Abstract
Women’s empowerment is associated with engagement in some areas of healthcare, but its role in prevention of mother-to-child HIV transmission (PMTCT) services has not been previously considered. In this secondary analysis, we investigated the association of women’s decision-making and uptake of health services for PMTCT. Using data from population-based household surveys, we included women who reported delivery in the 2-year period prior to the survey and were HIV-infected. We measured a woman’s self-reported role in decision-making in her own healthcare, making of large purchases, schooling of children, and healthcare for children. For each domain, respondents were categorized as having an “active” or “no active” role. We investigated associations between decision-making and specific steps along the PMTCT cascade: uptake of maternal antiretroviral drugs, uptake of infant HIV prophylaxis, and infant HIV testing. We calculated unadjusted and adjusted odds ratios via logistic regression. From March to December 2011, 344 HIV-infected mothers were surveyed and 276 completed the relevant survey questions. Of these, 190 (69%) took antiretroviral drugs during pregnancy; 175 (64%) of their HIV-exposed infants received antiretroviral prophylaxis; and 160 (58%) had their infant tested for HIV. There was no association between decision-making and maternal or infant antiretroviral drug use. We observed a significant association between decision-making and infant HIV testing in univariate analyses (OR 1.56–1.85; p<0.05); however, odds ratios for the decision-making indicators were no longer statistically significant predictors of infant HIV testing in multivariate analyses. In conclusion, women who reported an active role in decision-making trended toward a higher likelihood of uptake of infant testing in the PMTCT cascade. Larger studies are needed to evaluate the impact of empowerment initiatives on the PMTCT service utilization overall and infant testing in particular.
Keywords: Decision-making, PMTCT Cascade, infant HIV testing, Africa, Zambia
Introduction
Significant strides have been made in the prevention of mother-to-child transmission of HIV (PMTCT) over the past decade (UNAIDS, 2016). Provision of universal, lifelong antiretroviral therapy (ART) for all HIV-infected pregnant and breastfeeding women has been adopted by many national programs in sub-Saharan Africa including Zambia. We have seen a precipitous decrease in pediatric HIV infections from these programs; however, access to such services remains inconsistent in many settings (Barker, Mphatswe, & Rollins, 2011).
To better understand the coverage of PMTCT programs, numerous “cascade” frameworks have been proposed to evaluate the extent of healthcare engagement (Chi et al., 2015; McNairy, Teasdale, El-Sadr, Mave, & Abrams, 2015; Stringer et al., 2008). Several steps are commonly described. For pregnant women, the cascade includes HIV testing, receipt of HIV results, and – for those who test positive – initiation of ART and retention in care. Infant engagement includes antiretroviral prophylaxis in the first six weeks of life, HIV testing within the first 6 weeks of life and throughout the breastfeeding period for early infant diagnosis, and immediate ART initiation among those who test HIV-positive. When each of these steps are met, perinatal transmission rates are as low as 1–2% (de Vincenzi, 2011; Kilewo et al., 2009; Shapiro et al., 2010; Thomas et al., 2011). In “real world” settings, however, there is often attrition along the cascade and this attenuates the impact of PMTCT programs at the individual and population level (Barker et al., 2011).
Women’s decision-making – measuring a woman’s control over her own life and environment – may influence women to participate in their own healthcare and the healthcare of their children (Kishor & Subaiya, 2008). Previous studies have shown that women demonstrating higher levels of decision-making, and more broadly empowerment, were more likely to engage in health-seeking behaviors. A study of the 2010 Tanzanian Demographic and Health Survey, for example, found that women actively involved in decision-making and those who agreed that women can refuse sex were more likely to test for HIV (Bashemera, Nhembo, & Benedict, 2013). In Zimbabwe, women who scored higher on an empowerment questionnaire were more likely to use dual methods for family planning with their partners, though overall reporting was still low (Mutowo, Kasu, & Mufunda, 2014). Qualitative studies have similarly shown an association between women’s empowerment and PMTCT service utilization, providing a theoretical framework for future potential interventions (Ngarina, Popenoe, Kilewo, Biberfeld, & Ekstrom, 2013; Zhou, 2016). However, supporting quantitative data is needed in the context of PMTCT, a gap we sought to address in the current analysis. Our central hypothesis was that active involvement in decision-making would be associated with positive health-seeking behaviors in an HIV-positive pregnant population.
Methods
Setting
From April 2009 to January 2011, we implemented a pilot program to introduce universal combination antiretroviral regimens during pregnancy and breastfeeding to reduce vertical transmission of HIV. This pilot program was supported by the Zambian Ministry of Health at a time when the feasibility and safety of combination regimens during breastfeeding was not yet fully established. Details about the health services provided are described elsewhere (Gartland et al., 2013). Briefly, in accordance with Zambian HIV treatment guidelines at the time, women testing HIV-positive were screened for antiretroviral therapy eligibility via clinical staging and CD4 screening. Those who required HIV treatment were immediately started on antiretroviral therapy; those who did not meet criteria were offered a three-drug combination regimen starting at 28 weeks gestation, which was continued until the cessation of breastfeeding. Women who refused to participate in the pilot program were offered antenatal zidovudine beginning at 28 weeks gestation and provided two-dose peripartum nevirapine around the time of delivery, the standard of PMTCT in Zambia at the time (Gartland et al., 2013). This pilot program was implemented at four peri-urban/rural clinics, selected in coordination with the Kafue District Health Management Team.
