Abstract
To investigate the mechanism of azalomycin F5a against methicillin-resistant Staphylococcus aureus (MRSA), the conductivity of MRSA suspension and the adenylate kinase activity of MRSA culture were determined with the intervention of azalomycin F5a, which were significantly increased compared to those of blank controls. This inferred that azalomycin F5a could lead to the leakage of cellular substances possibly by increasing permeability to kill MRSA. As phospholipid bilayer was mainly responsible for cell-membrane permeability, the interaction between azalomycin F5a and cell-membrane lipids was further researched by determining the anti-MRSA activities of azalomycin F5a combined with cell-membrane lipids extracted from test MRSA or with 1,2-dipalmitoyl-sn-glycero-3-phospho-glycerol (DPPG) for possible molecular targets lying in MRSA cell-membrane. The results indicated that the anti-MRSA activity of azalomycin F5a remarkably decreased when it combined with membrane lipids or DPPG. This indicated that cell-membrane lipids especially DPPG might be important targets of azalomycin F5a against MRSA.
1. Introduction
Azalomycin F, a complex of polyhydroxy macrolides including three main components azalomycins F5a, F4a, and F3a, was first isolated from the broth of Streptomyces hygroscopicus var. azalomyceticus [1, 2]. Unlike macrolide antibiotics as erythromycin and polyene macrolides as amphotericin B, it presented significant antimicrobial and antifungal activities [1–3]. As Sugawara reported, azalomycin F could lead to the leakage of cellular substances to kill Bacillus subtilis, while detailed mechanism had not been further reported because their chemical structures were not clear at that time [4]. Following studies indicated that many streptomycetes such as S. hygroscopicus and S. malaysiensis also had great potential to produce these compounds [3, 5]. Recently, twelve 36-membered macrocyclic lactones including azalomycins F5a, F4a, and F3a were also isolated from the broth of Streptomyces sp. 211726, and their planar structures and relative configurations were elucidated by us in 2011 to 2013 [6–8]. Considering the research and development of new antimicrobial drugs were desperate [9], the inhibitory activities of azalomycins F5a, F4a, and F3a against multidrug-resistant organisms were further tested, and the results showed they had significant antagonistic activities to methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococci [10]. Moreover, 23-demalonyl azalomycin F obtained by hydrolysis of azalomycin F presented more remarkable anti-MRSA activity and better aqueous solubility and stability than azalomycin F [11]. Thereby, azalomycin F5a as a representative of 36-membered macrolides was worthy of further researching, especially for its anti-MRSA mechanism.
2. Materials and Methods
2.1. MRSA Strain
MRSA ATCC 33592 obtained from American Type Culture Collection, Manassas, VA, USA, was inoculated in 20 mL of Mueller Hinton Broth (MHB) (Haibo Biotechnology Co., Ltd., Qingdao, China) and cultured at 37°C for 24 h on a rotary shaker (160 rpm). The cultures were diluted with MHB (1 : 100) and were incubated at 37°C for 8 h on a rotary shaker (160 rpm) to obtain MRSA cultures at exponential phase (approx. 1 × 108 cfu/mL) for following experiments.
2.2. Azalomycin F5a
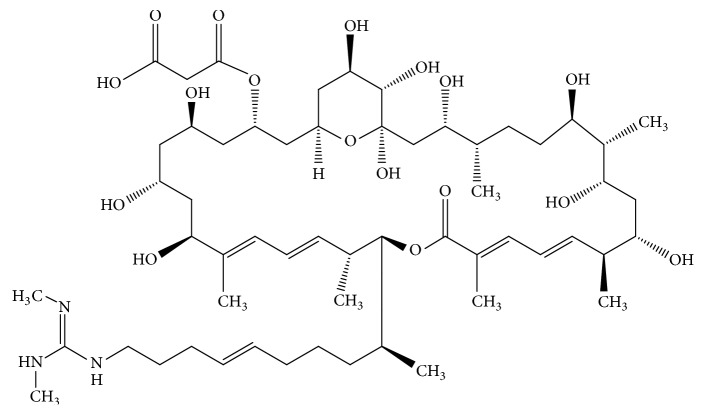
Azalomycin F5a (Figure 1) was isolated from the broth of Streptomyces hygroscopicus var. azalomyceticus according to our previous method [6], and its purity (98.2%) was analyzed by Waters 2695 Alliance HPLC System. It was dissolved in DMSO to obtain the concentration of 2048 μg/mL before use and made sure that the concentration of DMSO was less than 1.6% in test system. The minimum inhibitory concentration (MIC) of azalomycin F5a against MRSA ATCC 33592 was 4.0 μg/mL, which was determined in our published work [12].
