Abstract
Maternal stress during pregnancy has been linked to premorbid abnormalities associated with depression (e.g., difficult temperament, cognitive deficits) in offspring. However, few studies have looked across developmental periods to examine maternal stress during pregnancy and offspring depression during adolescence and whether these associations differ by sex. The current study used data from 1,711 mother-offspring dyads (offspring sex: 49.8% male) in a longitudinal birth cohort study. Maternal narratives collected during pregnancy were qualitatively coded for stress-related themes by independent raters. Latent class analysis (LCA) identified distinct subgroups of offspring based on exposure to maternal prenatal stress and other developmental factors from the prenatal, childhood, and adolescent periods that have been associated with depression and/or maternal prenatal stress. LCA identified subgroups that were compared to determine whether and to what extent they differed on adolescent depressive symptoms. LCA revealed a subgroup of “high-risk” individuals, characterized by maternal factors during pregnancy (higher ambivalence/negativity and lower positivity towards the pregnancy, higher levels of hassles, lower maternal education and higher maternal age at birth, higher pre-pregnancy BMI) and offspring developmental factors (decreased cognitive functioning during childhood and adolescence, lower perceived parental support during adolescence, and higher levels of maternal depression during adolescence). High-risk females exhibited elevated conduct symptoms and higher birth order, while high-risk males exhibited decreased internalizing symptoms and lower birth order. Both high-risk males and females reported elevated depressive symptoms during adolescence relative to their “low-risk” counterparts.
Keywords: prenatal stress, adolescence, depression, sex differences, developmental risk factors
A small but growing literature suggests an association between maternal psychological experiences during pregnancy and risk for depression among offspring during adolescence (Betts, Williams, Najman, & Alati, 2014; Pawlby, Hay, Sharp, Waters, & O'Keane, 2009). While the preponderance of prenatal studies have shown sex differences between maternal stress during pregnancy and offspring developmental outcomes, some studies have found no association (Agnafors et al., 2013; Agnafors et al., 2016). Despite the associated high disease burden, risk of recurrence, and risk of suicide (Stikkelbroek et al., 2013), depression often goes undiagnosed in adolescence (Fletcher, 2008). Research has shown that depression rates spike during adolescence, particularly in females (Hankin et al., 1998; Nolen-Hoeksema, 2001), and that adolescent onset of clinical depression is associated with more persistent and more severe depressive symptoms (Harrington, Fudge, Rutter, Pickles, & Hill, 1990; Kim-Cohen et al., 2003; Wickramaratne, Greenwald, & Weissman, 2000). Early intervention is essential, as the duration of untreated depression is associated with a worsened clinical course (Fletcher, 2008). Enhancing our understanding of the influence of early developmental factors—beginning as early as the prenatal period—on risk for subsequent depression represents a promising avenue for early identification of at-risk individuals.
Maternal Stress During Pregnancy & Risk for Offspring Depression
Although few studies have directly examined the association between prenatal maternal stress and adolescent depression, previous research has linked maternal stress during pregnancy to adverse childhood outcomes often found in the premorbid period of depression (Hankin & Abramson, 2001; Koenen et al., 2009; Lahey, Loeber, Burke, Rathouz, & McBurnett, 2002), including: shyness/inhibition (Talge et al., 2007), decreased intelligence scores (Slykerman et al., 2005); and increased behavioral/emotional problems (O'Connor, Heron, Golding, Beveridge, & Glover, 2002), such as childhood anxiety (Davis & Sandman, 2012), attention deficit hyperactivity disorder (ADHD; Loomans et al., 2011) and conduct problems (Rodriguez & Bohlin, 2005). These findings underscore the importance of not just examining a link between prenatal maternal stress and offspring depression, but also considering factors across multiple developmental periods that are associated with both prenatal maternal stress and offspring depression that may contribute to this association.
Among ecologic studies, one study found that adult offspring of women who were pregnant during Holland's “hunger winter” were at increased risk of developing unipolar and bipolar major affective disorder during adulthood (Brown, van Os, Driessens, Hoek, & Susser, 2000). Another study of women who were pregnant during a severe earthquake found that 13.3% of their 18-year-old offspring had severe depression, compared with only 5.5% of offspring born a year later (Watson, Mednick, Huttunen, & Wang, 1999). Nevertheless, the implications and generalizability of these studies are limited by their examination of severe and relatively rare traumatic stressors, as well as a presumed, but not assessed, uniform level of stress based on an adverse event or exposure experienced by the population. Recent work in a large, prospective Australian longitudinal birth cohort found that the combination of maternal anxiety, depression, and stress during pregnancy, assessed using self-report measures, was associated with increased risk of internalizing behavior problems in adolescence (Betts et al., 2014) and adulthood (Betts Williams, Najman, & Alati, 2015). Although evidence for a link between prenatal maternal stress and offspring depression is mounting, there are still unexplored gaps in the literature, including whether a combination of prenatal and childhood risk factors increases risk for depressive symptoms in adolescence.