The Kafue PMTCT Household Survey
In this secondary analysis, we analyzed data from a repeat cross-sectional household survey to evaluate this pilot program. The methods and primary results have been reported elsewhere (Chi et al., 2014; Escamilla et al., 2015; Turnbull et al., 2011). This household survey sought to measure the community impact of universal ART on infant HIV-free survival using a two-round, repeat cross-sectional “before/after” design. The first survey round was performed from November 2008 to May 2009; the second round – the data from which the current analysis is based – was conducted from March 2011 to December 2011.
Sampling was conducted at two levels. First, we randomly selected zones within the government-demarcated catchment area for each of the four health facilities. Within each zone, a central point was identified and – using a preferred spacing interval based on the established population – residences were sampled in a spiraling clockwise direction until the entire area had been canvassed. Selected households were deemed eligible for the study if they reported a household member who had given birth in the last 24 months, regardless of HIV status or reported use of local health services.
We obtained written informed consent prior to participation in the study. For those who agreed, a 165-question survey was administered to mother(s) in the household reporting a birth in the last 24 months. If the mother had died, the child’s guardian or head of household were given the option of completing the survey. Blood specimens were collected from both mother and child (if alive) for later HIV testing. The receipt of HIV test results was voluntary. Participants were provided an unlinked study number that could be used to obtain HIV results at the local clinic from trained counselors. Infants who tested HIV-positive were traced and referred to their local HIV clinic.
In this analysis, we included women who reported a delivery in the prior two years and tested positive for HIV. For each participant, we included only data from the most recent pregnancy. This study was approved by ethical review committees at the University of Zambia (Lusaka, Zambia) and the University of North Carolina (Chapel Hill, NC, USA).
Study Outcomes and Exposures
Our primary outcomes were the self-reported uptake of health services at three steps along the PMTCT cascade. This included maternal initiation of antiretroviral drugs during pregnancy (i.e., of either short-course zidovudine with intrapartum nevirapine or three-drug combination regimens), infant initiation of antiretroviral drugs for prophylaxis (i.e., zidovudine or nevirapine syrup following delivery), and HIV testing for the HIV-exposed infant.
Our primary exposure was the woman’s role in decision-making. Women were asked who makes decisions in the family regarding: (1) her healthcare, (2) large household purchases, (3) schooling of children, and (4) healthcare of children. Responses for each included “you,” “you and your husband/partner,” “husband/partner,” “someone else,” “respondent and husband/partner,” or “decision not made/not applicable.” These responses were categorized a priori to either “active role in decision-making” (where women reported full or partial role – “you” or “you and your husband/partner”) or “no active role in decision-making” (all other responses). We also considered a composite index where women with an active role in any of the four areas were considered to have an active decision-making role overall.
Statistical Analysis
We used Pearson’s Chi-squared tests to consider demographic characteristics, stratified by women’s decision-making role. We conducted univariate analyses to identify demographic characteristics that might be important predictors of maternal decision-making. Demographic characteristics that were significant at p<0.20 in univariate analyses were included in multivariate analyses. We tested for possible associations between each of our selected PMTCT outcomes and the different maternal decision-making domains (including our composite outcome). We constructed separate unadjusted and adjusted logistic regression models with clustered robust standard errors to adjust for the sampling design. All statistical analysis was conducted using Stata version 14.1 (Stata Corporation, Texas, TX, USA).
Results
Overall, 5801 households were approached and 2441 (42%) households were found to be eligible. A total of 2444 mother-infant pairs from eligible households were enrolled, 365 (15%) infants were HIV-exposed at time of birth. Twenty-one HIV-infected mothers gave birth to more than one infant during the study period; for those, only the most recent pregnancy was considered. Of the 344 mother-infant pairs included in this analysis, 276 (80%) had outcome information available about antiretroviral use during pregnancy, infant antiretroviral use, and infant HIV testing. Women who answered survey questions about antiretroviral use and HIV testing were similar in terms of their role in decision-making and most demographic characteristics, although they were more likely to be unmarried (p<0.01) and somewhat more likely to be unemployed (p = 0.08; see Supplemental Table 1).
Responses to the decision-making questions are shown in Figure 1. Overall, those reporting an active role in decision making varied for separate domains: 218 (79%) women reported an active role in their own healthcare decisions, 157 (57%) reported an active role in large household purchase decisions, 163 (59%) reported an active role in children’s school decisions, and 164 (59%) reported an active role in children’s healthcare decisions. In our composite outcome, 264 (77.4%) reported an active role in at least one of the four domains; 77 (22.6%) said they have no active decision-making role in any of the studied areas. Demographic and socioeconomic characteristics of the overall study population, as well as stratified by decision-making role, are shown in Table 1.
Figure 1.
