diego, USA) staining was used to observe the induction of apoptosis. Cells in the lower and upper right quadrant indicated early apoptosis (annexin-positive/PI-negative) and late apoptosis (annexin-positive/PI-positive), respectively. The percentage of apoptosis = (early apoptosis + late apoptosis)/total cells.
2.14. Protein Microarray
To screen for the secreted cytokines that may play an important role in the interaction between HA-SMCs and THP-1 monocytes, protein microarray analysis was performed using a commercially available array kit according to the manufacturer's protocol (RayBiotech, QAH-IFN-3). The multiplexed technologies allowed simultaneously detection of 40 different cytokines. Fluorescent signals were detected using a laser scanner (Axon Genepix; Molecular Devices, Sunny-vale, CA) set at 555 nm excitation and 565 nm emission. To determine the relative concentrations of cytokines in the supernatant, the densities of individual spots were measured using Image J software. The kit was used for visual detection and all the images of membranes after digital scanning were edited in the gray-scale 8-bit map. Results were obtained according to the instruction of the manufacturer in percentage of signal intensity. The membranes were compared together; the integral positive controls of each membrane reached the 100% of intensity and thus no other image transformation was necessary.
2.15. PathScan Stress and Apoptosis Signaling
To determine the signaling molecules that were involved in the regulation of TNF-sRII or glucocorticoids on the apoptosis of HA-SMCs in cellular interaction, the PathScan stress and apoptosis signaling antibody array kit (Cell Signaling Technology, 12923, Danvers, MA, USA) based upon the sandwich immunoassay principle was used. The array kit allowed for the simultaneous detection of 19 signaling molecules that were involved in the regulation of the stress response and apoptosis. The fluorescent image of the slide was captured with LI-COR imaging system, and spot intensities quantified using image studio software.
2.16. Western Blot Analysis
Proteins were extracted from the HA-SMCs using cell lysis buffer (Cell Signaling Technology, 7018) containing 1 μg/mL protease inhibitor cocktail (Biotool, B14001, Houston, TX, USA; containing 104 mM AEBSF, 80 μM aprotinin, 5 mM bestatin, 1.5 mM E-64, 2 mM leupeptin, 1.5 mM pepstatin A). Cells were lysed on ice for 2 min and scraped from the transwell plate, then clarified by centrifugation at 14,000g for 10 min at 4 °C. Equal amounts of proteins (20 μg) were loaded into each lane of a 10% SDS-PAGE gel for protein separation in every experiment. The protein bands were transferred to Polyvinylidene Difluoride membrane (0.2 μm, Millipore, USA). Nonspecific bindings were blocked by incubation in 3% BSA for 2 h. The membranes were incubated with primary antibodies (1:1000) overnight at 4 °C and washed for three times with Tris Buffered Saline with Tween. The following Cell Signaling Technology primary antibodies were used: bad antibody (9292), phospho-bad (Ser136) antibody (4366), NF-kB p65 antibody (6956), phospho-NF-kB p65 (Ser468) antibody (3039), p38 MAPK antibody (9212), phospho-p38 MAPK (Thr180/Tyr182) antibody (4511), heat shock protein (HSP)27 antibody (2402), phospho-HSP27 (Ser82) antibody (2401), GAPDH antibody (5174). Anti-Tubulin were from Abcam (ab179513). After incubation with secondary antibodies and washing for three times, the bands were visualized using enhanced chemiluminescence detection substrates (Biotool, B18005). Densitometric analysis of the positive bands was performed using Image J software.
2.17. Statistical Analysis
Data analyses were performed using Prism version 6.0 (GraphPad Software, San Diego, CA, USA) and IBM SPSS 19.0 (IBM Corporation, Armonk, NY). The values were expressed as numbers, percentages and mean ± standard deviation (SD) unless otherwise stated. Categorical variables were compared using chi-squared or Fisher's exact test, and a two-group Student's t-test or one-way ANOVA was used to compare the continuous variables. Kaplan-Meier analysis was used to estimate freedom from death, with log-rank test used to discriminate between Kaplan-Meier curves. Landmark analysis was performed according to a breakpoint at 12 days after AngII intervention. The probability values were two-tailed and the null hypothesis was rejected for values of P < 0.05.
3. Results
3.1. Serum Cortisol Increases in Aortic Dissection Patients
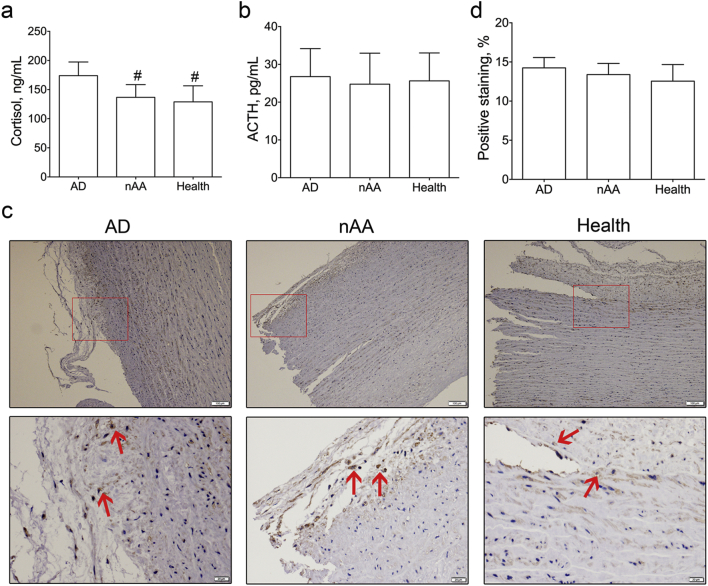
The demographic characteristics and comorbidities of healthy volunteers and patients with aortic dissection or non-ruptured aortic aneurysm were presented in Supplemental Table 1. There were no significant differences in average age, ratio of men to women, or prevalence of major risk factors among these groups except hypertension. As shown in Fig. 1a, the serum cortisol in aortic dissection patients (173.98 ± 23.44 ng/mL) was significantly higher than that in non-ruptured aortic aneurysm patients (136.60 ± 21.83 ng/mL, P < 0.001) or healthy volunteers (129.00 ± 27.56 ng/mL, P < 0.001) by radioimmunoassay. However, the plasma ACTH were 26.76 ± 7.41 pg/mL, 24.75 ± 8.21 pg/mL, and 25.64 ± 7.38 pg/mL for aortic dissection patients, aortic aneurysm patients, and healthy volunteers, respectively (Fig. 1b). No significant differences were observed among them using one-way ANOVA anaylsis.