Offspring Sex Differences in Response to Maternal Stress During Pregnancy
Human studies suggest that males may be more vulnerable than females to the deleterious influences of maternal stress during pregnancy, as they appear to be at increased risk for mortality, morbidity, reduced viability, delayed neonatal maturity, neurological problems, and perhaps even risk of schizophrenia spectrum disorders (Ellman et al., 2008; Fineberg et al., 2016; Sandman, Glynn, & Davis, 2013). Although these findings were previously thought to suggest that females are protected against the adverse influences of early life stress, emerging evidence suggests a “viability-vulnerability tradeoff,” such that for females, prenatal maternal stress is associated with subtle but persistent influences on temperament, behavior, and brain development that may predispose females to later affective problems (Sandman et al., 2013). In support of this theory, a growing body of work suggests that fetal exposure to maternal psychosocial distress and cortisol during pregnancy is linked to temperaments associated with affective disorders, childhood anxiety, and changes in brain regions associated with mood and emotional problems that could selectively put females compared to males at risk for future psychopathology, such as depression (Sandman et al., 2013). Despite known sex differences in the prenatal stress literature (Sandman et al., 2013) and in rates of depression during adolescence (Alloy & Abramson, 2007), the literature is agnostic about whether there are sex differences in risk for offspring depression after exposure to prenatal maternal stress
Developmental Models of Maternal Stress During Pregnancy & Offspring Depression
The question of how prenatal maternal stress exposure relates to adolescent depression is complex, given the myriad influences across developmental periods (i.e., in utero, childhood, adolescence) that could influence this association and the potentially differing characteristics that increase risk for adolescent depression among individuals or groups of individuals (Cicchetti & Rogosch, 1996). One possibility is that these developmental risk factors work in conjunction to influence later risk for depression. Support for this possibility comes from the relatively small effect sizes of many of these developmental variables, suggesting that their individual impact on the risk for the disorder is small, but may additively or interactively influence the etiology for depression (Gale & Martyn, 2004; Patton et al., 2004). Another possibility (that does not preclude the first possibility) is that maternal stress during pregnancy leads to a cascade of developmental difficulties that begin in early life (Davis & Sandman, 2012; Sandman et al., 2013; Talge et al., 2007). While both models are feasible, the present study sought to analyze a large pregnancy/birth cohort to identify distinct profiles of individuals who might be at increased risk for adolescent depression based on exposure to a variety of distal and proximal developmental risk factors, as a first step to understanding important developmental precursors for the disorder.
Present Study Aims and Hypotheses
Given our interest in characterizing developmental patterns associated with risk of depression among adolescents, we used latent class analysis (LCA)—a person-centered approach that groups individuals based on similarities and differences across a number of indicator variables—to explore patterns among known correlates of risk for depression across childhood and adolescence, as well as contemporaneously reported prenatal maternal stress and other obstetric variables that have been linked to risk of offspring depression. Although LCA is an exploratory data analysis technique, we anticipated based on previous studies that the following variables would collectively identify a subgroup at increased risk for adolescent depression: pregnancy and other maternal factors, such as maternal stress during pregnancy (Betts et al., 2014; Brown et al., 2000; Buss, Davis, Hobel, & Sandman, 2011; Da Costa, Brender, & Larouche, 1998; Pritchard & Teo, 1994; Shah et al., 2011; Watson et al., 1999), obstetric risk factors (e.g., lower birth weight), increased maternal age (Braithwaite, Murphy, & Ramchandani, 2014; Cleary-Goldman et al., 2005), increased maternal worry during the offspring's childhood (Entringer, Buss, & Wadhwa, 2015), and higher levels of maternal depressive symptoms during offspring adolescence (Murray et al., 2011); and offspring developmental factors, such as childhood internalizing symptoms and conduct problems (Talge et al., 2007), decreased cognitive functioning during childhood and adolescence (Koenen et al., 2009), and lower levels of perceived parental support in adolescence (Stice, Ragan, & Randall, 2004). Given known sex differences in the prenatal stress literature (Sandman et al., 2013) and research suggesting that pathways to depression differ for males and females (Piccinelli & Wilkinson, 2000), we chose to examine males and females separately in supplementary analyses after conducting LCA of the entire analytic sample. We hypothesized that females would be particularly at risk for symptoms of adolescent depression, given past research suggesting that the incidence of depression is higher among female adolescents (Alloy & Abramson, 2007) and a recent study that found an association between prenatal maternal anxiety and offspring depressive symptoms during adolescence that was mediated by offspring cortisol in females, but not in males (Van den Bergh, Van Calster, Smits, Van Huffel, & Lagae, 2008).
