Abstract
Anhedonia is a severe condition that describes a near-complete absence of enjoyment, motivation, and interest. A core feature of depression, clinical manifestations of anhedonia can include deficits in experiencing pleasure, approach-related motivated behavior, and learning how to match expectations to the environment. To date, the precise neurobiological mechanisms of anhedonia in major depression are still poorly understood. We have previously argued that contradictory findings and the inability to identify specific neurobiological substrates for anhedonic symptoms may result from sample heterogeneity, suboptimal methods of assessment, and the challenge of dissociating between different components of anhedonia. Recently, however, computational advances to the operationalization of psychiatric symptoms have enhanced the ability to evaluate the neurobiology of constituent elements of this symptom domain. In this paper, we review (1) advances in behavioral and computational methods of assessing reward processing and motivation and (2) the development of new self-report, neurological, and biological methods of subtyping that may be useful in future pursuits to expand our understanding of the neurobiology of anhedonia in depression.
Anhedonia, a core feature of depression, is a multi-faceted symptom that includes deficits in the experience of pleasure, reduced approach-related motivated behavior, and/or impaired learning about rewards in the environment (see Box 1). [1]. We have previously argued that the elusiveness of neurobiological substrates for anhedonia in depression results from the use of suboptimal methods of assessment, which fail to dissociate between these different components of anhedonia and result in pathophysiological heterogeneity. Anhedonia in mood disorders has long been hypothesized to be related to a reduction in dopamine (DA) transmission [2–4]. While neuroimaging and pharmacological manipulations have provided some support for the hypothesis that DA may be affected in at least some individuals with major depression [5–7], findings remain mixed (For a review, see [1]), and more work is needed to understand the neurobiology of anhedonia in depression.
Box 1. Anhedonia – What's in a name?
Despite significant progress in the study of reward-related symptoms, there remains considerable disagreement regarding the precise definition of anhedonia and its degree of conceptual overlap with other commonly used terms (e.g., avolition, anergia, apathy, alexithymia, etc.). This lack of clarity is likely due at least in part to the DSM, which offers two distinct definitions for anhedonia depending on whether the diagnostic context is depression or schizophrenia. In the case of the schizophrenia spectrum, anhedonia is defined narrowly as “the decreased ability to experience pleasure from positive stimuli or a degradation in the recollection of pleasure previously experienced” (DSM V p. 88), and is included among 4 other symptoms (alogia, avolition, asociality, and diminished emotional expression) that together comprise the broader “negative symptom” domain. In other words, anhedonia is a subordinate construct within the negative symptom criterion for schizophrenia spectrum disorders. In the context of major depression, however, anhedonia is a supraordinate construct; here it is used as a general criterion that may be satisfied through different clinical presentations, such as the loss of motivation or interest in hobbies (‘wanting’), or the ability to enjoy activities (‘liking’) and so on (DSM V p. 163). Consequently, the term “anhedonia” in depression is more akin to the term “negative symptoms” in the schizophrenia spectrum in that both are the supraordinate labels for a general domain. These competing definitions for anhedonia have caused confusion in the literature, especially for the translation of preclinical models [33]. Moreover, the use of anhedonia as an “umbrella term” in the nosology of major depression is inconsistent with its greek etymology implying a specific deficit related to the “absence of pleasure”. We have previously suggested that new clinical terminology be introduced in subsequent versions of the DSM to facilitate transdiagnostic definitions of symptoms in the anhedonia domain of depression [1]. Until such changes are enacted, however, we have chosen to remain aligned to the current nomenclature defining anhedonia as a supraordinate construct that is comprised of distinct features related to both motivation and pleasure that may satisfy the A2 anhedonia criterion in the diagnosis of depression.