Fig. 1.
Comparison of serum cortisol, adrenocorticotropic hormone and aortic glucocorticoid receptor in aortic dissection patients, non-ruptured aortic aneurysm patients and healthy volunteers.
a, Serum cortisol in aortic dissection patients (n = 82) was significantly higher than that in non-ruptured aortic aneurysm patients (n = 68) and healthy volunteers (n = 76), #, P < 0.001 vs. aortic dissection group.
b, No significance of plasma ACTH was observed among the three groups.
c, Immunohistochemistry of glucocorticoid receptor in aortic dissection aortas (n = 8), non-ruptured aortic aneurysm aortas (n = 8) and healthy aortas (n = 8). (Scale bars: above panel, 100 μm; below panel, 20 μm.) Red arrow, positive staining.
d, No statistical difference of positive staining of glucocorticoid receptor was observed among the three groups.
ACTH = adrenocorticotropic hormone; AD = aortic dissection; nAA = non-ruptured aortic aneurysm.
To explore the potential relationship between serum cortisol and GCR in aorta, immunohistochemistry was performed in eight aortas in each group (Fig. 1c). The percentages of GCR-positive staining were 14.25 ± 1.31% in aortic dissection group, 13.39 ± 1.42% in non-ruptured aortic aneurysm group, and 12.55 ± 2.12% in healthy group. No statistical difference was observed among them, either (Fig. 1d).
3.2. Serum Cortisol Correlates With Tear Number
To explore the potential correlation of serum cortisol, the clinical characteristics of male, age, height, weight, systolic blood pressure, diastolic blood pressure, blood glucose, triglyceride, and tear number in aortic dissection patients were collected. We found the tear number was associated with serum cortisol by performing the multivariable linear regression (b = 0.920, 95% CI 0.095–1.745, P = 0.029, Supplemental Table 2).
3.3. Glucocorticoids Inhibit the Formation of Aortic Dissection in Modified Mouse Model
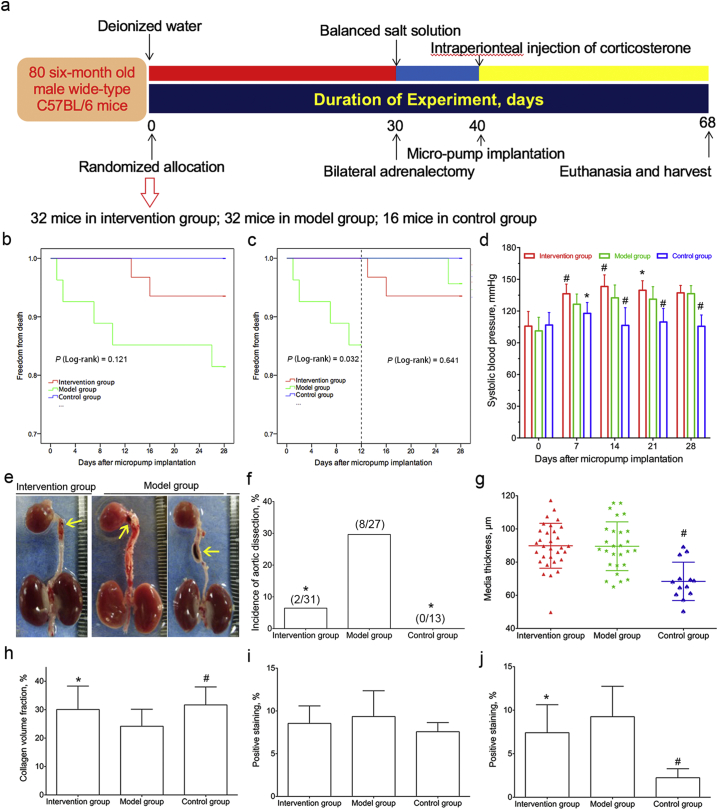
Based on the finding that serum cortisol was higher in aortic dissection patients, we sought to determine its role in the development and progression of aortic dissection in vivo by developing a mouse model. The experimental preparation of aortic dissection animal model was depicted in Fig. 2a. A total of 80 mice were randomly allocated into intervention group (n = 32), model group (n = 32), or control group (n = 16). Nine mice (one in intervention group, five in model group, and three in control group) died before micro-osmotic pumps implantation, and 71 mice were analyzed in the experiment.
Fig. 2.
Glucocorticoids intervention in modified C57BL/6 mouse model of aortic dissection.
a, The experimental study layout was shown in the flow diagram.
b, Cumulative proportions of survival were calculated by Kaplan-Meier analysis in intervention group (n = 31), model group (n = 27) and control group (n = 13). No differences were observed between model and intervention or control groups by log-rank test (P = 0.121).
c, Landmark analysis was performed with a breakpoint at 12-day after AngII intervention. Exogenous corticosterone intervention lowered the mortality of mice at 12-day time point.
d, The systolic blood pressure of mice at different time-point was presented. The systolic blood pressure in intervention group was significantly higher than that in model group at 7-, 14-, 21-day after micropump implantation, and the systolic blood pressure in control group was lower than that in model group at 7-, 14-, 21-, 28-day after micropump implantation.
e, Dissected aortas were photographed and presented. Yellow arrow, aortic dissection.
f, The incidence of aortic dissection in model group was pronounced higher than that in intervention group or control group.
g, Media thickness of aorta in model group (n = 27) was significantly higher than that in control group (n = 13). No difference was observed between intervention group (n = 31) and model group.
h, Collagen volume fraction in model group was significantly lower than that in intervention group or control group.
i, No statistical significances of positive staining of glucocorticoid receptor were observed between model and intervention or control groups.
j, Positive staining of macrophage in model group was statistically higher than that in intervention group or control group.
*, P < 0.05 vs. model group, #, P < 0.001 vs. model group.
The cumulative proportions of survival were calculated by the Kaplan-Meier curve in Fig. 2b. The cumulative proportions of survival with landmark analysis at 12-day breakpoint were presented in Fig. 2c. The exogenous corticosterone intervention improved the survival of mice at 12-day time point. Given the blood pressure elevation was considered as a major risk factor for aortic dissection, we measured the blood pressure at baseline and after AngII infusion every week. The blood pressure in model group was significantly higher than that in control group at 7-, 14-, 21-, 28-day, and lower than that in intervention group at 7-, 14-, 21-day time point (Fig. 2d). The dissected aortas were photographed and presented in Fig. 2e. Aortic dissection was defined as coexist of true lumen and false lumen, even the thrombus (yellow arrow) in false lumen by HE staining (Supplemental Fig. III). The incidence of aortic dissection in model group was significantly higher than that in intervention group (29.6% vs. 6.5%, P = 0.034), or control group (29.6% vs. 0%, P = 0.037) (Fig. 2f).
