Abstract
Objectives
To test the hypothesis that there is no difference in the in vivo maximum wear of enamel opposing monolithic zirconia crowns, enamel opposing porcelain fused to metal crowns and enamel opposing enamel.
Methods
Thirty patients needing single crowns were randomized to receive either a monolithic zirconia or metal-ceramic crown. Two non-restored opposing teeth in the same quadrants were identified to serve as enamel controls. After cementation, quadrants were scanned for baseline data. Polyvinylsiloxane impressions were obtained and poured in white stone. Patients were recalled at six-months and one-year for re-impression. Stone models were scanned using a tabletop laserscanner to determine maximum wear. Statistical analysis was performed using Mann-Whitney U to determine any significant differences between the wear of enamel against zirconia and metal-ceramic crowns.
Results
Sixteen zirconia and 14 metal-ceramic crowns were delivered. There were no statistical differences in mean wear of crown types (p = 0.165); enamel antagonists (p = 0.235) and enamel controls (p = 0.843) after one year.
Conclusion
Monolithic zirconia exhibited comparable wear of enamel compared with metal-ceramic crowns and control enamel after one year.
Significance
This study is clinically significant because the use of polished monolithic zirconia demonstrated comparable wear of opposing enamel to metal-ceramic and enamel antagonists.
Keywords: Monolithic zirconia, Wear of enamel, Laserscanner
1. Introduction
Zirconia became popular in dentistry because of this material’s excellent mechanical properties [1], which include high strength, fracture toughness [2–4], and biocompatibility [5,6]. Zirconia was mainly used as a substructure for ceramic-ceramic restorations and required veneering ceramics to obtain proper esthetics because of their high opacity. In general, these ceramic-ceramic restorations exhibited superior esthetic properties compared with their metal-ceramic counterparts [7–9].
Despite the excellent physical properties of zirconia, veneer chipping has been identified as a major cause of failure. A systematic analysis of zirconia-based FDPs shows a survival rate of 94.3% [10]. However, when technical complications such as chipping of the veneer ceramic are included, their survival decreases to 76.4% [10]. Heintze [11] performed a systematic review to analyze the survival of zirconia (90%) and metal (97%) supported FDPs after three years. He concluded that veneer chipping was a major cause of failure. The mean long-term survival rate of zirconia frameworks at 10 years is 91.5% [12] with failures attributed to marginal deficiencies and veneer chipping.
To overcome veneer chipping, dental manufacturers developed monolithic zirconia prostheses, which rely on the toughness and strength of the material to eliminate the need for the fracture-susceptible veneering ceramic. Veneer fractures in ceramic-ceramic restorations are believed to be the result of differences in the thermal expansion coefficients of the core ceramic and veneer ceramic and nonuniformity of condensation during ceramic build-up [13].
One major concern with the use of monolithic zirconia as a restorative material is the abrasive nature against opposing enamel because of this material’s hardness and surface roughness [14–19]. Several in vitro and in vivo studies were conducted to determine the wear of enamel against zirconia. Numerous in vitro studies showing wear of zirconia against different antagonists, including enamel, have shown zirconia to be comparable to other restorative materials in terms of wear of opposing enamel [17,20–24]. However, in vitro studies are hard to compare with each other because of differences in surface finish of material, type of material, method of wear and type of wear analysis used. The limited clinical studies which have been published describe how monolithic zirconia is a viable restorative material in that the wear of antagonist enamel is within the range of acceptable limits [25–28]. However, since there is a limited number of clinical studies available, there is need for more clinical analyses to further validate the wear compatibility of zirconia with enamel.
The purpose of this study was to test the hypothesis that there is no difference in the in vivo maximum wear of enamel opposing monolithic zirconia crowns, enamel opposing porcelain fused to metal crowns and enamel opposing enamel.
2. Materials and methods
2.1. Study design
A randomized, controlled, clinical trial was designed to analyze the wear of enamel by opposing polished monolithic zirconia crowns and by the polished veneer surfaces of metal-ceramic crowns. This single-blind pilot study involved a total of 30 teeth that required full coverage crowns that opposed natural antagonist teeth.
2.2. Study intervention
2.2.1. Participant recruitment
Participants that needed full coverage crowns were randomly assigned to receive either a polished (nonglazed) zirconia crown (Lava™ Plus, 3M ESPE, PZ), or a polished (nonglazed) veneer of metal-ceramic crown (GC Initial™, GC America; Argedent 62, Argen, USA, PV). All participants were over 21 years old with no contraindications to dental treatment. These participants were screened for low caries risk, the absence of periodontal disease and no temporomandibular disorders. Each participant needed a crown on either a first or second premolar or first or second molar in any arch. Inclusion criteria for abutment teeth included: [1] restorability with a crown:root ratio of at least 1:1; [2] presence of an opposing natural tooth which was non-restored or minimally restored; [3] the presence of two non-restored or minimally restored teeth opposing each other on the same quadrants as the crowned tooth and the opposing to serve as enamel controls. Minimally restored was defined as teeth which have no restoration greater than a Class II amalgam restoration. The opposing arch did not have a full coverage restoration or a partial denture. Two crowns were the maximum number of crowns for each participant. A random number table was formulated by the statistician to facilitate assignment of teeth to either material group. The clinical coordinator assigned to the study enrolled the participants and assigned them to the material groups. Patients were treated at the University of Florida College of Dentistry Dental Clinical Research Unit. Institutional Review Board approval for treating human subjects using the research protocol was obtained. All participants were required to sign an informed consent form prior to initiating the study.