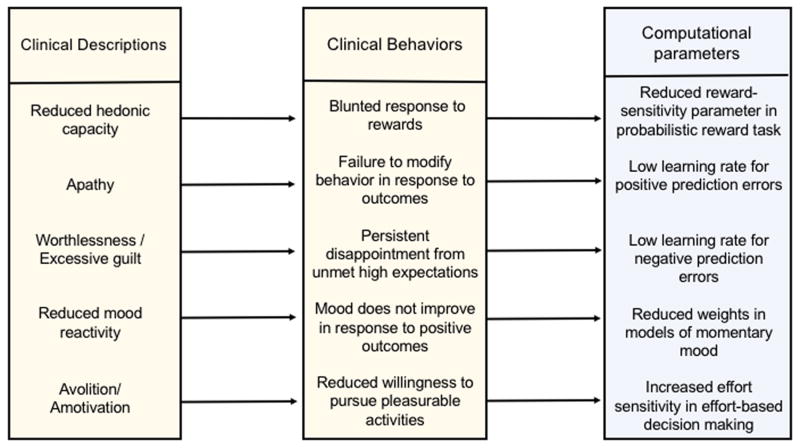
Within the last five years the field of psychiatry has moved in several new directions that hold promise for improving methods of assessment and increasing our understanding of the underlying neurobiology, including the possible role of DAergic deficits. In this review, we outline two new lines of research: First, the emergence of computational psychiatry [8] has encouraged the application of computational methods to improve our understanding of mental illness, including the use of computational modeling for making inferences regarding the underlying mechanisms that generate observed behavior in psychiatric groups [9,10] (Figure 1). This includes the use of behavioral paradigms and computational models that have been previously linked to DAergic signaling in animal models and human subjects, providing opportunities to evaluate the prevalence of DA-related deficits in patients with depression and anhedonia. Here we focus on advances in the use of reinforcement learning and effort-based choice to evaluate reward processing and motivational deficits.
Figure 1.
The computational approach to assessing anhedonia and related symptoms. This conceptual diagram outlines the hypothetical operationalization of behavioral manifestations of anhedonia and related symptoms within a computational psychiatry framework. Clinically defined facets of anhedonia have typically been associated with behavioral manifestations that are difficult to quantify or measure directly and are often confused with other related symptoms. However, computational approaches allow for objective assessment through association with computational parameters. To date, there is insufficient data to support specific mapping between behavioral and computational terms, but we present this hypothetical example as a representation of the potential to operationalize clinical behaviors using computational approaches.
Second, recent work has also encouraged the identification of subgroups within heterogeneous disorders for which individualized treatments can be developed [9], and has resulted in increased efforts to identify behaviorally, neurologically, or biologically distinct subgroups within and across diagnostic categories. One candidate sub-group with growing empirical support is the so-called “inflammatory sub-type” [11], which may be driven by immune-induced alterations in DAergic tone and basal ganglia function. In the sections that follow, we outline recent work in these domains and advocate for further integration of these lines of research to extend our understanding of neurobiological mechanisms associated with anhedonia in depression.
Computational Psychiatry in Depression: Reinforcement Learning
A fundamental premise of computational psychiatry is the idea that behavioral manifestations of clinical symptoms may be best conceptualized in terms of computational components that can be used to infer underlying mechanisms [1,8] (See Figure 1). In the case of depression, anhedonic symptoms have long been viewed in terms of failure within reinforcement systems (e.g. a fundamental deficit in response to positive reinforcement [12]). This idea, coupled with the early advances linking reinforcement learning (RL) models to midbrain DA neurons [13], presented an initial opportunity for the study of anhedonia using computational approaches. This body of research has focused primarily on the role of DAergic mesocorticolimbic pathways, including ventral and dorsal striatal targets for midbrain DA neurons, in the signaling of reward prediction errors (RPEs). More recently, an important direction in computational assessment has been to link behaviorally or computationally-derived measures of reward reactivity, learning, and decision-making to emotional experience and mood states that may be disrupted in mood disorders [14,15]. Specifically, Rutledge et al. (2014) modeled subjective feelings of happiness in healthy volunteers from the combined influence of recent reward expectations and associated (plausibly DAergic) prediction errors during a risky decision task [16] and showed that pharmacologically manipulating dopamine affected both choices and happiness ratings [17]. Thus, mechanisms of reinforcement learning provide a potential means of linking DAergic response to rewards to disrupted mood regulation as well as a mechanism of examining aberrant reinforcement processing in depression (See [18], for a review).
To date, however, evidence supporting the link between anhedonic symptoms and failures within reward learning has been mixed. One widely-used paradigm, the probabilistic reward task (PRT), employs a signal-detection methodology to test the development of an implicit bias towards rewarding stimuli. Patients with major depression exhibit reduced ability to modulate behavior in response to rewards [19,20]—a pattern that persists even after reported remission [21]. Further, depressed patients with high symptoms of anhedonia show diminished reward learning on the PRT relative to those with low symptoms [19,20]. A recent re-analysis of these data using an RL framework [22] compared healthy controls to both individuals with high symptoms of anhedonia and patients with MDD and found that the primary difference in behavior for both groups was related to reductions in reward sensitivity, and not reductions in learning from rewards per se [22]. This distinction is significant, as it is learning behavior in this task that has been clearly linked to both manipulations of and individual differences within the mesolimbic DA system [23]. Consequently, the question of whether associations between anhedonic symptoms and PRT performance in patients with depression are exclusively linked to a DAergic deficit remains unresolved.
