Abstract
Background
Although everolimus potentially improves long-term heart transplantation (HTx) outcomes, its early postoperative safety profile had raised concerns and needs optimization.
Methods
This 6-month, open-label, multicenter randomized trial was designed to compare the cumulative incidence of a primary composite safety endpoint comprising wound healing delays, pericardial effusion, pleural effusion needing drainage, and renal insufficiency events (estimated glomerular filtration rate ≤30/mL/min per 1.73 m2) in de novo HTx recipients receiving immediate everolimus (EVR-I) (≤144 hours post-HTx) or delayed everolimus (EVR-D) (4-6 weeks post-HTx with mycophenolate mofetil as a bridge) with reduced-dose cyclosporine A. Cumulative incidence of biopsy-proven rejection ≥ 2R, rejection with hemodynamic compromise, graft loss, or death was the secondary composite efficacy endpoint.
Results
Overall, 181 patients were randomized to the EVR-I (n = 89) or EVR-D (n = 92) arms. Incidence of primary safety endpoint was higher for EVR-I than EVR-D arm (44.9% vs 32.6%; P = 0.191), mainly driven by a higher rate of pericardial effusion (33.7% vs 19.6%; P = 0.04); wound healing delays, acute renal insufficiency events, and pleural effusion occurred at similar frequencies in the study arms. Efficacy failure was not significantly different in EVR-I arm versus EVR-D arm (37.1% vs 28.3%; P = 0.191). Three patients in the EVR-I arm and 1 in the EVR-D arm died. Incidence of clinically significant adverse events leading to discontinuation was higher in EVR-I arm versus EVR-D arm (P = 0.02).
Conclusions
Compared with immediate initiation, delayed everolimus initiation appeared to provide a clinically relevant early safety benefit in de novo HTx recipients, without compromising efficacy.
The 6-month, open-label, multicenter randomized trial is designed to compare primary safety endpoints in de novo heart transplantation and delayed everolimus initiation seems to provide a clinically relevant early safety benefit compared to immediate initiation without compromising efficacy. Supplemental digital content is available in the text.
Modern immunosuppressive regimens have enabled 1-year survival rates up to 85% after heart transplantation (HTx).1 However, incidences of long-term complications, such as cardiac allograft vasculopathy (CAV), malignancy, and chronic drug-induced renal impairment, have not improved meaningfully, with 5-year survival rates remaining at approximately 69%, with median survival of 11 years.1-4
Everolimus—a mammalian target of rapamycin inhibitor (mTORi)—with low-dose cyclosporine A (CsA) has demonstrated long-term efficacy in multiple studies by preventing acute rejection and reducing CAV progression.5-8 In the pivotal B253 study, where everolimus was superior to azathioprine in reducing CAV incidence, the need for therapeutic drug monitoring and CsA dose reduction were not considered; thus, patients were exposed to synergistic toxicity of standard-dose CsA (sCsA) and everolimus and exhibited renal dysfunction.5 The subsequent A2403, A2411, and A2310 studies comparing everolimus plus reduced-dose CsA (rCsA) and mycophenolate mofetil (MMF) plus sCsA further confirmed the immunosuppressive efficacy of everolimus, but did not improve renal function probably because of insufficient protocol-defined CsA reduction.7,9,10 The A2310 study further revealed a high incidence of pleural effusions (PLEs) and pericardial effusions (PCEs) and early mortality from perioperative infections in high-risk patients receiving everolimus-based regimen along with rabbit antithymocyte globulin (ATG) as induction therapy.7 Moreover, other mTORi-based regimens were found to promote wound healing delays and PLEs/PCEs probably due to high sCsA loading doses. Because these regimens are inapplicable to currently prevalent everolimus-based regimens,11,12 everolimus effect on wound healing delays remains uncertain.13
Since the approval of everolimus for HTx in over 90 countries, a need emerged to define a therapeutic regimen that could manage its early postoperative side effects without compromising long-term benefits on CAV, cytomegalovirus (CMV) infections, and malignancies. Delayed everolimus initiation has been explored in kidney transplant recipients,14 but not in HTx recipients who are particularly at risk of surgical wound complications and fluid retention.15 Therefore, the EVERHEART trial was designed to evaluate whether delayed (4-6 weeks post-HTx) versus early (≤144 hours) everolimus initiation was superior in terms of reducing the incidences of postoperative wound healing delays, PLEs/PCEs, and acute renal insufficiency events, while maintaining efficacy in terms of graft rejection and mortality.
MATERIALS AND METHODS
Study Design
EVERHEART was a 6-month, phase IIIB prospective, open-label, randomized, parallel-group study conducted across 12 HTx centers in Italy between September 2009 and December 2013. The open-label design was selected to allow therapeutic drug monitoring of everolimus and CsA. Patients were screened at transplant and randomized 24 to 144 hours (days 1-5) after graft reperfusion in a 1:1 ratio via a Web-based system to either immediate everolimus (EVR-I) or delayed everolimus (EVR-D) arms and stratified by baseline estimated glomerular filtration rate (eGFR) (≤60 or >60 mL/min per 1.73 m2; Modification of Diet in Renal Disease scale) and pretransplant diabetes status. In the EVR-D arm, MMF was replaced with everolimus at 4 to 6 weeks to prevent potentially adverse conditions. Patients were followed up at weeks 2, 4, and 6 and months 2, 3, and 6 (Figure 1A).
FIGURE 1.
