ABSTRACT
Purpose
The presence of bone metastases has excluded participation of cancer patients in exercise interventions and is a relative contraindication to supervised exercise in the community setting because of concerns of fragility fracture. We examined the efficacy and safety of a modular multimodal exercise program in prostate cancer patients with bone metastases.
Methods
Between 2012 and 2015, 57 prostate cancer patients (70.0 ± 8.4 yr; body mass index, 28.7 ± 4.0 kg·m−2) with bone metastases (pelvis, 75.4%; femur, 40.4%; rib/thoracic spine, 66.7%; lumbar spine, 43.9%; humerus, 24.6%; other sites, 70.2%) were randomized to multimodal supervised aerobic, resistance, and flexibility exercises undertaken thrice weekly (EX; n = 28) or usual care (CON; n = 29) for 3 months. Physical function subscale of the Medical Outcomes Study Short-Form 36 was the primary end point as an indicator of patient-rated physical functioning. Secondary end points included objective measures of physical function, lower body muscle strength, body composition, and fatigue. Safety was assessed by recording the incidence and severity of any adverse events, skeletal complications, and bone pain throughout the intervention.
Results
There was a significant difference between groups for self-reported physical functioning (3.2 points; 95% confidence interval, 0.4–6.0 points; P = 0.028) and lower body muscle strength (6.6 kg; 95% confidence interval, 0.6–12.7; P = 0.033) at 3 months favoring EX. However, there was no difference between groups for lean mass (P = 0.584), fat mass (P = 0.598), or fatigue (P = 0.964). There were no exercise-related adverse events or skeletal fractures and no differences in bone pain between EX and CON (P = 0.507).
Conclusions
Multimodal modular exercise in prostate cancer patients with bone metastases led to self-reported improvements in physical function and objectively measured lower body muscle strength with no skeletal complications or increased bone pain.
Trial Registration: ACTRN12611001158954.
Key Words: BONE METASTASES, PROSTATE CANCER, RANDOMIZED CONTROLLED TRIAL, EXERCISE
The clinical course of metastatic bone disease in prostate cancer (PCa) patients is relatively long, with a 5-yr survival rate of approximately 30% (1). Metastases to bone occurs in approximately 80% of men with advanced PCa (2), and most of these patients are at risk for developing pathological fractures, hypercalcemia, bone marrow suppression, and nerve compression or spinal cord compression that results in significant morbidity, limited function, and decreased quality of life (3–5). Consequently, improving physical function and delaying or preventing skeletal complication in patients with bone metastases can provide clinically meaningful benefits to patients.
Over the past decade, numerous clinical trials investigating the efficacy of exercise in men with PCa, including our previous work, excluded patients with bone metastases (6–11), or bone lesions deemed clinically “unstable” (12) because of the potential risk of skeletal fractures, spinal compression, or exacerbation of bone pain. Nevertheless, international exercise oncology guidelines suggest that cancer patients, including those with bone metastases, should avoid inactivity (13,14). However, it is unknown if exercise is efficacious at this stage of the disease and whether it can be tolerated by this patient group given the current absence of clinical data on exercise safety, tolerability, and efficacy (15).
In this clinical trial, we tested the efficacy and safety of a modular multimodal exercise program (M3EP) comprising resistance, aerobic, and flexibility training taking into consideration the location and extent of bone metastases as a strategy to maintain or enhance physical function in this group of patients with advanced PCa. Consequently, the program was based on a mechanical perspective to avoid direct loading to the metastatic lesions. Change in the physical function subscale of the Medical Outcomes Study Short-Form 36 (SF-36) over 3 months served as the primary study end point. Secondary end points included safety and objective measures of physical function, muscle strength, body composition, and fatigue. We hypothesized that a short-term modular multimodal exercise regimen would preserve physical function in patients with bone metastases without increasing skeletal complications.
METHODS
Patients
One hundred three patients with PCa were referred by their oncologist or urologist in Perth, Western Australia, from August 2012 to August 2015. Inclusion criteria included established bone metastases according to their most recent bone scan, and this included at any time point of their bone metastatic disease (e.g., at onset of bone metastatic diagnosis or at any time point thereafter), no acute illness, and no significant bone pain as assessed by their clinician. Exclusion criteria included the presence of any musculoskeletal, cardiovascular, or neurological disorders that could inhibit patients from exercising, and if patients had undertaken structured and supervised aerobic and/or resistance training two or more times per week within the past 3 months (15). The study was approved by the University Human Research Ethics Committee, and all participants provided written informed consent.