A parallel body of work has suggested that depression-related differences in reinforcement learning may be driven by disruptions in goal-directed reasoning that depend on formulating a model of the reward environment (often referred to as “model-based” valuation) rather than prediction error signaling (also known as “model free”) [15,24]. In support of this idea, Rutledge et al. (2017) used a mixed gambles task designed to elicit RPEs in the absence of learning and found that patients with moderate depression showed no difference from healthy controls in striatal reward prediction error signaling, and that symptoms of anhedonia were not related to striatal RPE signaling. Moreover, an analysis using a similar task in a large community sample of individuals with varying self-reported symptoms of depression as measured by the Beck Depression Inventory [25] found no effect of depressive or anhedonic severity on the relationship between momentary mood and outcome, though baseline mood did correspond to depression severity [15]. These results were interpreted as suggesting that the integrity of DAergic reward prediction error signaling is intact in depressive anhedonia, and that the previously-observed attenuations in ventral-striatal signaling during reinforcement learning in patients with depression may be related to impaired model-based valuation (For a review of model-based vs. model-free decision-making in depression, see [25]).
While this hypothesis is certainly plausible and worthy of pursuit in future studies, an alternative explanation for these null findings is that deficits in DAergic model-free signaling may be a marker of particular sub-types of depression. Consequently, the ability to detect alterations in RL signaling may depend on symptom severity of the sample populations, sample inclusion criteria, and symptom heterogeneity. These paradigms will be critical for evaluating and quantifying reward processing and reward learning in heterogeneous samples with anhedonia or motivational impairments that are thought to be related to DAergic functioning, ideally among individuals with similar behavioral manifestations.
Computational Psychiatry in Depression: Motivation and Effort-based Decision-Making
In addition to reinforcement learning, another common behavioral manifestation of anhedonia in depression is reduced motivation [26]. In depressed patients, several laboratory paradigms have thus been developed to explore reward motivation and its relationship with anhedonia. One such paradigm developed by our group, the Effort-Expenditure for Rewards Task (EEfRT) [27], has shown reduced willingness to expend effort for reward in patients with subsyndromal depression, first-episode depression, and remitted depression compared to controls [28,29]. Additionally, self-reported symptoms of anhedonia have been found to correlate with willingness to exert effort in patients [29] and in undergraduates with a wide range of trait anhedonia [27]. Other effort-based decision-making paradigms have shown similar associations between willingness to exert effort for rewards among patients with unipolar depression [30] and in correlation with measures of apathy among otherwise healthy participants [31]. Taken together, studies using these measures in depressed patients are consistent with clinical observations of reduced motivation as a core feature of the disorder. Like measures of RL, effort-based decision-making paradigms can be readily analyzed using computational models [32] that may provide objective methods of quantifying reduced motivation associated with anhedonia.
As with RL processes, studies of the willingness to expend greater effort in order to obtain larger or preferred rewards have repeatedly implicated disruption of corticostriatal DA as a critical substrate [33,34]. In both humans and animals, potentiation or attenuation of DA signaling respectively increases or decreases effort expenditure for rewards [35–38], and intra-individual variation in striatal DA availability predicts individual differences in effort-based discounting [39,40]. In addition to striatal DA, human and animal studies suggest several potential regions of interest for future studies examining the neurobiology of reduced motivation in anhedonia. Specifically, functional magnetic resonance imaging (fMRI) studies in humans have identified a role for the dorsal anterior cingulate cortex (dACC) and anterior insula (aI) in the subjective discounting of rewards as a function of required effort [41], as well as activity in ventromedial prefrontal cortex (vmPFC) and supplementary motor area (SMA) that drive behavior toward reward maximization or effort minimization, respectively [42]. Finally, the dorsomedial and dorsolateral prefrontal cortices have been shown to play a role in encoding both effort devaluation [41] and effort learning signals [43]. Further, these studies have also indicated that reduced motivation may be linked to decreased connectivity between SMA and ACC [44] as well as decreased striatal activation. Taken together, these studies highlight the importance of cortical networks in guiding effortful behavior. Future studies are needed to test this corticostriatal network as a substrate for reductions in effortful behavior associated with anhedonic symptoms in depression.