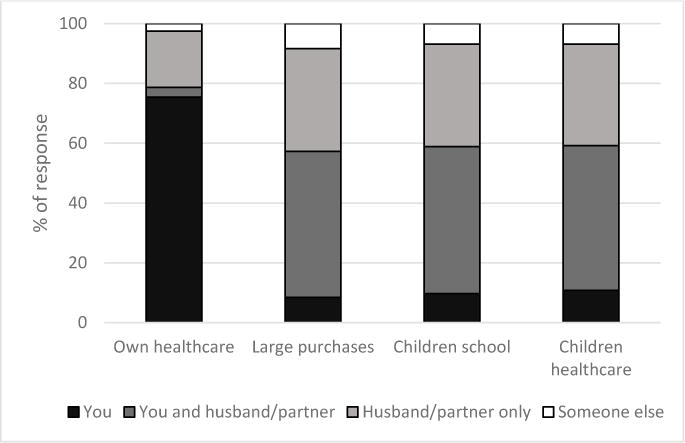
Distribution of responses when participants asked about their role in decision-making in Kafue District, Zambia. The black and grey bars (you, you and husband/partner) represent an “active” role in decision-making. The striped and white bars (husband/partner only, someone else) represent women with “no active” role in decision-making in the selected domain.
Table 1.
Household and demographic characteristics of participating HIV-infected women across 4 communities in the Kafue District, Zambia
| Overall study population | Mother’s Healthcare | Household Purchases | Children’s School | Children’s Healthcare | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active Role N =218 n (%) |
No Active Role N = 59 n (%) |
p | Active Role N = 157 n (%) |
No Active Role N = 117 n (%) |
p | Active Role N = 163 n (%) |
No Active Role N = 114 n (%) |
p | Active Role N = 164 n (%) |
No Active Role N = 113 n (%) |
p | ||
| Maternal age, in years | 0.22 | 0.07 | 0.21 | 0.30 | |||||||||
| 15 to <25 yrs | 67 (25.6%) | 50 (74.6) | 17 (25.4) | 30 (44.8) | 37 (55.2) | 33 (49.3) | 34 (50.8) | 34 (50.8) | 33 (49.3) | ||||
| 25 to <35 yrs | 147 (56.1%) | 114 (77.6) | 33 (22.5) | 88 (60.7) | 57 (39.3) | 90 (61.2) | 57 (38.8) | 90 (61.2) | 57 (38.8) | ||||
| 35 yrs or older | 48 (18.3%) | 42 (87.5) | 6 (12.5) | 29 (61.7) | 18 (38.3) | 30 (62.5) | 18 (37.5) | 30 (62.5) | 18 (37.5) | ||||
| Parity | 0.51 | 0.03 | 0.16 | 0.15 | |||||||||
| 0–1 | 69 (24.8%) | 51 (73.9) | 18 (26.1) | 30 (43.5) | 39 (56.5) | 34 (49.3) | 35 (50.7) | 34 (49.3) | 35 (50.7) | ||||
| 2–3 | 133 (48.0%) | 106 (79.7) | 27 (20.3) | 80 (61.1) | 51 (38.9) | 81 (60.9) | 52 (39.1) | 82 (61.7) | 51 (38.4) | ||||
| 4 or more | 75 (27.1%) | 61 (81.3) | 14 (18.7) | 47 (63.5) | 27 (36.5) | 48 (64.0) | 27 (36.0) | 48 (64.0) | 27 (36.0) | ||||
| Marital status | 0.28 | 0.31 | 0.88 | 0.92 | |||||||||
| Married or cohabitating | 235 (85.5%) | 182 (77.5) | 53 (22.6) | 136 (58.6) | 96 (41.4) | 138 (58.7) | 97 (41.3) | 139 (59.2) | 96 (40.9) | ||||
| Other | 40 (14.6%) | 34 (85.0) | 6 (15.0) | 20 (50.0) | 20 (50.0) | 24 (60.0) | 16 (40.0) | 24 (60.0) | 16 (40.0) | ||||
| Educational level | 0.32 | 0.51 | 0.43 | 0.43 | |||||||||
| Primary schooling or less | 97 (40.6%) | 79 (81.4) | 18 (18.6) | 56 (59.0) | 39 (41.1) | 59 (60.8) | 38 (39.2) | 59 (60.8) | 38 (39.2) | ||||
| Secondary or higher | 142 (59.4%) | 108 (76.1) | 34 (23.9) | 77 (54.6) | 64 (45.4) | 79 (55.6) | 63 (44.4) | 79 (55.6) | 63 (44.4) | ||||
| Currently employed | 0.18 | 0.12 | 0.19 | 0.15 | |||||||||
| No | 156 (56.7%) | 118 (85.6) | 38 (24.4) | 82 (52.9) | 73 (47.1) | 86 (55.1) | 70 (44.9) | 86 (55.1) | 70 (44.9) | ||||
| Yes | 119 (43.3%) | 98 (82.4) | 21 (17.7) | 73 (62.4) | 44 (37.6) | 75 (63.0) | 44 (37.0) | 76 (63.9) | 43 (36.1) | ||||
| Institutional Delivery | 0.39 | 0.31 | 0.29 | 0.32 | |||||||||
| No | 78 (28.3%) | 59 (75.6) | 19 (24.4) | 48 (62.3) | 29 (37.7) | 50 (64.1) | 28 (35.9) | 50 (64.1) | 28 (35.9) | ||||
| Yes | 198 (71.7%) | 159 (80.3) | 39 (19.7) | 109 (55.6) | 87 (44.4) | 113 (57.1) | 85 (42.9) | 114 (57.6) | 84 (41.4) | ||||
Of the 276 women included in this analysis, 190 (69%) reported taking antiretroviral drugs during pregnancy; 175 (64%) reported giving their HIV exposed infant antiretroviral prophylaxis, and 160 (58%) took their infant to be tested for HIV. There were no statistically significant associations between women’s decision-making and either maternal antiretroviral use during pregnancy or infant antiretroviral use. However, all five decision-making domains were associated with infant HIV testing. Those reporting an active role in medical decision-making (61% vs 46% for no active role, p=0.04), household purchases (63% vs 50% for no active role, p=0.04), children’s schooling (63% vs 51% for no active role, p=0.05), children’s healthcare (62% vs 51% for no active role, p=0.07), and the composite of an active role in any decision-making domain (61% vs 46% for no active role, p=0.04) were all more likely to report HIV testing for their newborn infants (Table 2).