Method
Participants
The study was approved by the Institutional Review Boards of the New York State Psychiatric Institute, Public Health Institute, and Temple University. Data for the current study were drawn from the Child Health and Development Study (CHDS), a large pregnancy cohort comprised of nearly all pregnant women receiving health care through the Kaiser Foundation Health Plan (KFHP) at its hospital and clinics in Alameda County, California from 1959-1966 (live births N = 19,044) (van den Berg, Christianson, & Oechsli, 1988). Approximately 30 percent of the population of Alameda County, including the cities of Oakland, Berkeley, and Hayward, received care through KFHP at the time. KFHP membership was demographically similar to the region's population, albeit with slightly elevated socioeconomic status, as eligibility the KFHP typically depended on employment (Krieger, 1992). To encourage participation, routine prenatal lab work was provided free of charge to participants.
CHDS has continued to follow these mothers and their offspring over multiple time points throughout development. The present study analyzed data from Kaiser medical records and from interviews at three study time points (described further in Measures): 1) interviews of the mother during pregnancy, completed in or around the second trimester, as well as extensive medical information collected about the mother just before, during, and after the pregnancy (e.g., during the early neonatal period), 2) interviews and questionnaires given to the mother when her offspring was aged 9 to 11, as well as cognitive testing administered to the child and, 3) separate interviews with the mother and child when the child was aged 15 to 17, as well as cognitive testing administered to the child. Data were included in the present analyses only if the mother and child participated in all three time points. Of the 2,518 offspring eligible for the Adolescent Study—by virtue of continued residence in the San Francisco Bay area, participation in all previous study time points, and birth dates between March 1, 1960 and March 31, 1963—the CHDS enrolled 2,020 (80.2%; see Online Supplementary Figure). In cases where financial hardship would have prevented a family from bringing a child to an examination, the CHDS paid the cost of the visit. Compared to the initial CHDS pregnancy cohort, the offspring in the Adolescent Study had a smaller proportion of firstborn offspring and a greater proportion of offspring whose mothers were 1) married and living with a husband at the original intake, 2) white, and 3) high school graduates, though these differences were small (see van den Berg et al., 1988 for details).
Of the 2,020 adolescent cohort members, 1,964 (97.2%) had data on the pregnancy variables of interest (see Online Supplementary Figure 2 for comparative demographics between our sample of 1,964 and the remainder CHDS live births). For mothers who had more than one child in the Adolescent Study, we selected one child at random using a random number generator to avoid potential statistical complications associated with non-independent observations among siblings. As such, 253 siblings were removed from the analytic dataset, leaving a final analytic sample of 1,711 mother-offspring dyads with data available at all relevant time points (see Table 1 for sample demographics)..
Table 1. Demographic Characteristics of the Sample (N = 1711).
| Demographics | Overall | Males | Females | p-value |
|---|---|---|---|---|
| Offspring Sex, n (%) | ||||
| Male | 859 (49.8) | |||
| Female | 852 (50.2) | |||
| Maternal Race, n (%) | 0.55 | |||
| Caucasian | 1304 (76.3) | 659 (76.7) | 645 (75.8) | |
| African American | 317 (18.5) | 152 (17.7) | 165 (19.4) | |
| Asian | 89 (5.2) | 48 (5.6) | 41 (4.8) | |
| Maternal Education, n (%) | 0.99 | |||
| Less than or equal to H.S. | 782 (45.8) | 393 (45.8) | 389 (45.8) | |
| Greater than H.S. | 927 (54.2) | 466 (54.3) | 461 (54.2) | |
| Birth Weight (g), M (SD) | 3365.1 (516.3) | 3444.5 (514.8) | 3285.0 (505.62) | <0.01* |
| Gestational Age at Interview (weeks), M (SD) | 15.3 (6.8) | 15.2 (6.7) | 15.4 (6.9) | 0.45 |
| Gestational Age at Birth (weeks), M (SD) | 40.0 (2.2) | 40.0 (2.1) | 40.1 (2.4) | 0.78 |
| Maternal Age, M (SD) | 29.0 (6.0) | 29.0 (5.9) | 29.0 (6.2) | 0.86 |
| Maternal smoking during pregnancy n (%) | 589 (34.4) | 316 (36.8) | 273 (32.0) | 0.04* |
| Clinical Characteristics | ||||
|
| ||||
| Ambivalent Feelings About Pregnancy, n (%) | 205 (12.0) | 99 (11.5) | 106 (12.4) | 0.55 |
| Ailments of Pregnancy, n (%) | 63 (3.7) | 33 (3.8) | 30 (3.5) | 0.72 |
| Medical Life Events, n (%) | 55 (3.2) | 27 (3.1) | 28 (3.3) | 0.87 |
| Negative Affect About Preg Ailments, n (%) | 40 (2.3) | 19 (2.2) | 21 (2.5) | 0.73 |
| Negative Feelings About Pregnancy, n (%) | 189 (11.1) | 102 (11.9) | 87 (10.2) | 0.27 |
| Non-traumatic Life Events, n (%) | 432 (25.3) | 211 (24.6) | 221 (25.9) | 0.51 |