2.2.2. Crown fabrication
One investigator prepared all the teeth to receive crowns based on design criteria for crown preparation. Provisional material (Protemp™ Plus, 3 M ESPE) was used to fabricate provisionals. Prepared teeth were scanned using a chairside oral scanner (3M True Definition™ Chairside Oral Scanner Digital Impression System, 3M, ESPE). Scans were sent to one laboratory for crown fabrication.
Crowns were received from the laboratory with a polished surface. All crowns were polished using porcelain polishers impregnated with diamond abrasives (Shofu Dura Polish Dia, Shofu Dental Corporation). Try-in and adjustment, if necessary, of each crown were made with a fine diamond bur (8369DF.31.025 FG Fine Football Dialite Diamond, Brassler, USA) on a high-speed handpiece. Crowns were polished with diamond impregnated porcelain polishers in the order of coarse, medium, and fine points (Dialite, Brassler, USA) until a fine lustre was achieved. All crowns were cemented with a resin cement (Rely X™ Unicem Self-Adhesive Resin Cement, 3M ESPE). Participants were not made aware of the type of crown they received.
A baseline examination was performed one week after cementation to ensure that the patient was comfortable with the crown and no further adjustments were needed. When no further adjustments were necessary, teeth were cleaned to remove plaque and saliva. A vinylpolysiloxane impression (Imprint 3, 3M ESPE) was made of the maxillary and mandibular quadrants, where the crown and the opposing tooth are located, to record the occlusal surfaces of each cemented crown and its antagonist tooth. These are the same quadrants where the enamel controls are also located. Photographs of the quadrants were made with occlusal contacts marked by articulating paper (Accufilm®II double sided articulating film, Parkell Prod Inc). The red paper was used to indicate maximum intercuspation while the black paper was used to indicate contacts in excursive movements. The post-cementation casts were poured with a white gypsum material (GC Fujirock, GC America) to enable optimal scanned image contrast. The participants were asked to return at 6 months and one year. Quadrants were re-impressed during both time periods.
2.2.3. Wear quantification
The maximum wear was quantified as the maximum loss in height. A 3D Laserscanner (CS2, Straumann, Germany) was used to scan the casts at baseline, six months and one year along the x, y and z axes of the casts. These period scans were superimposed against one another using tripodization by identifying three points on the occlusal anatomy which are expected to remain stable (i.e. marginal ridges). The matching of the two scans was conducted by the software to achieve a match with a standard deviation (SD) less than 25 μm. The scanning accuracy for this type of scanner is reported to be 20 μm [29,30]. The matching process was repeated until an acceptable SD was achieved. After proper matching was achieved between the period scans, the maximum wear of the crowns and teeth at these time periods were compared and recorded. The wear areas were compared with the clinical photographs to confirm intra-oral contact areas.
2.3. Statistical analysis
All analyses were performed using the R statistical software package (V3.2.4, The R Foundation for Statistical Computing, Vienna, Austria). Since the sample size was small (N < 15 per group for all comparisons), the non-parametric Mann-Whitney U test was used to compare wear between the zirconia and metal-ceramic crown types at six months and one year. To compare antagonist wear to control wear between the two groups, the difference between antagonist wear and control wear as the outcome for each patient was calculated. The mean wear of the two control teeth was used as the control wear for that patient.