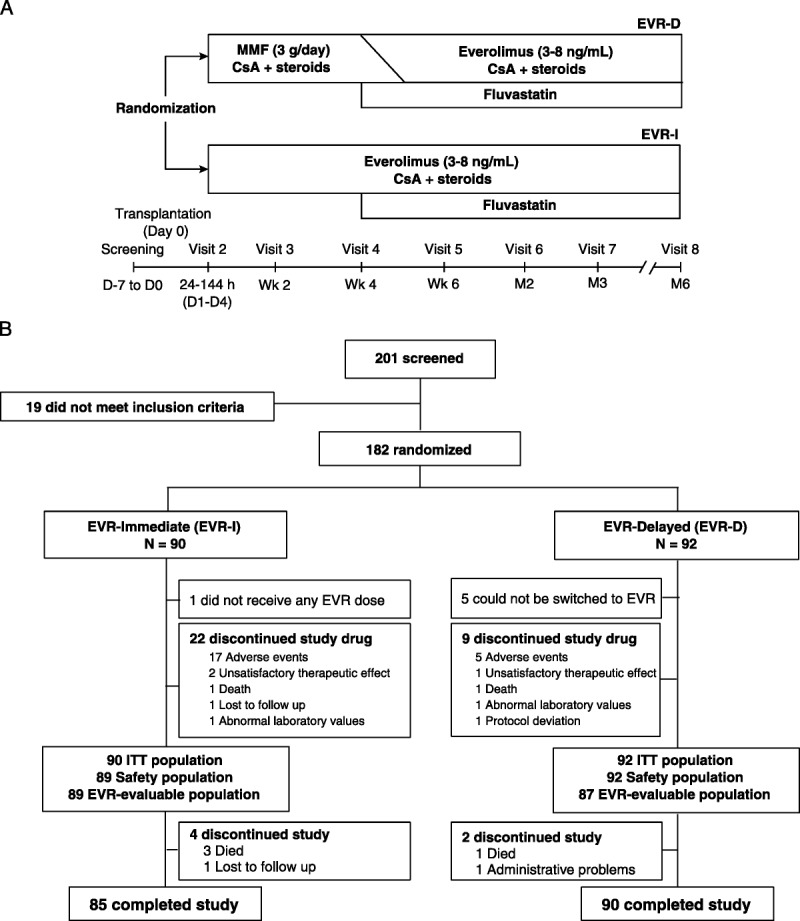
A, Study design and (B) Patient enrollment and disposition. ITT, intention to treat.
Study participants provided written informed consent, and study protocol was approved by each center’s institutional review board in accordance with the European Community Guidance on Good Clinical Practice guidelines and the Declaration of Helsinki.16,17
Study Population
Male or female de novo HTx recipients aged 18 years or older were eligible. Main exclusion criteria at screening were intolerance to CsA/statins; seropositivity for human immunodeficiency virus, hepatitis C virus, or hepatitis B surface antigen; hepatitis C virus- and hepatitis B surface antigen-positive donors; and panel reactive antibodies greater than 30% as assessed by cytotoxicity assay. To be randomized, patients had to meet the following criteria between days 1 and 5 after graft reperfusion: eGFR ≥40 mL/min per 1.73 m2, platelet count ≥40 000/mm3, white blood cell count >4000/mm3; absence of a clinically significant systemic infection; and cold ischemia time less than 6 hours.
Treatment Regimen
Patients in the EVR-I arm received everolimus (trough level [C0], 3-8 ng/mL [5-10 ng/mL by Innofluor Certican® assay kit, Seradyn Inc.]18) 0.75 mg bid orally at 12-hour intervals, with dose initiation of 144 hour or less after graft reperfusion. The everolimus dose was reduced to 0.50 mg bid if platelet count was less than 100 000/mm3; white blood cell count, less than 5000/mm3; body mass index (BMI), greater than 30 kg/m2; body weight, less than 60 kg; eGFR, less than 60 mL/min per 1.73 m2; or if diabetes was present.
Patients in the EVR-D arm received the first MMF dose of 1 g or greater within 144 hours after graft perfusion; the target maintenance dose of 3 g daily was achieved by the first week. At 4 to 6 weeks, MMF was replaced with 0.5 mg bid everolimus. The dose was reduced to 0.25 mg bid if one of the conditions reported for the EVR-I arm was present. MMF was discontinued when everolimus C0 reached 3 ng/mL (5 ng/mL, Certican assay kit kit).18
Patients in both arms received oral CsA (Neoral) per the prespecified target trough levels (C0; Table S1, SDC, http://links.lww.com/TP/B489). During the first 4 to 8 weeks after transplant, CsA C0 levels were lower in the EVR-I arm versus EVR-D arm. In the EVR-D arm, CsA C0 levels were lowered when MMF was substituted by everolimus.
The use and choice of induction therapy, steroid downtitration protocol, and prophylactic/preemptive treatment of CMV infections were in accordance with the centers’ standard practice. Fluvastatin 40 mg was administered to all patients as a lipid-lowering agent, starting at week 4. At month 3, the dose was increased to 80 mg in patients with low-density lipoprotein of 100 mg/dL or greater.19,20 Acute rejection was monitored by endomyocardial biopsies and treated with intravenous pulse steroids if graded 2R or higher.
Primary Endpoints
The primary composite safety endpoint was the 6-month cumulative incidence of surgical wound healing delays, PLEs, PCEs, and acute renal insufficiency (eGFR ≤30 mL/min/1.73 m2). Any sign of wound dehiscence and need for surgical revision of lymphoceles recorded at weeks 2, 4, and 6 and month 6 were considered as events concurring with the composite endpoint. Need for surgical drainage tubes for more than 7 days after surgery and/or PLE leading to drainage were considered as events concurring with the composite endpoint. Any PCE recorded on echocardiograms obtained at days 1 to 5, weeks 2 and 4, and months 3 and 6 and defined as at least moderate (ie, ≥2.0 cm in diastole), with/without signs of hemodynamic compromise, or leading to drainage/prolonged hospitalization met the criteria as events concurring with the composite endpoint. Echocardiograms were reviewed and assigned as PCEs by treatment-blinded data monitoring committee members. Patients lost to follow-up without experiencing any of the above events were considered as failures.
Secondary Endpoints and Adverse Events
Secondary efficacy endpoint was assessed as the cumulative incidence of biopsy-proven acute rejection (BPAR) ≥2R,21 rejection with hemodynamic compromise, graft loss, and death. Hemodynamic compromise was defined as the need of inotrope therapy or vasoactive treatment. The incidences of adverse events (AEs), serious AEs (SAEs), AEs leading to discontinuation, CMV infections and disease, and low-density lipoprotein levels of 100 ng/mL or greater were separately monitored.