Figure 1.
Chemical structure of azalomycin F5a.
2.3. Conductivity of MRSA Suspension
One hundred milliliters of MRSA cultures at exponential phase was centrifuged for 10 min at 5,600g, and the cells were suspended in 100 mL of 20 mM phosphate buffer (pH 6.0). Referring to the previous method [13], the conductivity of MRSA suspension with the intervention of azalomycin F5a was determined in triplicate. Briefly, the MRSA suspension was divided into three equal ones, two of which contained, respectively, azalomycin F5a to obtain concentrations of 0.5 MIC (2.0 μg/mL) and 1.0 MIC (4.0 μg/mL). The third one without azalomycin F5a was used as blank control. Then, the conductivity of each suspension was, respectively, determined at 0, 20, 40, 60, 80, 120, and 180 min after azalomycin F5a was added.
2.4. Adenylate Kinase Activity
Referring to the previous method [14], adenylate kinase activity was determined in triplicate using 96-well plates. In brief, fifty milliliters of MRSA cultures at exponential phase (approx. 1 × 108 cfu/mL) was added to each well containing 50 μL MHB with different concentrations of azalomycin F5a. The final concentrations of azalomycin F5a in the wells of lines C to F were, respectively, 0.25, 0.5, 1.0, and 2.0 of MIC, and the wells of line B without azalomycin F5a were used for blank controls. Next, the plates were incubated at 37°C for 6 h on a rotary shaker (220 rpm) and then were equilibrated for 30 min at room temperature. Finally, 100 μL of adenylate kinase reagent (Lonza Rockland, Inc., USA) was added to each well according to the instruction of ToxiLight® BioAssay Kit and incubated 30 min at room temperature in darkness. The relative luminescence unit (RLU) of each well was measured using a SpectraMax M5 microplate reader.
2.5. Influences of MRSA Cell-Membrane Lipids on Azalomycin F5a against MRSA
MRSA cells at exponential phase were collected by centrifugation at 4000 rpm for 20 min and washed three times in 10 mM Tris-HCl, pH 3.0. Then, MRSA cell-membrane lipids were extracted by modified Bligh-Dyer method [15]. Briefly, 15 mL of CHCl3-CH3OH (2 : 1, v/v) was added to 4 mL of MRSA suspension and then extracted on a vortex mixer for 30 min. Next, 5 mL of CHCl3 and 5 mL of 10 mM Tris-HCl (pH 3.0) were successively added to the mixture for further extraction of phospholipids. Finally, the organic phase was collected and concentrated in vacuum on a rotary evaporator to obtain MRSA cell-membrane lipids.
Next, MRSA cultures at exponential phase were diluted with MHB (1 : 100) for anti-MRSA activity test. With the intervention of 128 or 512 μg/mL of MRSA cell-membrane lipids, the MICs of azalomycin F5a against MRSA ATCC 33592 were, respectively, measured in triplicate on a 96-well plate using broth microdilution method [12, 16]. MHB that only contained azalomycin F5a and 128 or 512 μg/mL of MRSA cell-membrane lipids were, respectively, used as controls; the influences of MRSA cell-membrane lipids on azalomycin F5a against MRSA were investigated.