Table 2.
Role in decision-making at different points in the prevention of mother-to-child HIV transmission (PMTCT) cascade
| Maternal antiretroviral use in pregnancy | Infant antiretroviral prophylaxis | Infant testing | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes N=190 n (%) |
No N=86 n (%) |
p | Yes N=175 n (%) |
No N=100 n (%) |
p | Yes N=160 n (%) |
No N=117 n (%) |
p | |
| Mother’s healthcare | 0.66 | 0.87 | 0.04 | ||||||
| Active role | 148 (68.2) | 69 (31.8) | 138 (63.9) | 78 (36.1) | 133 (61.0) | 85 (39.0) | |||
| No active role | 42 (71.2) | 17 (28.8) | 37 (62.7) | 22 (37.3) | 27 (45.8) | 32 (54.2) | |||
| Household purchases | 0.62 | 0.96 | 0.04 | ||||||
| Active role | 110 (70.1) | 47 (29.9) | 100 (64.1) | 56 (35.9) | 99 (63.1) | 58 (36.9) | |||
| No active role | 78 (67.2) | 38 (32.8) | 74 (63.8) | 42 (36.2) | 59 (50.4) | 58 (49.6) | |||
| Children’s school | 0.64 | 0.98 | 0.05 | ||||||
| Active role | 114 (69.9) | 49 (30.1) | 103 (63.6) | 59 (36.4) | 102 (62.6) | 61 (37.4) | |||
| No active role | 76 (67.3) | 37 (32.7) | 72 (63.7) | 41 (36.3) | 58 (50.9) | 56 (49.1) | |||
| Children’s healthcare | 0.58 | 0.95 | 0.07 | ||||||
| Active role | 115 (70.1) | 49 (29.9) | 104 (63.8) | 59 (36.2) | 102 (62.2) | 62 (37.8) | |||
| No active role | 75 (67.0) | 37 (33.0) | 71 (63.4) | 41 (36.6) | 58 (51.3) | 55 (48.7) | |||
| Composite outcome | 0.66 | 0.82 | 0.04 | ||||||
| Active role in at least one domain | 146 (68.2) | 68 (31.8) | 137 (64.3) | 76 (35.7) | 131 (60.9) | 84 (39.1) | |||
| No active role reported in any domain | 42 (71.2) | 17 (28.8) | 37 (62.7) | 22 (37.3) | 27 (45.8) | 32 (54.2) | |||
We conducted unadjusted and adjusted logistic regression to further assess the association between decision-making and the PMTCT cascade clinical points. In unadjusted analyses, women reporting an active role in decision-making had a greater likelihood of having infant HIV testing performed: medical decision-making (OR 1.85, 95% CI 1.01–3.41), household purchases (OR 1.68, 95% CI 1.09–2.58), children’s schooling (OR 1.61, 95% CI 1.09–2.40), children’s healthcare (OR 1.56, 95% CI 1.05–2.32). Women who reported an active role in at least one decision-making domain also have higher likelihood for infant HIV testing compared to those who reported none at all (OR 1.85, 95% CI 1.01–3.38). In univariate analyses (not shown) maternal age and institutional delivery were significantly associated with infant HIV testing, so they were included in multivariate analyses. When we adjusted for potential confounders, the trend remained; however, they were no longer statistically significant at p<0.05. No significant differences were observed in the adjusted or unadjusted models for either maternal or infant antiretroviral use (Table 3).
Table 3.