Sample Size and Statistical Analyses
Based on the empirical incidence of 60% for the primary endpoint on introducing everolimus immediately after surgery (EVR-I arm),5-7,9 we hypothesized that the primary endpoint would occur in 40% of patients with delayed everolimus introduction. Thus, a recruitment target of 194 patients (97 per arm) was aimed to determine statistically significant superiority of EVR-D, with a 5% 2-tailed alpha error and 80% power.
All analyses were conducted on the safety population (intention-to-treat population including patients who could not be switched to everolimus). The hazards ratios (HRs) with 95% confidence intervals (CIs) for occurrence of primary and secondary endpoints were determined using the Cox regression model. Effects of potential confounders were analyzed between the composite safety endpoints and baseline clinical or demographic variables using odds ratios (ORs).
RESULTS
Study Population and Interventions
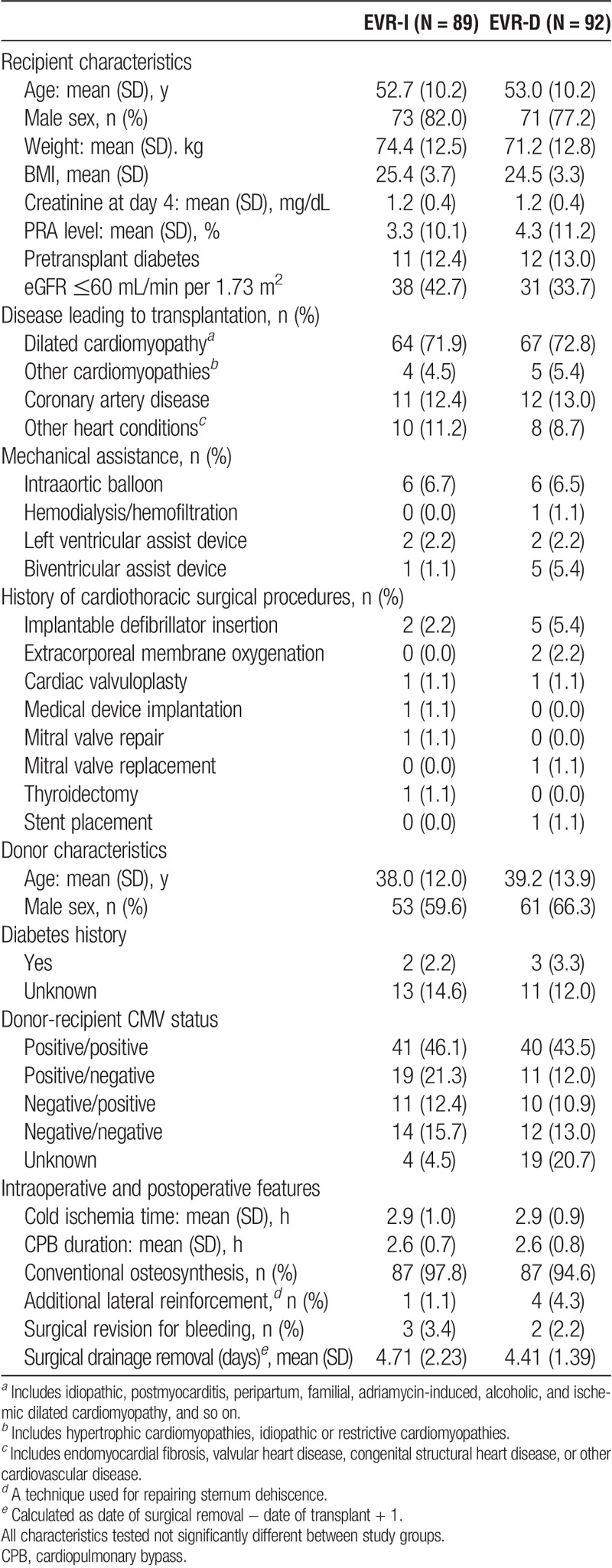
Overall, 201 patients were screened; of these, 182 were randomized to the EVR-I (n = 90) and EVR-D (n = 92) arms; of these, 1 patient assigned to the EVR-I arm did not receive any everolimus dose. Randomized patients corresponded to 93% of the planned population with a 77% power against the initial study assumption of 80%. In all, 175 patients completed the study (EVR-I, 85 and EVR-D, 90; Figure 1B). The baseline characteristics of randomized patients and donors were mostly balanced between the 2 arms (Table 1). The therapeutic switch from MMF to everolimus was successful in 87 (94.6%) patients. The median time-to-switch was 6.0 weeks (range, 3.4-14.1 weeks). The delayed approach was associated with a lower rate of study drug discontinuation (24.7%, EVR-I vs 9.8%, EVR-D; P = 0.008; Figure 1B).
TABLE 1.
Baseline demographics
Majority of patients in both arms received induction therapy (EVR-I, 77/89 [86.5%] vs EVR-D, 71/92 [77.2%]). Of these, 9 (11.7%) of 77 patients and 6 (8.5%) of 71 patients in EVR-I versus EVR-D arm received basiliximab, respectively. Alternatively, 45 (58.4%) of 77 patients and 39 (54.9%) 71 patients in EVR-I and EVR-D arms received ATG, respectively. Antilymphocyte immunoglobulin was administered to 18 (23.4%) 77 patients and 22 (31.0%) 71 patients in EVR-I and EVR-D arms, respectively. Steroids were administered at comparable doses during induction and maintenance.
The recommended CsA levels were more frequently adhered to in the EVR-D versus EVR-I arms (Figure S1A-D, SDC, http://links.lww.com/TP/B489). At month 6, CsA C0 levels were above the protocol-defined target range in 43 (62.3%) of 69 patients and 35 (47.9%) of 73 patients in the EVR-I and EVR-D arms, respectively (P = 0.085; Figure S1D, SDC, http://links.lww.com/TP/B489).