2.6. Influences of DPPG on Azalomycin F5a against MRSA
MRSA cultures at exponential phase (approx. 1 × 108 cfu/mL) were diluted with MHB (1 : 100) for anti-MRSA activity test. With the intervention of 32 and 64 μg/mL of 1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol (DPPG) purchased from Avanti Polar Lipids, (USA), the MICs of azalomycin F5a against MRSA ATCC 33592 were measured in triplicate on a 96-well plate using broth microdilution method [12, 16]. MHB that only contained azalomycin F5a and 32 or 64 μg/mL of DPPG were, respectively, used as controls; the influences of DPPG on azalomycin F5a against MRSA were further investigated.
2.7. Statistical Analysis
All statistical analyses and diagrams were performed with Microsoft Office Excel 2007. The results were expressed as , and t-test was used for the comparison between two groups. P ≤ 0.05 indicates a significant difference between blank control and azalomycin F5a group with specific concentration.
3. Results
3.1. Conductivity of MRSA Suspension
Treated with different concentrations of azalomycin F5a, the conductivities of MRSA suspensions were showed in Figure 2. Being relative to blank control, the conductivities of suspensions containing azalomycin F5a significantly increased, while the increasing rates and final conductivities were different along with different concentrations of azalomycin F5a. Moreover, treated with 4.0 μg/mL of azalomycin F5a, the suspension conductivity increased rapidly in 20 to 60 min, and the following conductivity tended to be stable. These indicated that the cellular substances of MRSA leaked rapidly in 20 to 60 min and finally led to the cell lysis of MRSA.
Figure 2.

Conductivities of MRSA suspensions treated with different concentrations of azalomycin F 5a. MRSA ATCC 33592 suspensions were, respectively, treated with 0.5 and 1.0 MIC of azalomycin F5a, and their conductivities (n = 3) were recorded at 0, 20, 40, 60, 80, 120, 160, and 180 min using a conductivity meter. ∗ indicates a significant difference between blank control and azalomycin F5a group with specific concentration (P ≤ 0.05).
3.2. Adenylate Kinase Activity
The adenylate kinase activities of MRSA cultures treated with different concentrations of azalomycin F5a were showed in Figure 3. The results indicated that luminescence remarkably increased as the concentration of azalomycin F5a increased. The cultures presented highest luminescence when the concentration of azalomycin F5a was 4.0 μg/mL (equal to MIC), and this deduced that azalomycin F5a could lead to the leakage of adenylate kinase from MRSA cell. Moreover, the luminescence decreased slightly when the concentration of azalomycin F5a was 8.0 μg/mL, which might be attributed to the decreased cell numbers at this concentration (2 MIC) of azalomycin F5a.
Figure 3.

Adenylate kinase activities of MRSA cultures treated with different concentrations of azalomycin F5a. MRSA ATCC 33592 cultures, respectively, treated with 1.0, 2.0, 4.0, and 8.0 μg/mL of azalomycin F5a, were incubated at 37°C for 6 h, and their luminescence (n = 3) was recorded using a SpectraMax M5 microplate reader. ∗ indicates a significant difference between blank control and azalomycin F5a group with specific concentration (P ≤ 0.05).
3.3. Influences of MRSA Cell-Membrane Lipids on Azalomycin F5a against MRSA
The MICs of azalomycin F5a against MRSA ATCC 33592 were, respectively, 16 and 32 μg/mL with the intervention of 128 and 512 μg/mL of cell-membrane lipids extracted from test MRSA, and that without the intervention of lipids was 4.0 μg/mL. These indicated that the anti-MRSA activity of azalomycin F5a could be weakened by cell-membrane lipids extracted from test MRSA and further deduced that there were some interactions between azalomycin F5a and cell-membrane lipids.
3.4. Influences of DPPG on Azalomycin F5a against MRSA
Both of the MICs of azalomycin F5a against MRSA ATCC 33592 were 32 μg/mL with the intervention of 32 and 64 μg/mL of DPPG, while that without the intervention of DPPG was 4.0 μg/mL. These indicated that the anti-MRSA activity of azalomycin F5a could be weakened by DPPG and deduced that there were some interactions between azalomycin F5a and DPPG.