The location and scope of hematoma was presented in Supplemental Table 3. The external diameter of ascending aorta in model group was significantly bigger than that in control group or intervention group (1458.49 ± 257.96 μm vs. 1094.61 ± 154.57 μm or 1279.17 ± 204.24 μm, P < 0.001 or 0.005, respectively). The external diameter differences of aortic arch and descending aorta between model and control groups were pronounced (aortic arch: 1449.48 ± 290.34 μm vs. 986.38 ± 151.16 μm, P < 0.001; descending aorta: 1343.47 ± 238.48 μm vs. 980.36 ± 98.48 μm, P < 0.001) (Supplemental Table 4).
3.4. Glucocorticoids Inhibit the Collagen Degradation and Macrophage Activation in Mice Aortas
Based on our finding that exogenous glucocorticoids lowered the incidence of aortic dissection in vivo, histological examination was performed in mice aortas to determine the pathological differences. Media thickness was measured on H&E staining using image pro-plus software (Supplemental fig. IVa). We found the media thickness in model group was significantly higher than that in control group (89.6 ± 14.7 μm vs. 68.4 ± 11.6 μm, P < 0.001). No difference was observed between intervention and model groups (89.8 ± 13.5 μm vs. 89.6 ± 14.7 μm, P = 0.937) (Fig. 2g). The collagen volume fraction was analyzed by Masson's trichrome staining (Supplemental fig. IVb). The difference was significant between model and control (24.2 ± 6.0% vs. 31.7 ± 6.3%, P = 0.001) or intervention groups (24.2 ± 6.0% vs. 30.1 ± 8.2%, P = 0.003) (Fig. 2h). No significant difference of positive-GCR staining was observed between model group and intervention or control group (9.3 ± 3.0% vs. 8.5 ± 2.1% or 7.6 ± 1.1%, P = 0.235 or P = 0.115, respectively) by immunohistochemistry (Supplemental fig. IVc; Fig. 2i). But the positive-macrophage staining in model group was higher than that in control group or intervention group (9.3 ± 3.5% vs. 2.2 ± 1.0% or 7.4 ± 3.2%, P < 0.001 or P = 0.041, respectively) (Supplemental fig. IVd; Fig. 2j).
3.5. Cellular Protective Mechanism of Glucocorticoids in Collagen Degradation
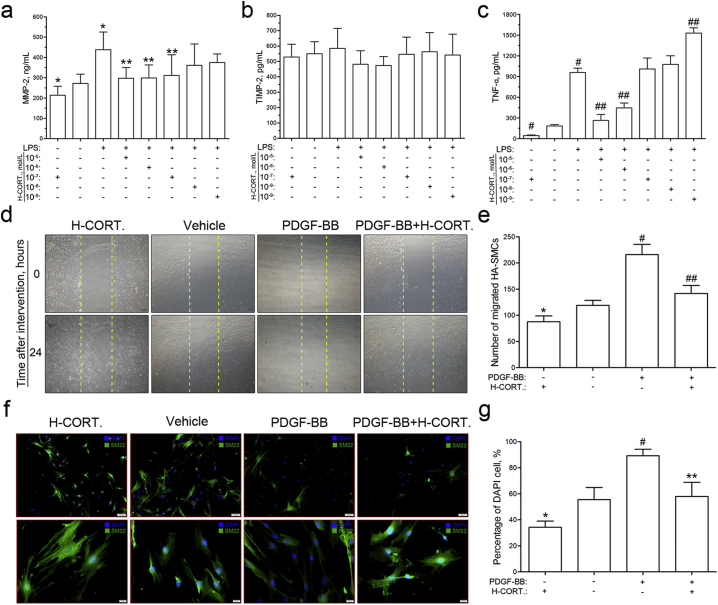
To explore the cellular mechanism that exogenous glucocorticoids protected the collagen from degradation, the supernatants of MMP-2, TIMP-2, and TNF-α were detected by ELISA in THP-1 cells. The results indicated that glucocorticoids inhibited the LPS-induced MMP-2 secretion (Fig. 3a). However, no regulation role that glucocorticoids played on TIMP-2 secretion was observed (Fig. 3b). In addition, high concentration of glucocorticoids (10− 5 and 10− 6 mol/L) inhibited the TNF-α secretion, and low concentration of glucocorticoids (10− 9 mol/L) promoted the TNF-α secretion (Fig. 3c).
Fig. 3.
Glucocorticoids regulate macrophage secretion, human aortic smooth muscle cell migration and phenotype switch in vitro.
a–c, Macrophages were starved for 24 h. Secretions of MMP-2, TIMP-2 and TNF-α were measured by ELISA after LPS stimulation and glucocorticoids intervention for 48 h.
a, Glucocorticoids inhibited LPS-induced MMP-2 secretion at the concentrations of 10− 5, 10− 6 and 10− 7 mol/L in macrophages (n = 6). *, P < 0.05 vs. Blank; **, P < 0.05 vs. LPS.
b, No regulatory role of glucocorticoids in TIMP-2 secretion was observed in macrophages (n = 6).
c, Glucocorticoids inhibited LPS-induced TNF-α secretion at high concentration (10− 5 and 10− 6 mol/L) and stimulated at low concentration (10− 9 mol/L) (n = 4). #, P < 0.001 vs. Blank; ##, P < 0.001 vs. LPS.
d, Human aortic smooth muscle cells were starved for 24 h, and the migration was determined by scratch wound assay after PDGF-BB and glucocorticoid intervention for another 24 h.
e, Migrated cells were quantitated, and the results indicated that glucocorticoids inhibited PDGF-BB-induced migration of human aortic smooth muscle cells. *, P < 0.05 vs. blank; #, P < 0.001 vs. blank; ##, P < 0.001 vs. PDGF-BB or H-CORT. group (n = 4).
f, Human aortic smooth muscle cells were starved for 24 h, and the phenotype switch was determined by cellular immunofluorescence after PDGF-BB and glucocorticoid intervention for another 48 h. SM22 was used as the contraction phenotype marker (green), and the nuclei were counterstained with DAPI (blue) (Scale bars: above panel, 100 μm; below panel, 20 μm.).
g, Glucocorticoids inhibited human aortic smooth muscle cells phenotype switch from contractile type to synthetic type (n = 4). *, P < 0.05 vs. blank; #, P < 0.001 vs. blank; **, P < 0.05 vs. PDGF-BB or H-CORT. group. DAPI = 4′,6-diamidino-2-phenylindole; H-CORT. = human cortisol; LPS = lipopolysaccharides; MMP-2 = matrix metalloprotein-2; PDGF-BB = platelet derived growth factor-BB; TIMP-2 = tissue inhibitor of metalloproteinase-2; TNF-α = tumor necrosis factor-α.