Study design and random assignment
This was a two-armed prospective randomized controlled trial (RCT) (15). Potential patients were referred to the study coordinator to confirm eligibility, describe the study, and obtain informed consent. Patients underwent a familiarization session followed by baseline testing comprising physical and functional tests, a dual-energy x-ray absorptiometry scan, questionnaires, and a blood draw. After the baseline assessment, patients were randomly assigned to exercise (EX) or usual care controls (CON) in a ratio of 1/1 using a computer random assignment program subject to maintaining approximate balance regarding stratification for current chemotherapy. The allocation sequence was concealed from the project coordinator and exercise physiologist involved in assigning patients to groups. Patients in CON were asked not to change their physical activity and were offered the same exercise program after the 3-month control period.
Exercise training program
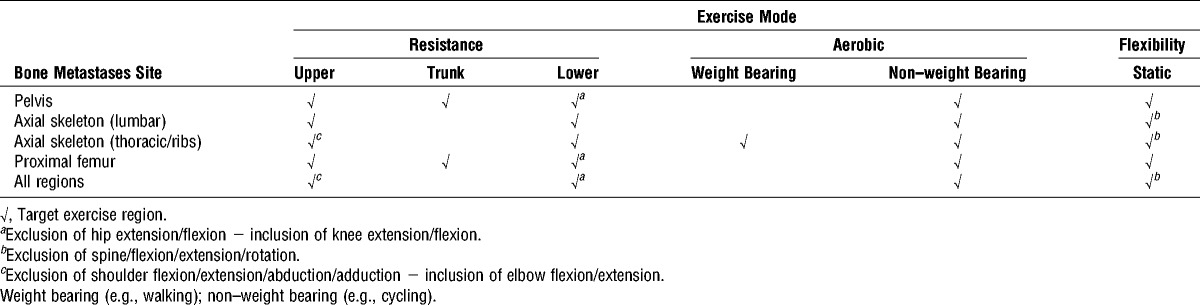
The M3EP comprised resistance, aerobic, and flexibility exercises undertaken three times per week in a university exercise clinic supervised by an exercise physiologist and has been described in detail elsewhere (15). In brief, the exercise prescription was based on location/extent of bone metastases to avoid specific loading of the sites (Table 1), with exercise sessions lasting ~60 min. The resistance exercise component targeted the major trunk and upper and lower body muscle groups, which we have used in previous studies (8,16,17), at a moderate intensity ranging from 10- to 12-repetition maximum (RM; e.g., maximal weight that can be lifted 10–12 times) for three sets per exercise. Exercises were performed at a set cadence of 2 s for both eccentric and concentric phases, minimizing peak forces transmitted to the skeleton (18,19). To ensure progression, resistance was increased by 5%–10% for the next set/training session when the RM values were exceeded during a set. The aerobic exercise component included 20–30 min of cardiovascular exercise using various modes such as walking on a treadmill, cycling, or rowing on a stationary ergometer on the basis of disease extent with a target intensity of 60%–85% estimated maximal heart rate. The flexibility component involved static stretching, 2–4 repetitions for 30–60 s per stretch for all major joints considered important for maintaining function (20). Each session commenced with a 10-min warm-up comprising low-level aerobic activities such as treadmill walking and stationary cycling as determined by bone metastases site, as well as stretching, and concluded with a 5-min cool-down period of stretching activities.
TABLE 1.
M3EP for PCa with bone metastases.
Primary and secondary study end points
Study end points were assessed at baseline and after the 12-wk intervention. The primary study end point was the physical function subscale from the SF-36 questionnaire (Table 2) used as an indicator of patient-rated physical functioning (21). Secondary end points included objective measures of physical function assessed by the timed up-and-go test, 6-m usual and fast walk using electronic timing gates, and the 400-m walk test (8,17). Tests were performed in triplicate (except 400 m) with recovery time between trials. Dynamic muscle strength was determined for the leg extension exercise and the chest press exercise using the 1-RM method (22). Patients with proximal femur bone lesions were excluded from the leg extension 1-RM and 400-m walk test, whereas those with rib/thoracic spine lesions and humerus lesions were excluded from the chest press 1-RM. Balance was assessed by the sensory organization test (NeuroCom International Inc, Clackamas, OR) (8). Whole-body lean mass and fat mass were assessed by dual-energy x-ray absorptiometry (Hologic Discovery A, Waltham, MA). Fatigue was assessed using the Functional Assessment of Chronic Illness Therapy—Fatigue questionnaire (23). Prostate-specific antigen was measured commercially by a nationally accredited laboratory (8).