3. Results
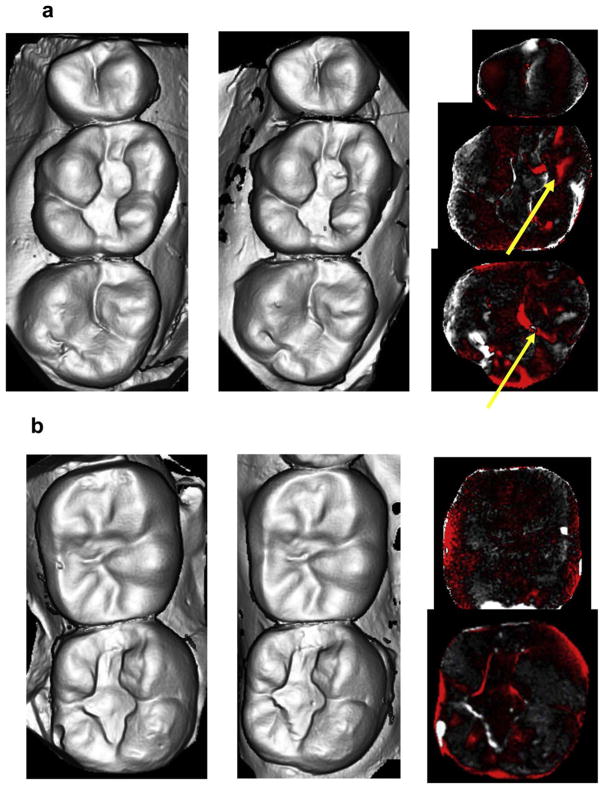
Thirty [30] teeth in 25 enrolled participants (20 females, 5 males and no more than two crowns per participant), were included in this study and were seen from 2013 to 2017. There were 16 monolithic zirconia crowns (PZ) and 14 metal-ceramic crowns (PV) analyzed. The consort diagram (Fig. 1) shows a more detailed distribution of the participants. Wear between the monolithic zirconia and metal-ceramic crowns were compared at six months and one year (Fig. 2). There were no significant differences observed at any time point (6 months p = 0.958; 1 year p = 0.367). The wear of the enamel opposing both types of crowns was also compared to determine if one material wore the opposing enamel more than the other (Fig. 3). There were no significant differences observed for antagonist enamel wear across all time periods (6 months p = 0.776; 1 year p = 0.534). The opposing enamel wear was then compared to the wear between two opposing enamel surfaces (control wear) to determine if either material caused an increase in opposing enamel wear. This was computed for by the difference between antagonist wear and control wear for each participant. The mean between the two controls were subtracted from the antagonist enamel wear of the crowns. In Fig. 4, negative numbers indicate more control wear than antagonist wear and positive values indicate more antagonist tooth wear. The p values are 6 months p = 0.864 and 1 year p = 0.093 indicating no significant difference between the control enamel wear and the antagonist enamel wear. Greater opposing enamel wear was observed for the metal ceramic than the zirconia crown for the first six months. This trend changed for the first-year data with an increase in wear for the enamel opposing zirconia crowns. Fig. 5a and b are representative images retrieved from the laserscanner for wear comparison at one year. Fig. 5b shows the crowned tooth on the lower left second molar, the left first molar is serving as the enamel control. The left most image is the baseline image while the middle image is the one year image. The right most image is the superimposed image of the two scans and red marks indicate differences between the two images or possibly wear. In this superimposed image, the red marks are located in peripheral areas which are not indicative of wear because the teeth do not contact in those areas. Fig. 5a shows the antagonist teeth to the crown and the control. The superimposed image shows more distinct red marks which are possible areas of wear (arrows). No wear facets are visible on any of these scans.
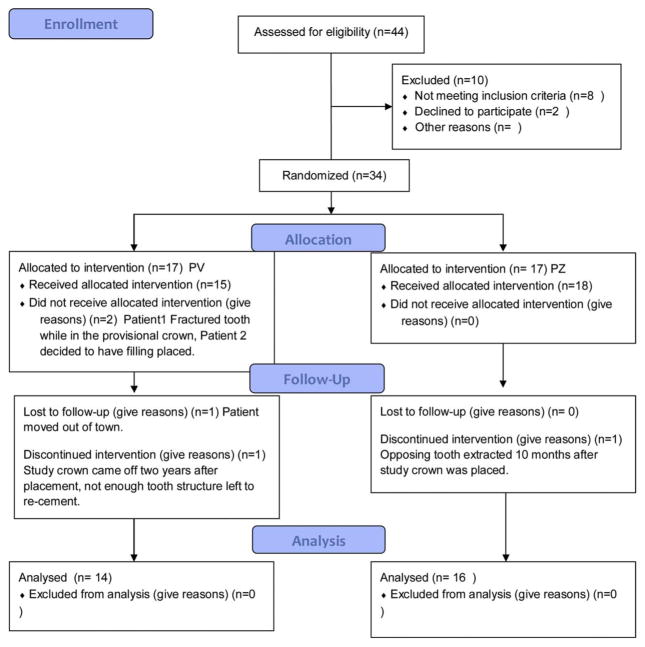
Fig. 1.
Consort diagram showing enrollment, allocation, follow up and analysis of participants.
Fig. 2.
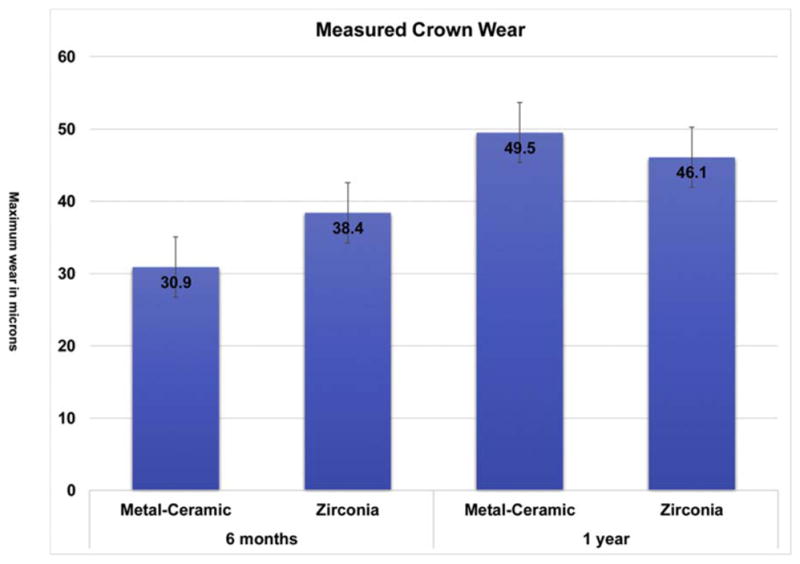
Comparison of wear between metal-ceramic (PFM) and monolithic zirconia crowns at 6 months and one year.