Association between active role in maternal decision-making indicators and key steps along the prevention of mother-to-child HIV transmission (PMTCT) cascade
| Crude Odds Ratioa | Adjusted Odds Ratioa, b | |
|---|---|---|
| Maternal antiretroviral use in pregnancy | ||
| Mother’s healthcare | 0.87 (0.50 – 1.52) | 0.77 (0.43 – 1.38) |
| Household purchases | 1.14 (0.76 – 1.73) | 1.11 (0.70 – 1.75) |
| Children’s school | 1.13 (0.78 – 1.65) | 1.14 (0.77 – 1.66) |
| Children’s healthcare | 1.16 (0.80 – 1.69) | 1.17 (0.79 – 1.73) |
| Active role in any domain | 0.87 (0.49 – 1.53) | 0.77 (0.43 – 1.38) |
| Infant antiretroviral prophylaxis | ||
| Mother’s healthcare | 1.05 (0.59 – 1.86) | 0.80 (0.42 – 1.51) |
| Household purchases | 1.01 (0.65 – 1.57) | 1.01 (0.64 – 1.58) |
| Children’s school | 0.99 (0.62 – 1.59) | 1.01 (0.65 – 1.58) |
| Children’s healthcare | 1.02 (0.64 – 1.62) | 1.04 (0.66 – 1.63) |
| Active role in any domain | 1.07 (0.60 – 1.91) | 0.81 (0.43 – 1.55) |
| Infant testing | ||
| Mother’s healthcare | 1.85 (1.01 – 3.41) | 1.76 (0.89 – 3.46) |
| Household purchases | 1.68 (1.09 – 2.58) | 1.58 (0.94 – 2.66) |
| Children’s school | 1.61 (1.09 – 2.40) | 1.56 (0.97 – 2.53) |
| Children’s healthcare | 1.56 (1.05 – 2.32) | 1.52 (0.94 – 2.44) |
| Active role in any domain | 1.85 (1.01 – 3.38) | 1.75 (0.89 – 3.43) |
Odds Ratios (OR) from logistic regression models with clustered robust standard errors.
Models adjusted for maternal age (15–25, 25–35, and 35 years and older) and institutional delivery (which were significantly associated with HIV testing in bivariate models). Marital status, maternal education, parity, and employment were not associated with HIV testing and were not included in multivariate models.
Discussion
We investigated the association between a woman’s role in decision-making and reported engagement at key steps along the PMTCT cascade. An active role in decision-making was positively associated with only one of our three steps (i.e., infant HIV testing). When we controlled for potential confounders, the positive trend remained; however, it no longer met our a priori threshold for statistical significance.
We adapted our indices about decision-making from the Demographic and Health Survey, which includes questions about one’s own healthcare, large household purchases, purchases for daily household needs, and visits to family and relatives (Central Statistical Office/Zambia et al., 2015). In DHS reports, these decisions are thought to provide insight into the level of control of a woman’s daily life (Kishor & Subaiya, 2008). We included the first two questions in our survey; the latter two were replaced with others (i.e., children’s schooling, children’s healthcare) thought to be more relevant to maternal-child health.
In our study, a high proportion of women reported an active role in decision-making. Overall, 57 to 78% of women reported an active role according to at least one indicator. This is in-line with the 2014 Demographic and Health Survey in Zambia, which showed that 66 to 86% of women participate in at least one of the four decisions (Central Statistical Office/Zambia et al., 2015). The minor differences in proportion could be attributed to our focus on an HIV population in a single rural area, without exclusions based on marital status. Regionally, a woman’s reported involvement in at least one decision varies greatly, from 46–72% in Tanzania to 84–89% in Zimbabwe (Health, Social Services - MoHSS/Namibia, & ICF International, 2014; Ministry of Health et al., 2016; National Statistical Office - NSO/Malawi & ICF Macro, 2011; Zimbabwe National Statistics Agency - ZIMSTAT & ICF International, 2012). These greater differences may result from cultural, educational, economic, and religious differences, as well as the status of women in the various countries in the region.
We observed similar or higher levels of engagement of maternal antiretroviral uptake (69%), infant antiretroviral uptake (63%), and infant HIV testing (58%), compared to other studies in the medical literature. In a meta-analysis of the PMTCT cascade uptake in sub-Saharan Africa, Wettstein and colleagues reported maternal antiretroviral coverage of 70% (95% CI 64–76%) during pregnancy and infant HIV testing rates of 64% (95% CI 48–81%) (Wettstein et al., 2012). More recent studies from South Africa, Cote d’Ivoire, and Malawi confirm this level of engagement (Granato et al., 2016; Sinunu et al., 2014; Woldesenbet et al., 2015). However, a recent analysis of the four-country PEARL study found that only 42% of HIV-positive women started maternal antiretroviral drugs and 36% of infants received antiretroviral prophylaxis after birth (Chi et al., 2015). Factors associated with completion of the cascade included HIV diagnosis prior to pregnancy and concordant HIV-positive partners (Dionne-Odom et al., 2016). We did not have analogous information in our current study, but such factors could have influenced PMTCT health seeking behaviors.
We found an association between active decision-making and HIV infant testing; however, such associations were not present in the uptake of antiretroviral regimens for mother or infant. It is unclear why there was this difference, but there may be inherent characteristics of the health-seeking behaviors themselves. Provision of antiretroviral drugs for HIV-infected pregnant women is common. In one study, up to 80% for both maternal and infant antiretroviral use (Woldesenbet et al., 2015). This broad coverage can limit the statistical power to detect smaller incremental differences. Also, infant HIV testing is associated with a health outcome. Women may be willing to risk accidental disclosure and the effects of stigma to know their child’s status (Aliyu et al., 2014; Cromwell et al., 2015). Follow-on qualitative research may shed light on how empowerment relates to these different types of decisions.
