Abstract
Langerhans cells (LCs) are antigen-presenting cells in the epidermis whose roles in antigen-specific immune regulation remain incompletely understood. Desmoglein 3 (Dsg3) is a keratinocyte cell-cell adhesion molecule critical for epidermal integrity and an autoantigen in the autoimmune blistering disease pemphigus. Although antibody-mediated disease mechanisms in pemphigus are extensively characterized, the T cell aspect of this autoimmune disease still remains poorly understood. Herein, we utilized a mouse model of CD4+ T cell-mediated autoimmunity against Dsg3 to show that acquisition of Dsg3 and subsequent presentation to T cells by LCs depended on the C-type lectin langerin. The lack of LCs led to enhanced autoimmunity with impaired Dsg3-specific regulatory T cell expansion. LCs expressed the IL-2 receptor complex and the disruption of IL-2 signaling in LCs attenuated LC-mediated regulatory T cell expansion in vitro, demonstrating that direct IL-2 signaling shapes LC function. These data establish that LCs mediate peripheral tolerance against an epidermal autoantigen and point to langerin and IL-2 signaling pathways as attractive targets for achieving tolerogenic responses particularly in autoimmune blistering diseases such as pemphigus.
Keywords: Langerhans cells, Regulatory T cells, Autoimmune disease, Pemphigus
Highlights
-
•
Langerhans cells take up a keratinocyte-expressed autoantigen, desmoglein 3, via langerin.
-
•
Langerhans cells suppress autoimmunity by expanding regulatory T cells.
-
•
IL-2 receptor signaling occurs in Langerhans cells, conditioning them to mediate peripheral tolerance.
Lymphocytes are critical for combating pathogens, but they can cause autoimmune diseases when misdirected against autoantigens. While past experimental models have provided detailed mechanisms utilizing neo-antigens, immune regulation against naturally-expressed autoantigen(s) remains largely unexplored. Herein, we studied immune responses against desmoglein 3, a bona fide autoantigen in pemphigus, and demonstrated that epidermal Langerhans cells (antigen-presenting cells) take up the autoantigen from surrounding keratinocytes via a C-type lectin receptor to induce regulatory T cells, which are critical for immune suppression. IL-2 signaling in Langerhans cells was required to preferentially expand regulatory T cells, providing new insights into mechanisms that regulate autoimmunity.
1. Introduction
Langerhans cells (LCs) are unique antigen-presenting cells (APC) in the epidermis whose roles in host defense have become clearer over the last few years. We previously demonstrated that LCs take up protein antigens and bacterial toxins by extending their dendrites through intact tight junction barriers (Kubo et al., 2009) to initiate protective humoral responses (Nagao et al., 2009, Ouchi et al., 2011). This non-redundant function for LCs in vivo was observed by controlling the distribution of antigens and utilizing protein antigens that do not penetrate epidermal tight junctions and thus are accessible to only LCs (Ouchi et al., 2011). On the other hand, when epidermal barriers are breached during epicutaneous Candida albicans infection, LCs are capable of inducing Th17-mediated cellular responses (Igyártó et al., 2011). The route of antigen delivery that allows for natural antigen uptake by LCs was an important factor in determining LC function in these studies.
Langerin is a C-type lectin required for the formation of Birbeck granules (Kissenpfennig et al., 2005a, Valladeau et al., 2000) and was demonstrated to be an endocytic receptor in in vitro propagated LCs and in fibroblasts transfected with Langerin (Valladeau et al., 2000). However, the genomic ablation of langerin did not result in any obvious immune phenotypes (Kissenpfennig et al., 2005a), and its function(s) in vivo had remained elusive. Human LCs have been shown to scavenge HIV via langerin (de Witte et al., 2007), but functional contributions of langerin during immune responses have not been demonstrated.
Whether LCs are capable of suppressing immunity has been a topic of debate. Loss of LCs leads to attenuated disease in leishmaniasis with decreased numbers of regulatory T (Treg) cells (Kautz-Neu et al., 2011), and the treatment of mice with antigen-conjugated anti-langerin antibodies results in enhanced Treg cell expansion (Flacher et al., 2014, Idoyaga et al., 2013). LCs have also recently been shown to induce the expansion of Treg cells in response to ionizing irradiation (Price et al., 2015). However, the physiological setting in which LCs mediate immuno-regulatory responses and whether this occurs in an antigen-specific manner has yet to be clearly demonstrated.
Past models including contact hypersensitivity responses, intradermal injection of pathogens and transgenic mice expressing neo-autoantigens have been utilized to explore LC function. However, the route of antigen delivery or the superphysiological load of antigens may lead to experimental outcomes that do not reflect physiological LC function. This issue may be avoided by studying immune responses against keratinocyte-associated autoantigens that are physiologically expressed.
Desmoglein 3 (Dsg3) is a classic cadherin family cell adhesion molecule and a major desmosomal glycoprotein that is expressed by keratinocytes (Amagai et al., 1991). Dsg3 is not only critical for maintaining epidermal integrity, it is also a bona fide autoantigen that is targeted in pemphigus vulgaris, an autoimmune blistering disease (Amagai et al., 1991). While mechanisms regarding T cell immunity against Dsg3 remain incompletely characterized, a mouse model has helped provide some insight (Takahashi et al., 2009). Experimental autoimmune dermatitis (EAD) is a mouse model in which CD4+ T cells target Dsg3 to mediate autoimmune skin inflammation (Takahashi et al., 2011) and represents a unique model in which autoimmunity against a physiologically expressed, functional self-antigen can be studied. Herein, we utilized in vitro and in vivo systems and determined that langerin-mediated acquisition of Dsg3 by LCs leads to the expansion of antigen-specific Treg cells. We also demonstrate that LCs expanded Treg cells via a mechanism that involves direct IL-2 signaling in LCs.
2. Materials and Methods
2.1. Mice
C57BL/6J and C57BL/6J Rag2−/− mice were purchased from CLEA Japan, Inc. and the Central Institute for Experimental Animals (Tokyo, Japan), respectively. Langerin-DTA mice (Kaplan et al., 2005) were crossed with the C57BL/6J Rag2−/− background. Homozygous or heterozygous Langerin-DTR mice (Bennett et al., 2005) in the C57BL/6SJL (CD45.1) background were obtained by breeding homozygous Langerin-DTR mice with each other or with C57BL/6SJL wild-type mice. Dsg3−/− mice were crossed with K5-Dsg1 transgenic mice, which express another desmosomal protein, Dsg1, in keratinocytes (Hata et al., 2011). The Dsg1 expression driven by the K5 promoter compensates for the loss of Dsg3 and ameliorates the mucosal erosions that Dsg3−/− mice are prone to develop, thereby enhancing the viability of Dsg3−/− mice (Hata et al., 2011). For the sake of simplicity, the K5-Dsg1 transgenic Dsg3−/− mice have been referred to as Dsg3−/− mice throughout this manuscript. The Dsg3-specific T cell receptor transgenic mice (H1 mice) in the C57BL/6J background were previously generated (Takahashi et al., 2011). CD25−/− mice were kindly provided by Tai Xuguang and Alfred Singer (National Cancer Institute, National Insitutes of Health, Bethesda).
To generate K5-Dsg3-eGFP mice, a transgene vector pGEM3Z-hK5-mDsg3-EGFP containing the human keratin 5 (K5) promoter [which was kindly provided by Dr. Junji Takeda (Osaka University)], a full length mouse Dsg3 (mDsg3) and enhanced GFP (eGFP) were constructed. Full length mDsg3 fused with eGFP was subcloned between the β-globin cassette and BGHpA of the modified K14pNotIpGEM3Z vector (Hata et al., 2011). Then, the K14 promoter was replaced with the K5 promoter as previously described (Hata et al., 2011). The nucleotide region from the K5 promotor to BGHpA was excised and microinjected into the pronuclei of the C57BL/6J mice zygotes. The zygotes were implanted into pseudopregnant foster C57BL/6J mice to generate mDsg3-eGFP transgenic mice.
All mice were bred and housed in specific pathogen-free facilities. All animal procedures and study protocols were approved by the Keio University Ethics Committee for Animal Experiments.
2.2. Antibodies
Anti-mouse langerin (clone L31, eBioscience) was used either purified or in conjugated forms labeled in house with Alexa Fluor 647 (Invitrogen). Additional anti-mouse mAbs obtained from BioLegend (except otherwise noted) were used for flow cytometry or immunofluorescence staining: CD122 (TM-β1), CD132 (TUGm2), CD25 (PC61), CD3ε (145-2C11), CD4 (GK1.5), CD45 (30F-11), CD80 (16-10A1), CD86 (GL-1), EpCAM (G8.8), Foxp3 (FJK-16s; eBioscience), IL-2 (JES6-1A12), MHC II (anti-IA/IE; M5/114.15.2), Vβ6 (RR4-7; BD Bioscience), CD11b (M1/70; eBioscience) and pSTAT5 (pY694: BD Biosciences).