3.6. Glucocorticoids Inhibit the Migration and Phenotype Switch of HA-SMCs
In this study, scratch wound assay was performed to evaluate the regulation of glucocorticoids on HA-SMCs migration (Fig. 3d). We found that glucocorticoids significantly inhibited the PDGF-BB-induced HA-SMCs migration (Fig. 3e). In the cellular immunofluorescence, SM-22 was used as the marker of contractile phenotype, and the percentage of DAPI cell, which was calculated as following formula: Percentage of DAPI cell = DAPI/(DAPI + SM-22) × 100%, represented the amount of synthetic phenotype cells (Fig. 3f). Our results showed that glucocorticoids promoted the differentiation of HA-SMCs from synthetic to contractile phenotype and maintain the homeostasis of aortic wall (Fig. 3g).
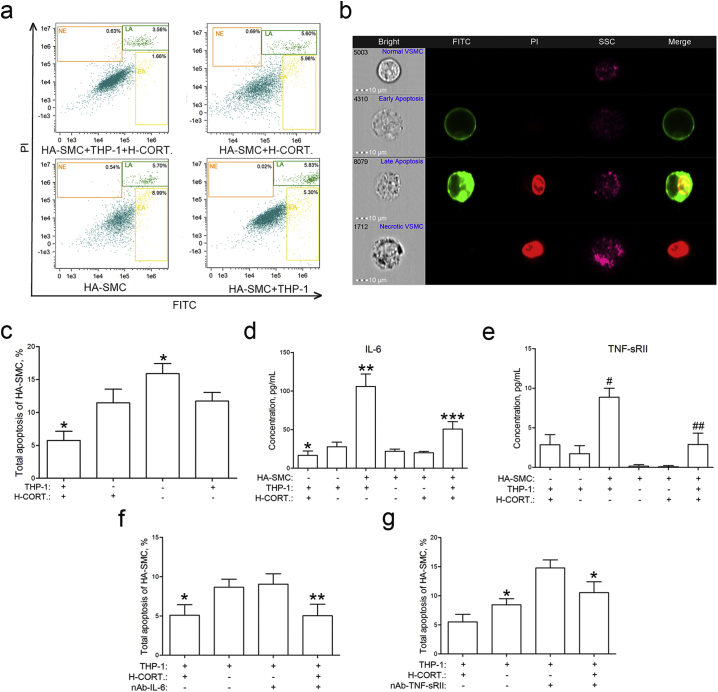
3.7. Glucocorticoids Enhance the Inhibitory Effect on Apoptosis of HA-SMCs in Co-culture With THP-1 Monocytes Model
To explore the role of HA-SMC and THP-1 co-culture in vascular remodeling, the apoptosis of HA-SMCs was detected using ImageStreamχ MK II imaging cytometer. We found that co-culture of HA-SMCs with THP-1 cells decreased the apoptosis and glucocorticoids enhanced the inhibitory effect (Fig. 4a, b and c). The multiplexed protein microarray analysis was performed to find the key cytokines that regulate the apoptosis of HA-SMC in co-culture experiment. We found the IL-6 and TNF-sRII levels in co-culture group were significantly elevated than that in THP-1 or HA-SMC group (Fig. 4d and e). The data indicated that IL-6 or TNF-sRII might play a significant role in the cellular interaction. Therefore, the monoclonal mouse anti human IL-6 or TNF-sRII antibody was used to neutralize the cytokines. We found the inhibitory effect on HA-SMC apoptosis induced by co-culture was eliminated by TNF-sRII antibody, not by the IL-6 antibody (Fig. 4f and g).
Fig. 4.
Glucocorticoids regulate the apoptosis of human aortic smooth muscle cells in co-culture with macrophages system in vitro.
a, Human aortic smooth muscle cells were starved for 24 h. Total apoptosis of HA-SMCs was determined by ImageStreamχ imaging flow cytometry after glucocorticoids intervention for another 48 h.
b, Single normal, early apoptosis, late apoptosis, and necrotic human aortic smooth muscle cells were presented.
c, Co-culture with macrophages caused a decreased apoptosis of human aortic smooth muscle cells, and glucocorticoids enhanced the inhibitory effect (n = 4). *, P < 0.05 vs. THP-1 or H-CORT. group.
d, The concentration of IL-6 in co-culture system was significantly elevated than that in single-cell group (n = 4). *, P < 0.05 vs. THP-1 group; **, P < 0.05 vs. THP-1 or HA-SMC group; ***, P < 0.05 vs. THP-1 + H-CORT., HA-SMC + THP-1 or HA-SMC + H-CORT. group.
e, The concentration of TNF-sRII in co-culture system was pronounced higher than that in single-cell groups (n = 4). #, P < 0.001 vs. THP-1 or HA-SMC group; ##, P < 0.001 vs. HA-SMC + THP-1 or HA-SMC + H-CORT. group.
f, Total apoptosis of human aortic smooth muscle cell was determined by flow cytometry after glucocorticoids intervention and IL-6 antibodies neutralization (n = 4). *, P < 0.05 vs. THP-1 group; **, P < 0.05 vs. THP-1 + nAb-IL-6 group.
g, Total apoptosis of human aortic smooth muscle cell A-SMC was determined by flow cytometry after glucocorticoids intervention and TNF-sRII antibodies neutralization (n = 4). *, P < 0.05 vs. THP-1 + H-CORT. or THP-1 + nAb-TNF-sRII group.
HA-SMC = human aortic smooth muscle cell; H-CORT. = human cortisol; IL-6 = interleukin-6; nAb-IL-6 = neutralizing antibody of IL-6; nAb-TNF-sRII = neutralizing antibody of TNF-sRII; TNF-sRII = soluble tumor necrosis factor receptor II.