| Positive Feelings About Pregnancy, n (%) | 756 (44.2) | 373 (43.4) | 383 (45.0) | 0.52 |
| Perceived Stress, n (%) | 365 (21.3) | 189 (22.0) | 176 (20.7) | 0.50 |
| Pregnancy-Specific Anxiety, n (%) | 251 (14.7) | 126 (14.7) | 125 (14.7) | 0.99 |
| Reported Anxiety, n (%) | 571 (33.4) | 286 (33.3) | 285 (33.5) | 0.94 |
| Reported Depression, n (%) | 117 (6.8) | 60 (7.0) | 57 (6.7) | 0.81 |
| Routine Daily Hassles, n (%) | 570 (33.3) | 269 (31.3) | 301 (35.3) | 0.08 |
| Traumatic Life Events, n (%) | 96 (5.6) | 52 (6.1) | 44 (5.2) | 0.42 |
| Childhood Internalizing Symptoms, M (SD) | 2.7 (2.3) | 2.6 (2.3) | 2.8 (2.3) | 0.20 |
| Childhood Conduct Symptoms, M (SD) | 51.3 (9.7) | 51.4 (9.5) | 51.3 (9.9) | 0.87 |
| Childhood Peabody Scores, M (SD) | 23.4 (5.1) | 23.2 (5.1) | 23.6 (5.1) | 0.12 |
| Adolescent Depression Scores, M (SD) | 114.8 (15.4) | 116.5 (14.3) | 113.1 (16.2) | <0.01* |
| Adolescent Peabody Scores, M (SD) | 205.0 (12.0) | 99.0 (11.5) | 106.0 (12.4) | 0.55 |
Note. For males, the following variables had missing data: gestational at interview (n = 7); gestational age (n = 7); maternal age (n = 2); childhood inhibition symptoms (n = 518); childhood Peabody scores (n = 14); adolescent depression scores (n = 70), adolescent Peabody scores (n = 21).
For females, the following variables had missing data: maternal education (n = 2); gestational age at interview (n = 3); gestational age (n = 3); maternal age (n = 2); childhood inhibition symptoms (n = 517); childhood Peabody scores (n = 18); adolescent depression scores (n = 41); adolescent Peabody scores (n = 8).
P-values correspond to tests of sex differences (t-tests for continuous or count variables and chi-square tests for categorical variables).
Measures (for examples of questionnaire items see Online Supplementary Materials)
Pregnancy Interviews
Pregnancy interviews included a detailed reproductive history and questions about her health, the health of her husband and children, her attitudes toward the current pregnancy, and her health-related behaviors. While these interviews often took 4-6 hours to complete, the questions pertaining to the stress themes analyzed in the present study typically were completed in 10 minutes or less. The majority of the interviews were conducted during the second trimester (M=15.32, SD=6.76), as calculated from gestational age (GA) at the time of interview. (See Supplementary Figure 1 for full study timeline.)
Maternal Psychosocial Stress during Pregnancy
Maternal psychosocial stress during pregnancy was measured by extracting stressful life events and stress-related themes from narratives generated in response to interview questions such as “What kinds of things have been worrying you recently?” For most of the interviews (n=1440, or 84.2%), interviewers were instructed to “Encourage gravida to talk freely” and gently probe for detail, but there were no specific follow up questions. For the remaining interviews (n=271, or 15.8%), in addition to the “worrying” question, women were asked whether they had experienced “any nervousness, anxiety, or depression” or “any family troubles.” Given that the presence of these additional stress-theme-related questions could lead to greater endorsement of stress themes, a dichotomous interview version variable was created for analyses.
Typical responses in the maternal narratives included descriptions of financial and relationship stress and stressful life events (see Online Supplementary Table2 for examples of stress coding and Online Supplementary Manual for our stress theme coding manual). Two independent raters followed a detailed coding manual to extract stress-related themes. To minimize bias, inter-rater reliability was established after coders analyzed 50 maternal interviews chosen at random from a previous CHDS subsample that did not overlap with participants from the current study. Reliability was evaluated using intraclass correlations (ICCs) calculated from frequency of stress-related themes (e.g., the number of narrative references to each theme), which are the most appropriate statistic for continuous data (Shrout & Fleiss, 1979). ICCs of .70 or above on the aforementioned test cases were required before a coder began to code study interviews; ICCs ranged from .72 to 1.00 (mean ICC = 0.89; substantial to almost perfect reliability). To assess the possibility of coder drift during coding, 75 interviews from the analytic sample were selected at random to be coded by both coders independently throughout the coding process. ICCs for this second reliability check ranged from .76 to 1.00 (mean ICC = .81; almost perfect reliability). After all interviews were coded initially, an additional 325 interviews were selected at random to be double coded to further to assess the possibility of coder drift; this third reliability check yielded ICCs ranging from .71 to .87 (mean ICC = 0.76; substantial reliability).