Evidence for pathophysiologically distinct anhedonic sub-types
As noted in the prior sections, a clear role for DAergic impairments as a primary cause for deficits in reinforcement learning or effort expenditure in anhedonia in depression has yet to be established. One explanation for this body of inconsistent findings is the presence of distinct subtypes. Indeed, growing appreciation for the multi-faceted nature of individual symptoms and diagnostic categories (see Box 1) has spurred the development of increasingly sophisticated methods of assessment. Self-report measures of anhedonia have expanded to separately capture aspects of motivation and pleasure [45], and anticipatory and consummatory aspects of pleasure [46]. The development of assessment methods has also advanced to include the application of computational methods to identify different profiles within a heterogeneous symptom domain. For example, the newly developed Apathy Motivation Index was created and validated using factor analysis and latent profile analysis to distinguish aspects of apathy associated with behavioral, social, and emotional domains and profiles associated with depression, anhedonia, and fatigue [47]. These methods may be useful in identifying specific components of anhedonia and associating them with comorbid symptoms and diagnoses. Additionally, subtyping efforts have also benefitted from the use of machine learning techniques. Applied to resting fMRI data of patients with depression, these techniques have been used to identify anhedonia-related patterns of connectivity and have potential to aid in the identification of subgroups that may benefit from targeted treatments (see [48]).
While innovative self-report methods and neurologically-based classification efforts show great promise in identifying subtypes of patients within and across diagnostic categories, recent studies of brain-immune interactions in depression have also highlighted the existence of heterogeneous pathophysiologies for anhedonic symptoms in depression. Peripheral markers of inflammation are frequently increased in depressed patients [49–51]. Moreover, a wealth of data has established that in both humans and laboratory animals, chronic administration of cytokines or cytokine-inducers is associated with decreased striatal dopamine release and blunted striatal responsivity to reward [52–54] as well as reports of anhedonic symptoms [55,56]. Indeed, it is increasingly understood that DA acts as major regulator of immune cell function within the brain [57] and conversely, the elevation of cytokines has been found to down-regulate processes that govern pre-synaptic DA availability and function [52,54].
Interestingly, the possible contribution of inflammation to the etiopathophysiology of depression may help resolve some of the inconsistencies in detection of DAergic alterations in depressed samples. While administration of cytokine inducers like interferon-alpha (IFN) therapy can produce severe depressive episodes in individuals with no prior history of depression, this response only occurs in approximately 30%–50% of drug recipients [58]. In a further study, IFN therapy was found to blunt DA synthesis capacity as well as striatal BOLD responses to reward [53]. Importantly, the magnitude of this effect varied across patients, and IFN-induced change correlated with change in motivational symptoms as measured by the Multidimensional Fatigue Inventory [53]. Given that substantial increases in inflammation occur in almost everyone receiving interferon-alpha therapy, this finding suggests that the onset of depressive symptoms may reflect a particular vulnerability to the down-stream effects of increased immune signaling.
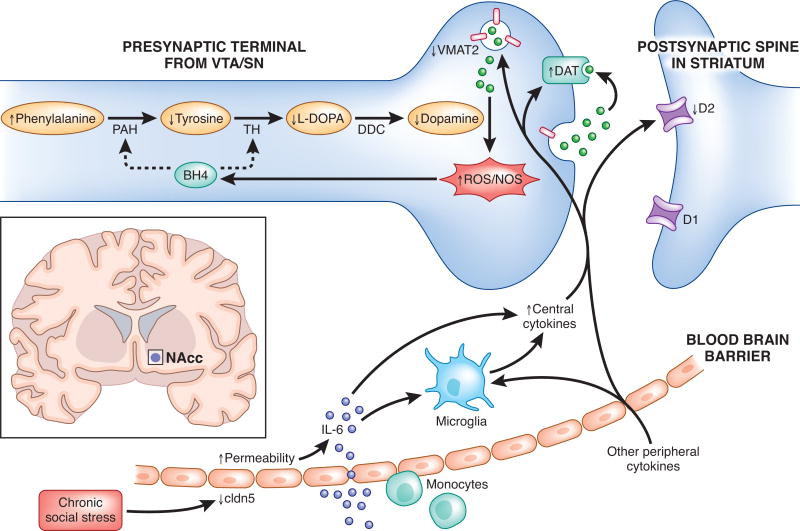
Intriguingly, a recent study by Menard and colleagues found direct evidence for such a vulnerability. Following exposure to chronic social stress, mice that went on to develop a depressive phenotype–but not stress resilient mice–were found to have developed a “leaky” blood-brain barrier (BBB) in the NAcc that permitted increased trafficking of the inflammatory cytokine interleukin-6 (IL-6) from the periphery into the CNS [59]. Critically, this enhanced permeability was detected around the NAcc, but not prefrontal cortex or hypothalamus, providing a possible explanation for the potential selectivity of the “inflammatory subtype” and DA-linked anhedonia symptoms (See Figure 2). Given that periods of significant life stress are one of the major risk factors for the development of a first depressive episode [60], sensitivity to stress-induced vulnerability of the BBB may be a critical moderating variable in determining whether markers of abnormal striatal DA function will be present. Moreover, the importance of BBB permeability as a potential mediator for vulnerability to inflammation may help explain inconsistencies in the effects of inflammatory challenges on reward behavior in healthy individuals (e.g [61]).