TABLE 2.
Physical functional questions from the SF-36.

Safety of the program
Safety of the exercise program was assessed by recording the incidence and severity of any adverse events, including skeletal complications, throughout the intervention. Skeletal complications included pain at known bone metastases sites and pathological skeletal fractures (24). Bone pain was monitored according to the Common Terminology Criteria of the National Cancer Institute: grade 1, mild, not interfering with function; grade 2, moderate pain, interfering with function but not interfering with the activities of daily life; and grade 3, severe pain, severely interfering with the activities of daily living. The Functional Assessment of Cancer Therapy—Bone Pain was used to assess bone pain (25). Tolerance of the exercise program was evaluated by recording patients’ ratings of perceived exertion, with the ratings of perceived exertion assessed at the end of each session to represent the cumulative effect of the exercise session (26). In addition, exercise tolerance was assessed before the first exercise session each week using a 0- to 7-point scale, with higher scores indicating greater tolerance. The number of patients completing the intervention and sessions attended was recorded.
Statistical analyses and sample size calculation
The sample size estimate for the RCT was based on projected changes in the primary outcome of self-reported physical function from the SF-36. A priori, 36 subjects per group were required to achieve 80% power at an alpha level of 0.05 (two-tailed) to detect a 3-point difference between groups at the end of the 3-month intervention. To account for projected dropout, our goal was to recruit 90 patients in total. Data were analyzed using IBM SPSS Version 24 (IBM Corp, Armonk, NY). Normality of the distribution for outcome measures was tested using the Kolmogorov–Smirnov test. Between-group differences in baseline characteristics for continuous data were assessed using independent t tests or the Mann–Whitney U test, as appropriate, and chi-square test for categorical data. Analysis of covariance adjusted for baseline values was used to assess the intervention effects on the primary and secondary study end points. Tests were two-tailed with an alpha level of 0.05 applied as the criterion for statistical significance. Because of the variation in number of patients able to undertake objective assessments due to bone metastases, we used complete cases for the analyses.
RESULTS
Patients characteristics
A total of 103 patients with PCa bone metastatic disease were screened for participation and 57 patients were randomly assigned to the two study arms, and progress through the study is shown in Figure 1. Forty-six patients declined participation or were excluded (Fig. 1). Patient characteristics are presented in Table 3. There were no significant differences between groups at baseline. Patients were 70.0 ± 8.4 yr of age (mean ± SD) with a body mass index of 28.7 ± 4.0 kg·m−2 and had extensive bone disease with metastatic lesions present in the pelvis (75.4%), femur (40.4%), rib/thoracic spine (66.7%), lumbar spine (43.9%), humerus (24.6%), and other sites (70.2%). Ninety-five percent of patients were undertaking androgen deprivation therapy (ADT) and 16% of patients were on current or previous chemotherapy. During the course of the trial, five men withdrew from the exercise program, whereas three men withdrew from the usual care group (Fig. 1). There was no significant change in prostate-specific antigen (P = 0.567) during the study period.
FIGURE 1.