LEAF™ Purified anti-mouse I-A/I-E (M5/114.15.2, BioLegend) and the appropriate isotypes were used for functional assays. Mouse CD4 MicroBeads (L3T4, Miltenyi Biotec) were used for CD4+ T cell isolation. Anti-Cy7 and anti-Biotin MicroBeads were used for the CD11b+ dermal DC isolation via a MACS cell separation system.
Primary antibodies were detected with Alexa Fluor-labeled secondary antibodies (Invitrogen). Cell nuclei were stained with Hoechst 33258 dye (Invitrogen). NAK5 (anti-Dsg3 IgG1 mAb) was used to stain epidermal sheets.
For in vitro blocking assays, anti-mouse antibodies against I-A/I-E (M5/114.15.2), CD25 (PC61), CD80 (16-10A1), CD86 (GL-1), CD275 (HK5.3), PD-1 (29F.1A12 and RMP1-14), PD-L1 (10F.9G2), IL-2 (JES6-1A12), IL-6 (MP5-20F3) and IL-10 (JES5-16E3) from BioLegend, IL-15 (AIO.3) from eBioscience and TGF-beta (1D11) from R&D were used.
2.3. Epidermal Sheet and Cryosection Preparation
Epidermal sheets were prepared as previously described (Nagao et al., 2009). Briefly, mouse ears were divided into dorsal and ventral halves with forceps and incubated for 15 min at 37 °C on 3.8% ammonium thiocyanate (Wako Pure Chemical Industries) in phosphate buffer (pH 7.0). The epidermal sheets were manually detached from the dermis under a dissecting microscope (Olympus). Tissue samples for cryosections were embedded in O.C.T. compound (Tissue Tek) and frozen at − 80 °C.
2.4. Immunofluorescence Microscopy
Staining of the epidermal sheets and cryosections was performed as previously described. Briefly, the epidermal sheets or cryosections were fixed in acetone at − 20 °C for 15 min and were rehydrated in PBS for at least 5 min (3 times). The samples were blocked with 3% dry milk (Morinaga) and 5% goat serum (Dako) in PBS for an hour at room temperature. The primary antibodies were incubated overnight at 4 °C and washed and detected with appropriate secondary antibodies. Nuclei were stained with Hoechst 33258 (Invitrogen). Image acquisition was performed with an Axio Observer.Z1 (Zeiss) and the AxioVision software (ver. 4.8), with a Leica confocal microscope (TCS-SP5, Leica) or with a Zeiss confocal microscope (LSM880, Zeiss) and the Zeiss Zen (v2.1) software. The levels of the images were linearly adjusted using Photoshop CS5 (Adobe) when necessary. 3D reconstruction images were built using sp5 software (Leica) and movies were processed using iMovie (Apple).
2.5. Cell Suspension Preparation
Epidermal cell suspensions were prepared as previously described with slight modifications (Nagao et al., 2009). The shaved mouse trunk skins were floated on RPMI 1640 medium containing 0.15% trypsin and 0.27 mM EDTA at 37 °C for 30 or 45 min. The epidermis was mechanically scraped off in 5% FCS-PBS and washed and filtered through cell strainers (BD Falcon). To obtain the dermal cell suspension, the dermis was minced with scissors or scalpels and incubated in RPMI 1640 medium containing 0.25 mg/ml of Liberase TL Research grade (Roche) and 200 U/ml of DNase I (Sigma) at 37 °C for 60 min. Skin-draining lymph nodes (brachial, axiliary, cervical and inguinal) and spleens were excised and disrupted through a cell strainer (BD Falcon) in 5% FCS-PBS to generate single cell suspensions.
2.6. Flow Cytometry
Anti-mouse CD16/32 (clone 93, BioLegend) was routinely used to block Fcγ receptors. Non-viable cells were excluded with PI (Sigma-Aldrich), 7AAD (BioLegend) or LIVE/DEAD cell (Invitrogen). Intracellular staining for Foxp3 (FJK-16s) as well as for langerin (L31) was performed after cell surface staining using Foxp3 staining buffer (eBioscience). Intracellular staining for langerin was performed because there is no detectable cell-surface staining after trypsinization of epidermal cells (data not shown). Intracellular staining for pSTAT5 was performed by fixing cells with BD Phosflow™ Fix buffer I (BD Biosciences) followed by permeabilizing cells with BD Phosflow™ Perm Buffer III (BD Biosciences). Data acquisition was performed on a FACS Canto II (BD Biosciences) or LSR II (BD Biosciences) and analyzed using FlowJo (Tree star).
2.7. ELISA
For cytokine analysis, cells harvested from epidermis, lymph nodes and spleens were incubated at a concentration of 2 × 106/ml for 6 h in complete RPMI 1640 medium supplemented with PMA (5 ng/ml, Sigma-Aldrich) and ionomycin (500 ng/ml, Sigma-Aldrich). Quantification of cytokines in cell culture supernatants was performed using Mouse IL-17A or IFN-γ ELISA MAX™ Standard Sets (BioLegend) according to the manufacturer's instructions.
2.8. Preparation of Skin Dendritic Cells
LCs that migrated from skin explants were isolated as previously described (Nagao et al., 2009). In short, mouse ears were divided into dorsal and ventral halves, cartilage was removed, and the ear halves were then floated on 40 ml of complete RPMI 1640 medium supplemented with Amphotericin B at 37 °C in 10-cm petri dishes. The cells that migrated into the culture medium were collected after 24 h and were stored at 4 °C. These cells were pooled with cells that were harvested after an additional 24 h of skin explant incubation in newly replaced culture medium. EpCAM− CD11b+ dermal DCs were isolated from dermal cell suspensions using a MACS cell separation system (Miltenyi Biotec).
2.9. In vitro T Cell Proliferation Assay
To generate pure and naïve H1 cells, 2 × 106 bone marrow cells from Rag2−/− H1 mice were transferred into lethally irradiated (9.5 Gy) Dsg1-transgenic Dsg3−/− mice. CD4+ T cells were isolated from spleen and lymph nodes of the bone marrow chimeric mice using a MACS cell separation system (Miltenyi Biotec). H1 or WT polyclonal CD4+ T cells (2 × 105 CFSE-labeled) (Life Technologies) were co-cultured with 1 × 104 LCs in 96-well round-bottom plates. The cells were harvested for analysis on day 3. For in vitro blocking assays, 10 μg/ml of anti-mouse monoclonal antibodies were added. 10 ng/ml of recombinant mouse IL-2 (R&D) in PBS was used for IL-2 treatment experiments.
2.10. Experimental Autoimmune Dermatitis
CD4+ T cells were isolated from spleens and lymph nodes of H1 mice via a MACS cell separation system (Miltenyi Biotec), and 5 × 106 cells were injected intravenously into Rag2−/− and Langerin-DTA Rag2−/−.
2.11. Statistical Analysis
Statistical analyses were performed with Student's t-test using Prism version 5 for MAC OS X (GraphPad Software), and p values ≤ 0.05 were regarded as significant.
2.12. Nanostring Analysis
Skin-explant LCs were treated with 10 ng/ml of rIL-2 for 8 h and RNA was subsequently extracted. Nanostring analysis was performed according to the manufacturer's instructions.
Raw data from the rIL-2 treated skin-explant LCs were uploaded to nSolver Nanostring software and processed with default quality control settings. Per sample transcript counts were normalized to the six positive controls in the nCounter panel and six housekeeping genes (Gusb, Oaz1, Polr2a, Ppia, Rpl19, Tubb5) and samples were subsequently partitioned by treatment group (WT or WT + rIL-2). Ratios for the comparison of treatment groups and associated p-values were generated by the nSolver software.
A heatmap was generated in nSolver using normalized expression values for the 19 genes that were significantly different (p < 0.05) in both experiments.
A scatterplot for expression data was generated using group expression values exported from nSolver into Excel, where values were log-transformed and subsequently plotted in Prism. Genes were chosen for display out of the 561 total genes analyzed based on their statistical or biological significance.
2.13. Ingenuity Pathway Analysis
Gene expression data from the nCounter Mouse Immunology Panel were analyzed using Ingenuity Pathway Analysis Version 01-07 (Qiagen). Uploaded ratios from nSolver software were converted to fold change values in IPA for core analysis and used to calculate a z-score and p-value corresponding to the prediction of up- or downregulation in Canonical Pathways and Diseases or Functions annotations in the IPA database. Canonical Pathways with significant (p < 0.05) up- or downregulation in rIL-2-treated LCs were filtered by | z-score | > 1 and assessed for biological relevance. Diseases and Functions were assessed based on their IPA-predicted activation state (| z-score | > 2).