Significant investments have been made to support women’s and girl’s empowerment in sub-Saharan Africa. There are varying definitions of empowerment, but all seek to improve the degree of control a woman has over her life, body, and environment. Decision-making is an important aspect of this control (Kishor & Subaiya, 2008) and should be considered in empowerment strategies. Initiatives have focused on economic improvement, political engagement, and education, including a growing number at the intersection of women’s and population health. For example, a recent South African study showed that school attendance – an important form of girls’ empowerment – reduced the risk of HIV acquisition among adolescent girls (Pettifor et al., 2016). To date, few studies have investigated the impact of empowerment interventions on health or health behavior outcomes in PMTCT (Ambia & Mandala, 2016), though some are now underway (Woelk, Kieffer, Walker, Mpofu, & Machekano, 2016). Theoretical frameworks are needed to better describe the approach, along with carefully considered metrics for empowerment and its impact.
We acknowledge several limitations. First, this study had a relatively small sample size that may be insufficient to show marginal differences between comparison groups. Second, we used decision-making indicators as a proxy for empowerment. There may be aspects of empowerment that these questions did not capture (Central Statistical Office/Zambia et al., 2015; Kishor & Subaiya, 2008). Inclusion of gender role indicators would have enhanced this analysis (and others like it), as has been done in other health sectors evaluating empowerment. Third, we relied solely on self-report for the cascade outcomes and this could lead to an inherent reporting bias. Misclassification of responses based on social desirability could favor the null hypothesis and thus show no effect of decision-making roles on the PMTCT cascade. Finally, the health-seeking behaviors we considered along the cascade have different characteristics and this might have implications for our analysis. Medication use, for example, is a frequent and repetitive behavior that occurs mostly outside of the health facility. Infant HIV testing, on the other hand, is episodic and conducted in the clinic setting. Such differences could influence recall and result in differential misclassification, particularly when self-reported outcomes are used.
In conclusion, we observed a positive association between an active role in decision-making and infant HIV testing. Interestingly, similar trends were not observed for antiretroviral uptake for either the HIV-infected mother or her HIV-exposed infant. Larger studies are under way to evaluate whether improving community interventions by empowering women will improve uptake of infant testing (Woelk et al., 2016). Regardless of their findings, the impact of women’s empowerment outside of health (i.e., economic, political, educational) is substantial and these benefits should be fully considered.
Supplementary Material
Supplemental Table 1: Comparison of women who did and did not have complete information about health utilization at key points along cascade for the prevention of mother-to-child HIV transmission (PMTCT)
Figure 2.
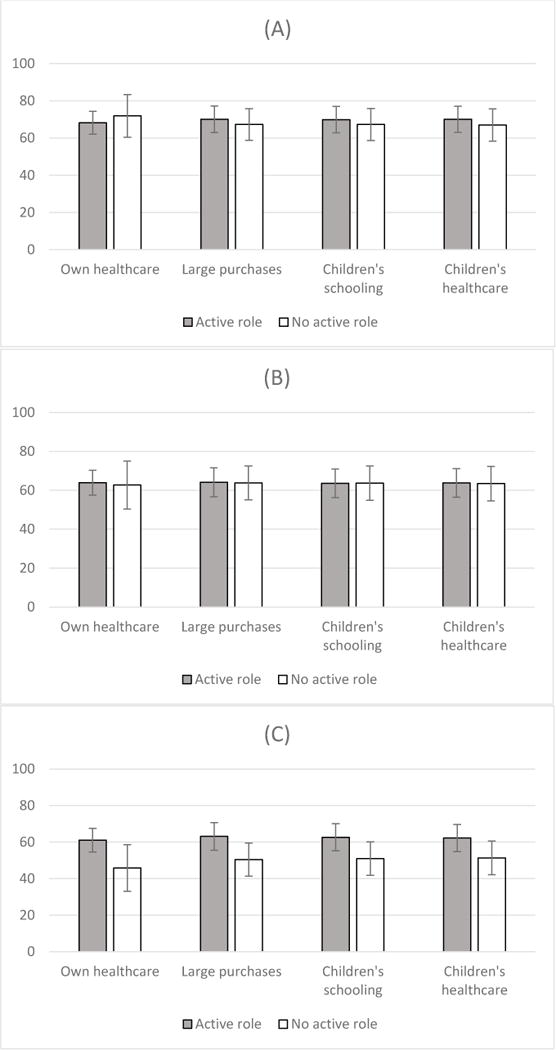
Uptake of prevention of mother-to-child HIV transmission (PMTCT) services according to participant’s self-reported role in decision-making. Comparisons are made at three points along the PMTCT cascade: uptake of maternal antiretroviral drugs (A), use of infant HIV prophylaxis (B), and testing infant for HIV (C).