4. Discussion
Considering that antibiotic resistance spread widely, the research and development of new antibiotics against multidrug-resistant organisms were desperate [9, 17]. After the planar structure and relative configuration of azalomycin F5a were elucidated by us, its anti-MRSA and anti-VRE activities were discovered in our previous works [10, 12]. Inspired by the fact that azalomycin F could lead to the leakage of cellular substances of B. subtilis [4], the interactions between azalomycin F5a, as a representative of these compounds, and MRSA cell membrane were researched. The results showed that azalomycin F5a could significantly increase the conductivity of MRSA suspensions when its concentration increased to 4.0 μg/mL (equal to MIC). This indicated that a large amount of cellular substances leaked from MRSA cell treated with azalomycin F5a and further deduced that that azalomycin F5a killed MRSA likely by damaging cell membrane or increasing permeability. As adenylate kinase was an intracellular substance, the fact that extracellular adenylate kinase activity remarkably increased when the concentration of azalomycin F5a increased also proved above inferences.
The MRSA cell membrane mainly contains lipids and proteins, and the former is an important factor for cell-membrane integrity, stability, and permeability. To explore the interactions between azalomycin F5a and cell membrane, the anti-MRSA activity of azalomycin F5a against MRSA ATCC 33592 was determined with the intervention of cell-membrane lipids extracted from test MRSA. The results showed the anti-MRSA activity of azalomycin F5a could be weakened by cell-membrane lipids isolated from test MRSA strain. Thereby, some interactions between azalomycin F5a and MRSA cell-membrane lipids were deduced, which possibly caused the molecular numbers of azalomycin F5a interacting with the membrane lipids bilayer of MRSA to decrease. To confirmed this and discover more detailed information, the anti-MRSA activity of azalomycin F5a against MRSA was further determined with the intervention of DPPG which is a main component of MRSA cell-membrane lipid [18]. Excitedly, the results indicated that the anti-MRSA activity of azalomycin F5a could be significantly weakened by DPPG, which deduced that there were some interactions between azalomycin F5a and DPPG. Thus, DPPG lying in cell membrane might be an important molecular target of azalomycin F5a against MRSA.
The resistance mechanism of MRSA is very complex and mainly involves various proteins expressed from resistant genes [19, 20]. These proteins mostly embed in cell membrane, and their functions accordingly depend on the integrity and liquidity of cell membrane. As it is difficult for MRSA to modify the drug-resistant proteins lying in the cell membrane in a short time, new antibiotics targeting MRSA cell membrane become an important field on the research and development of anti-MRSA drugs [21]. Among them, daptomycin, a lipopeptide antibiotic approved by FDA in 2003, was generally used for treating infection caused by MRSA being resistant to vancomycin [22, 23]. It kills Gram-positive pathogens in a strictly calcium dependent manner by perturbing the integrity and electrochemical gradient of cell membrane [24]. Recent reports indicated that the change of cell-membrane lipid profiles was a key factor for the resistance of MRSA to daptomycin [18, 25]. Thereby, some interactions between azalomycin F5a and cell-membrane lipids especially DPPG indicated that cell-membrane lipids especially DPPG might be an important molecular target of azalomycin F5a against MRSA and that the molecular mechanism of azalomycin F5a interacting with DPPG and cell-membrane lipids of MRSA was worth being further detailed using 31P NMR, ATR-FT-IR, DSC, and Raman technologies with the help of models of biological membranes and molecular dynamics simulation.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (nos. 81460529, 81660578, and 81260476).
Contributor Information
Ganjun Yuan, Email: gyuan@jxau.edu.cn.