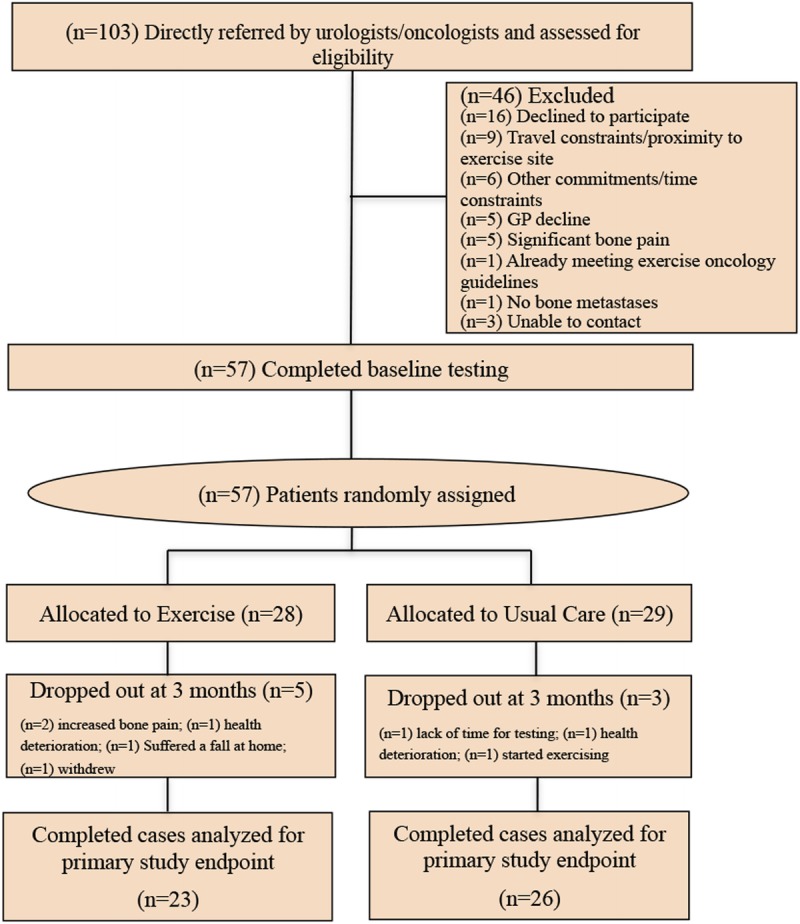
CONSORT diagram.
TABLE 3.
Patient characteristics.
Self-reported physical function
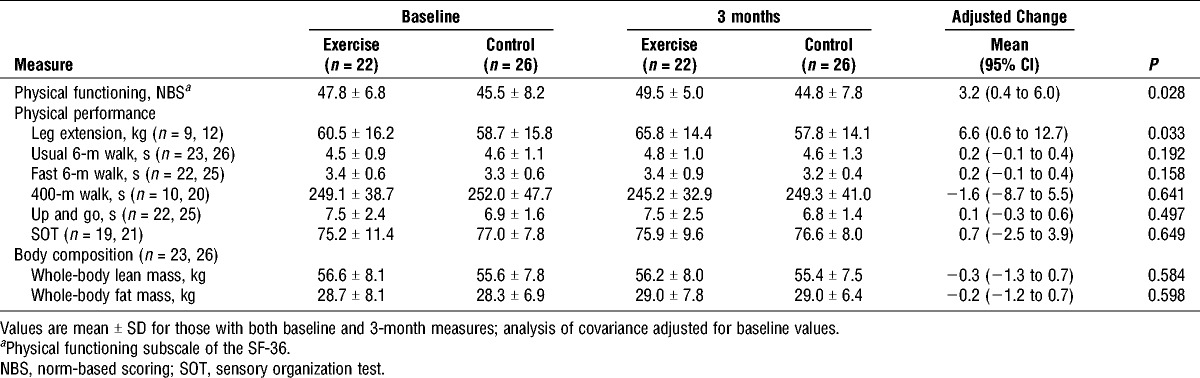
There was no difference between groups for self-reported physical function (P = 0.682) at baseline (Table 4). After 3 months of the intervention, there was a significant difference between groups for self-reported physical function favoring EX (3.2 points; 95% confidence interval (CI), 0.4–6.0 points; P = 0.028).
TABLE 4.
Physical functioning, physical performance, and body composition at baseline and 3 months.
Muscle strength, body composition, objective measures of physical function, and fatigue
There were no differences between groups at baseline for leg extension muscle strength, physical function, body composition, or fatigue (P = 0.214–0.824). Muscle strength differed significantly between groups for the leg extension exercise as a result of the intervention, with the EX group improving at 3 months with an adjusted mean difference of 6.6 kg (95% CI, 0.6–12.7; P = 0.033; Table 4). However, on the basis of spine and upper body lesions, only four patients were able to complete both baseline and 12-wk chest press assessments, and as a result, data are not shown. There were no changes for objective measures of physical function, balance, lean mass, or total body fat mass. Furthermore, there was no change in fatigue (P = 0.964) assessed by the Functional Assessment of Chronic Illness Therapy—Fatigue during the study period.