Primary Safety Endpoint
The 6-month cumulative incidence of composite safety endpoints was 44.9% (n = 40) versus 32.6% (n = 30) in the EVR-I vs EVR-D arms, respectively (log-rank test: P = 0.1913; Figure 2A). This accounted for a 48% nonsignificant increase in relative risk in the EVR-I arm (HR, 1.48; 95% CI, 0.92-2.38; P = 0.104; Table 2). The difference in study arms was driven by the increased incidence of PCEs in the EVR-I arm, which had an 80% significant risk of PCEs (HR, 1.84; 95% CI: 1.02-3.30; log-rank test: P = 0.041; Figure 2B). Furthermore, among the 30 patients with PCEs in the EVR-I arm, 10 (33.3%) needed pericardiocentesis, as opposed to only 1 (5.5%) of the 18 patients with PCEs in the EVR-D arm (P = 0.03). On the other hand, wound healing delays, episodes of renal insufficiency, and PLEs needing drainage were not significantly different between the 2 study groups (Table 2).
FIGURE 2.
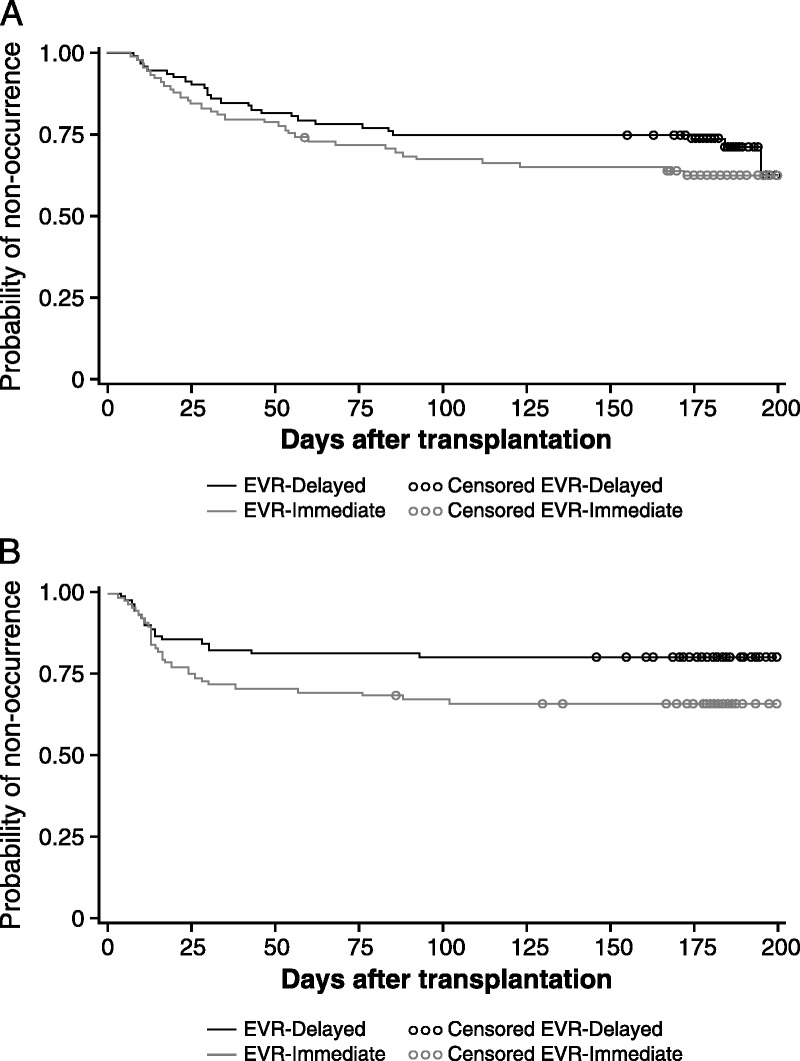
Kaplan-Meier estimates of probability of nonoccurrence of (A) composite safety endpoint and (B) PCE/PLE.
TABLE 2.
Incidence and relative risk of safety endpoints in safety population
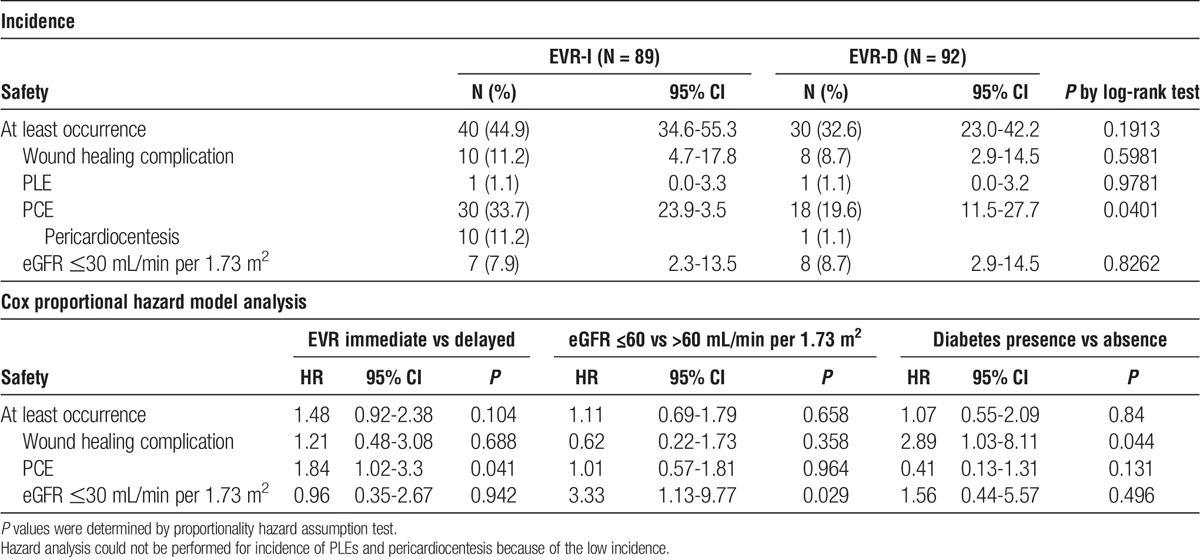
During the study follow-up, eGFR (Figure S2, SDC, http://links.lww.com/TP/B489) levels were comparable, indicating no impairment in renal function with the delayed approach. Nevertheless, baseline eGFR less than 60 mL/min per 1.73 m2 was a significant risk factor for post-HTx renal insufficiency (HR, 3.33; 95% CI, 1.13-9.77; P = 0.029; Table 2).