Figure 2.
Potential signaling pathways linking peripheral inflammation to disruption of dopaminergic function. Adapted from [66]. As suggested by one recent study [59], individuals who go on to develop a depressive phenotype following stress show increased permeability of the blood brain barrier (BBB) to peripheral cytokines such as IL-6. The peripheral cytokines that cross the blood brain barrier, as well as central cytokines produced by activated microglia, may contribute to oxidative stress and reactive oxygen species (ROS) generation. This, in turn, may increase the oxidation of tetrahydrobiopterin (BH4), a cofactor required for the conversion of phenylalanine to tyrosine and tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA), thereby impeding DA synthesis. Additionally, central inflammatory cytokines may decrease the expression or function of the vesicular monoamine transporter 2 (VMAT2) as well as increase the expression or function of the dopamine transporter (DAT), increasing DA and leading to increased generation of ROS. Finally, inflammatory cytokines may also decrease DA signaling by reducing DA D2 receptors. D1, dopamine 1 receptor 1; D2, dopamine 2 receptor; DDC, dopamine decarboxylase; NOS, nitric oxide synthase; PAH, phenylalanine hydroxylase; ROS, reactive oxygen species; SN; substantia nigra; TH, tyrosine hydroxylase; VTA, ventral tegmental area.
Consistent with the results of Menard et al., a recent study by our group also observed that individual differences in the effects of an acute stressor on NAcc prediction error signals measured with fMRI were dependent on the magnitude of change in IL-6 following acute stress [62]. Finally, blockade of inflammation using the tumor necrosis factor (TNF-alpha) antagonist infliximab was found to relieve reward-related symptoms in depressed patients with high–but not low–inflammation [63]. Taken together, these data suggest the possibility that neurobiological sensitivity to inflammatory stimuli–possibly mediated by BBB integrity around the NAcc–may drive the link between stress and anhedonic symptoms for a subset of patients, thereby forming the basis for an “inflammatory subtype”. While this tantalizing model needs to be translated to further clinical studies, it suggests the possibility that patients with depression in the context of high inflammation may show selective benefit from treatments aimed at reducing inflammation or increasing DAergic tone [64,65].
Summary and Future Directions
We have highlighted several recent advances in the assessment of reward processing and motivational deficits in depression and anhedonia through behavioral and computational methodology, and have reviewed recent efforts to identify neurologically and biologically distinct subtypes within the diagnostic construct of depression. We are optimistic that this work will progress toward the identification of neurobiology associated with subtypes within anhedonia and lead to targeted treatment approaches. While both of these fields have led to novel and informative work, we advocate that further integration of these research trajectories will be critical for increasing our understanding of the neurobiology of anhedonia and depression, specifically regarding reward processing and motivational deficits, and for developing targeted treatment strategies.
Highlights.
Discrete cognitive processes underlying anhedonia can be computationally operationalized
Approaches include models of reinforcement learning and effort-based decision-making
Mixed findings in both domains may reflect the presence of pathophysiological subtypes
One candidate sub-type is the presence of chronic inflammation
Acknowledgments
This work was supported by the National Institutes of Health [grant numbers MH102355, MH108605] to MTT and the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-1444932 to ARA. We thank Katie Ris-Vicari for her help in creating Figure 2.