Safety, tolerance, and attendance of the exercise program
EX completed a mean of 32 ± 10 of the 36 exercise sessions (89% attendance) with no exercise-related adverse events or skeletal fractures. Reasons for missed sessions were medical appointments, travel, and social commitments. Session perceived exercise intensity (6–20 scale) was 12.7 ± 1.2, perceived tolerance (0–7 scale) was 5.5 ± 1.1, and Common Terminology Criteria pain grade (0–3 scale) was 0.2 ± 0.3. There were no changes in bone pain assessed by the Functional Assessment of Cancer Therapy—Bone Pain (P = 0.507).
DISCUSSION
This study examined the efficacy and safety of an M3EP in PCa patients with bone metastases. There were four important findings: 1) self-reported physical functioning (primary study end point) and lower body muscle strength were significantly increased in EX compared with CON; 2) neither bone pain nor skeletal events were exacerbated in EX as a result of the intervention; and 3) the program was well tolerated, and there was high attendance and compliance by this group of patients who have extensive disease burden; however, 4) body composition, objective measures of physical function, and fatigue were not different between groups.
Patients with bone metastases experience considerable morbidity resulting from skeletal complications and decline in physical function, with increased fatigue secondary to chemotherapy for those with castrate-resistance PCa (4,27). Our results demonstrated notable changes in self-reported physical functioning of 3.2 points between study arms at 12 wk, indicating significant and clinically relevant improvements in physical functioning for the EX group. Declines of 3 points reflect clinically meaningful changes (28), and we previously showed similar changes of ~3.5 in patients with localized PCa previously treated with ADT/radiation (17). We also provide further evidence of muscle strength improvements in this group of patients with advanced PCa, which extends previous work in PCa patients with localized disease (8,17) and our pilot work in those with bone metastases undertaking resistance training as the sole intervention mode (29).
We found that neither bone pain nor skeletal events were exacerbated in the EX group as a result of the intervention. This is an important finding given the absence of clinical data on exercise safety, tolerability, and efficacy in PCa patients with bone metastases undertaking aerobic, resistance, and flexibility modes of exercise, and that international guidelines have endorsed exercise training for cancer patients (13) including those with bone metastases without supporting empirical evidence. Specifically, our modular multimodal targeted exercise program was designed to minimize compressive and shear loads on affected skeletal sites to account for the reduced load-bearing capabilities of bone due to metastatic disease in specific regions (15). However, because PCa primarily generates sclerotic (osteoblastic) lesions that commonly metastasize to the pelvis and axial skeleton (30), our findings are limited to PCa patients with sclerotic bone metastases. Ongoing studies are extending this work by examining the safety and preliminary efficacy of controlled spinal isometric training in PCa patients with sclerotic lesions on tumor morphology and circulating metastatic tumor biomarkers (31).
We also observed high attendance and compliance and low attrition to the intervention, similar to patients with localized PCa undertaking short-term exercise programs (8). Although the intervention in this study was highly supervised, the nature of the modular prescription approach is patient inclusive, such that all patients can be prescribed some amount of exercise, which therefore has the potential to be performed and implemented in different centers reaching a significant number of patients. This is particularly important given the increased number of cohort studies suggesting an association between increased exercise and PCa-specific and overall survival (32,33). Furthermore, our findings contribute substantially to identifying strategies to prescribe exercise for patients with advanced PCa, particularly with recent developments and changes in the clinical practice landscape with novel pharmaceutical interventions (i.e., abiraterone, enzalutamide) for metastatic castrate resistance PCa (mCRPC). These are of clinical importance given that many mCRPC patients will develop or have bone metastatic lesions. Furthermore, the multimodal and modular components of the exercise prescription support the exercise intervention of a phase 3 trial in mCRPC, for which the primary outcome is overall survival (34,35).
Although patients improved their perceived physical functioning and objective muscle strength, the lack of changes in objective measures of physical function, body composition, or fatigue is intriguing. This could be explained by the use of very conservative modular exercise prescriptions so that all patients received some form of intervention, and as a result, the exercise stimulus may not have been sufficient to induce changes in objective measures of physical function, lean mass, or fat mass, or for fatigue. Furthermore, patients were intentionally excluded from several measures on the basis of bone metastases location, hence reducing the statistical power to detect changes in some of these secondary study end points. As a result, we intentionally used self-reported physical function as our a priori primary study end point to capture all patients with complete data.