None of the baseline and procedural variables was associated with significant risk of a composite safety endpoint, except for borderline effects of donor older than 35 years and female donors (P = 0.063; Figure 3A). Within the patient subgroups, the risk of safety endpoint was significantly lower in the EVR-D versus EVR-I arms for patients with BMI of 30 kg/m2 or less, without diabetes, and with 35 year or younger donors (Figure 3B).
FIGURE 3.
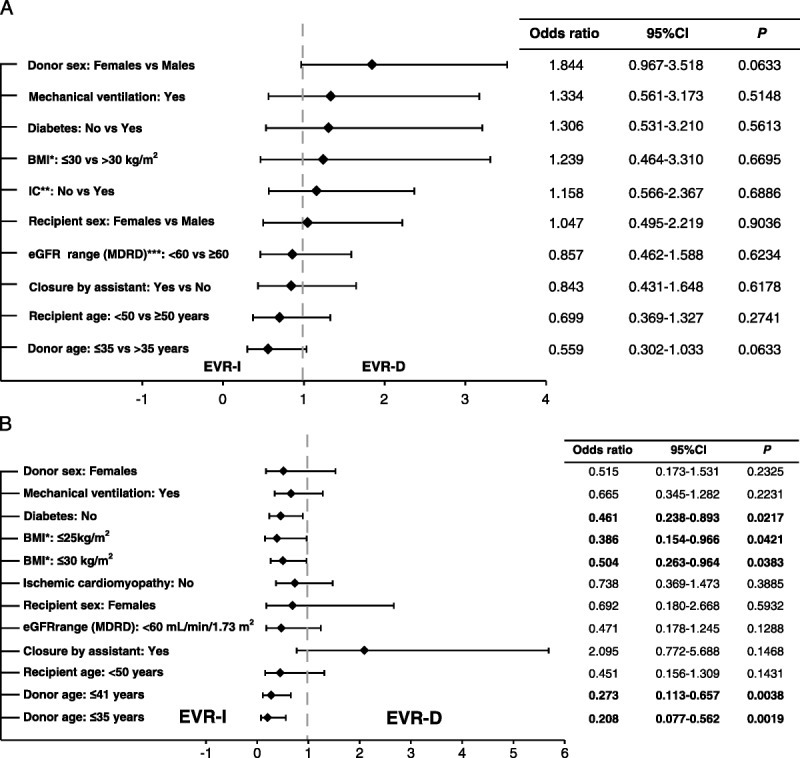
A, Prognostic factors of primary composite endpoint by baseline characteristics. B, Forest plot depicting occurrence of composite safety endpoint by baseline characteristics between delayed and immediate everolimus initiation arms. *BMI, body mass index; **IC, ischemic cardiomyopathy; ***eGFR, estimated glomerular filtration rate (mL/min per 1.73 m2).
Secondary Efficacy Endpoint
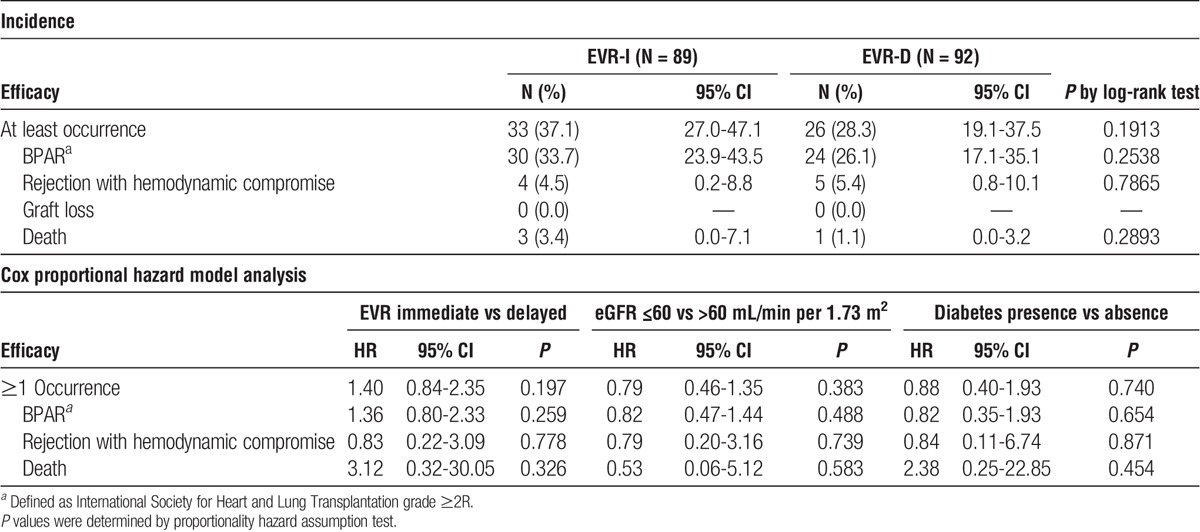
Composite efficacy failure events were higher, but not significantly different, in the EVR-I arm (37.1% [n = 33], EVR-I vs 28.3% [n = 26], EVR-D; HR, 1.40; 95% CI: 0.84-2.35; log-rank test: P = 0.191; Table 3). During the 6-month period, 4 patients died: 3 in the EVR-I arm (1 each due to congestive heart failure/multiple organ failure, pulmonary infection, and arrhythmia), and 1 in the EVR-D arm (gastric adenocarcinoma).
TABLE 3.