Raters, blind to offspring characteristics, coded interviews for the following themes: reported anxiety, reported depression, perceived stress, pregnancy-specific anxiety, positive/negative/ambivalent feelings about pregnancy, traumatic/severe life events, non-traumatic life events, routine daily hassles, medical life events, ailments of pregnancy, and negative affect about pregnancy ailments. These themes were chosen because they are representative of constructs that previously have been associated with depression or with obstetric/childhood risk factors associated with depression (Betts et al., 2014, 2015; Brown et al., 2000; Buss et al., 2011; Da Costa et al., 1998; Huizink, Robles de Medina, Mulder, Visser, & Buitelaar, 2003; Huizink, Robles De Medina, Mulder, Visser, & Buitelaar, 2002; Pritchard & Teo, 1994; Shah et al., 2011; Watson et al., 1999). Stress-related themes were operationally defined and coded based on well-established definitions of the constructs (Cohen, Kessler, & Gordon, 1997; Khashan et al., 2008; Serido, Almeida, & Wethington, 2004) and/or by using validated measures of the construct as a guide (Cohen, Kamarck, & Mermelstein, 1983; Radloff, 1977; Rini, Dunkel-Schetter, Wadhwa, & Sandman, 1999; Spielberger, 1983).
Reported depression and anxiety were coded based on the mother's use of words consistent with depression or anxiety, using the CES-D scale and the State-Trait Anxiety Inventory as models (Radloff, 1977; Spielberger, 1983). Perceived stress was coded if the woman made appraisals of situations in her life as stressful, as defined in the Perceived Stress Scale (Cohen et al., 1983). Pregnancy-specific anxiety was coded if the woman reported worries or concerns about the pregnancy, such as concerns about labor and delivery or health of the baby (Rini et al., 1999). Positive, negative, and ambivalent feelings about the pregnancy were coded based on responses addressing wantedness of the pregnancy (Herman et al, 2006). Non-traumatic life events were coded as present if the pregnant woman reported discrete and observable events that required an adjustment in life routines and were impactful enough to not be considered routine daily hassles (Cohen et al., 1997). Routine daily hassles were coded if a women reported relatively minor events arising out of day-to-day living and/or small, unexpected events that disrupt daily life (Serido et al., 2004). Medical life events were coded if a concern related to an ongoing medical condition was endorsed. Ailments of pregnancy were coded when women reported physical discomfort related to pregnancy (e.g., morning sickness), and negative affect about pregnancy ailments was coded when women reported negative feelings towards pregnancy-related physical discomfort. Severe/traumatic life events were coded as present if there was actual or threatened death or serious injury, threat to physical integrity of self or others, and/or loss or diagnosis with cancer, acute myocardial infarction, or cerebrovascular accident of a close relative, based on previous research of traumatic life events during pregnancy (Khashan et al., 2008) (See Online Supplementary Table 2 for examples of stress themes).
Although interview coding documented the frequency with which women reported a given stress theme (e.g., how often daily stress was mentioned in a given narrative), the stress themes were reduced to dichotomous variables (present/absent) prior to analyses because of limited variability in frequency data found in a previous study of CHDS women (data available upon request) (Fineberg et al., 2016). Stress themes were coded using ATLAS.ti 6 (ATLAS.ti Scientific Software Development GmbH, Berlin, Germany).
Other Prenatal Factors
Of the potential socioeconomic status (SES) variables, maternal education had the most complete data (n = 1709, or 99.88%), has been correlated with other measures of socioeconomic status in this sample, and is often used to account for the potential contributions of postnatal adversity to risk of negative developmental sequelae (Schlotz & Phillips, 2009); therefore, maternal education was treated as representative of SES for the present study. Although a variety of pre- and perinatal factors are thought to influence offspring development (Siegel, 1982), variables that have previously been associated with depression, or with obstetric risk factors associated with depression, were chosen for inclusion in the present study (Braithwaite et al., 2014), specifically: maternal age (Cleary-Goldman et al., 2005), maternal pre-pregnancy body mass index (BMI) (Abenhaim, Kinch, Morin, Benjamin, & Usher, 2007), maternal smoking during pregnancy (Menezes et al., 2013), gestational age (Patton, Coffey, Carlin, Olsson, & Morley, 2004), and birth weight (Gale & Martyn, 2004).
Age 9-11 Childhood Visit
At the child's age 9-11 year visit, mothers answered true or not true or uncertain to 100 questions about their child's behavior, including behaviors related to internalizing symptoms and conduct problems. The 100-item questionnaire was based on a 46-question inventory of child behavior that had been used in previous studies at the University of California, Berkeley (see Tuddenham, Brooks, & Milkovich, 1974 for additional detail). Using as a guide Achenbach Child Behavior Checklist (CBCL), a well-validated and commonly used measure among children (Achenbach, 1991), we identified items that corresponded to childhood internalizing symptoms and conduct symptoms as follows (see Online Supplementary Table 3 for items from unpublished scales).