3.8. Molecular Mechanism of TNF-sRII and Glucocorticoids Regulation
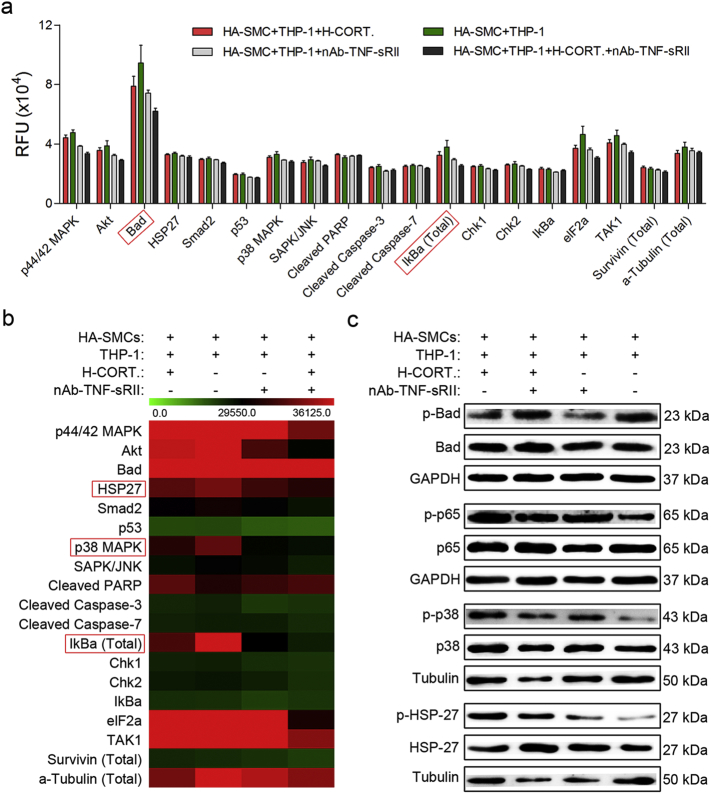
To interpret the molecular mechanism underlying the TNF-sRII and glucocorticoids affected the apoptosis of HA-SMCs in co-culture model, stress and apoptosis related signaling antibody array experiments were performed. We identified significant differences in the protein levels of bad, HSP27, p38 MAPK and total IkBa between the groups (Fig. 5a and b). Further, western blot analysis was used to verify the changes (Fig. 5c). We found neutralizing antibody of TNF-sRII and glucocorticoids affected phosphorylation of p38 and HSP27.
Fig. 5.
Total of 19 signaling molecules were detected using pathscan stress and apoptosis signaling antibody array kit.
a, Quantified fluorescence intensity (RFU) for each target was shown using the LI-COR image studio array analysis software (n = 4).
b, Heat map of 19 signaling molecules that are involved in stress and apoptosis signaling (n = 4).
c, Western blot analysis was used to verify the change of bad, HSP27, p38 MAPK and total IkBa (n = 4).
HA-SMC = human aortic smooth muscle cell; H-CORT. = human cortisol; HSP27 = heat shock protein 27; MAPK = mitogen-activated protein kinase; nAb-TNF-sRII = neutralizing antibody of TNF-sRII.
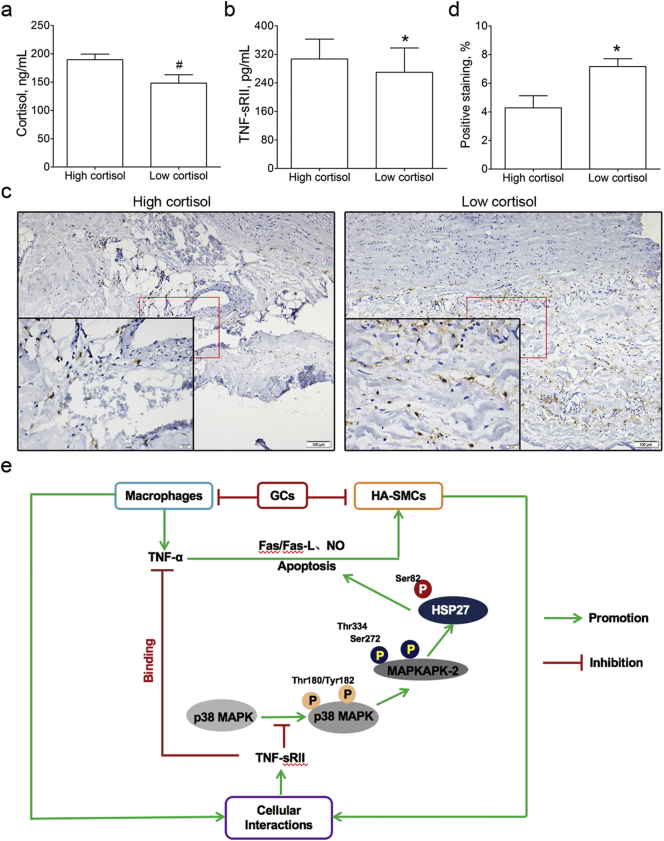
3.9. The Correlation of Serum Cortisol and TNF-sRII in Aortic Dissection Patients
The 82 aortic dissection patients were stratified into high (189.67 ± 9.70 ng/mL, n = 51) and low (148.16 ± 14.83 ng/mL, n = 31) cortisol groups according to the mean concentration of serum cortisol (Fig. 6a). We found the concentration of TNF-sRII in high cortisol group was significantly higher than that in low cortisol group (307.14 ± 55.72 vs. 269.61 ± 68.15 pg/mL, P = 0.008, Fig. 6b). To understand the difference of serum TNF-sRII in high and low cortisol groups, the percentage of macrophages in aortic dissection aorta was performed by immunohistochemistry (Fig. 6c). We found the positive staining of macrophages in high cortisol group was pronounced lower than that in low cortisol group (4.28 ± 0.85% vs. 7.17 ± 0.55%, P = 0.002, Fig. 6d).
Fig. 6.
Correlation of serum cortisol and TNF-sRII in aortic dissection patients and the potential mechanism involved.
a, The aortic dissection patients were stratified into high (n = 51) and low (n = 31) cortisol groups by the mean concentration of serum cortisol. #, P < 0.001 vs. high cortisol group.
b, Serum TNF-sRII was detected using ELISA method and the TNF-sRII in high cortisol group was pronounced higher than that in low cortisol group. *, P < 0.05 vs. high cortisol group.
c, Aortic macrophages were detected by immunohistochemistry (Scale bars, 100 μm and 20 μm, respectively).
d, The percentage of macrophage in high cortisol group (n = 5) was significantly lower than that in low cortisol group (n = 3). *, P < 0.05 vs. high cortisol group.
e, Role of glucocorticoids in the development of aortic dissection.
TNF-sRII = soluble tumor necrosis factor receptor II.