Fig. 3.
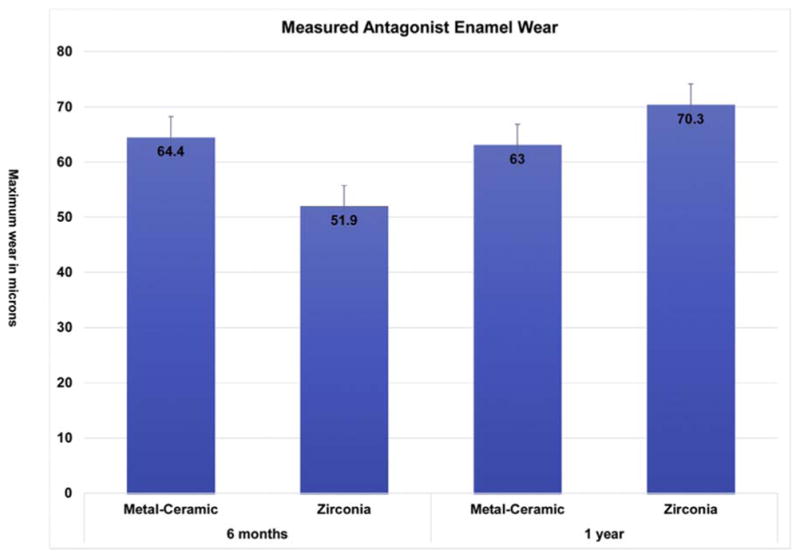
Comparison of antagonist enamel wear between metal ceramic and monolithic zirconia crowns at 6 months and one year.
Fig. 4.
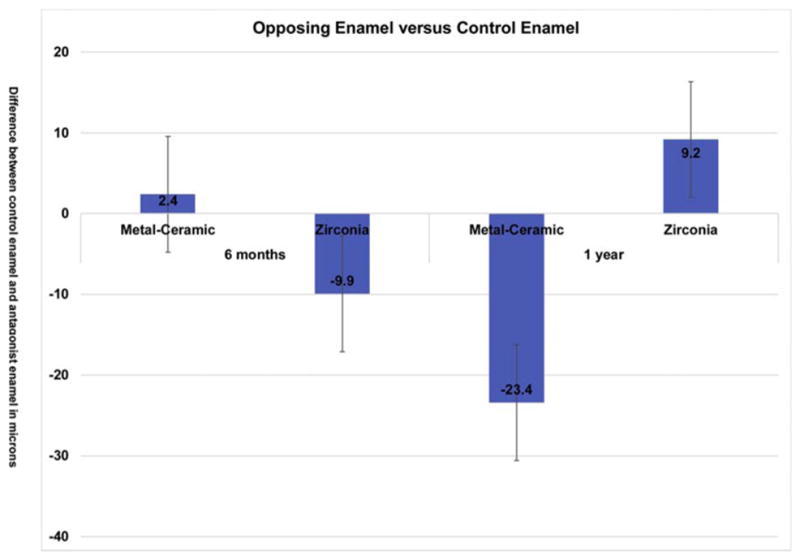
Comparison of enamel wear between crown antagonist enamel and control enamel. Negative values indicate greater control enamel wear while positive values indicate greater antagonist enamel wear.
Fig. 5.
(a) Scanned image of casts of antagonist enamel and control enamel at baseline (left), one year (middle) and superimposed image showing wear areas in red (right); (b) Scanned image of casts from the same patient of crowned tooth and antagonist enamel at baseline (left), one year (middle) and superimposed image showing wear areas in red (right). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Wear is a complicated phenomenon to measure. As a result of veneer chipping associated with zirconia substrates, dental manufacturers are marketing monolithic zirconia restorations for full coverage restorations. This has brought about wide concern of excessive enamel wear opposing this hard and possibly abrasive dental material. However, as of 2015, there have only been in vitro studies analyzing the wear of enamel against zirconia antagonists. The problem with in vitro wear analysis is wear machines cannot replicate complex masticatory movements. Chewing patterns vary between individuals and are dependent on multiple factors such as muscle tone, joint dyscrasia, oral health, etc. [31]. There is a need for long-term clinical studies to determine the wear potential of a dental material.