3. Results
3.1. Langerhans Cells Take Up and Present Desmoglein 3
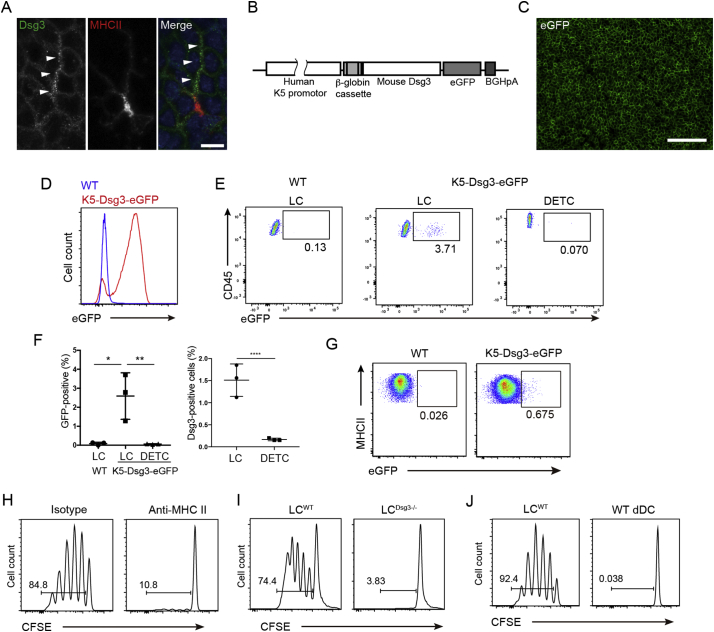
To determine if LCs take up keratinocyte-associated autoantigens, we first examined epidermal sheets from wild-type mice and stained them for class II MHC (MHC II) and Dsg3. The conformation of the extracellular portion of Dsg3 relies on an optimal calcium concentration (Amagai et al., 1995), which is unlikely to be retained after its internalization within LCs. Utilizing an anti-Dsg3 mAb that recognizes non-conformational epitopes in a calcium-independent manner (Kawasaki et al., 2006), we detected punctate Dsg3 signals within LCs which were distinct from signals of cytoplasmic MHC II (Fig. 1A). To confirm that this observation represented Dsg3 uptake by LCs from keratinocytes, we generated transgenic mice that expressed a Dsg3-eGFP fusion protein driven by the keratin 5 promoter (Fig. 1B). Only epidermal keratinocytes express Dsg3-eGFP in the skin of these mice and the eGFP signal was detected in epidermal sheets and CD45− MHC II− keratinocytes via flow cytometry (Fig. 1C,D). Interestingly, we found that up to 5% of LCs were eGFP+ via flow cytometry analysis of epidermal cell suspensions, while eGFP signals were not found in dendritic epidermal T cells (Fig. 1E,F), indicating that a subset of LCs had taken up Dsg3-eGFP fusion protein within the epidermis. Similarly, Dsg3+ LCs were detected in WT mice by flow cytometry analysis via anti-Dsg3 antibody staining (Fig. 1F, right panel). Rare Langerin− CD11b+ dermal DCs in Dsg3-eGFP mice also contained eGFP (Fig. 1G), thereby demonstrating that LCs were the predominant but not the sole APC subset that had access to keratinocyte-expressed Dsg3-eGFP fusion protein.
Fig. 1.
LCs acquire Dsg3 from keratinocytes and activate Dsg3-specific T cells. (A) Dsg3 co-stained with MHC II in epidermal sheets. Bar = 10 μm. (B) Construct map for K5-Dsg3-eGFP mice generation. (C) eGFP visualization in epidermal sheets from K5-Dsg3-eGFP transgenic mice. Bar = 100 μm. (D) eGFP expression in CD45− MHC II− keratinocytes from K5-Dsg3-eGFP and WT mice via flow cytometry. (E) eGFP detection in CD45+ MHC IIhi EpCAM+ LCs and CD45hi CD3εhi MHCII− EpCAM− dendritic epidermal T cells (DETC). (F) Quantification of eGFP+ LCs and DETCs in WT and K5-Dsg3-eGFP mice (left panel) and quantification of Dsg3+ LCs and DETCs in WT mice after staining with anti-Dsg3 (NAK5) and detection by flow cytometry (right panel). (G) Detection of eGFP+ CD45+ MHC II+ CD11b+ EpCAM− dermal cells from K5-Dsg3-eGFP mice. (H) CFSE-dilution assay for H1Rag cells co-cultured with LCs in the presence of an anti-MHC II blocking antibody or an isotype control. As in (H) H1Rag cells co-cultured with (I) skin-explant LCs from WT (LCWT) or Dsg3 −/− (LCDsg3 −/−) mice and (J) skin-explant LCWT or CD11b+ dermal DCs from WT mice. Data are representative of three independent experiments (n = 3). p values: *<0.05, **<0.01, ****<0.0001.
3.2. LCs Present Dsg3 in the Context of MHC Class II
To functionally evaluate the immunological consequences of Dsg3 uptake by LCs, we collected LCs from skin explants of unmanipulated WT mice (LCWT) to be used as APCs. A subset of them would be expected to have taken up Dsg3 from surrounding keratinocytes (Fig. 1A,E,F). To generate responder T cells, Rag2−/− mice were crossed to TCR (Vβ6) transgenic mice in which CD4+ T cells express a Dsg3-specific TCR (H1 cells) (Takahashi et al., 2011). Bone marrow from these mice was transferred into lethally irradiated Dsg3−/− mice (Hata et al., 2011). This procedure enabled the development of Dsg3-reactive T cells in a Dsg3-deficient environment and resulted in high ratios of naïve, Vβ6-expressing H1 cells (hereafter referred to as H1Rag cells) in the recipient mice (data not shown) (Takahashi et al., 2011).
When LCWT were co-cultured with carboxyfluorescein succinimidyl ester (CFSE)-labeled H1Rag cells, H1Rag cells underwent robust proliferation, which was completely blocked by antibodies against MHC II (Fig. 1H). LCs collected from the skin explants of Dsg3−/− mice (LCDsg3 −/−) failed to activate H1 cells (Fig. 1I), indicating that H1Rag cell proliferation occurred in a Dsg3-specific manner. These data demonstrated that the LCs acquired Dsg3 from surrounding keratinocytes and presented it in the context of MHC II to H1Rag cells. In contrast to LCs, EpCAM− CD11b+ dermal DCs did not activate H1Rag cells (Fig. 1J). This can be explained by the small number of dermal DCs that had acquired Dsg3 and in aggregate demonstrate that LCs were the predominant APC that took up and presented Dsg3.
3.3. Langerin Is Required for the Uptake of Dsg3
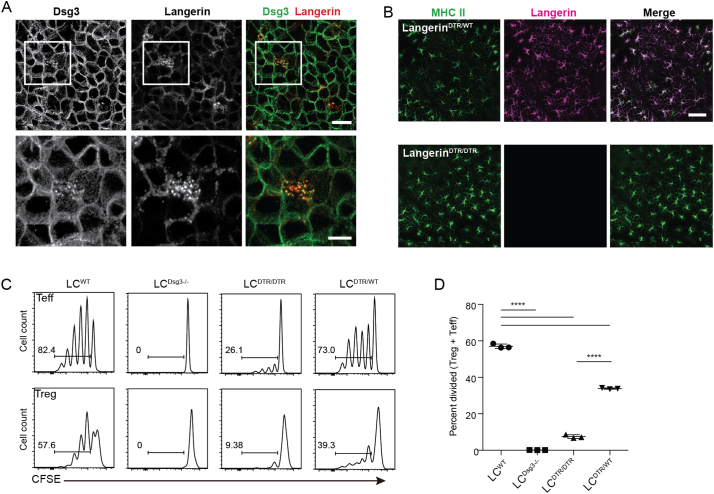
Langerin binds carbohydrate residues (Valladeau et al., 2000). We hypothesized that the uptake of Dsg3 by LCs, a highly glycosylated protein, may be mediated by langerin. When we co-stained langerin and Dsg3 in epidermal sheets prepared from WT mice, we observed that the punctate Dsg3 signal in LC cytoplasm co-localized with langerin (Fig. 2A, Supplementary Movie 1). Based on the predominantly cytoplasmic localization of MHC II, these LCs exhibited an immature phenotype (data not shown). To explore the role of langerin in immune responses, we utilized mice in which the extracellular domain of the langerin gene is replaced by the simian diphtheria toxin receptor (DTR) (Langerin-DTR mice) (Bennett et al., 2005). Mice homozygous for the langerin-DTR allele (LangerinDTR/DTR) lack functional langerin. Indeed, staining of epidermal sheets from LangerinDTR/DTR mice for MHC II and langerin revealed that LCs existed in a normal distribution within the epidermis but displayed an absence of langerin expression (Fig. 2B). Apart from their lack of langerin, LCs (LCDTR/DTR) from LangerinDTR/DTR mice were phenotypically indistinguishable from LCWT with regard to MHC II, CD80 and CD86 expression (data not shown).