Acknowledgments
Funding acknowledgements: The parent study was funded by a Clinical Scientist Development Award from the Doris Duke Charitable Foundation (2007061). Additional support was provided by the National Institutes of Health (R25 TW009340, T32 HD075731, K24 AI120796, P30 AI050410). PM would like to acknowledge support from the Research Council of Norway and its Centres of Excellence scheme to the Centre for Intervention Science in Maternal and Child Health (CISMAC; project number 223269).
References
- Aliyu MH, Blevins M, Megazzini KM, Audet CM, Dunlap J, Sodangi IS, Vermund SH. Correlates of suboptimal entry into early infant diagnosis in rural north central Nigeria. J Acquir Immune Defic Syndr. 2014;67(1):e19–26. doi: 10.1097/qai.0000000000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambia J, Mandala J. A systematic review of interventions to improve prevention of mother-to-child HIV transmission service delivery and promote retention. J Int AIDS Soc. 2016;19(1):20309. doi: 10.7448/IAS.19.1.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker PM, Mphatswe W, Rollins N. Antiretroviral drugs in the cupboard are not enough: the impact of health systems’ performance on mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2011;56(2):e45–48. doi: 10.1097/QAI.0b013e3181fdbf20. [DOI] [PubMed] [Google Scholar]
- Bashemera DR, Nhembo MJ, Benedict G. The role of women’s empowerment in influencing HIV testing. 2013 Retrieved from Calverton, Maryland, USA: http://dhsprogram.com/pubs/pdf/WP101/WP101.pdf.
- Central Statistical Office/Zambia, Ministry of Health/Zambia, University of Zambia Teaching Hospital Virology Laboratory, University of Zambia Department of Population Studies, Tropical Diseases Research Centre/Zambia, & ICF International. Zambia Demographic and Health Survey 2013–14. 2015 Retrieved from Rockville, Maryland, USA: http://dhsprogram.com/pubs/pdf/FR304/FR304.pdf.
- Chi BH, Musonda P, Lembalemba MK, Chintu NT, Gartland MG, Mulenga SN, Stringer JS. Universal combination antiretroviral regimens to prevent mother-to-child transmission of HIV in rural Zambia: a two-round cross-sectional study. Bull World Health Organ. 2014;92(8):582–592. doi: 10.2471/BLT.13.129833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi BH, Tih PM, Zanolini A, Stinson K, Ekouevi DK, Coetzee D, Stringer JS. Implementation and Operational Research: Reconstructing the PMTCT Cascade Using Cross-sectional Household Survey Data: The PEARL Study. J Acquir Immune Defic Syndr. 2015;70(1):e5–9. doi: 10.1097/QAI.0000000000000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell EA, Dow AE, Low D, Chirambo C, Heyderman RS, Dube Q, Van Rie A. Barriers to successful early infant diagnosis of HIV infection at primary care level in Malawi. Pediatr Infect Dis J. 2015;34(3):273–275. doi: 10.1097/inf.0000000000000625. [DOI] [PubMed] [Google Scholar]
- de Vincenzi I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11(3):171–180. doi: 10.1016/s1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- Dionne-Odom J, Welty TK, Westfall AO, Chi BH, Ekouevi DK, Kasaro M, Tita AT. Factors Associated with PMTCT Cascade Completion in Four African Countries. AIDS Res Treat. 2016;2016:2403936. doi: 10.1155/2016/2403936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escamilla V, Chibwesha CJ, Gartland M, Chintu N, Mubiana-Mbewe M, Musokotwane K, Chi BH. Distance from household to clinic and its association with the uptake of prevention of mother-to-child HIV transmission regimens in rural Zambia. J Acquir Immune Defic Syndr. 2015 doi: 10.1097/qai.0000000000000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartland MG, Chintu NT, Li MS, Lembalemba MK, Mulenga SN, Bweupe M, Chi BH. Field effectiveness of combination antiretroviral prophylaxis for the prevention of mother-to-child HIV transmission in rural Zambia. AIDS. 2013;27(8):1253–1262. doi: 10.1097/QAD.0b013e32835e3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato SA, Gloyd S, Robinson J, Dali SA, Ahoba I, Aka D, Kone A. Results from a rapid national assessment of services for the prevention of mother-to-child transmission of HIV in Cote d’Ivoire. J Int AIDS Soc. 2016;19(5 Suppl 4):20838. doi: 10.7448/IAS.19.5.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health, M. o., Social Services - MoHSS/Namibia, & ICF International. Namibia Demographic and Health Survey 2013. 2014 Retrieved from Windhoek, Namibia: http://dhsprogram.com/pubs/pdf/FR298/FR298.pdf.
- Kilewo C, Karlsson K, Ngarina M, Massawe A, Lyamuya E, Swai A, Biberfeld G. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr. 2009;52(3):406–416. doi: 10.1097/QAI.0b013e3181b323ff. [DOI] [PubMed] [Google Scholar]
- Kishor S, Subaiya L. Understanding women’s empowerment: a comparative analysis of demographic and health surveys (DHS) data. 2008 Retrieved from Calverton, Maryland, USA: http://dhsprogram.com/pubs/pdf/CR20/CR20.pdf.