To date, there are five clinical studies which examined the wear potential of monolithic zirconia against different antagonists, including this current study (Table 1) [25–27]. This table compares all clinical studies in terms of the different variables employed in each. For surface finish of zirconia, all clinical studies, except one, utilized a polished surface. The preference for the surface finish was, at least for this study, based on literature findings. In vitro studies have shown that polished zirconia produces less wear on enamel antagonists than glazed zirconia [14–17,22]. Kim et al. [17] reported that polished zirconia showed less enamel wear than feldspathic porcelain and heat-pressed ceramics. In addition, the rate of enamel wear was dependent on the surface roughness of the zirconia. Jung et al. [18] reported that polished zirconia showed less enamel wear than feldspathic porcelain and polished zirconia with glazing. Park et al. [23] concluded that polished zirconia showed the least volume loss of enamel while the stained and glazed zirconia showed the highest volume loss. These studies indicate that polished zirconia full coverage crowns, without glazing, cause less wear the antagonist enamel. In addition, the glazed zirconia surface has been shown to have significant wear after 6 months [18,23,32]. Contrasting studies reveal that zirconia against enamel causes more wear compared with zirconia against gold or against zirconia [24]. In vitro wear analysis of zirconia revealed that zirconia demonstrated less wear compared with lithium disilicate ceramic [33]. Enamel wear for both these types of ceramics was comparable with the enamel-enamel wear observed. Further, glazed zirconia causes more material loss on the surface. These two occurrences can be explained by the fact that a polished zirconia surface produces a quantifiably smoother surface than the glazed zirconia, therefore proving to be less abrasive to the opposing enamel. Rougher surfaces have been correlated with increased wear of the opposing dentition [34]. The increased material loss is explained by the glaze layer wearing away from the surface, leaving a rougher surface, which perpetuates greater antagonist enamel wear.
Table 1.
Comparison of published clinical studies documenting the wear of enamel against monolithic zirconia.
| Author | surface treatment |
replica | device | material | antanogist | Wear (a. mean volume loss, b. mean wear, c. mean maximum vertical loss) |
control | duration (time) |
Is zirconia acceptable after duration of observa- tion? |
|---|---|---|---|---|---|---|---|---|---|
| Lohbauer U. and Reich S. | polished | epoxy resin AlphaDie | 3D-non contact profilometer (CT-100, Cybertechnologies, Ingolstadt, Germany). Samples were scanned with lateral step size of 5 μm. | monolithic zirconia premolar and molar crown (LAVA Plus, 3 M ESPE) (n = 14) | enamel (n = 7) ceramic (n = 10) ceramic/enamel (n = 2) |
enamel a3.61E8 μm3, c204 μm ceramic a3.33E8 μm3, c145 μm ceramic/enamel a115 μm3, c163 μm |
none | 2 years | monolithic zirconia crowns were acceptable after 2 years |
| Mundhe K. et. al | polished-zirconia glazed-metal ceramic | gypsum | 3D white light scanner (SmartSCAN3D HE scanner; Breukmann), precision: ± 9 μm | enamel monolithic yttrium-stabilized zirconium oxide crown (Y-TZP; LAVA; 3 M ESPE) (n = 10) metal ceramic (65.2% Ni, 22.5% Cr, and 9.5% Mo (Bellabond Plus; BEGO) (n = 10) |
enamel | enamel b17.30 μm –premolar region enamel b35.10 μm-molar region enamel b42.10 μm for premolar teeth enamel b127.00 μm for molar teeth enamel b69.2 μm – premolar teeth enamel b179.70 μm – molar teeth |
enamel vs. enamel; enamel vs. metal-ceramic | 1 year | Wear of enamel caused by metal ceramic crown > zirconia crown > natural enamel |
| Stober T. et al. | polished | stone | Laserscan 3D and Match 3D, version 1.6; Willytec, Gräfelfing, Germany), resolution: 20 μm | yttrium-stabilised zirconia crown (Zenostar Zr Translucent; Wieland Dental) (n = 12) contralateral teeth (n = 24) | enamel | zirconia b14 μm, maximum vertical loss 60 μm enamel b46 μm, maximum vertical loss 151 μm enamel b19 ± 9 μm, b26 ± 13 μm, maximum vertical loss 75 μm, maximum vertical loss 115 μm |
enamel vs. enamel | 2 year | The wear of enamel antagonist caused by monolithic zirconia crowns was more than natural teeth |
| Cardelli P. et al. | glazed | polyurethane resin | 3D scanner (Echo2 Scanner; Sweden & Martina, Due Carrare, Italy), accuracy: 10 μm | yttria-stabilized zirconia (Y-TZP; NexxZr; Sagemax, Federal Way, WA) (n = 47) | enamel nanohybrid composite |
zirconia b63 μm, enamel b76 μm zirconia b19 μm, composite b70 μm |
none | 1 year | Wear of enamel or composite antagonists caused by zirconia was acceptable after 1 year. |
| Esquivel-Upshaw et al. | polished | stone | 3D Laserscanner (CS2, Straumann, Germany), accuracy: 20 μm | monolithic zirconia (LavaTM Plus, 3 M ESPE, PZ) (n = 16) metal-ceramic (GC InitialTM, GC America; Argedent 62, Argen, USA, PV) (n = 14) contralateral enamel |
enamel | Zirconia c38.4 μm (6 months), c46.1 μm (1 year) enamel c51.9 μm (6 months), c70.3 μm (1 year) metal-ceramic c30.9 μm (6 months), c49.5 μm (1 year) Enamel c64.4 μm (6 months), c63 μm (1 year) contralateral enamel (zirconia vs. enamel) c61.8 μm (6 months), c61.1 μm (1 year) contralateral enamel (metal-ceramic vs. enamel) c62 μm (6 months), c86.4 μm (1 year) |
enamel vs. enamel | 6 months, 1 year | Wear of enamel caused by monolithic zirconia was comparable with metal-ceramic. |
All the clinical studies utilized an indirect method for measuring wear which required making an impression of the teeth and developing a replica either in acrylic or gypsum. These replicas were then compared using either a 3D non-contact profilometer or a 3D laserscanner. As a result of the two-step process involved with creating a replica, there can be inherent errors in the data which can produce inconsistencies or inaccuracies [35]. The setting expansion for the stone used for this study was 0.12%, and the linear dimensional change for the impression used was 1.5%. Additionally, the accuracy for the scanners/profilometer used were in the range of 5 μm–20 μm This can account for slight differences in wear values measured (Table 1).