Incidence and risk of efficacy endpoints in safety population
Other Safety Events
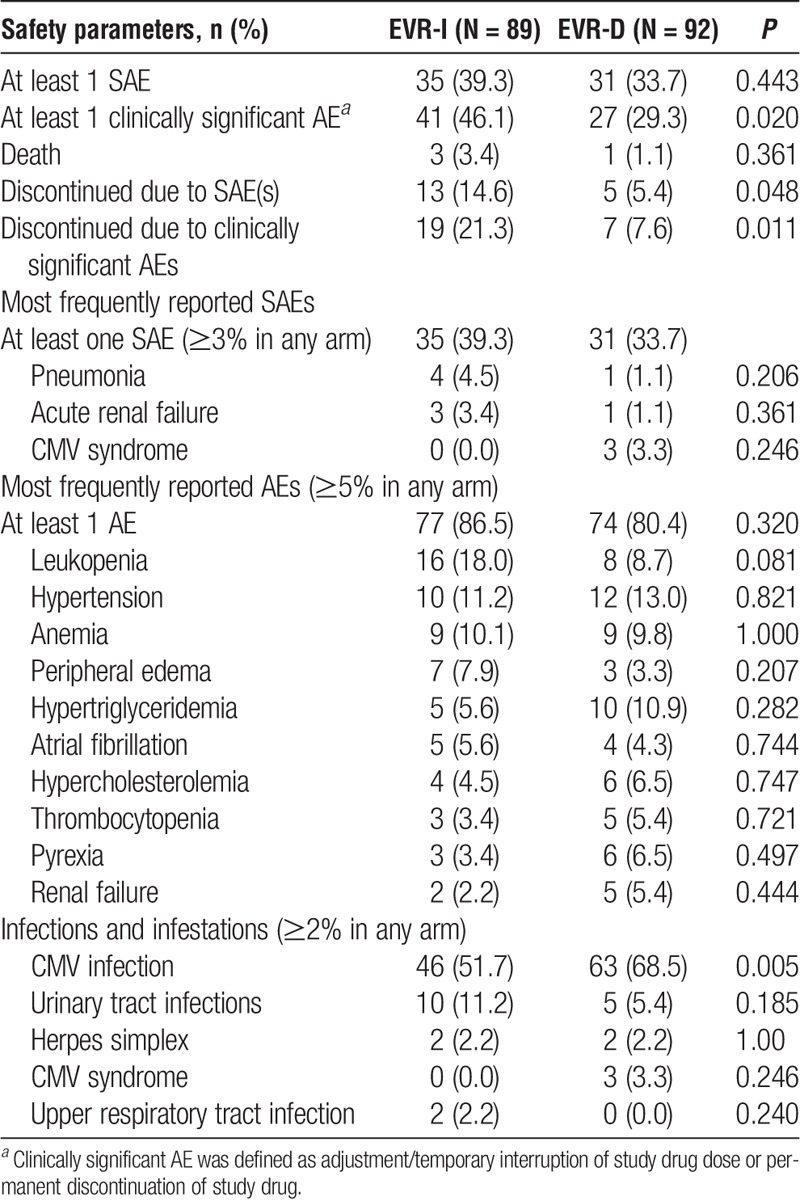
At least 1 AE occurred in 86.5% (n = 77) and 80.4% (n = 74) of patients in the EVR-I and EVR-D arms, respectively. Overall, clinically significant AEs and AEs leading to drug discontinuation were significantly higher in the EVR-I arm than in the EVR-D arm (Table 4). Discontinuations due to SAEs were also significantly higher in the EVR-I versus EVR-D arm (P = 0.048). Incidence of patients requiring fluvastatin dose adjustment was comparable between the 2 arms at month 1 (46.1% [n = 41], EVR-I vs 41.8% [n = 38], EVR-D), month 3 (43.0% [n = 37], EVR-I vs 40.2% [n = 37], EVR-D), and month 6 (38.2% [n = 34], EVR-I vs 39.1% [n = 36], EVR-D). Risk of CMV disease/infection was significantly lower in the EVR-I versus EVR-D arms (OR, 0.414; 95% CI, 0.223-0.771; P = 0.005).
TABLE 4.
Incidence of adverse and serious AEs
DISCUSSION
This multicenter, prospective, randomized, open-label study designed to systematically evaluate early postoperative complications in de novo HTx recipients22 provides suggestive evidence that the timing of everolimus initiation after HTx may influence the risk of AEs, mainly moderate PCEs and PCEs leading to pericardiocentesis. In addition, everolimus initiation between 4 and 6 weeks after surgery appears to provide a better safety profile as compared with immediate initiation, retaining antirejection efficacy, whereas everolimus discontinuation due to AEs appears to be more frequent when the drug is initiated in the immediate postoperative period. On the other hand, CMV infection was more common with delayed rather than early everolimus initiation.
To date, 4 multicenter randomized studies have evaluated the efficacy of everolimus combined with CsA in de novo HTx recipients with major differences in everolimus dosing and administration strategies.5-7,9,23 PCEs and PLEs were reported more frequently in the everolimus arm in 2 of the 4 studies,7,9 which is consistent with the higher incidence in the EVR-I arm (44.9%, EVR-I arm vs 32.6%, EVR-D arm), for which everolimus was introduced within 5 days of HTx. Therefore, we suggest that delayed everolimus initiation could markedly reduce the incidence of critical AEs, like PCEs/PLEs, which can compromise safety in heart transplant recipients.
Previous studies in which everolimus was introduced with sCsA in the immediate postoperative period,7,9 impaired renal function appeared as a concern.6,7,9 In contrast, renal insufficiency events in the present study were low; moreover, eGFR was maintained and not significantly different between study arms probably because everolimus initiation was concomitant with CsA reduction in both the arms. In this context, a recent randomized study demonstrated that CsA discontinuation combined with everolimus and MMF at weeks 7 to 11 after transplantation improved renal function versus sCsA and MMF,24 but was associated with higher rate of BPAR. In our study, both everolimus arms with reduced-dose CsA showed stable renal function during the study period (Figure S2, SDC, http://links.lww.com/TP/B489), suggesting a strategy of effective compromise between the risk of BPAR and the need to preserve renal function.
Previous studies5-7,9 and a meta-analysis by Zuckermann et al13 revealed a high incidence of wound healing delays in the everolimus arms compared with the standard calcineurin inhibitor (CNI) arms. A systematic review by Pengel et al,25 involving 4 HTx studies, found that patients receiving mTORis experienced more wound complications (OR, 1.82, 95% CI, 1.15-2.87). However, sirolimus was used in 2 of these studies,12,26 and wound infections alone were categorized as wound healing complications in 1 study involving everolimus.9 Although none of these studies was statistically powered to determine significant differences in early AE occurrence, there existed a rather homogeneous understanding among clinicians that surgical trauma and patient frailty seem to amplify wound complications with an everolimus-based regimen. In the present study, however, the frequency of wound healing delays in the EVR-D arm (11.2%, EVR-I vs 8.7%, EVR-D) was lower than that reported previously as AEs,7,25 thereby indicating that delayed everolimus initiation could help prevent this complication.