- McNairy ML, Teasdale CA, El-Sadr WM, Mave V, Abrams EJ. Mother and child both matter: reconceptualizing the prevention of mother-to-child transmission care continuum. Curr Opin HIV AIDS. 2015;10(6):403–410. doi: 10.1097/coh.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health, C. D., Gender, Elderly, Children - MoHCDGEC/Tanzania Mainland, Ministry of Health - MoH/Zanzibar, National Bureau of Statistics - NBS/Tanzania, Office of Chief Government Statistician - OCGS/Zanzibar, & ICF. Tanzania Demographic and Health Survey and Malaria Indicator Survey 2015–2016. 2016 Retrieved from Dar es Salaam, Tanzania: http://dhsprogram.com/pubs/pdf/FR321/FR321.pdf.
- Mutowo J, Kasu CM, Mufunda E. Women empowerment and practices regarding use of dual protection among family planning clients in urban Zimbabwe. Pan Afr Med J. 2014;17:300. doi: 10.11604/pamj.2014.17.300.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Statistical Office - NSO/Malawi, & ICF Macro. Malawi Demographic and Health Survey 2010. 2011 Retrieved from Zomba, Malawi: http://dhsprogram.com/pubs/pdf/FR247/FR247.pdf.
- Ngarina M, Popenoe R, Kilewo C, Biberfeld G, Ekstrom AM. Reasons for poor adherence to antiretroviral therapy postnatally in HIV-1 infected women treated for their own health: experiences from the Mitra Plus study in Tanzania. BMC Public Health. 2013;13:450. doi: 10.1186/1471-2458-13-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettifor A, MacPhail C, Hughes JP, Selin A, Wang J, Gomez-Olive FX, Kahn K. The effect of a conditional cash transfer on HIV incidence in young women in rural South Africa (HPTN 068): a phase 3, randomised controlled trial. Lancet Glob Health. 2016;4(12):e978–e988. doi: 10.1016/s2214-109x(16)30253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C, Essex M. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362(24):2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinunu MA, Schouten EJ, Wadonda-Kabondo N, Kajawo E, Eliya M, Moyo K, Kellerman SE. Evaluating the impact of prevention of mother-to-child transmission of HIV in Malawi through immunization clinic-based surveillance. PLoS One. 2014;9(6):e100741. doi: 10.1371/journal.pone.0100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer EM, Chi BH, Chintu N, Creek TL, Ekouevi DK, Coetzee D, Stringer JS. Monitoring effectiveness of programmes to prevent mother-to-child HIV transmission in lower-income countries. Bull World Health Organ. 2008;86(1):57–62. doi: 10.2471/BLT.07.043117. doi:S0042-96862008000100016 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas TK, Masaba R, Borkowf CB, Ndivo R, Zeh C, Misore A, Fowler MG. Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding–the Kisumu Breastfeeding Study, Kenya: a clinical trial. PLoS Med. 2011;8(3):e1001015. doi: 10.1371/journal.pmed.1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull E, Lembalemba MK, Guffey MB, Bolton-Moore C, Mubiana-Mbewe M, Chintu N, Chi BH. Causes of stillbirth, neonatal death and early childhood death in rural Zambia by verbal autopsy assessments. Trop Med Int Health. 2011;16(7):894–901. doi: 10.1111/j.1365-3156.2011.02776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. FACT SHEET 2017. 2017 http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf Retrieved August 21, 2017.
- UNAIDS. On the fast-track to an AIDS-free generation. Geneva: World Health Organization; 2016. [Google Scholar]
- Wettstein C, Mugglin C, Egger M, Blaser N, Vizcaya LS, Estill J, for the Ie, D. E. A. S. A. C. Missed opportunities to prevent mother-to-child-transmission: systematic review and meta-analysis. AIDS. 2012;26(18):2361–2373. doi: 10.1097/QAD.0b013e328359ab0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woelk GB, Kieffer MP, Walker D, Mpofu D, Machekano R. Evaluating the effectiveness of selected community-level interventions on key maternal, child health, and prevention of mother-to-child transmission of HIV outcomes in three countries (the ACCLAIM Project): a study protocol for a randomized controlled trial. Trials. 2016;17:88. doi: 10.1186/s13063-016-1202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldesenbet S, Jackson D, Lombard C, Dinh TH, Puren A, Sherman G, South African, P. E. T. Missed Opportunities along the Prevention of Mother-to-Child Transmission Services Cascade in South Africa: Uptake, Determinants, and Attributable Risk (the SAPMTCTE) PLoS One. 2015;10(7):e0132425. doi: 10.1371/journal.pone.0132425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A. The uncertainty of treatment: Women’s use of HIV treatment as prevention in Malawi. Soc Sci Med. 2016;158:52–60. doi: 10.1016/j.socscimed.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Zimbabwe National Statistics Agency - ZIMSTAT & ICF International. Zimbabwe Demographic and Health Survey 2010–11. 2012 Retrieved from Calverton, Maryland, USA: http://dhsprogram.com/pubs/pdf/FR254/FR254.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Comparison of women who did and did not have complete information about health utilization at key points along cascade for the prevention of mother-to-child HIV transmission (PMTCT)