Fig. 2.
Langerin is required for Dsg3 uptake. (A) Confocal microscopy of epidermal sheets co-stained with anti-Dsg3 (NAK5) and anti-langerin mAbs. Lower panels show high magnification of the outlined area in the upper panels. Bars = 20 μm (upper) and 10 μm (lower). (B) Immunofluorescence microscopy of epidermal sheets from LangerinDTR/WT or LangerinDTR/DTR mice co-stained with anti-MHC II and anti-langerin mAbs. (C) CFSE-labeled H1 cells co-cultured with skin-explant LCs from WT (LCWT), Dsg3 −/− (LCDsg3 −/−), LangerinDTR/DTR (LCDTR/DTR) or LangerinDTR/WT (LCDTR/WT) mice. (D) Y-axis, percent divided, represents the frequency of cells among the original input population of CD4+ Vβ5+ T cells that have undergone division. p values: ****<0.0001.
We utilized skin-explant LCWT and LCDTR/DTR as APCs in an in vitro proliferation assay with CFSE-labeled H1Rag cells as responder T cells. We also phenotypically evaluated H1 cells that had proliferated into Treg and effector T cells (Teff cells). LCWT induced the proliferation of both T cell subsets (Fig. 2C). Notably, Treg cells were generated despite the absence of Rag2, a condition that does not favor Treg cell generation (Szymczak-Workman et al., 2009).
In contrast to LCWT, LCDTR/DTR were markedly impaired in their ability to activate H1Rag Treg and Teff cells (Fig. 2C,D), indicating that langerin mediated Dsg3 uptake prior to antigen presentation. LCs that were heterozygous for the langerin-DTR allele, LCDTR/WT, were modestly impaired in activating H1Rag cells (Fig. 2D), likely reflecting decreased Dsg3 acquisition and presentation of fewer Dsg3 peptide-MHC II complexes to T cells. These results demonstrated a critical role for langerin during the acquisition of a keratinocyte-derived autoantigen.
3.4. LCs Are Required for Treg Cell Expansion In Vivo
Because skin-explant LCWT expanded both Treg and Teff H1Rag cells in vitro (Fig. 2C), we sought to confirm the role of LCs in EAD, a CD4+ T cell-mediated autoimmune response against Dsg3 (Takahashi et al., 2011). In this model, when H1 cells are adoptively transferred into Rag2−/− mice, they readily infiltrate the skin and elicit IFN-γ-dependent responses directed against keratinocytes (which express Dsg3), causing apoptosis and obscuring the dermo-epidermal junction (Takahashi et al., 2011).
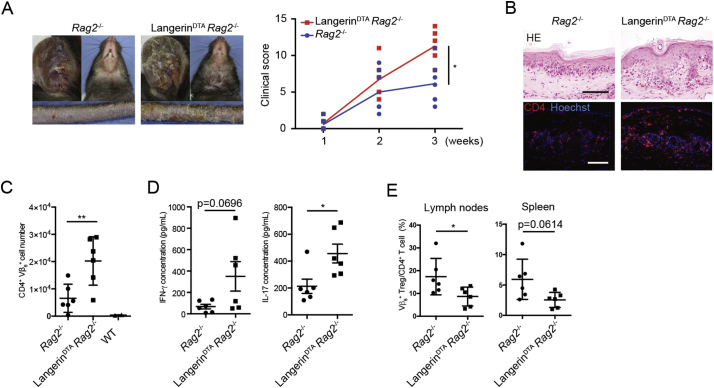
We crossed Langerin-DTA (diphtheria toxin fragment A) mice (Kaplan et al., 2005), which constitutively lack LCs, to the Rag2−/− background and adoptively transferred CD4+ T cells that were collected from Rag2-sufficient H1 mice (30–40% H1 cells). The utilization of H1 cells in the Rag2-sufficient background facilitates Treg cell analysis (Szymczak-Workman et al., 2009). EAD developed in Langerin-DTA Rag2−/− mice (Fig. 3A), consistent with a previous demonstration of autoimmune T cell activation in the absence of DCs (Teichmann et al., 2010). Interestingly, Langerin-DTA Rag2−/− mice exhibited an aggravated skin phenotype with significantly elevated clinical scores (Fig. 3A). Histological analysis revealed a more prominent interface dermatitis in Langerin-DTA Rag2−/− mice that was accompanied by an increased number of CD4+ Vβ6+ (Dsg3-specific) cells in the epidermis (Fig. 3B,C).
Fig. 3.
The loss of LCs leads to exacerbated autoimmunity. H1 T cells were adoptively transferred into Rag2−/− mice to generate EAD. (A) Phenotype (3 weeks post transfer) and clinical scores of EAD in mice that have (Rag2−/−) or lack (Langerin-DTA Rag2−/−) LCs. p = 0.0215. (B) H&E (upper panels) and frozen sections stained for CD4 (lower panels) from lesional skin of EAD in indicated mice. (C) Quantification of CD45+ CD3+ CD4+ Vβ6+ cells in the epidermis via flow cytometry. (D) Supernatants of in vitro stimulated (PMA/ionomycin) epidermal cell suspensions were analyzed for IFN-γ or IL-17 concentration via ELISA. (E) Quantification of Vβ6+ Treg cells in skin-draining lymph nodes and spleen in indicated mice via flow cytometry. p values: *<0.05, **<0.01.
Re-stimulation of CD4+ T cells from lesional epidermis in vitro revealed an increased production of IL-17 as well as a trend toward increased IFN-γ (Fig. 3D). Flow cytometry analysis of the skin-draining lymph nodes revealed a decreased expansion of Treg cells in mice that lacked LCs (Fig. 3E). In aggregate, although LCs activated both Teff and Treg H1 cells in vitro, the lack of LCs led to impaired expansion of Treg cells and exacerbated inflammation in vivo, highlighting their role in suppressing autoimmunity.
3.5. LCs Preferentially Expand Polyclonal Treg Cells
Human LCs are capable of expanding polyclonal Treg cells in vitro in the absence of exogenous antigens or stimulation (Seneschal et al., 2012). To explore the mechanism by which skin explant LCs expand Treg cells, we utilized CSFE-labeled syngeneic WT CD4+ T cells as responder cells rather than H1Rag cells because the activation of Teff cells and cytokines that they produce may have secondary effects on Treg cell expansion.
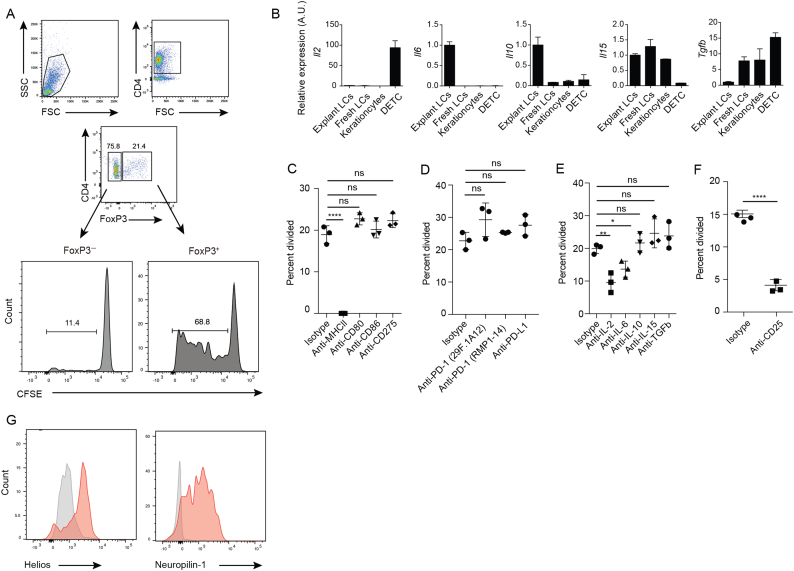
Consistent with the human setting, a significant portion of Treg cells, but not Teff cells, underwent proliferation (Fig. 4A), demonstrating that LCs preferentially expanded Treg cells in the absence of autoimmune T cells. The Treg cells that proliferated in this experiment may be specific for various epidermal self-antigens that were acquired by LCs.
Fig. 4.