Childhood Internalizing Symptoms
Thirteen items related to internalizing symptoms (depression and anxiety) were drawn from the 100-item questionnaire and summed to create a scale with items broadly representative of the internalizing behaviors on the CBCL (Achenbach, 1991). The resultant internalizing symptoms scale has moderate internal consistency (KR-20 α = .70) and was correlated with adolescent depression scores in our sample (p < 0.001), suggesting that it represents a uniform construct and that it is predictive of related outcomes found in previous studies (described below). This variable was treated as continuous.
Childhood Conduct Symptoms
Fifteen items related to conduct problems were drawn from the 100-item maternal report questionnaire and summed to create a conduct symptom scale with items broadly representative of the externalizing behaviors on the CBCL (Achenbach, 1991). The conduct problems scale has good internal consistency (KR-20 α = .73). This variable was treated as continuous.
Childhood conduct symptoms, were included in the analyses given associations with both prenatal maternal stress (Loomans et al., 2011) and later development of depression (Lahey et al., 2002). Further, in another study of the same cohort, conduct problems predicted alcohol abuse in adolescence, suggesting good external validity (Keyes, Keyes, March, & Susser, 2011).
Maternal Worry during Childhood
At the 9-11 visit mothers were asked to answer “Yes” or “No” to whether any or all of their finances, marital relations, employment, or health had been worrying them recently. These variables were included to account for the influence of postnatal stress on offspring development (Entringer et al., 2015). Birth order, which was assessed at the 9-11 visit and is highly correlated with family size in this sample (Keyes et al., 2011), and whether the child lived in a single parent household were included to tap variables related to family structure during childhood, as certain aspects of family structure (e.g., smaller family size, two parents) have been positively associated with offspring well-being (Amato, 2005). Because firstborn status has been associated with lower depression scores (e.g., Gates, Lineberger, Crockett, & Hubbard, 1988), and because there is evidence that birth order can have non-normal relationships with developmental outcomes, we dichotomized birth order into firstborn (n = 427) and second born or later (n = 1264) (Haukka, Suvisaari, & Lönnqvist, 2004).
Childhood and Adolescent Cognitive Variables
The Peabody Picture Vocabulary Test (PPVT) is a commonly used receptive vocabulary measure that can be administered to individuals aged 2.5 years and above and is correlated with IQ (Lezak, Howieson, & Loring, 2004). The PPVT has high test-retest reliability (>0.90) and does not require reading, writing, or verbal responses. These data were included based on past research linking prenatal maternal stress exposure to cognitive problems, including decreased IQ (Slykerman et al., 2005), which has been found to occur during the premorbid period of depression (Koenen et al., 2009; Zammit et al., 2004). PPVT standardized scores from the 9-11 and adolescent visits were used.
Adolescent Depressive Symptoms
Adolescents, ages 15-17, completed a self-report inventory about their lives and beliefs about themselves, which included depressive symptom items. The adolescent depression symptom scale included 14 items related to depressive symptomatology and assessed affective and cognitive symptoms of depression on a 4-point Likert scale. The internal consistency of the depression scale is high (Cronbach's α = .81); the depression scale was treated as a continuous variable.
A number of follow-up studies of the CHDS cohort have used semi-structured interviews, including the Structured Clinical Interview for DSM-IV-TR Disorders and the Diagnostic Interview for Genetic Studies (First, Spitzer, Gibbon, & Williams, 1994; Nurnberger et al., 1994; Parboosing, Bao, Shen, Schaefer, & Brown, 2013; Susser et al., 2011) to ascertain diagnoses of mental disorders. While most of the participants of these follow-up studies were not overlapping with the Adolescent Study, we sought to determine whether scoring high on the depression scale was predictive of major depressive disorder (MDD) diagnoses among those who participated in both the Adolescent study and one of these follow up studies. Analyses suggest that the adolescent depression scale has excellent predictive validity with semi-structured interviews conducted in 20-30 year follow-up studies, as well as with well-known questionnaires administered in an ongoing follow-up study. Adolescent Study members who received a MDD diagnosis in these follow-up studies (n = 13) had significantly higher scores on the adolescent depression scale compared to those who did not receive a MDD diagnosis (n = 39), and these differences were associated with a large effect size (p = .04; Cohen's d = .62, unpublished data, available upon request). Additionally, there was 72% specificity in predicting MDD among those who scored in the upper quartile on the adolescent depression scale (46% sensitivity). Although the adolescent depression scale does not capture everyone who will later develop depression, those scoring high on the scale appear to be at increased risk for a depression diagnosis.