4. Discussion
In the current study, we demonstrated that serum cortisol was elevated in aortic dissection patients, but not in non-ruptured aortic aneurysm patients or healthy volunteers. However, no statistical differences of plasma ACTH were found among the three groups. Given these findings from human specimens, a mouse model of aortic dissection was developed to elucidate the potential effect of increasing glucocorticoids on the development and progression of aortic dissection. The reported incidences of aortic dissection in optional models were 35% and 100%, respectively (Tieu et al., 2009, Kurihara et al., 2012). It was inappropriate if we chose the latter model, because the increasing glucocorticoids might promote the development of aortic dissection as a harmful factor. Therefore, the former model was selected and modified by prolonging the AngII infusion from 6/10 to 28 days maintaining the same dose of 2500 ng/kg/min.
The present study demonstrated that exogenous corticosterone lowered the mortality rate at 12-day time point and prolonged the survive time in mouse model. It should be noted that the role of AngII in triggering aortic dissection onset is not only the result of vasopressor effect, but also by activating the vascular inflammation, such as neutrophils infiltration into the aorta and pro-MMP-9 release (Kurihara et al., 2012). The result that the incidence of aortic dissection was lower in intervention group might be relevant to the anti-inflammatory property of glucocorticoids. Moreover, we found corticosterone attenuated the collagen degradation induced by AngII. Collagen, which was critical for maintaining vessel wall integrity, was disrupted by AngII infusion leading to the generation of mechanically fragile aortas that contributed to medial degeneration and aortic dissection development.
Macrophages are thought to play a major role in vascular inflammation, which is a complex and multistep process of circulating mononuclear leukocytes into the vessel wall (Ross, 1999). Previous studies have demonstrated subcutaneous AngII infusion increased macrophage recruitment and resulted in vascular remodeling (Tieu et al., 2009, Moore et al., 2015). As we supposed previously, the exogenous corticosterone intervention alleviated the vascular inflammation by decreasing the inflammatory infiltration of macrophages, which provided a reasonable explanation for the lower incidence of aortic dissection in animal study.
MMPs are zinc-dependent proteolytic enzymes that carry out highly selective cleavage of specific substrates, degrading collagen, extracellular matrix components and are essential players in tissue remodeling. MMP-2, belonging to the gelatinases, could degrade collagen I, IV, V, VII, X, XI, XIV, gelatin, elastin, fibronectin, laminin, aggrecan, versican, osteonectin, proteoglycan (Rodriguez et al., 2008). Many studies confirmed that the unbalance between MMPs and their inhibitors (TIMPs) was involved in the process of vascular remodeling (Ceron et al., 2013, Lin et al., 2015), as well as the growth, destabilization, and the eventual rupture of atherosclerotic lesions (Rodriguez et al., 2008). In this study, glucocorticoids inhibited the MMP-2 secretion from macrophages in vitro, but had no effect on TIMP-2 secretion. Therefore, glucocorticoids decreased the ratio of MMP-2/TIMP-2 and protected the collagen and extracellular matrix from degradation, which was accordance with the results of animal study in vivo.
Interestingly, TNF-α secretion from macrophages was reduced in high concentration of glucocorticoids, and stimulated in low concentration. This reversal effect suggested that glucocorticoids not only had anti-inflammatory effect, but also could enhance the expression of inflammatory cytokines (Cox, 1995), recognized as a concentration dependent manner (Munck et al., 1984). Previous studies considered that the reversal effect was related to the different affinities of glucocorticoid receptor and mineralocorticoid receptor to glucocorticoids, in which the mineralocorticoid receptor was characterized as high affinity and the glucocorticoid receptor as low affinity (Baker et al., 2013, Poleti et al., 2015). Glucocorticoids combined to mineralocorticoid receptor and played the pro-inflammatory role at low concentration, while glucocorticoids served as an anti-inflammatory character when combined with glucocorticoid receptor at high concentration.
Vascular smooth muscle cells (VSMCs) are the main source of extracellular matrix proteins in aortic media, and the interplay between VSMCs and extracellular matrix proteins is critical for the structural and functional integrity of the aortic wall (Wang et al., 2012). The mature VSMCs maintain a quiescent differentiated state to perform contractile function at homeostasis (Owens et al., 2004). Sometimes, the contractile phenotype of VSMCs can differentiate to synthetic type and produce extracellular matrix proteins and proteases in pathological state (Sansilvestri-Morel et al., 2005). The quantity and phenotype changes of VSMCs in aortic media were important pathological process of vascular remodeling, resulting in aortic dissection. In the present study, we found glucocorticoids inhibited the VSMCs migration and the phenotype switch from contractile to synthetic type, maintaining the quantitative and functional homeostasis.
In addition, the finding that THP-1 monocytes and VSMCs co-culture inhibited the apoptosis of VSMCs further suggested the cellular interactions played more important role in the process of vascular remodeling than single cells. We also found that IL-6 or TNF-sRII might be the potential intermediate cytokine in VSMCs-macrophages interactions after protein microarray analysis of 40 cytokines. Neutralizing anti-TNF-sRII antibody not the anti-IL-6 antibody abrogated the inhibitory effect on HA-SMCs apoptosis in co-culture model.
As a pleiotropic cytokine with a wide range of biological activities, TNF-α mediates its effects by binding to two distinct cell surface receptors, namely, TNF-RI and TNF-RII. TNF-sRII are released by proteolytic cleavage of the extracellular domains of the membrane-associated forms, acting as blockers through neutralizing the function of TNF without invoking inflammatory effects (Marchi et al., 2012). Boyle et al. had demonstrated that TNF-α contributed to macrophage-induced human VSMC apoptosis by autocrine and direct pathways (Boyle et al., 2001, Boyle et al., 2002, Boyle et al., 2003). Previous study had confirmed that TNF-α participated in variety of cellular functions by activating different intracellular signaling components, such as enhancing the phosphorylation of p38 (Chen et al., 2003). On the other hand, enough evidence suggested that HSP27 was phosphorylated at serine 15, 78 and 82 by MAPKAP kinase 2 resulting from the activation of the p38 MAPK pathway. Combining with TNF-α, TNF-sRII decreased the apoptosis of VSMCs in co-culture model as a result of inhibiting the TNF-α activity and the phosphorylation of p38 and HSP27.