Footnotes
Financial Disclosures
The authors report no conflicts of interest. In the past 3 years, MTT has served as a paid consultant to Boston Consulting Group, NeuroCog Trials, Avanir Pharmaceuticals, and Blackthorn Therapeutics. MTT is a co-inventor of the EEfRT, which is discussed in this review. Emory University and Vanderbilt University licensed this software to BlackThorn Therapeutics. Under the IP Policies of both universities, Dr. Treadway receives licensing fees and royalties from BlackThorn Therapeutics. Additionally, Dr. Treadway has a paid consulting relationship with BlackThorn. The terms of these arrangements have been reviewed and approved by Emory University in accordance with its conflict of interest policies. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willner P. Dopamine and depression: a review of recent evidence. I. Empirical studies. Brain Res. 1983;287:211–224. doi: 10.1016/0165-0173(83)90005-x. [DOI] [PubMed] [Google Scholar]
- 3.Willner P. Dopamine and depression: a review of recent evidence. III. The effects of antidepressant treatments. Brain Res. 1983;287:237–246. doi: 10.1016/0165-0173(83)90007-3. [DOI] [PubMed] [Google Scholar]
- 4.Willner P. Dopamine and depression: a review of recent evidence. II. Theoretical approaches. Brain Res. 1983;287:225–236. doi: 10.1016/0165-0173(83)90006-1. [DOI] [PubMed] [Google Scholar]
- 5.Yadid G, Friedman A. Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog Brain Res. 2008;172:265–286. doi: 10.1016/S0079-6123(08)00913-8. [DOI] [PubMed] [Google Scholar]
- 6.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 7.Tremblay LK, Naranjo CA, Cardenas L, Herrmann N, Busto UE. Probing brain reward system function in major depressive disorder: altered response to dextroamphetamine. Arch Gen Psychiatry. 2002;59:409–416. doi: 10.1001/archpsyc.59.5.409. [DOI] [PubMed] [Google Scholar]
- 8.Montague PR, Dolan RJ, Friston KJ, Dayan P. Computational psychiatry. Trends Cogn Sci. 2012;16:72–80. doi: 10.1016/j.tics.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephan KE, Mathys C. Computational approaches to psychiatry. Curr Opin Neurobiol. 2014;25:85–92. doi: 10.1016/j.conb.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Huys QJ, Maia TV, Frank MJ. Computational psychiatry as a bridge from neuroscience to clinical applications. Nat Neurosci. 2016;19:404–413. doi: 10.1038/nn.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. This paper reviews the mechanisms by which immune systems interact with neurotransmitters and neurocircuits to influence the risk for depression. Importantly, it points to an inflammatory subtype of depression which may be driven by immune-induced alterations in dopaminergic tone and basal ganglia function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewinsohn PM. A behavioral approach to depression. Essential papers on depression. 1974:150–172. [Google Scholar]
- 13.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 14.Eldar E, Rutledge RB, Dolan RJ, Niv Y. Mood as Representation of Momentum. Trends Cogn Sci. 2016;20:15–24. doi: 10.1016/j.tics.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Rutledge RB, Moutoussis M, Smittenaar P, Zeidman P, Taylor T, Hrynkiewicz L, Lam J, Skandali N, Siegel JZ, Ousdal OT, et al. Association of Neural and Emotional Impacts of Reward Prediction Errors With Major Depression. JAMA Psychiatry. 2017;74:790–797. doi: 10.1001/jamapsychiatry.2017.1713. This work utilized non-learning risky-decision tasks in patients with moderate depression to examine neural differences in reward prediction error (RPE) signaling and the association between symptoms of depression and the emotional impact of RPEs and outcomes. Participants with depression showed intact ventral striatal RPE signaling. Participants with depression and participants with high self-reported symptoms of depression in a large community sample also showed intact associations between RPEs and happiness in a computational model of subjective well-being. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutledge RB, Skandali N, Dayan P, Dolan RJ. A computational and neural model of momentary subjective well-being. Proc Natl Acad Sci U S A. 2014;111:12252–12257. doi: 10.1073/pnas.1407535111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutledge RB, Skandali N, Dayan P, Dolan RJ. Dopaminergic Modulation of Decision Making and Subjective Well-Being. J Neurosci. 2015;35:9811–9822. doi: 10.1523/JNEUROSCI.0702-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28:7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, Schmidt M, Claes S. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013;73:639–645. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pechtel P, Dutra SJ, Goetz EL, Pizzagalli DA. Blunted reward responsiveness in remitted depression. J Psychiatr Res. 2013;47:1864–1869. doi: 10.1016/j.jpsychires.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huys QJ, Pizzagalli DA, Bogdan R, Dayan P. Mapping anhedonia onto reinforcement learning: a behavioural meta-analysis. Biol Mood Anxiety Disord. 2013;3:12. doi: 10.1186/2045-5380-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaiser RH, Treadway MT, Wooten DW, Kumar P, Goer F, Murray L, Beltzer M, Pechtel P, Whitton A, Cohen AL, Alpert NM, El Fakhri G, Normandin MD, Pizzagalli DA. Frontostriatal and dopamine markers of individual differeneces in reinforcement learning: a multi-modal investigation. Cerebral Cortex. doi: 10.1093/cercor/bhx281. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huys QJ, Daw ND, Dayan P. Depression: a decision-theoretic analysis. Annu Rev Neurosci. 2015;38:1–23. doi: 10.1146/annurev-neuro-071714-033928. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Steer RA, Brown GK. Beck depression inventory. 1996 [Google Scholar]