Lambrechts, et al. [36], documented normal clinical enamel to enamel wear per year for molars (38 μm, 28 μm for steady state) and premolars (18 μm,15 μm for steady state). The authors describe that wear is critical during the first year where there is initial increased wear [36]. This wear then plateaus into a steady state where equilibrium is reached. However, this can vary depending on the patient’s occlusal condition, diet, quantity and quality of saliva, and the presence of parafunctional habits. This is the reason for incorporating an enamel-enamel control that is patient specific where the enamel antagonist wear for each material can be compared with the enamel-enamel wear within the same conditions. Three [26,27], including this study, out of the five studies employed enamel-enamel controls. For enamel versus enamel in patients with zirconia crowns, the enamel wear values are: 26.2 μm [27], 95 μm [26] and 61.6 μm for this study, However, there are differences among the three studies in that the time period is different with Stober’s [26] study at 2 years and the others at 1-year analysis and that the higher valued studies all report maximum wear whereas Munde’s [27] study reported mean wear.
A comparison of enamel wear against zirconia among the 1-year studies shows Munde at 84.5 μm, Cardelli [28] at 76 μm and the current study at 70.3 μm. These values are all comparable with each other. However, compared with Munde’s enamel-enamel control (26.2 μm) for the same patients, the enamel wear against zirconia is higher. There was no enamel-enamel control for Cardelli’s study. For this current study the enamel control was 61.6 μm which is comparable to the enamel-zirconia wear. For the studies reporting 2-year wear, the antagonist enamel wear reported for Lohbauer [25] was 204 μm and Stober was 151 μm. While these values by themselves seem comparable, Lohbauer’s conclusion that zirconia is enamel friendly is hard to validate because the study is missing enamel-enamel controls. In Stober’s study, they state that the wear of enamel vs. zirconia is greater than that compared with enamel vs. enamel (95 μm) for the same patient.
For the current study and Mundhe’s study, metal-ceramic crowns were used as a second control. They reported an average enamel wear of 124 μm (for molars and premolars) opposing metal-ceramic crowns while our study demonstrated wear of antagonist enamel to metal-ceramic at 68 μm after one year. For Munde’s study, the enamel vs. metal-ceramic wear was reported to be higher than zirconia vs. enamel wear (84.5 μm) or the enamel-enamel (26.2 μm) wear while for our study the enamel-enamel wear at 86.4 μm was comparable.
In general, the consensus among the studies, despite the differences in wear values, is that zirconia holds promise as a dental restorative material. The differences can be attributed to lack of enamel controls as well as to the inherent errors produced in creating replicas for measuring wear.
The phenomenon of transformation toughening occurs with Y-TZP ceramics when a reverse tetragonal to monoclinic transformation occurs within the crystalline phases. This is considered beneficial in that the material can actually “heal” itself. When tensile stresses are generated at the tip of a crack, the reverse tetragonal to monoclinic transformation occurs. This phase change at the tip of the crack is accompanied by volumetric expansion and subsequent compressive stresses around the crack tip. This volumetric expansion can result in partial closure of the crack and prevent its propagation through the entire structure [37]. Another phenomenon known as low-temperature degradation (LTD) induces tetragonal to monoclinic transformation at the surface of the specimen in the presence of moisture at 250 °C [38]. This can cause loss of strength and adverse effects on other mechanical properties such as roughening of the surface. Although this has not been shown to occur in vitro [37], long-term clinical studies need to be conducted to determine the effect LTD may have on the surface of these polished zirconia surfaces.
5. Conclusion
The results of this study demonstrate that polished monolithic zirconia does not cause accelerated wear of the opposing enamel. The wear of both metal-ceramic and monolithic zirconia is comparable and that there are no significant differences between the enamel antagonist wear and control enamel wear of the two materials. This is clinically significant because polished monolithic zirconia holds promise as a versatile restorative material because of this material’s high strength and esthetic properties.
Acknowledgments
3M ESPE, Seefeld, Germany and NIH-NIDCR Grant R01 DE025001 supported this study. All materials were provided by 3M ESPE and wear analysis was conducted at the University of Florida.
Footnotes
Conflict of interests
There are no conflicts of interest associated with this research project.