Yunqiu Qu, Email: quyunqiu@sina.com.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
Ganjun Yuan and Yunqiu Qu were responsible for conception and design of study. Li Xu, Xuejie Xu, Ganjun Yuan, Yimin Wang, and Erxiao Liu were responsible for acquisition of data, analysis, and/or interpretation of data. Li Xu and Ganjun Yuan were responsible for drafting the manuscript. Ganjun Yuan and Yunqiu Qu were responsible for revising the manuscript critically for important intellectual content.
References
- 1.Arai M., Hamano K. Isolation of three main components, F3, F4 and F5, from azalomycin f-complex. The Journal of Antibiotics. 1970;23(3):107–112. doi: 10.7164/antibiotics.23.107. [DOI] [PubMed] [Google Scholar]
- 2.Iwasaki S., Namikoshi M., Sasaki K., Fukushima K., Okuda S. Studies on macrocyclic lactone antibiotics. V. the structures of azalomycins F3a and F5a. Chemical & Pharmaceutical Bulletin. 1982;30(11):4006–4014. doi: 10.1248/cpb.30.4006. [DOI] [Google Scholar]
- 3.Chandra A., Nair M. G. Azalomycin F complex from streptomyces hygroscopicus, MSU/MN-4-75B. The Journal of Antibiotics. 1995;48(8):896–898. doi: 10.7164/antibiotics.48.896. [DOI] [PubMed] [Google Scholar]
- 4.Sugawara S. Effect of azalomycin f on bacteria. The Journal of Antibiotics. 1968;21(1):83–84. doi: 10.7164/antibiotics.21.83. [DOI] [PubMed] [Google Scholar]
- 5.Cheng J., Yang S. H., Palaniyandi S. A., et al. Azalomycin F complex is an antifungal substance produced by streptomyces malaysiensis mjm1968 isolated from agricultural soil. Journal of Applied Biological Chemistry. 2010;53(5):545–552. doi: 10.3839/jksabc.2010.084. [DOI] [Google Scholar]
- 6.Yuan G., Lin H., Wang C., Hong K., Liu Y., Li J. 1H and 13C assignments of two new macrocyclic lactones isolated from streptomyces sp. 211726 and revised assignments of azalomycins F3a, F4a and F5a. Magnetic Resonance in Chemistry. 2011;49(1):30–37. doi: 10.1002/mrc.2697. [DOI] [PubMed] [Google Scholar]
- 7.Yuan G., Li P., Pan W., Pang H., Chen S. The relative configurations of azalomycins F5a, F4a and F3a. Journal of Molecular Structure. 2013;1035:31–37. doi: 10.1016/j.molstruc.2012.09.024. [DOI] [Google Scholar]
- 8.Yuan G., Hong K., Lin H., She Z., Li J. New azalomycin F analogs from mangrove Streptomyces sp. 211726 with activity against microbes and cancer cells. Marine Drugs. 2013;11(3):817–829. doi: 10.3390/md11030817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boucher H. W., Talbot G. H., Bradley J. S., et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clinical Infectious Diseases. 2009;48(1):1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 10.Yuan G., Li P., Chen S., Zhu X., Zhang Q. Anti-methicillin-resistant Staphylococcus aureus activity of three main components of azalomycin F. Chinese Pharmaceutical Journal. 2014;49(8):644–648. [Google Scholar]
- 11.Wu X., Xu X., Yuan G., Xu L., Wang Y. Mutant selection windows of azalomycin F5a in Combination with vitamin K3 against methicillin-resistant Staphylococcus aureus. Journal of Biosciences and Medicines. 2016;04(12):162–174. doi: 10.4236/jbm.2016.412020. [DOI] [Google Scholar]
- 12.Yuan G.-J., Li P.-B., Yang J., Pang H.-Z., Pei Y. Anti-methicillin-resistant Staphylococcus aureus assay of azalomycin F5a and its derivatives. Chinese Journal of Natural Medicines. 2014;12(4):309–313. doi: 10.1016/S1875-5364(14)60061-3. [DOI] [PubMed] [Google Scholar]