LCs induce the expansion of polyclonal Treg cells (A) CFSE-dilution assay utilizing polyclonal CD4+ T cells that were isolated from syngeneic WT mice, co-cultured with skin-explant WT LCs. Dot plots show the gating strategy for CD4+ T cells after co-culture. Histograms show FoxP3+ or FoxP3− CD4+ T cells. (B) Real-time PCR analysis for indicated cytokine mRNA expressed by skin-explant LCs (Explant LCs), LCs (Fresh LCs), keratinocytes and dendritic epidermal T cells (DETCs) that were FACS sorted from freshly prepared epidermal cell suspensions. (C - F) CFSE-dilution assay as in (A) with antibodies or isotypes added to the co-culture as indicated. The Y-axis, percent divided, represents the frequency of CD4+ FoxP3+ T cells among original input population that have undergone division. (G) Flow cytometry analysis for cell-surface markers on Treg cells expanded by LCs in vitro, stained with antibodies against indicated molecules (red) or isotypes (gray). p values: *<0.05, **<0.01, ****<0.0001.
LCs in epidermal cell suspension as well as those from skin explants expressed a variety of molecules that play major roles in immune regulation, including MHC II, CD80, CD86 and PD-L1 (data not shown) as well as cytokines IL-6, 10, 15, and TGF-β1 (Fig. 4B). We added neutralizing antibodies against these and other molecules that are involved Treg cell induction (Busse et al., 2012, Fontenot et al., 2005), and found that only anti-MHC II and anti-IL-2 antibodies had notable effects on Treg cell expansion, with modest effects of IL-6 blockage (Fig. 4C,D,E). Blockage of IL-2 receptor alpha (CD25) had a similar effect than IL-2 neutralization (Fig. 4F).
The importance of TGF-β1 for induced Treg cells is well-established (Li and Rudensky, 2016). The observation that a blocking antibody against TGF-β1 had no effect on LC-mediated Treg cell expansion (Fig. 4E) was consistent with the down-regulation of Tgfb1 expression by skin-explant LCs, as compared with LCs sorted from freshly prepared epidermal cell suspensions (Fig. 4B). Furthermore, thymus-derived Treg cell markers Helios and Neuropilin-1 were expressed by LC-expanded Treg cells (Fig. 4G). These data suggested that LCs mediate the expansion of naturally-occurring Treg cells, rather than the expansion of induced Treg cells. Thus, LCs expand Treg cells that are likely to be naturally-occuring, via a mechanism that involves the IL-2-IL-2 receptor axis.
3.6. IL-2 Receptor Signaling in LCs
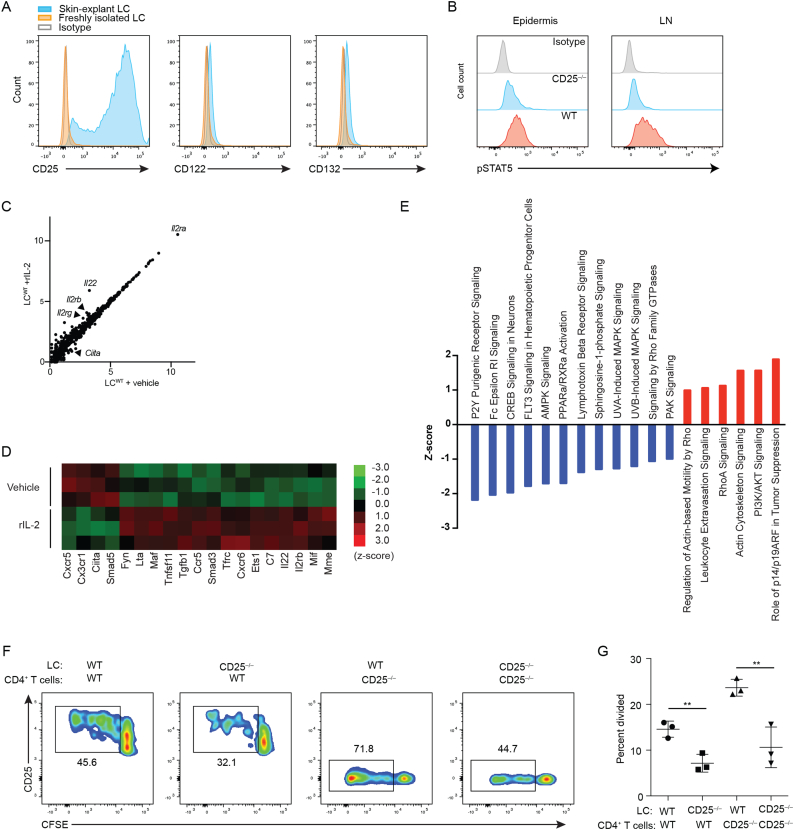
The effect of IL-2 and CD25 blockage on Treg cells could be explained by direct effects on T cells. Interestingly, upon emigration skin-explant LCs highly upregulated CD25 (IL2Rα), consistent with previous reports (Larsen et al., 1990, Steiner et al., 1986), and to a lesser extent IL-2Rβ and IL-2Rγ chains (Fig. 5A), suggesting that IL-2 might directly affect LCs.
Fig. 5.
IL-2 signaling occurs in LCs and is required for optimal LC-mediated Treg cell expansion (A) Expression of the IL-2 receptor complex by LCs from indicated sources. (B) Flow cytometry analysis for pSTAT5 in LCs from freshly prepared epidermal cell suspensions or lymph nodes from WT or CD25−/− mice. (C) Grouped expression values for rIL-2-treated skin explant LCs (WT + rIL-2) were plotted against vehicle-treated LCs (WT + vehicle) for all 561 nCounter Mouse Immunology Panel (Nanostring) genes. (D) Differential gene expression between rIL-2- and vehicle-treated LCs was significant (p < 0.05) for 19 genes in the Nanostring analysis. (E) Canonical IPA pathways with significant up- or downregulation in rIL-2-treated LCs. (F) CFSE-labeled syngeneic WT or CD25−/− CD4+ T cells were co-cultured with WT or CD25−/− LCs. (G) Quantification of the percentage of CD4+ FoxP3+ T cells from (F). The Y-axis, percent divided, represents the frequency of CD4+ FoxP3+ T cells among original input population that have undergone division. Data are representative of 3 independent experiments. p values: *<0.05, **<0.01, ****<0.0001.
Signal transducer and activator of transcription 5 (STAT5) undergoes phosphorylation (pSTAT5) upon engagement of IL-2 with the IL-2 receptor complex (O'Shea and Plenge, 2012). We therefore stained for pSTAT5 and detected it in LCs from both the epidermis and lymph nodes of WT mice. Furthermore, pSTAT5 levels were decreased in LCs from CD25−/− mice (Fig. 5B), indicating that IL-2 signaling takes place in LCs.
To further characterize the effect of IL-2 on LCs, skin-explant LCs obtained from WT mice (Fig. S1A,B) were exposed to recombinant IL-2 (rIL-2) in vitro for 8 h. RNA was extracted and transcripts for 561 immunology-related genes were quantified via Nanostring analysis. rIL-2 induced gene expression changes in LCs (Fig. 5C). Highly significant changes included decreased expression of Ciita, a regulator of MHC II, and increased Tgfb1 and Smad3 expression (Fig. 5D), both of which are associated with TGF-β signaling and LC migration (Mohammed et al., 2016). There was a trend for upregulated Il2rg expression as well as statistically significant upregulation of Il2rb in response to rIL-2 (Fig. 5C,D).
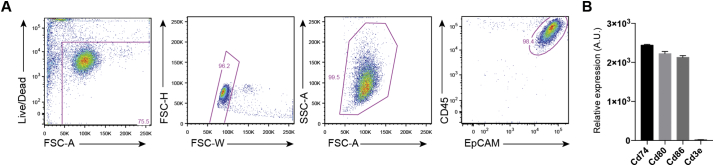
Fig. S1.
Purity of skin-explant LCs. (A) Gating strategy for skin-explant LCs from WT mice that were subsequently exposed to recombinant IL-2. (B) mRNA levels of LC-derived genes (Cd74, Cd80, Cd86) and T cell-derived Cd3e as determined by Nanostring analysis performed in Fig. 5C–E.
To predict the biological significance of rIL-2-induced overall gene expression changes in LCs, we performed Ingenuity Pathways Analysis. Whereas the mitogen-activated protein kinases (MAPK) signaling pathway has been reported to be activated in response to ultraviolet irradiation in LCs (Wang et al., 2011), rIL-2 exposed LCs exhibited downregulation of these pathways (Fig. 5E). Among upregulated pathways were those that related to cell motility and migration, as well as PI3K/AKT and p14/p19ARF pathways, both of which have been demonstrated to be required for LC homeostasis (Kellersch and Brocker, 2013, Sparber et al., 2014). Significant decreases in disease-associated pathways that reflect the activation of APCs were also detected (Table S1). Taken together, rIL-2 has effects on biological pathways that are known to be relevant in LC physiology, suggesting that IL-2 signaling in LCs evokes a less activated but motile phenotype that may represent homeostatic migration of LCs that carry autoantigens (Hemmi et al., 2001) and is consistent with a previous study reporting IL-2-mediated increased DC motility in rats (Kradin et al., 1996).