References
- 1.Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999 Janaury;20(1):1–25. doi: 10.1016/s0142-9612(98)00010-6. [DOI] [PubMed] [Google Scholar]
- 2.Chen YM, Smales RJ, Yip KHK, Sung WJ. Translucency and biaxial flexural strength of four ceramic core materials. Dent Mater. 2008;24(11):1506–1511. doi: 10.1016/j.dental.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Beuer F, Schweiger J, Eichberger M, Kappert HF, Gernet W, Edelhoff D. High-strength CAD/CAM-fabricated veneering material sintered to zirconia copings – a new fabrication mode for all-ceramic restorations. Dent Mater. 2009;25(1):121–128. doi: 10.1016/j.dental.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 4.Denry I, Kelly JR. State of the art of zirconia for dental applications. Dent Mater. 2008;24(3):299–307. doi: 10.1016/j.dental.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Akagawa Y, Hosokawa R, Sato Y, Kamayama K. Comparison between free-standing and tooth-connected partially stabilized zirconia implants after two years’ function in monkeys: a clinical and histologic study. J Prosthet Dent. 1998;80(5):551–558. doi: 10.1016/s0022-3913(98)70031-9. [DOI] [PubMed] [Google Scholar]
- 6.Ichikawa Y, Akagawa Y, Nikai H, Tsuru H. Tissue compatibility and stability of a new zirconia ceramic in vivo. J Prosthet Dent. 1992;68(2):322–326. doi: 10.1016/0022-3913(92)90338-b. [DOI] [PubMed] [Google Scholar]
- 7.Kelly JR, Nishimura I, Campbell SD. Ceramics in dentistry: historical roots and current perspectives. J Prosthet Dent. 1996 Janaury;75(1):18–32. doi: 10.1016/s0022-3913(96)90413-8. [DOI] [PubMed] [Google Scholar]
- 8.Heffernan MJ, Aquilino SA, Diaz-Arnold AM, Haselton DR, Stanford CM, Vargas MA. Relative translucency of six all-ceramic systems. Part II: core and veneer materials. J Prosthet Dent. 2002;88(1):10–15. [PubMed] [Google Scholar]
- 9.Baldissara P, Llukacej A, Ciocca L, Valandro FL, Scotti R. Translucency of zirconia copings made with different CAD/CAM systems. J Prosthet Dent. 2010;104(1):6–12. doi: 10.1016/S0022-3913(10)60086-8. [DOI] [PubMed] [Google Scholar]
- 10.Schley JS, Heussen N, Reich S, Fischer J, Haselhuhn K, Wolfart S. Survival probability of zirconia-based fixed dental prostheses up to 5 yr: a systematic review of the literature. Eur J Oral Sci. 2010 Oct;118(5):443–450. doi: 10.1111/j.1600-0722.2010.00767.x. [DOI] [PubMed] [Google Scholar]
- 11.Heintze SD, Rousson V. Survival of zirconia- and metal-supported fixed dental prostheses: a systematic review. Int J Prosthodont. 2010 Nov-Dec;23(6):493–502. [PubMed] [Google Scholar]
- 12.Sax C, Hammerle CH, Sailer I. 10-year clinical outcomes of fixed dental prostheses with zirconia frameworks. Int J Comput Dent. 2011;14(3):183–202. [PubMed] [Google Scholar]
- 13.Sailer I, Zembic A, Jung RE, Hammerle CH, Mattiola A. Single-tooth implant reconstructions: esthetic factors influencing the decision between titanium and zirconia abutments in anterior regions. Eur J Esthet Dent. 2007 Autumn;2(3):296–310. [PubMed] [Google Scholar]
- 14.Janyavula S, Lawson N, Cakir D, Beck P, Ramp LC, Burgess JO. The wear of polished and glazed zirconia against enamel. J Prosthet Dent. 2013;109(1):22–29. doi: 10.1016/S0022-3913(13)60005-0. [DOI] [PubMed] [Google Scholar]
- 15.Stawarczyk B, Özcan M, Schmutz F, Trottmann A, Roos M, Hämmerle CHF. Two-body wear of monolithic, veneered and glazed zirconia and their corresponding enamel antagonists. Acta Odontol Scand. 2013;71(1):102–112. doi: 10.3109/00016357.2011.654248. [DOI] [PubMed] [Google Scholar]
- 16.Mitov G, Heintze SD, Walz S, Woll K, Muecklich F, Pospiech P. Wear behavior of dental Y-TZP ceramic against natural enamel after different finishing procedures. Dent Mater. 2012;28(8):909–918. doi: 10.1016/j.dental.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Kim MJ, Oh SH, Kim JH, Ju SW, Seo DG, Jun SH, et al. Wear evaluation of the human enamel opposing different Y-TZP dental ceramics and other porcelains. J Dent. 2012 Nov;40(11):979–988. doi: 10.1016/j.jdent.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Jung YS, Lee JW, Choi YJ, Ahn JS, Shin SW, Huh JB. A study on the in-vitro wear of the natural tooth structure by opposing zirconia or dental porcelain. J Adv Prosthodont. 2010 Sep;2(3):111–115. doi: 10.4047/jap.2010.2.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Lin, Liu Yihong, Si Wenjie, Feng Hailan, Tao Yongqing, Ma Zhizuo. Friction and wear behaviors of dental ceramics against natural tooth enamel. J Eur Ceram Soc. 2012;32:2599–2606. [Google Scholar]