Composite efficacy endpoint of BPAR, rejection with hemodynamic compromise, graft loss, or death was not significantly different between the EVR-I and EVR-D arms. Nonetheless, the numerically higher incidence in the EVR-I arm was mainly driven by BPAR ≥ 2R, which was higher than that reported previously.7,9
Kobashigawa et al27 previously reported that an everolimus-based regimen initiated within 72 hours post-HTx is associated with a lower incidence of CMV infections compared with azathioprine- or MMF-based regimens. Indeed, the risk of CMV disease in our study was significantly higher in the EVR-D versus EVR-I arms, indicating that delayed initiation may not alleviate baseline CMV infection, most likely because preemptive strategy was usually preferred over prophylaxis in most centers, thereby exposing the patients to high risk of early reactivation during the initial phase of MMF intake.
Evidence from randomized trials and observational studies support the concept that everolimus improves long-term outcomes by reducing early CAV development and malignancy onset.7,9,10,21,28,29 Thus, if the goal of everolimus-based therapies is long-term benefit, a strategy that avoids early toxicity could improve patient management, reduce the chances of early discontinuation, and increase potential benefits for patients.
The main limitation of this study was a slightly underpowered sample size, resulting from dramatic decrease in the number of HTx procedures performed in Italy during the study period. Patient enrollment began in June 2009 and had to be repeatedly prolonged until December 2013. When the study was first designed, 320 to 350 HTx procedures/year were being performed in Italy.30 However, since initiation, the number of procedures dramatically reduced to less than 250 cases/year. To avoid excessive prolongation of study period, we terminated enrollment at 93% of the planned population, accounting for a 77% power for the initial assumptions. The study power was additionally affected by the discrepancy between expected versus observed incidences of the primary endpoint.5-7,9 The use of CsA instead of tacrolimus, the current CNI of choice, may appear as another study limitation. However, little clinical evidence of tacrolimus and everolimus was available when the study was designed; moreover, the objective of our study was evaluation of everolimus-related safety profile, rather than the CNI combination therapy. Finally, it must be noted that as in most of randomized controlled studies, the current cohort of patients represents a healthier subgroup when compared with the overall population of patients undergoing HTx. It is possible that this selection may be driven by the need for patient stability between screening and randomization at day 5, with unstable patients unlikely to get enrolled.
In conclusion, in the context of an immunosuppressive regimen based on everolimus and reduced-dose CsA, delayed initiation of everolimus with MMF as a bridge appears to provide a better safety profile than immediate initiation, by reducing the incidence of PCEs, especially those requiring pericardiocentesis, and by improving overall drug tolerability, with less AE-driven discontinuations, without compromising antirejection efficacy.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Raffaella Guarisco, Vincenza Vinaccia, and Marta Bartezaghi, Novartis Pharma, Italy, and Prachiti Narvekar, Novartis Healthcare Pvt. Ltd., India, for editorial assistance. The authors also wish to thank the following data monitoring committee and study team members:
Data Monitoring Committee Members: Luca Salvatore De Santo: Hospital Monaldi, Naples; Francesco Tona: Hospital of Monza, Monza; Daniele Poggio: Academic Hospital of Padova, Padova; Carlo Savini: Academic Hospital S. Orsola-Malpighi, Bologna; and Federico Ambrogi: University of Milan, Milan.
Study Team Members: Sonia Bernazzali: Heart Transplant Surgery Unit, Academic Hospital Senese, Siena; Andrea Maria D’Armini and Gabriella Mattiucci: Cardiac Surgery Department, University of Pavia-Hospital Policlinico, San Matteo, Pavia, Italy; Mauro Rinaldi and Marco Ribezzo: Cardiac Surgery Unit, Hospital Molinette, Torino; Maurizio Porcu: Cardiology Unit, Hospital G. Brotzu - San Michele, Cagliari; Francesco Musumeci: Cardiac Surgery Unit, Hospital San Camillo Forlanini, Rome; Antonio Gambino: Cardiac Surgery Unit, Academic Hospital of Padova, Padova; Ciro Maiello: Transplant Surgery Department, Hospitals Colli-Monaldi, Naples; Maria Frigerio: Cardiology 2-Cardiac Insufficiency and Transplantation Department, Academic Hospital Niguarda, Milan; Giorgio Guzzi: Cardiothoracic Surgery Department, Academic Hospital S. Maria della Misericordia, Udine; Alberto Forni: Cardiac Surgery Unit, Academic Hospital Civile Maggiore, Verona; and Giuseppe Capone: Cardiac Surgery Unit, Hospital of Bari, Bari.
Footnotes
The study was funded by Novartis Pharma, Italy.
L.P. consulted for Biotest and Qiagen, has received institutional grants from Qiagen, and was a consultant for Novartis in 2008 as a coauthor for the study protocol. F.G. received travel grants, speaker’s honoraria, and research grants from Novartis. C.P. and P.L.M. received travel grants from Novartis. C.A., U.L., M.M., G.M., G.F., G.G., N.M., M.C., and M.B. have no conflicts of interest to disclose.
All authors were involved in conception, design, and conduct of the trial and in writing, reviewing, and approval of the final draft of the article.