- 13.Li Y.-Q., Han Q., Feng J.-L., Tian W.-L., Mo H.-Z. Antibacterial characteristics and mechanisms of ε-poly-lysine against Escherichia coli and Staphylococcus aureus. Food Control. 2014;43:22–27. doi: 10.1016/j.foodcont.2014.02.023. [DOI] [Google Scholar]
- 14.Jacobs A. C., DiDone L., Jobson J., Sofia M. K., Krysan D., Dunman P. M. Adenylate kinase release as a high-throughput-screening-compatible reporter of bacterial lysis for identification of antibacterial agents. Antimicrobial Agents and Chemotherapy. 2013;57(1):26–36. doi: 10.1128/AAC.01640-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domenech O., Dufrêne Y. F., Van Bambeke F., Tukens P. M., Mingeot-Leclercq M.-P. Interactions of oritavancin, a new semi-synthetic lipoglycopeptide, with lipids extracted from Staphylococcus aureus. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2010;1798(10):1876–1885. doi: 10.1016/j.bbamem.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Wayne P. A., editor. Clinical and Laboratory Standards Institute, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: Approved Standard. 9th. Clinical and Laboratory Standards; 2012. [Google Scholar]
- 17.Infectious Diseases Society of America. The 10 x '20 initiative: pursuing a global commitment to develop10 new antibacterial drugs by 2020. Clinical Infectious Diseases. 2010;50(8):1081–1083. doi: 10.1086/652237. [DOI] [PubMed] [Google Scholar]
- 18.Mishra N. N., Bayer A. S. Correlation of cell membrane lipid profiles with daptomycin resistance in methicillin-resistant staphylococcus aureus. Antimicrobial Agents and Chemotherapy. 2013;57(2):1082–1085. doi: 10.1128/AAC.02182-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiramatsu K. Elucidation of the mechanism of antibiotic resistance acquisition of methicillin-resistant Staphylococcus aureus (MRSA) and determination of its whole genome nucleotide sequence. Japan Medical Association Journal. 2004;47(4):153–159. [Google Scholar]
- 20.Bernal P., Lemaire S., Pinho M. G., Mobashery S., Hinds J., Taylor P. W. Insertion of epicatechin gallate into the cytoplasmic membrane of methicillin-resistant Staphylococcus aureus disrupts penicillin-binding protein (PBP) 2a-mediated β-lactam resistance by delocalizing PBP2. The Journal of Biological Chemistry. 2010;285(31):24055–24065. doi: 10.1074/jbc.M110.114793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bambeke F. V., Mingeot-Leclercq M.-P., Struelens M. J., Tulkens P. M. The bacterial envelope as a target for novel anti-MRSA antibiotics. Trends in Pharmacological Sciences. 2008;29(3):124–134. doi: 10.1016/j.tips.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Raja A., LaBonte J., Lebbos J., Kirkpatrick P. Daptomycin. Nature Reviews Drug Discovery. 2003;2(12):943–944. doi: 10.1038/nrd1258. [DOI] [PubMed] [Google Scholar]
- 23.Steenbergen J. N., Alder J., Thorne G. M., Tally F. P. Daptomycin: a lipopeptide antibiotic for the treatment of serious gram-positive infections. Journal of Antimicrobial Chemotherapy. 2005;55(3):283–288. doi: 10.1093/jac/dkh546. [DOI] [PubMed] [Google Scholar]
- 24.Baltz R. H. Daptomycin: mechanisms of action and resistance, and biosynthetic engineering. Current Opinion in Chemical Biology. 2009;13(2):144–151. doi: 10.1016/j.cbpa.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 25.Mishra N. N., Yang S.-J., Sawa A., et al. Analysis of cell membrane characteristics of in vitro-selected daptomycin-resistant strains of methicillin-resistant Staphylococcus aureus. Antimicrobial Agents and Chemotherapy. 2009;53(6):2312–2318. doi: 10.1128/AAC.01682-08. [DOI] [PMC free article] [PubMed] [Google Scholar]