- 20.Passos SP, Torrealba Y, Major P, Linke B, Flores-Mir C, Nychka JA. In vitro wear behavior of zirconia opposing enamel: a systematic review. J Prosthodont. 2014 Dec;23(8):593–601. doi: 10.1111/jopr.12167. [DOI] [PubMed] [Google Scholar]
- 21.Sripetchdanond J, Leevailoj C. Wear of human enamel opposing monolithic zirconia, glass ceramic, and composite resin: an in vitro study. J Prosthet Dent. 2014 Nov;112(5):1141–1150. doi: 10.1016/j.prosdent.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Zandparsa R, El Huni RM, Hirayama H, Johnson MI. Effect of different dental ceramic systems on the wear of human enamel: an in vitro study. J Prosthet Dent. 2016 Feb;115(2):230–237. doi: 10.1016/j.prosdent.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Park JH, Park S, Lee K, Yun KD, Lim HP. Antagonist wear of three CAD/CAM anatomic contour zirconia ceramics. J Prosthet Dent. 2014 Janaury;111(1):20–29. doi: 10.1016/j.prosdent.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Kwon MS, Oh SY, Cho SA. Two-body wear comparison of zirconia crown, gold crown, and enamel against zirconia. J Mech Behav Biomed Mater. 2015 Jul;47:21–28. doi: 10.1016/j.jmbbm.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 25.Lohbauer U, Reich S. Antagonist wear of monolithic zirconia crowns after 2 years. Clin Oral Invest. 2016 Jun;:09. doi: 10.1007/s00784-016-1872-6. [DOI] [PubMed] [Google Scholar]
- 26.Stober T, Bermejo JL, Schwindling FS, Schmitter M. Clinical assessment of enamel wear caused by monolithic zirconia crowns. J Oral Rehabil. 2016 Aug;43(8):621–629. doi: 10.1111/joor.12409. [DOI] [PubMed] [Google Scholar]
- 27.Mundhe K, Jain V, Pruthi G, Shah N. Clinical study to evaluate the wear of natural enamel antagonist to zirconia and metal ceramic crowns. J Prosthet Dent. 2015 Sep;114(3):358–363. doi: 10.1016/j.prosdent.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Cardelli P, Manobianco FP, Serafini N, Murmura G, Full-Arch Beuer F. Implant-Supported monolithic zirconia rehabilitations: pilot clinical evaluation of wear against natural or composite teeth. J Prosthodont. 2016 Dec;25(8):629–633. doi: 10.1111/jopr.12374. [DOI] [PubMed] [Google Scholar]
- 29.Hack GD, Sebastian BMP. Evaluation of the accuracy of six intraoral.scanning devices. An in-vitro investigation. ADA Professional Product Review. 2015;10:1–5. [Google Scholar]
- 30.Mehl A, Gloger W, Kunzelmann KH, Hickel R. A new optical 3-D device for the detection of wear. J Dent Res. 1997 Nov;76(11):1799–1807. doi: 10.1177/00220345970760111201. [DOI] [PubMed] [Google Scholar]
- 31.Okeson JP. Etiology and treatment of occlusal pathosis and associated facial pain. J Prosthet Dent. 1981 Feb;45(2):199–204. doi: 10.1016/0022-3913(81)90340-1. [DOI] [PubMed] [Google Scholar]
- 32.Etman MK. Confocal examination of subsurface cracking in ceramic materials. J Prosthodont. 2009 Oct;18(7):550–559. doi: 10.1111/j.1532-849X.2009.00447.x. [DOI] [PubMed] [Google Scholar]
- 33.Lawson NC, Janyavula S, Syklawer S, McLaren EA, Burgess JO. Wear of enamel opposing zirconia and lithium disilicate after adjustment, polishing and glazing. J Dent. 2014;42(12):1586–1591. doi: 10.1016/j.jdent.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Ghazal M, Yang B, Ludwig K, Kern M. Two-body wear of resin and ceramic denture teeth in comparison to human enamel. Dent Mater. 2008;24(4):502–507. doi: 10.1016/j.dental.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez JM, Bartlett DW. A comparison of two-dimensional and three-dimensional measurements of wear in a laboratory investigation. Dent Mater. 2010;26(10):e221–e225. doi: 10.1016/j.dental.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Lambrechts P, Braem M, Vuylsteke-Wauters M, Vanherle G. Quantitative in vivo wear of human enamel. J Dent Res. 1989;68:1752–1754. doi: 10.1177/00220345890680120601. [DOI] [PubMed] [Google Scholar]
- 37.Papanagiotou HP, Morgano SM, Giordano RA, Pober R. In vitro evaluation of low-temperature aging effects and finishing procedures on the flexural strength and structural stability of Y-TZP dental ceramics. J Prosthet Dent. 2006 Septmber;96(3):154–164. doi: 10.1016/j.prosdent.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Lilley E, Tressler RE, MM, editors. Review of Low Temperature Degradation in Y-TZPs. American Ceramics Society; Westerville: 1990. [Google Scholar]