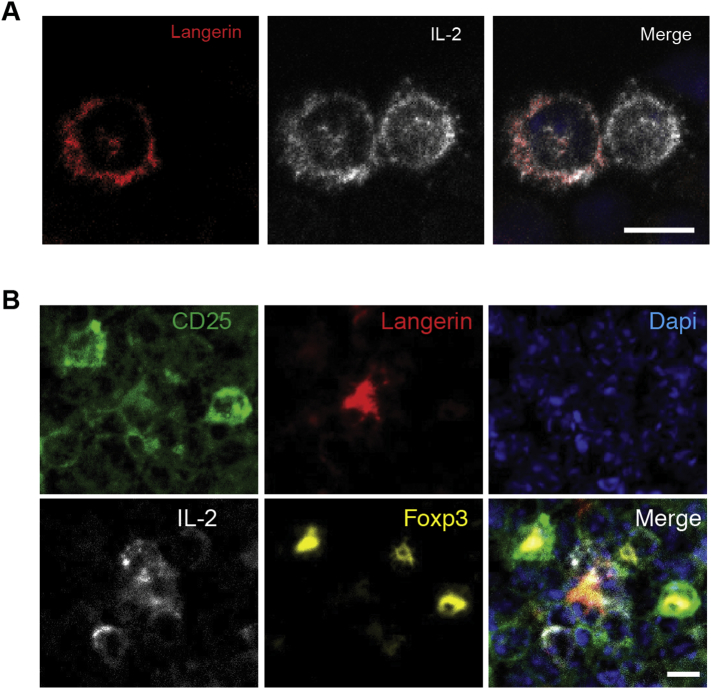
In contrast to human in vitro derived DCs that produce IL-2 to activate T cells (Wuest et al., 2011), LCs did not express Il2 (Fig. 4B). We therefore hypothesized that LCs may acquire IL-2 from Teff cells, a major source of IL-2. Indeed, immunofluorescence analysis of cytospin preparation of LCs and syngeneic WT CD4+ T cells that were co-cultured for 4 days revealed that LCs were in close proximity to IL-2+ FoxP3− T cells, and that LCs had acquired IL-2 protein on their cell surfaces (Fig. S2A). Similarly, IL-2-bearing LCs were observed near IL-2+ cells in the T cell zone in skin draining lymph nodes (Fig. S2B). These data suggested that LCs acquire IL-2 from IL-2-producing T cells nearby. Given that dendritic epidermal T cells express Il2 (Fig. 4B) and that pSTAT5 is decreased in epidermal LCs of CD25−/− mice (Fig. 5B), dendritic epidermal T cells may represent the source of IL-2 for LCs that reside within the epidermis.
Fig. S2.
LCs acquire IL-2 from neighboring IL-2-producing, non-Treg cells. (A) WT CD4+ T cells and WT LCs were co-cultured for 3 days followed by preparation of cytospins and staining for IL-2 and langerin. Bar = 10 μm. (B) Frozen sections from inguinal lymph nodes of WT C57/BL6 mice were stained for CD25, IL-2, langerin and FoxP3. Bar = 10 μm. All data are representative of two independent experiments (n = 3).
3.7. IL-2-Mediated LC Conditioning Is Required for Treg Cell Expansion
Finally, to determine the consequence of impaired IL-2 signaling in LCs during Treg cell proliferation, we co-cultured WT syngeneic T cells with skin explant LCWT and LCs from CD25−/− mice (LCCD25 −/−) and found that LCCD25 −/− were impaired in their capability to induce the proliferation of CD25+ FoxP3+ Treg cells (Fig. 5F,G). To eliminate the effect of CD25 expressed on T cells, we co-cultured LCWT or LCCD25 −/− with CD25−/− CD4+ T cells. This experiment is valid because FoxP3+ Treg cells are able to develop in CD25−/− mice (Fontenot et al., 2005). Indeed, CD25−/− CD4+ FoxP3+ T cells co-cultured with LCWT underwent robust proliferation, likely reflecting their hyperactivated state in vivo (Willerford et al., 1995). However, consistent with the results obtained with WT CD4+ Treg cells, co-culture with LCCD25 −/− led to a decreased proliferation of CD25−/− CD4+ FoxP3+ Treg cells (Fig. 5F,G). These data collectively demonstrate that CD25 on LCs was required for optimal Treg cell expansion and that the IL-2-IL-2 receptor axis in LCs conditioned them to expand Treg cells.
4. Discussion
Pemphigus vulgaris is an autoantibody-mediated blistering skin disease, which can be debilitating and potentially life-threatening if not properly treated (Stanley and Amagai, 2006). Although IgG-mediated inhibition of Dsg3 function is the major mechanism that leads to the loss of keratinocyte adhesion and blister formation, autoreactive T cells participate upstream in the immune response to help B cells to produce autoantibodies (Takahashi et al., 2009), and further upstream, it is likely that APCs acquire and present autoantigens to activate autoreactive T cells. Better understanding of mechanisms by which APCs take up an epidermal autoantigen such as Dsg3, and better characterization of subsequent immune responses would provide a foundation for the development of novel therapeutic strategies against not only pemphigus vulgaris, but also other autoimmune blistering diseases that target epidermal antigens, such as bullous pemphigoid, epidermal bullosa acquisita, and perhaps other T cell-mediated diseases such as lichen planus and erythema multiforme, in which T cells directly mediate the cell death of keratinocytes (Gru and Salavaggione, 2017). In this work, we asked whether LCs, given their proximity to keratinocyte-associated autoantigens, are the major APC to regulate immune responses against keratinocyte-expressed Dsg3.
In previous work, functional analyses of LCs had utilized infectious agents or haptens, and the conclusion of experiments that explored the tolerogenic aspects of LCs had been somewhat variable (Kaplan et al., 2008, Kautz-Neu et al., 2010). The nature of antigens, how and where they are captured, as well as the location and extent of inflammation may influence experimental outcomes. The experimental systems utilized herein were unique in that we did not utilize any exogenous antigens, neoantigens or adjuvants, which may alter the homeostatic function of LCs. Dsg3 is expressed exclusively by epidermal keratinocytes in the skin. Owing to this, no manipulations were required to observe LCs' immune responses toward the antigen, ensuring that LCs captured Dsg3 in the absence of external cues, and importantly, at physiological doses.
The discovery of langerin reoriented the LC field, allowing for the identification of migrated LCs and the generation of in vivo depletion models (Bennett et al., 2005, Kaplan et al., 2005, Kissenpfennig et al., 2005b, Valladeau et al., 2000). However, despite its demonstration as an endocytic receptor in vitro (Valladeau et al., 2000), langerin-deficient mice failed to exhibit any obvious phenotypes (Kissenpfennig et al., 2005a). Furthermore, although langerin functions as a scavenging receptor against HIV (de Witte et al., 2007), its roles in immune responses had remained incompletely characterized. The co-localization of langerin with non-conformational Dsg3 epitopes within LCs prompted us to address the role of langerin during self-antigen uptake. This hypothesis, that the C-type lectin langerin could bind Dsg3, was reasonable because Dsg3 is highly glycosylated (Amagai et al., 1991). Because langerin is essential for the formation of Birbeck granules (Valladeau et al., 2000) and Birbeck granules are reportedly involved in the routing of antigen for antigen processing (van der Vlist et al., 2011), more work is needed to unequivocally show that langerin mediates the uptake of, rather than the proper routing of, acquired antigens.