Further, during the adolescent visit maternal responses to the question, “Overall, how happy would you say your child is?” were significantly related to their adolescent's overall depression score, F(3, 1520) = 39.95, p < .001. Mothers who responded with answers of not too happy or very unhappy had adolescents with significantly higher depression scores, M = 25.95, SD = 5.15 and M = 30.63, SD = 5.29, respectively, than adolescents of mothers who reported their child was fairly happy or very happy, M = 23.98, SD = 5.00 and M = 21.59, SD = 4.75, respectively.
Maternal Depressive Symptoms during Adolescence
Mothers completed a self-report inventory of their own depressive symptoms that mirrored the self-report inventory completed by adolescents at the same time point. The scale has good internal consistency (Cronbach's α =.76). Previous work has linked maternal depression to increased rates of depressive symptoms in childhood and adolescence (Murray et al., 2011).
Perceived Maternal and Paternal Support during Adolescence
Adolescents were asked a number of questions about family support. The Adolescent Personal Questionnaire asked questions about the adolescent's relationship with their parents along with a variety of statements related to their relationship with their parents that were scored on a 4-point Likert scale. Each scale consisted of 5 items; the Perceived Maternal and Paternal Support scales have moderate internal consistency (Cronbach's αs = .77 and .72, respectively). Perceived maternal and paternal support during adolescence were included in the analyses because they have been identified as protective factors associated with decreased risk for depression (Stice et al., 2004).
Statistical Analyses
Preliminary analyses, including descriptive and bivariate analyses, were conducted using SAS version 9.4. Primary analyses were conducted using Mplus statistical software package, Version 7.3 (Muthén & Muthén, 2012). Mplus addresses missing data using Full-Information Maximum Likelihood (FIML) estimation, a robust and commonly accepted way to handle missing data (Graham, 2009; Muthén & Shedden, 1999; Schafer & Graham, 2002). See Table 1 for information about missing data. LCA, which classifies individuals from a heterogeneous population into smaller, more homogenous subgroups (Nylund, Asparouhov, & Muthén, 2007), was conducted with the full, analytic sample to determine whether there were profiles of maternal stress during pregnancy and other developmental variables that were associated with increased risk of offspring depression. Percentages of sexes within LCA classes were examined to ascertain whether there were descriptive differences in the proportions of females and males between classes. Next, the hypothesis that there are sex-specific profiles that increase risk for offspring depression was tested using LCA analyses conducted separately for male and female offspring. Prior to running LCA, continuous variables were converted to z-scores (M = 0.00, SD = 1.00), first in the full analytic sample and then separately for males and females, to put all indicator variables on the same scale.
In LCA, selection of the best-fitting model in LCA is based on both statistical indicators and conceptual (i.e., practical) considerations (Nylund, Bellmore, Nishina, & Graham, 2007). While there is no definitive fit index to determine the best-fitting model, a simulation study suggested that the Bayesian Information Criterion (BIC) and bootstrap likelihood ratio test (BLRT) were the most consistent statistical indicators of relative fit across models (Nylund et al., 2007). The BLRT evaluates whether adding an additional class significantly improves model fit by comparing model fit with k classes to the model fit with k-1 classes (Nylund et al., 2007). Other fit indices—the Akaike Information Criterion (AIC) and the sample-size adjusted BIC (ABIC) were also evaluated (Muthén & Muthén, 2000). Both entropy and posterior probability values provide information about the precision of assigning latent class membership, with values closer to 1 indicating greater precision (Muthén & Muthén, 2000). Statistical measures of fit were assessed accordingly and models were evaluated to determine whether adding a class was both substantively meaningful and consistent with the underlying conceptual model.
Once the best-fitting models were selected, we applied a model-based approach to LCA with distal outcomes (Lanza, Tan, & Bray, 2013) to assess class differences in levels of adolescent depressive symptoms. Specifically, we added as auxiliary variables the adolescent depressive symptoms score and a dichotomous variable indicating whether given depressive symptoms score was above the 75th percentile of the sample, and equality of means chi-square tests and chi-square tests of independence were used to compare the latent classes on these variables. Finally, an auxiliary variable was added to assess potential differences by interview version. To assess differences potentially related to verbosity, we added word count of the maternal stress narratives during pregnancy as an auxiliary variable in our analyses.
Results
Demographics and mean scores for clinical characteristics are displayed in Table 1 (full analytic sample and by sex).