- 26.Klein DN. Depression and Anhedonia. In: Clark DC, Fawcett J, editors. Anhedonia and Affect Deficit States. PMA Publishing Corporation; 1987. [Google Scholar]
- 27.Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the 'EEfRT'? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One. 2009;4:e6598. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol. 2012;121:553–558. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang XH, Huang J, Zhu CY, Wang YF, Cheung EF, Chan RC, Xie GR. Motivational deficits in effort-based decision making in individuals with subsyndromal depression, first-episode and remitted depression patients. Psychiatry Res. 2014;220:874–882. doi: 10.1016/j.psychres.2014.08.056. [DOI] [PubMed] [Google Scholar]
- 30.Hershenberg R, Satterthwaite TD, Daldal A, Katchmar N, Moore TM, Kable JW, Wolf DH. Diminished effort on a progressive ratio task in both unipolar and bipolar depression. Journal of affective disorders. 2016;196:97–100. doi: 10.1016/j.jad.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonnelle V, Veromann KR, Burnett Heyes S, Lo Sterzo E, Manohar S, Husain M. Characterization of reward and effort mechanisms in apathy. J Physiol Paris. 2015;109:16–26. doi: 10.1016/j.jphysparis.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein-Flugge MC, Kennerley SW, Saraiva AC, Penny WD, Bestmann S. Behavioral modeling of human choices reveals dissociable effects of physical effort and temporal delay on reward devaluation. PLoS Comput Biol. 2015;11:e1004116. doi: 10.1371/journal.pcbi.1004116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Treadway MT, Zald DH. Parsing Anhedonia Translational Models of Reward-Processing Deficits in Psychopathology. Current Directions in Psychological Science. 2013;22:244–249. doi: 10.1177/0963721412474460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2008;33:1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- 36.Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H. Amping up effort: effects of d-amphetamine on human effort-based decision-making. J Neurosci. 2011;31:16597–16602. doi: 10.1523/JNEUROSCI.4387-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- 38.Robles CF, Johnson AW. Disruptions in effort-based decision-making and consummatory behavior following antagonism of the dopamine D2 receptor. Behav Brain Res. 2017;320:431–439. doi: 10.1016/j.bbr.2016.10.043. [DOI] [PubMed] [Google Scholar]
- 39.Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Kessler RM, Zald DH. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci. 2012;32:6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Randall PA, Pardo M, Nunes EJ, Lopez Cruz L, Vemuri VK, Makriyannis A, Baqi Y, Muller CE, Correa M, Salamone JD. Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow feeding choice task: pharmacological studies and the role of individual differences. PLoS One. 2012;7:e47934. doi: 10.1371/journal.pone.0047934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chong TT, Apps M, Giehl K, Sillence A, Grima LL, Husain M. Neurocomputational mechanisms underlying subjective valuation of effort costs. PLoS Biol. 2017;15:e1002598. doi: 10.1371/journal.pbio.1002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Klein-Flugge MC, Kennerley SW, Friston K, Bestmann S. Neural Signatures of Value Comparison in Human Cingulate Cortex during Decisions Requiring an Effort-Reward Trade-off. J Neurosci. 2016;36:10002–10015. doi: 10.1523/JNEUROSCI.0292-16.2016. This study provides evidence for the strength of computational modeling in characterizing effort discounting behavior in effort-based decision-making. The results of this study provide the first evidence for the dissociation of effort discounting from delay discounting, enabling accurate modeling of cost-benefit decisions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hauser TU, Eldar E, Dolan RJ. Separate mesocortical and mesolimbic pathways encode effort and reward learning signals. Proc Natl Acad Sci U S A. 2017;114:E7395–E7404. doi: 10.1073/pnas.1705643114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonnelle V, Manohar S, Behrens T, Husain M. Individual Differences in Premotor Brain Systems Underlie Behavioral Apathy. Cereb Cortex. 2016;26:807–819. doi: 10.1093/cercor/bhv247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Llerena K, Park SG, McCarthy JM, Couture SM, Bennett ME, Blanchard JJ. The Motivation and Pleasure Scale-Self-Report (MAP-SR): reliability and validity of a self-report measure of negative symptoms. Compr Psychiatry. 2013;54:568–574. doi: 10.1016/j.comppsych.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: a scale development study. Journal of research in personality. 2006;40:1086–1102. [Google Scholar]