Our study has several strengths and limitations worthy of comment. This is the largest RCT evaluating the effects of exercise in PCa patients with bone metastases or in any other cancer group with bone metastatic disease, resulting to a paradigm shift in relation to exercise prescription in advanced PCa. Our novel but conservative approach with M3EP may have limited our ability to observe changes in the patients’ achievable gains in our secondary end points. We were only able to recruit 57 patients as opposed to 90 patients we originally planned; however, this was a very challenging trial to recruit given the high disease burden of patients, and as such, recruitment pathways were different from those in patients with localized disease with referrals largely dependent on a small team of oncologists. Nevertheless, we have shown significant and clinically meaningful improvements in self-reported physical function (primary study end point) with as little as 12-wk training undertaken three times a week with high compliance and low attrition, and this remains the largest exercise trial in patients with bone metastases. Lastly, our protocol could be easily replicated on a larger scale using the modular targeted exercise program where exercise can be performed without the bone metastatic lesions being loaded.
CONCLUSIONS
Exercise improved self-reported physical functioning in PCa patients with bone metastases with no exercise-related adverse events, skeletal fractures, or increased bone pain. In addition, the program was well perceived and tolerated by the patients. These changes are likely to provide clinically meaningful benefits to PCa patients with bone metastases.
Acknowledgments
This study was funded by Movember New Directions Development Award obtained through Prostate Cancer Foundation of Australia’s Research Program. Daniel A. Galvão is funded by a Cancer Council Western Australia Research Fellowship. Suzanne Chambers is supported by an Australian Research Council Professorial Future Fellowship. Daniel A. Galvão, Suzanne Chambers, Nigel Spry, and Robert Newton are funded by a National Health and Medical Research Council Centre for Research Excellence in Prostate Cancer Survivorship.
The sponsors did not participate in the design or conduct of the study; collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. All authors had no conflict of interest, including relevant financial interests, activities, relationships, and affiliations to declare relating to this manuscript. The results of the study are presented clearly, honestly, without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Daniel A. Galvão had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. [DOI] [PubMed] [Google Scholar]
- 2.Small EJ, Smith MR, Seaman JJ, Petrone S, Kowalski MO. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003;21(23): 4277–84. [DOI] [PubMed] [Google Scholar]
- 3.Fizazi K, Beuzeboc P, Lumbroso J, et al. Phase II trial of consolidation docetaxel and samarium-153 in patients with bone metastases from castration-resistant prostate cancer. J Clin Oncol. 2009;27(15):2429–35. [DOI] [PubMed] [Google Scholar]
- 4.Carlin BI, Andriole GL. The natural history, skeletal complications, and management of bone metastases in patients with prostate carcinoma. Cancer. 2000;88(12 Suppl):2989–94. [DOI] [PubMed] [Google Scholar]
- 5.Saad F, Olsson C, Schulman CC. Skeletal morbidity in men with prostate cancer: quality-of-life considerations throughout the continuum of care. Eur Urol. 2004;46(6):731–9; discussion 9–40. [DOI] [PubMed] [Google Scholar]
- 6.Galvao DA, Nosaka K, Taaffe DR, et al. Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sports Exerc. 2006;38(12):2045–52. [DOI] [PubMed] [Google Scholar]
- 7.Galvao DA, Nosaka K, Taaffe DR, et al. Endocrine and immune responses to resistance training in prostate cancer patients. Prostate Cancer Prostatic Dis. 2008;11(2):160–5. [DOI] [PubMed] [Google Scholar]
- 8.Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28(2):340–7. [DOI] [PubMed] [Google Scholar]
- 9.Segal RJ, Reid RD, Courneya KS, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27(3):344–51. [DOI] [PubMed] [Google Scholar]
- 10.Taaffe DR, Newton RU, Spry N, et al. Effects of different exercise modalities on fatigue in prostate cancer patients undergoing androgen deprivation therapy: a year-long randomised controlled trial. Eur Urol. 2017;72(2):293–9. [DOI] [PubMed] [Google Scholar]
- 11.Wall BA, Galvao DA, Fatehee N, et al. Exercise improves V˙O2max and body composition in androgen deprivation therapy–treated prostate cancer patients. Med Sci Sports Exerc. 2017;49(8):1503–10. [DOI] [PubMed] [Google Scholar]