In summary, increased glucocorticoids decreased the margination, extravasation, and local activation of macrophages, resulting in the inhibition of MMP-2 secretion to protect the collagen from degradation. Glucocorticoids inhibited the migration, the phenotype switch from contractile to synthetic, and the apoptosis of HA-SMCs induced by TNF-α to maintain the homeostasis of aortic wall. In addition, glucocorticoids suppressed the TNF-α secretion and increased the uncombined TNF-sRII level, which played an important role in cellular interactions and inhibited the activation of p38 MAPK-HSP27 pathway (Fig. 6e).
4.1. Limitations
Although the present study demonstrates that glucocorticoids lower the incidence of aortic dissection and prolong the survival time, it is important to note that these results are based on data from pre-clinical animal model of aortic dissection. Although our study tries to elucidate the potential cellular and molecular mechanisms underlying glucocorticoids regulate the process of vascular remodeling, it is important to note that these results are insufficient to completely interpret the pathological and physiological process of aortic dissection. As we all know, glucocorticoids were used in many diseases such as systemic lupus erythematosus, rheumatoid arthritis, and Takayasu's Arteritis. A large-scale study in glucocorticoids use and non-use populations is necessary to demonstrate that our findings have efficacy in the clinic.
5. Conclusions
Glucocorticoids play an important protective role in vascular remodeling of aortic dissection via the p38 MAPK-HSP27 pathway mediated by TNF-sRII. These findings suggest that glucocorticoids or TNF-sRII may be used as an attractive target for the intervention of aortic dissection in the future.
Acknowledgments
Acknowledgements
None
Funding Sources
This work was partly supported by the National Natural Science Foundation of China [81700430 to L.Z, 81273522 to J.Z.].
Conflict of Interest
None declared.
Author Contributions
Conception and design of the study: Z.P.J., J.Z.
Acquisition of data, or analysis and interpretation of data: L.Z., Y.X., Y.D.S., H.Y.S., Y.N.W.
Drafting the article or revising it critically for important intellectual content: L.Z., J.Z., Z.P.J., H.Y.S.
Final approval of the version to be submitted: all authors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2017.12.002.
Contributor Information
Jian Zhou, Email: zhoujian1-2@163.com.
Zaiping Jing, Email: xueguanky@163.com.
Appendix A. Supplementary data
Supplementary material
References
- Ait-Oufella H., Wang Y., Herbin O., Bourcier S., Potteaux S., Joffre J., Loyer X., Ponnuswamy P., Esposito B., Dalloz M., Laurans L., Tedgui A., Mallat Z. Natural regulatory T cells limit angiotensin II-induced aneurysm formation and rupture in mice. Arterioscler. Thromb. Vasc. Biol. 2013;33:2374–2379. doi: 10.1161/ATVBAHA.113.301280. [DOI] [PubMed] [Google Scholar]
- Baker M.E., Funder J.W., Kattoula S.R. Evolution of hormone selectivity in glucocorticoid and mineralocorticoid receptors. J. Steroid. Biochem. Mol. Biol. 2013;137:57–70. doi: 10.1016/j.jsbmb.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Boyle J.J., Bowyer D.E., Weissberg P.L., Bennett M.R. Human blood-derived macrophages induce apoptosis in human plaque-derived vascular smooth muscle cells by Fas-ligand/Fas interactions. Arterioscler. Thromb. Vasc. Biol. 2001;21:1402–1407. doi: 10.1161/hq0901.094279. [DOI] [PubMed] [Google Scholar]
- Boyle J.J., Weissberg P.L., Bennett M.R. Human macrophage-induced vascular smooth muscle cell apoptosis requires NO enhancement of Fas/Fas-L interactions. Arterioscler. Thromb. Vasc. Biol. 2002;22:1624–1630. doi: 10.1161/01.atv.0000033517.48444.1a. [DOI] [PubMed] [Google Scholar]
- Boyle J.J., Weissberg P.L., Bennett M.R. Tumor necrosis factor-alpha promotes macrophage-induced vascular smooth muscle cell apoptosis by direct and autocrine mechanisms. Arterioscler. Thromb. Vasc. Biol. 2003;23:1553–1558. doi: 10.1161/01.ATV.0000086961.44581.B7. [DOI] [PubMed] [Google Scholar]
- Ceron C.S., Rizzi E., Guimarães D.A., Martins-Oliveira A., Gerlach R.F., Tanus-Santos J.E. Nebivolol attenuates prooxidant and profibrotic mechanisms involving TGF-β and MMPs, and decreases vascular remodeling in renovascular hypertension. Free Radic. Biol. Med. 2013;65:47–56. doi: 10.1016/j.freeradbiomed.2013.06.033. [DOI] [PubMed] [Google Scholar]
- Chen Y., Ke Q., Yang Y., Rana J.S., Tang J., Morgan J.P., Xiao Y.F. Cardiomyocytes overexpressing TNF-alpha attract migration of embryonic stem cells via activation of p38 and c-Jun amino-terminal kinase. FASEB J. 2003;17:2231–2239. doi: 10.1096/fj.03-0030com. [DOI] [PubMed] [Google Scholar]
- Clouse W.D., Hallett J.W., Jr., Schaff H.V., Spittell P.C., Rowland C.M., Ilstrup D.M., Melton L.J., 3rd. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin. Proc. 2004;79:176–180. doi: 10.4065/79.2.176. [DOI] [PubMed] [Google Scholar]
- Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J. Immunol. 1995;154:4719–4725. [PubMed] [Google Scholar]
- Goldfinger J.Z., Halperin J.L., Marin M.L., Stewart A.S., Eagle K.A., Fuster V. Thoracic aortic aneurysm and dissection. J. Am. Coll. Cardiol. 2014;64:1725–1739. doi: 10.1016/j.jacc.2014.08.025. [DOI] [PubMed] [Google Scholar]
- Hagan P.G., Nienaber C.A., Isselbacher E.M., Isselbacher E.M., Bruckman D., Karavite D.J., Russman P.L., Evangelista A., Fattori R., Suzuki T., Oh J.K., Moore A.G., Malouf J.F., Pape L.A., Gaca C., Sechtem U., Lenferink S., Deutsch H.J., Diedrichs H., Marcos Y., Robles J., Llovet A., Gilon D., Das S.K., Armstrong W.F., Deeb G.M., Eagle K.A. The international registry of acute aortic dissection (IRAD): New insights into an old disease. JAMA. 2000;283:897–903. doi: 10.1001/jama.283.7.897. [DOI] [PubMed] [Google Scholar]