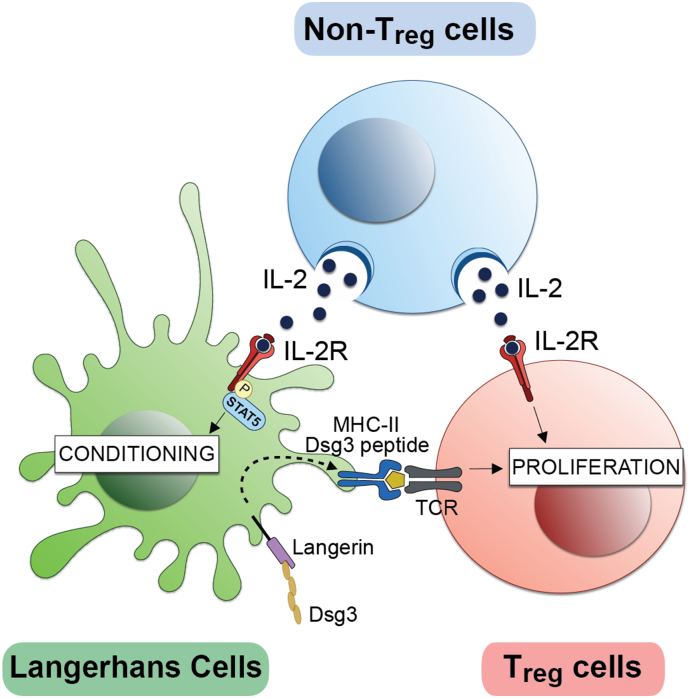
We observed an upregulation of the IL-2 receptor complex on skin-explant LCs. It has long been known that mature Langerhans cells express CD25 (IL2Rα) (Larsen et al., 1990, Steiner et al., 1986), however, the functional role of IL2Rα for LCs has remained largely unknown. Our data show that IL-2 signaling actually takes place within LCs and conditions them to optimally induce Treg cell expansion. In vitro-derived human myeloid DCs produce IL-2. Because blockage of CD25 on DCs impaired their ability to activate T cells, it was suggested that DCs present IL-2 in-trans via CD25 to activate T cells (Wuest et al., 2011). However, LCs did not express IL-2 mRNA before or after emigration from the skin. Although it remains to be investigated where this event occurs, gene expression analyses of LCs and data from in vitro co-culture experiments suggested that LCs and Treg cells may acquire biologically active IL-2 from nearby non-Treg cells in the epidermis and/or the lymph nodes. While IL-2 conditions LCs to induce Treg cell expansion, Treg cells also require the cytokine for proliferation. Furthermore, Treg cells additionally require antigen presentation by LCs, collectively forming a unique triangular relationship (Fig. 6). IL-2 signaling has not been previously demonstrated to take place in APCs, and it would be of interest to determine if this phenomenon can be extended to other APC subsets.
Fig. 6.
Triangular cell interaction supports Treg proliferation. Langerhans cells take up Desmoglein-3 via langerin and display it through MHC-II molecules to induce Treg proliferation. In addition, IL-2 produced by non-Treg cells is required for both conditioning of LC function and subsequent Treg cell expansion.
Naive Treg cells are thought to circulate until they are activated by DCs in the lymph node (Li and Rudensky, 2016). Some activated Treg cells can become memory Treg cells, whose presence has been shown in both mouse and human skin (Rosenblum et al., 2011, Sanchez Rodriguez et al., 2014). Treg cells have also been found in human epidermis (Seneschal et al., 2012) where they co-localized with LCs. It was postulated that the direct association of LCs and Treg cells in skin may be involved in memory responses. It is therefore possible that LCs regulate various aspects of the Treg cell life cycle. Although our study in mice cannot yet be directly translated to human diseases, it is reasonable to hypothesize that LC-Treg cell interactions are also relevant in various autoimmune and inflammatory skin diseases, thereby representing an attractive therapeutic target.
In conclusion, we have demonstrated that LCs take up the epidermal antigen Dsg3 via langerin and that direct IL-2-mediated signaling conditions them to optimally induce the expansion of Treg cells. Although LCs activated both Teff and Treg cells in the autoimmune setting, the loss of LCs in vivo led to exacerbated skin inflammation. Taken together, the finding that LCs preferentially induced the expansion of polyclonal Treg cells suggests that LCs suppress autoimmunity via Treg cell expansion in the steady state. This observation is consistent with a previous report, in which epicutaneously administered Dsg3 induced skin inflammation in MRL-lpr mice, a model of systemic autoimmunity (King et al., 2015). Further work should be conducted to determine the functional role of the IL-2 pathway in LCs in vivo and the mechanism by which IL-2-conditioned LCs induce the expansion of Treg cells. Considering previous studies that have demonstrated LCs' roles in protective immunity (Igyártó et al., 2011, Ouchi et al., 2011), LCs are likely to be versatile and context-dependent in their function (Doebel et al., 2017). Exploring environmental cues that govern LCs' immunological polarity should enable new strategies to control skin immunity and to achieve tolerogenic responses in autoimmune skin diseases.
The following are the supplementary data related to this article.
Functional predictions for transcriptional changes in rIL-2-treated LCs via Ingenuity Pathway Analysis.
Co-localization of langerin and Dsg3 signals in epidermal LCs.
Funding Sources
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (24390277 for K.Na. and 21229014 for M.A.), Research on Measures against Intractable Diseases, the Ministry of Health, Labour and Welfare of Japan (14427215) and the Intramural Research Program, Center for Cancer Research, National Cancer Institute, National Institute of Health (ZIA BC 011561). D.Y.K. received a scholarship from the Ministry of Education, Culture, Sports, Science and Technology. T.K. is a JSPS-NIH fellow. T. W. was funded by the NIH Medical Research Scholars Program, supported jointly by the NIH and contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, and other private donors.
Conflict of Interest
The authors declare no competing financial interests.
Author Contributions
D.Y.K. and T.K. performed the majority of experiments with assistance from K.I., H.T., T.A., T.O., T.D. and B.V. T.W. and T.K. performed bioinformatics analyses. K·Ni. and M.A. generated the K5-Dsg3-eGFP mice. B.E.C. provided the Langerin-DTR mice, and D.H.K. provided the Langerin-DTA mice. K·Na. conceived the experiments, oversaw the project. T.W., T.K. and K·Na. wrote the manuscript.
Acknowledgements
Authors thank Xuguang Tai and Alfred Singer for helpful discussions and for providing CD25−/− mice; Michael Kruhlak for assistance with confocal microscopy, and the CCR Genomics core for Nanostring analysis.
References
- Amagai M., Klaus-Kovtun V., Stanley J.R. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991;67:869–877. doi: 10.1016/0092-8674(91)90360-b. [DOI] [PubMed] [Google Scholar]
- Amagai M., Ishii K., Hshimoto T., Gamou S., Shimizu N., Nishikawa T. Conformational epitopes of pemphigus antigens (Dsg1 and Dsg3) are calcium dependent and glycosylation independent. J. Invest. Dermatol. 1995;105:243–247. doi: 10.1111/1523-1747.ep12317587. [DOI] [PubMed] [Google Scholar]
- Bennett C.L., van Rijn E., Jung S., Inaba K., Steinman R.M., Kapsenberg M.L., Clausen B.E. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J. Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse M., Krech M., Meyer-Bahlburg A., Hennig C., Hansen G. ICOS mediates the generation and function of CD4 + CD25 + Foxp3 + regulatory T cells conveying respiratory tolerance. J. Immunol. 2012;189:1975–1982. doi: 10.4049/jimmunol.1103581. [DOI] [PubMed] [Google Scholar]
- Doebel T., Voisin B., Nagao K. Langerhans cells - the macrophage in dendritic cell clothing. Trends Immunol. 2017;38:817–828. doi: 10.1016/j.it.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Flacher V., Tripp C.H., Mairhofer D.G., Steinman R.M., Stoitzner P., Idoyaga J., Romani N. Murine langerin + dermal dendritic cells prime CD8 + T cells while langerhans cells induce cross-tolerance. EMBO Mol. Med. 2014;6:1191–1204. doi: 10.15252/emmm.201303283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot J.D., Rasmussen J.P., Gavin M.A., Rudensky A.Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Gru A.A., Salavaggione A.L. Lichenoid and interface dermatoses. Semin. Diagn. Pathol. 2017;34:237–249. doi: 10.1053/j.semdp.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Hata T., Nishifuji K., Shimoda K., Sasaki T., Yamada T., Nishikawa T., Koyasu S., Amagai M. Transgenic rescue of desmoglein 3 null mice with desmoglein 1 to develop a syngeneic mouse model for pemphigus vulgaris. J. Dermatol. Sci. 2011;63:33–39. doi: 10.1016/j.jdermsci.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Hemmi H., Yoshino M., Yamazaki H., Naito M., Iyoda T., Omatsu Y., Shimoyama S., Letterio J.J., Nakabayashi T., Tagaya H. Skin antigens in the steady state are trafficked to regional lymph nodes by transforming growth factor-beta1-dependent cells. Int. Immunol. 2001;13:695–704. doi: 10.1093/intimm/13.5.695. [DOI] [PubMed] [Google Scholar]
- Idoyaga J., Fiorese C., Zbytnuik L., Lubkin A., Miller J., Malissen B., Mucida D., Merad M., Steinman R.M. Specialized role of migratory dendritic cells in peripheral tolerance induction. J. Clin. Invest. 2013;123:844–854. doi: 10.1172/JCI65260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igyártó B.Z., Haley K., Ortner D., Bobr A., Gerami-Nejad M., Edelson B.T., Zurawski S.M., Malissen B., Zurawski G., Berman J. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D.H., Jenison M.C., Saeland S., Shlomchik W.D., Shlomchik M.J. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Kaplan D.H., Kissenpfennig A., Clausen B.E. Insights into langerhans cell function from langerhans cell ablation models. Eur. J. Immunol. 2008;38:2369–2376. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- Kautz-Neu K., Meyer R.G., Clausen B.E., Von Stebut E. Leishmaniasis, contact hypersensitivity and graft-versus-host disease: understanding the role of dendritic cell subsets in balancing skin immunity and tolerance. Exp. Dermatol. 2010;19:760–771. doi: 10.1111/j.1600-0625.2010.01116.x. [DOI] [PubMed] [Google Scholar]