- 47*.Ang YS, Lockwood P, Apps MA, Muhammed K, Husain M. Distinct Subtypes of Apathy Revealed by the Apathy Motivation Index. PLoS One. 2017;12:e0169938. doi: 10.1371/journal.pone.0169938. This paper discusses the Apathy Motivation Index, a self-report measure designed to measure and assess motivational deficits. This measure distinguishes aspects of apathy associated with behavioral, social, and emotional domains and profiles associated with depression, anhedonia, and fatigue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48**.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, Fetcho RN, Zebley B, Oathes DJ, Etkin A, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38. doi: 10.1038/nm.4246. This important study used fMRI in a large multisite sample to subtype patients with depression based on their patterns of connectivity in limbic and frontostriatal networks. Clustering patients in this way enabled the development of highly sensitive and specific biomarkers for depression subtypes. These results pave the way for promising new methods of identifying subtypes and developming targeted treatments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain, Behavior and Immunity. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 50.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 51.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain, behavior, and immunity. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Felger JC, Mun J, Kimmel HL, Nye JA, Drake DF, Hernandez CR, Freeman AA, Rye DB, Goodman MM, Howell LL. Chronic Interferon-α Decreases Dopamine 2 Receptor Binding and Striatal Dopamine Release in Association with Anhedonia-Like Behavior in Nonhuman Primates. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH. Dopaminergic mechanisms of reduced Basal Ganglia responses to hedonic reward during interferon alfa administration. Archives of General Psychiatry. 2012;69:1044–1053. doi: 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yohn SE, Arif Y, Haley A, Tripodi G, Baqi Y, Müller CE, San Miguel N, Correa M, Salamone JD. Effort-related motivational effects of the pro-inflammatory cytokine interleukin-6: pharmacological and neurochemical characterization. Psychopharmacology. 2016;233:3575–3586. doi: 10.1007/s00213-016-4392-9. [DOI] [PubMed] [Google Scholar]
- 55.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dantzer R, Meagher MW, Cleeland CS. Translational approaches to treatment-induced symptoms in cancer patients. Nature Reviews Clinical Oncology. 2012;9:414–426. doi: 10.1038/nrclinonc.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57**.Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, Zhou R. Dopamine Controls Systemic Inflammation through Inhibition of NLRP3 Inflammasome. Cell. 2015;160:62–73. doi: 10.1016/j.cell.2014.11.047. This study provides evidence for the role of dopamine as a regulator of immune cell function in the brain. Specifically, the authors found that dopamine inhibits inflammasome activation via the dopamine D1 receptor. [DOI] [PubMed] [Google Scholar]
- 58.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH. Paroxetine for the prevention of depression induced by high-dose interferon alfa. New England Journal of Medicine. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 59**.Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, Takahashi A, Flanigan ME, Aleyasin H, LeClair KB. Social stress induces neurovascular pathology promoting depression. Nature neuroscience. 2017;20:1752. doi: 10.1038/s41593-017-0010-3. This exciting study suggests a biological mechanism through which heightened peripheral inflammation contributes to the pathogenesis of depression. The authors use a mouse model of depression to show that social stress downregulates tight junction protein claudin-5 (Cldn5) and increases blood brain barrier permeability in the nucleus accumbens of stress-susceptible, but not resilient, mice. This increase in permeability promotes increased passage of interleukin-6 across the blood brain barrier and induces depression-like behaviors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monroe SM, Harkness KL. Recurrence in major depression: a conceptual analysis. Psychological Review. 2011;118:655–674. doi: 10.1037/a0025190. [DOI] [PubMed] [Google Scholar]
- 61.Lasselin J, Treadway MT, Lacourt TE, Soop A, Olsson MJ, Karshikoff B, Paues-Göranson S, Axelsson J, Dantzer R, Lekander M. Lipopolysaccharide alters motivated behavior in a monetary reward task: a randomized trial. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62**.Treadway MT, Admon R, Arulpragasam AR, Mehta M, Douglas S, Vitaliano G, Olson DP, Cooper JA, Pizzagalli DA. Association Between Interleukin-6 and Striatal Prediction-Error Signals Following Acute Stress in Healthy Female Participants. Biological Psychiatry. 2017 doi: 10.1016/j.biopsych.2017.02.1183. This study found that stress-induced increases in an inflammtory cytokine, IL-6, were associated with decreased strital prediction error signals, as measured by fMRI. Importantly, this study provided evidence for a novel link between inflammation and striatal RPEs during reinforcement learning in the context of acute psychological stress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A Randomized Controlled Trial of the Tumor Necrosis Factor Antagonist Infliximab for Treatment-Resistant DepressionThe Role of Baseline Inflammatory BiomarkersInfliximab for Treatment-Resistant Depression. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Escalona R, Fawcett J. Pramipexole in Treatment Resistant-Depression, Possible Role of Inflammatory Cytokines. Neuropsychopharmacology. 2017;42:363–363. doi: 10.1038/npp.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Felger JC, Hernandez CR, Miller AH. Levodopa Reverses Cytokine-Induced Reductions in Striatal Dopamine Release. International Journal of Neuropsychopharmacology. 2015:pyu084. doi: 10.1093/ijnp/pyu084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Felger JC, Treadway MT. Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology. 2017;42:216–241. doi: 10.1038/npp.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]