- 12.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21(9):1653–9. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–26. [DOI] [PubMed] [Google Scholar]
- 14.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–74. [DOI] [PubMed] [Google Scholar]
- 15.Galvão DA, Taaffe DR, Cormie P, et al. Efficacy and safety of a modular multi-modal exercise program in prostate cancer patients with bone metastases: a randomized controlled trial. BMC Cancer. 2011;11:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Acute versus chronic exposure to androgen suppression for prostate cancer: impact on the exercise response. J Urol. 2011;186(4):1291–7. [DOI] [PubMed] [Google Scholar]
- 17.Galvao DA, Spry N, Denham J, et al. A multicentre year-long randomised controlled trial of exercise training targeting physical functioning in men with prostate cancer previously treated with androgen suppression and radiation from TROG 03.04 RADAR. Eur Urol. 2014;65(5):856–64. [DOI] [PubMed] [Google Scholar]
- 18.Cronin JB, McNair PJ, Marshall RN. Force–velocity analysis of strength-training techniques and load: implications for training strategy and research. J Strength Cond Res. 2003;17(1):148–55. [DOI] [PubMed] [Google Scholar]
- 19.Frost DM, Cronin J, Newton RU. A biomechanical evaluation of resistance: fundamental concepts for training and sports performance. Sports Med. 2010;40(4):303–26. [DOI] [PubMed] [Google Scholar]
- 20.Galvao DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol. 2005;23(4):899–909. [DOI] [PubMed] [Google Scholar]
- 21.Ware JE, Jr, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol. 1998;51(11):903–12. [DOI] [PubMed] [Google Scholar]
- 22.Galvao DA, Taaffe DR. Resistance exercise dosage in older adults: single- versus multiset effects on physical performance and body composition. J Am Geriatr Soc. 2005;53(12):2090–7. [DOI] [PubMed] [Google Scholar]
- 23.Reddy S, Bruera E, Pace E, Zhang K, Reyes-Gibby CC. Clinically important improvement in the intensity of fatigue in patients with advanced cancer. J Palliat Med. 2007;10(5):1068–75. [DOI] [PubMed] [Google Scholar]
- 24.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3): 165–76. [DOI] [PubMed] [Google Scholar]
- 25.Broom R, Du H, Clemons M, et al. Switching breast cancer patients with progressive bone metastases to third-generation bisphosphonates: measuring impact using the Functional Assessment of Cancer Therapy—Bone Pain. J Pain Symptom Manage. 2009;38(2): 244–57. [DOI] [PubMed] [Google Scholar]
- 26.Borg GA. Perceived exertion: a note on “history” and methods. Med Sci Sports. 1973;5(2):90–3. [PubMed] [Google Scholar]
- 27.Weinfurt KP, Li Y, Castel LD, et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol. 2005;16(4):579–84. [DOI] [PubMed] [Google Scholar]
- 28.Ware JE, Jr, Kosinski M, Bjorner JB, Turner-Bowker DM, Gandek B, Maruish ME. User’s Manual for the SF-36v2 Health Survey. 2nd ed Lincoln (RI): QualityMetric Incorporated; 2008. [Google Scholar]
- 29.Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvao DA. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16(4): 328–35. [DOI] [PubMed] [Google Scholar]
- 30.Lee RJ, Saylor PJ, Smith MR. Treatment and prevention of bone complications from prostate cancer. Bone. 2011;48(1):88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hart NH, Newton RU, Spry NA, et al. Can exercise suppress tumour growth in advanced prostate cancer patients with sclerotic bone metastases? A randomised, controlled study protocol examining feasibility, safety and efficacy. BMJ Open. 2017;7(5):e014458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedenreich CM, Wang Q, Neilson HK, Kopciuk KA, McGregor SE, Courneya KS. Physical activity and survival after prostate cancer. Eur Urol. 2016;70(4):576–85. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Jacobs EJ, Gapstur SM, et al. Recreational physical activity in relation to prostate cancer-specific mortality among men with nonmetastatic prostate cancer. Eur Urol. 2017; [Epub ahead of print]. doi: 10.1016/j.eururo.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 34.Newton RU, Galvão DA. Accumulating evidence for physical activity and prostate cancer survival: time for a definitive trial of exercise medicine? Eur Urol. 2016;70(4):586–7. [DOI] [PubMed] [Google Scholar]
- 35.Hart NH, Galvao DA, Newton RU. Exercise medicine for advanced prostate cancer. Curr Opin Support Palliat Care. 2017;11(3):247–57. [DOI] [PubMed] [Google Scholar]