- Howard D.P., Banerjee A., Fairhead J.F., Perkins J., Silver L.E., Rothwell P.M., Oxford Vascular Study Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the oxford vascular study. Circulation. 2013;127:2031–2037. doi: 10.1161/CIRCULATIONAHA.112.000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T., Shimizu-Hirota R., Shimoda M., Adachi T., Shimizu H., Weiss S.J., Itoh H., Hori S., Aikawa N., Okada Y. Neutrophil-derived matrix metalloproteinase 9 triggers acute aortic dissection. Circulation. 2012;126:3070–3080. doi: 10.1161/CIRCULATIONAHA.112.097097. [DOI] [PubMed] [Google Scholar]
- Lin Z.W., Wang Z., Zhu G.P., Li B.W., Xie W.L., Xiang D.C. Hypertensive vascular remodeling was inhibited by xuezhikang through the regulation of Fibulin-3 and MMPs in spontaneously hypertensive rats. Int. J. Clin. Exp. Med. 2015;8:2118–2127. [PMC free article] [PubMed] [Google Scholar]
- Luo F., Zhou X.L., Li J.J., Hui R.T. Inflammatory response is associated with aortic dissection. Ageing Res. Rev. 2009;8:31–35. doi: 10.1016/j.arr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Marchi E., Vargas F.S., Acencio M.M., Sigrist R.M., Biscaro M.D., Antonangelo L., Teixeira L.R., Light R.W. Proinflammatory and antiinflammatory cytokine levels in complicated and noncomplicated parapneumonic pleural effusions. Chest. 2012;141:183–189. doi: 10.1378/chest.10-3181. [DOI] [PubMed] [Google Scholar]
- Moore J.P., Vinh A., Tuck K.L., Sakkal S., Krishnan S.M., Chan C.T., Lieu M., Samuel C.S., Diep H., Kemp-Harper B.K., Tare M., Ricardo S.D., Guzik T.J., Sobey C.G., Drummond G.R. M2 macrophage accumulation in the aortic wall during angiotensin II infusion in mice is associated with fibrosis, elastin loss, and elevated blood pressure. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H906–H917. doi: 10.1152/ajpheart.00821.2014. [DOI] [PubMed] [Google Scholar]
- Munck A., Guyre P.M., Holbrook N.J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Nienaber C.A., Clough R.E. Management of acute aortic dissection. Lancet. 2015;385:800–811. doi: 10.1016/S0140-6736(14)61005-9. [DOI] [PubMed] [Google Scholar]
- Owens G.K., Kumar M.S., Wamhoff B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Owens C.D., Gasper W.J., Walker J.P., Alley H.F., Conte M.S., Grenon S.M. Safetey and feasibility of adjunctive dexamethasone infusion into the adventitia of the femoropopliteal artery following endovascular revascularization. J. Vasc. Surg. 2014;59:1016–1024. doi: 10.1016/j.jvs.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleti M.D., DeRijk R.H., Rosa A.F., Moncau C.T., Oliveira P.S., Coutinho L.L., Eler J.P., Balieiro J.C. Genetic variants in glucocorticoid and mineralocorticoid receptors are associated with concentrations of plasma cortisol, muscle glycogen content, and meat quality traits in male nellore cattle. Domest. Anim. Endocrinol. 2015;51:105–113. doi: 10.1016/j.domaniend.2014.12.004. [DOI] [PubMed] [Google Scholar]
- del Porto F., Proietta M., Tritapepe L., Miraldi F., Koverech A., Cardelli P., Tabacco F., de Santis V., Vecchione A., Mitterhofer A.P., Nofroni I., Amodeo R., Trappolini M., Aliberti G. Inflammation and immune response in acute aortic dissection. Ann. Med. 2010;42:622–629. doi: 10.3109/07853890.2010.518156. [DOI] [PubMed] [Google Scholar]
- Pross C., Farooq M.M., Angle N., Lane J.S., Cerveira J.J., Xavier A.E., Freischlag J.A., Law R.E., Gelabert H.A. Dexamethasone inhibits vascular smooth muscle cell migration via modulation of matrix metalloproteinase activity. J. Surg. Res. 2002;102:57–62. doi: 10.1006/jsre.2001.6220. [DOI] [PubMed] [Google Scholar]
- Rhen T., Cidlowski J.A. Antiinflammatory action of glucocorticoids — new mechanisms for old drugs. N. Engl. J. Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- Rodriguez J.A., Orbe J., Martinez, de Lizarrondo S., Calvayrac O., Rodriguez C., Martinez-Gonzalez J., Paramo J.A. Metalloproteinases and atherothrombosis: MMP-10 mediates vascular remodeling promoted by inflammatory stimuli. Front. Biosci. 2008;13:2916–2921. doi: 10.2741/2896. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Menocal L., Faridi M.H., Martinez L., Shehadeh L.A., Duque J.C., Wei Y., Mesa A., Pena A., Gupta V., Pham S.M., Vazquez-Padron R.I. Macrophage-derived IL-18 and increased fibrinogen deposition are age-related inflammatory signatures of vascular remodeling. Am. J. Physiol. Heart Circ. Physiol. 2014;306:H641–H653. doi: 10.1152/ajpheart.00641.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis—an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Sansilvestri-Morel P., Rupin A., Jullien N.D., Lembrez N., Mestries-Dubois P., Fabiani J.N., Verbeuren T.J. Decreased production of collagen type III in cultured smooth muscle cells from varicose vein patients is due to a degradation by MMPs: possible implication of MMP-3. J. Vasc. Res. 2005;42:388–398. doi: 10.1159/000087314. [DOI] [PubMed] [Google Scholar]
- Tieu B.C., Lee C., Sun H., Lejeune W., Recinos A., 3rd., Ju X., Spratt H., Guo D.C., Milewicz D., Tilton R.G., Brasier A.R. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J. Clin. Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher M.G., Duan S.Z., Ivaschenko C.Y., Frieler R.A., Berger S., Schütz G., Lumeng C.N., Mortensen R.M. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J. Clin. Invest. 2010;120:3350–3364. doi: 10.1172/JCI41080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhang J., Fu W., Guo D., Jiang J., Wang Y. Association of smooth muscle cell phenotypes with extracellular matrix disorders in thoracic aortic dissection. J. Vasc. Surg. 2012;56:1698–1709. doi: 10.1016/j.jvs.2012.05.084. [DOI] [PubMed] [Google Scholar]
- Zhou J., Li M., Sheng C.Q., Liu L., Li Z., Wang Y., Zhou J.R., Jing Z.P., Chen Y.Z., Jiang C.L. A novel strategy for development of glucocorticoids through non-genomic mechanism. Cell. Mol. Life Sci. 2011;68:1405–1414. doi: 10.1007/s00018-010-0526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material