Trial registration number: NCT01017029.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report—2014: focus theme: retransplantation. J Heart Lung Transplant. 2014;33:996–1008. [DOI] [PubMed] [Google Scholar]
- 2.Colvin-Adams M, Smith JM, Heubner BM, et al. OPTN/SRTR 2011 annual data report: heart. Am J Transplant. 2013;13(Suppl 1):119–148. [DOI] [PubMed] [Google Scholar]
- 3.Eisen HJ. Immunosuppression-state-of-the-art: anything new in the pipeline? Curr Opin Organ Transplant. 2014;19:500–507. [DOI] [PubMed] [Google Scholar]
- 4.Tonsho M, Michel S, Ahmed Z, et al. Heart transplantation: challenges facing the field. Cold Spring Harb Perspect Med. 2014;4:a015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisen HJ, Tuzcu EM, Dorent R, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349:847–858. [DOI] [PubMed] [Google Scholar]
- 6.Viganò M, Tuzcu M, Benza R, et al. Prevention of acute rejection and allograft vasculopathy by everolimus in cardiac transplants recipients: a 24-month analysis. J Heart Lung Transplant. 2007;26:584–592. [DOI] [PubMed] [Google Scholar]
- 7.Eisen HJ, Kobashigawa J, Starling RC, et al. Everolimus versus mycophenolate mofetil in heart transplantation: a randomized, multicenter trial. Am J Transplant. 2013;13:1203–1216. [DOI] [PubMed] [Google Scholar]
- 8.Kobashigawa JA, Pauly DF, Starling RC, et al. Cardiac allograft vasculopathy by intravascular ultrasound in heart transplant patients: substudy from the everolimus versus mycophenolate mofetil randomized, multicenter trial. JACC Heart Fail. 2013;1:389–399. [DOI] [PubMed] [Google Scholar]
- 9.Lehmkuhl HB, Arizon J, Vigano M, et al. Everolimus with reduced cyclosporine versus MMF with standard cyclosporine in de novo heart transplant recipients. Transplantation. 2009;88:115–122. [DOI] [PubMed] [Google Scholar]
- 10.Zuckermann A, Wang SS, Ross H, et al. Efficacy and safety of low-dose cyclosporine with everolimus and steroids in de novo heart transplant patients: a multicentre, randomized trial. J Transplant. 2011;2011:535983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuppahally S, Al-Khaldi A, Weisshaar D, et al. Wound healing complications with de novo sirolimus versus mycophenolate mofetil-based regimen in cardiac transplant recipients. Am J Transplant. 2006;6(5 Pt 1):986–992. [DOI] [PubMed] [Google Scholar]
- 12.Keogh A, Richardson M, Ruygrok P, et al. Sirolimus in de novo heart transplant recipients reduces acute rejection and prevents coronary artery disease at 2 years: a randomized clinical trial. Circulation. 2004;110:2694–2700. [DOI] [PubMed] [Google Scholar]
- 13.Zuckermann A, Arizon JM, Dong G, et al. Impact of de novo everolimus-based immunosuppression on incisional complications in heart transplantation. Transplantation. 2011;92:594–600. [DOI] [PubMed] [Google Scholar]
- 14.Albano L, Berthoux F, Moal MC, et al. Incidence of delayed graft function and wound healing complications after deceased-donor kidney transplantation is not affected by de novo everolimus. Transplantation. 2009;88:69–76. [DOI] [PubMed] [Google Scholar]
- 15.Zuckermann A, Barten MJ. Surgical wound complications after heart transplantation. Transpl Int. 2011;24:627–636. [DOI] [PubMed] [Google Scholar]
- 16.Research Quality Association. Good clinical practice (GCP) regulations and guidelines. http://www.therqa.com/committees-working-parties/good-clinical-practice/regulations-and-guidelines/. Updated 2017. Accessed February 7, 2017.
- 17.World Medical Association. World Medical Association Declaration of Helsinki. http://www.who.int/bulletin/archives/79(4)373.pdf. Published 2001. Accessed February 7, 2017.
- 18.Innofluor® Certican® Assay System. [package insert]Indianapolis, IN: Seradyn Inc; 2005. [Google Scholar]
- 19.Ballantyne CM, Corsini A, Davidson MH, et al. Risk for myopathy with statin therapy in high-risk patients. Arch Intern Med. 2003;163:553–564. [DOI] [PubMed] [Google Scholar]
- 20.Potena L, Grigioni F, Ortolani P, et al. Safety and efficacy of early aggressive versus cholesterol-driven lipid-lowering strategies in heart transplantation: a pilot, randomized, intravascular ultrasound study. J Heart Lung Transplant. 2011;30:1305–1311. [DOI] [PubMed] [Google Scholar]
- 21.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. [DOI] [PubMed] [Google Scholar]
- 22.Hirt SW, Bara C, Barten MJ, et al. Everolimus in heart transplantation: an update. J Transplant. 2013;2013:683964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arora S, Andreassen AK, Andersson B, et al. The effect of everolimus initiation and calcineurin inhibitor elimination on cardiac allograft vasculopathy in de novo recipients: one-year results of a Scandinavian randomized trial. Am J Transplant. 2015;15:1967–1975. [DOI] [PubMed] [Google Scholar]
- 24.Andreassen AK, Andersson B, Gustafsson F, et al. Everolimus initiation and early calcineurin inhibitor withdrawal in heart transplant recipients: a randomized trial. Am J Transplant. 2014;14:1828–1838. [DOI] [PubMed] [Google Scholar]
- 25.Pengel LH, Liu LQ, Morris PJ. Do wound complications or lymphoceles occur more often in solid organ transplant recipients on mTOR inhibitors? A systematic review of randomized controlled trials. Transpl Int. 2011;24:1216–1230. [DOI] [PubMed] [Google Scholar]
- 26.Kobashigawa JA, Miller LW, Russell SD, et al. Tacrolimus with mycophenolate mofetil (MMF) or sirolimus vs. cyclosporine with MMF in cardiac transplant patients: 1-year report. Am J Transplant. 2006;6:1377–1386. [DOI] [PubMed] [Google Scholar]
- 27.Kobashigawa J, Ross H, Bara C, et al. Everolimus is associated with a reduced incidence of cytomegalovirus infection following de novo cardiac transplantation. Transpl Infect Dis. 2013;15:150–162. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe T, Seguchi O, Nishimura K, et al. Suppressive effects of conversion from mycophenolate mofetil to everolimus for the development of cardiac allograft vasculopathy in maintenance of heart transplant recipients. Int J Cardiol. 2016;203:307–314. [DOI] [PubMed] [Google Scholar]
- 29.Rivinius R, Helmschrott M, Ruhparwar A, et al. Analysis of malignancies in patients after heart transplantation with subsequent immunosuppressive therapy. Drug Des Devel Ther. 2014;9:93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centro Nazionale Trapianti. Sistema informativo trapianti. https://trapianti.sanita.it/statistiche/. Updated August 30, 2017. Accessed February 7, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