- Kautz-Neu K., Noordegraaf M., Dinges S., Bennett C.L., John D., Clausen B.E., von Stebut E. Langerhans cells are negative regulators of the anti-leishmania response. J. Exp. Med. 2011;208:885–891. doi: 10.1084/jem.20102318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H., Tsunoda K., Hata T., Ishii K., Yamada T., Amagai M. Synergistic pathogenic effects of combined mouse monoclonal anti-desmoglein 3 IgG antibodies on pemphigus vulgaris blister formation. J. Invest. Dermatol. 2006;126:2621–2630. doi: 10.1038/sj.jid.5700450. [DOI] [PubMed] [Google Scholar]
- Kellersch B., Brocker T. Langerhans cell homeostasis in mice is dependent on mTORC1 but not mTORC2 function. Blood. 2013;121:298. doi: 10.1182/blood-2012-06-439786. [DOI] [PubMed] [Google Scholar]
- King J.K., Philips R.L., Eriksson A.U., Kim P.J., Halder R.C., Lee D.J., Singh R.R. Langerhans cells maintain local tissue tolerance in a model of systemic autoimmune disease. J. Immunol. 2015;195:464–476. doi: 10.4049/jimmunol.1402735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissenpfennig A., Aït-Yahia S., Clair-Moninot V., Stössel H., Badell E., Bordat Y., Pooley J.L., Lang T., Prina E., Coste I., Gresser O. Disruption of the langerin/CD207 gene abolishes birbeck granules without a marked loss of langerhans cell function. Mol. Cell. Biol. 2005;25:88–99. doi: 10.1128/MCB.25.1.88-99.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissenpfennig A., Henri S., Dubois B., Laplace-Builhé C., Perrin P., Romani N., Tripp C.H., Douillard P., Leserman L., Kaiserlian D. Dynamics and function of langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Kradin R.L., Xia W., Pike M., Byers H.R., Pinto C. Interleukin-2 promotes the motility of dendritic cells and their accumulation in lung and skin. Pathobiology. 1996;64:180–186. doi: 10.1159/000164033. [DOI] [PubMed] [Google Scholar]
- Kubo A., Nagao K., Yokouchi M., Sasaki H., Amagai M. External antigen uptake by langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 2009;206:2937–2946. doi: 10.1084/jem.20091527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C.P., Steinman R.M., Witmer-Pack M., Hankins D.F., Morris P.J., Austyn J.M. Migration and maturation of langerhans cells in skin transplants and explants. J. Exp. Med. 1990;172:1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.O., Rudensky A.Y. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat. Rev. Immunol. 2016;16:220–233. doi: 10.1038/nri.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed J., Beura L.K., Bobr A., Astry B., Chicoine B., Kashem S.W., Welty N.E., Igyarto B.Z., Wijeyesinghe S., Thompson E.A. Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-beta. Nat. Immunol. 2016;17:414–421. doi: 10.1038/ni.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao K., Ginhoux F., Leitner W.W., Motegi S., Bennett C.L., Clausen B.E., Merad M., Udey M.C. Murine epidermal langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3312–3317. doi: 10.1073/pnas.0807126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea J.J., Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi T., Kubo A., Yokouchi M., Adachi T., Kobayashi T., Kitashima D.Y., Fujii H., Clausen B.E., Koyasu S., Amagai M. Langerhans cell antigen capture through tight junctions confers preemptive immunity in experimental staphylococcal scalded skin syndrome. J. Exp. Med. 2011;208:2607–2613. doi: 10.1084/jem.20111718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.G., Idoyaga J., Salmon H., Hogstad B., Bigarella C.L., Ghaffari S., Leboeuf M., Merad M. CDKN1A regulates langerhans cell survival and promotes treg cell generation upon exposure to ionizing irradiation. Nat. Immunol. 2015;16:1060–1068. doi: 10.1038/ni.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum M.D., Gratz I.K., Paw J.S., Lee K., Marshak-Rothstein A., Abbas A.K. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538–542. doi: 10.1038/nature10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Rodriguez R., Pauli M.L., Neuhaus I.M., Yu S.S., Arron S.T., Harris H.W., Yang S.H., Anthony B.A., Sverdrup F.M., Krow-Lucal E. Memory regulatory T cells reside in human skin. J. Clin. Invest. 2014;124:1027–1036. doi: 10.1172/JCI72932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneschal J., Clark R.A., Gehad A., Baecher-Allan C.M., Kupper T.S. Human epidermal langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity. 2012;36:873–884. doi: 10.1016/j.immuni.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparber F., Scheffler J.M., Amberg N., Tripp C.H., Heib V., Hermann M., Zahner S.P., Clausen B.E., Reizis B., Huber L.A. The late endosomal adaptor molecule p14 (LAMTOR2) represents a novel regulator of langerhans cell homeostasis. Blood. 2014;123:217. doi: 10.1182/blood-2013-08-518555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J.R., Amagai M. Pemphigus, bullous impetigo, and the staphylococcal scalded-skin syndrome. N. Engl. J. Med. 2006;355:1800–1810. doi: 10.1056/NEJMra061111. [DOI] [PubMed] [Google Scholar]
- Steiner G., Tschachler E., Tani M., Malek T.R., Shevach E.M., Holter W., Knapp W., Wolff K., Stingl G. Interleukin 2 receptors on cultured murine epidermal langerhans cells. J. Immunol. 1986;137:155–159. [PubMed] [Google Scholar]
- Szymczak-Workman A.L., Workman C.J., Vignali D.A.A. Cutting edge: regulatory T cells do not require stimulation through their TCR to suppress. J. Immunol. 2009;182:5188–5192. doi: 10.4049/jimmunol.0803123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Kuwana M., Amagai M. A single helper T cell clone is sufficient to commit polyclonal naive B cells to produce pathogenic IgG in experimental pemphigus vulgaris. J. Immunol. 2009;182:1740–1745. doi: 10.4049/jimmunol.182.3.1740. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Kouno M., Nagao K., Wada N., Hata T., Nishimoto S., Iwakura Y., Yoshimura A., Yamada T., Kuwana M. Desmoglein 3–specific CD4 + T cells induce pemphigus vulgaris and interface dermatitis in mice. J. Clin. Invest. 2011;121:3677–3688. doi: 10.1172/JCI57379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann L.L., Ols M.L., Kashgarian M., Reizis B., Kaplan D.H., Shlomchik M.J. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity. 2010;33:967–978. doi: 10.1016/j.immuni.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladeau J., Ravel O., Dezutter-Dambuyant C., Moore K., Kleijmeer M., Liu Y., Duvert-Frances V., Vincent C., Schmitt D., Davoust J. Langerin, a novel C-type lectin specific to langerhans cells, is an endocytic receptor that induces the formation of birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- van der Vlist M., de Witte L., de Vries R.D., Litjens M., de Jong M.A., Fluitsma D., de Swart R.L., Geijtenbeek T.B. Human Langerhans cells capture measles virus through langerin and present viral antigens to CD4(+) T cells but are incapable of cross-presentation. Eur. J. Immunol. 2011;41:2619–2631. doi: 10.1002/eji.201041305. [DOI] [PubMed] [Google Scholar]
- Wang X., Bi Z., Wang Y., Wang Y. Increased MAPK and NF-κB expression of langerhans cells is dependent on TLR2 and TLR4, and increased IRF-3 expression is partially dependent on TLR4 following UV exposure. Mol. Med. Rep. 2011;4:541–546. doi: 10.3892/mmr.2011.450. [DOI] [PubMed] [Google Scholar]
- Willerford D.M., Chen J., Ferry J.A., Davidson L., Ma A., Alt F.W. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- de Witte L., Nabatov A., Pion M., Fluitsma D., de Jong M.A.W.P., de Gruijl T., Piguet V., van Kooyk Y., Geijtenbeek T.B.H. Langerin is a natural barrier to HIV-1 transmission by langerhans cells. Nat. Med. 2007;13:367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- Wuest S.C., Edwan J.H., Martin J.F., Han S., Perry J.S.A., Cartagena C.M., Matsuura E., Maric D., Waldmann T.A., Bielekova B. A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat. Med. 2011;17:604–609. doi: 10.1038/nm.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Functional predictions for transcriptional changes in rIL-2-treated LCs via Ingenuity Pathway Analysis.
Co-localization of langerin and Dsg3 signals in epidermal LCs.