LCA for overall sample
For the overall sample, LCAs were conducted for one, two, three, and four class models. LCA statistics are presented in Online Supplementary Table 4. Although the four-class model minimized the BIC, the smallest class in that model comprised only 2.6% of the sample, suggesting that the model was overfit to the sample. Statistical indices (i.e., lower BIC and significant BLRT) indicated that the three-class model was a better fit than the two-class model; however, conceptually, the three-class model did not differentiate the groups on a number of important variables (Masyn, Henderson, & Greenbaum, 2010). Specifically, the added class in the three-class model seemed to track closely with the larger class in the two-class model, particularly among maternal pregnancy stress characteristics, and the decrease in BIC between the two- and three-class models was marginal. The two-class model had higher entropy compared to the three-class model (0.765 vs. 0.652), indicating better precision of individuals being placed into latent classes. Relatedly, posterior probabilities were higher for the two-class model (range: 0.943 to 0.892) relative to the three-class model (range: 0.863 to 0.807), indicating clearer class separation for the two-class model. The two-class model produced classes that were not only substantively distinct but also conceptually meaningful; therefore this model was used in the present study (Masyn et al., 2010).
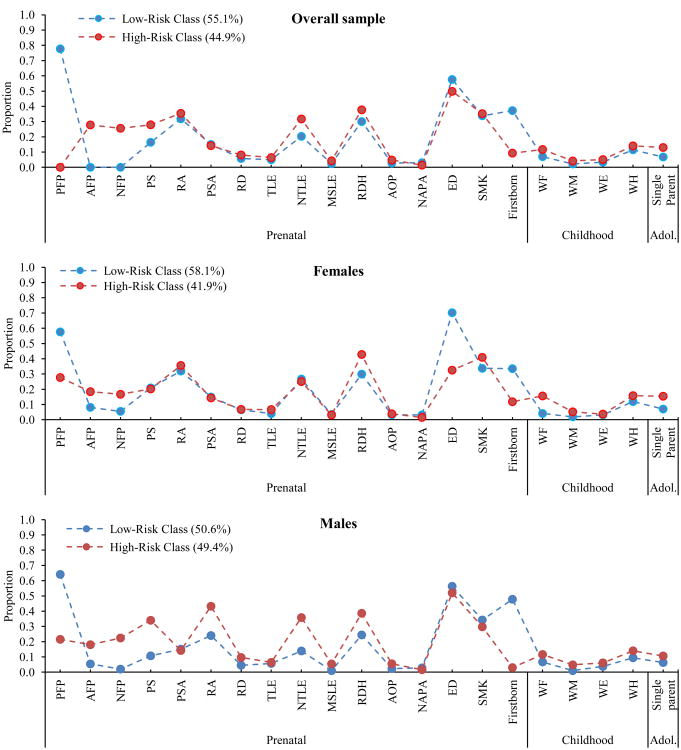
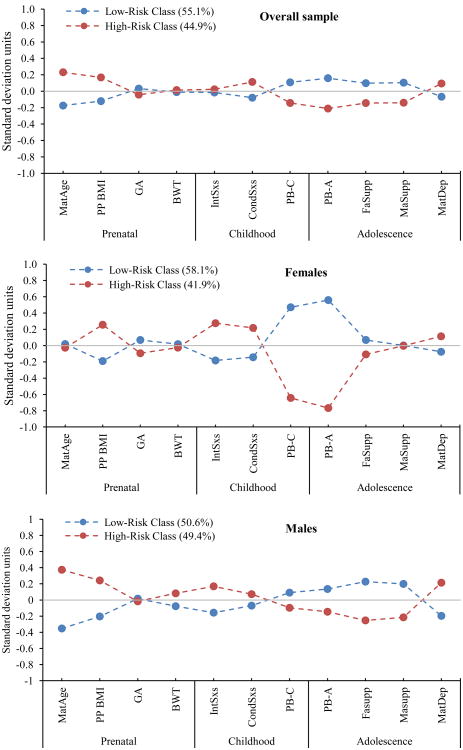
As Figures 1 and 2 show, the two-class model for the overall sample identified two subgroups that we have characterized as “low risk” (n = 943) and “high risk” (n = 768) groups. As compared to the low risk group, the high risk group was characterized by mothers with ambivalent or negative feelings about the pregnancy, mothers with an absence of positive feelings about the pregnancy, high maternal age at pregnancy, high pre-pregnancy BMI, and elevated maternal depressive symptoms during offspring adolescence. Further, offspring were characterized by elevated conduct symptoms in childhood, low offspring Peabody scores in childhood and adolescence, low perceived maternal and paternal support during, and a low probability of being firstborn. Post hoc analyses indicated that the high risk group had significantly higher levels of adolescent depressive symptom scores than the low risk group (X2(1) = 9.78, p = .002, Mhigh = 23.91, SEhigh = 0.22, Mlow = 22.95, SElow = 0.18, Cohen's d = 0.165). Further, offspring in the high risk group were 1.4 times more likely to have adolescent depressive symptom scores above the 75th percentile for the sample (X2(1) = 6.23, p = .013, OR = 1.41). The two risk groups did not differ significantly by sex.
Figure 1. Proportion of individuals in each latent class with presence (vs. absence) of each dichotomous variable by class.
Note. All variables refer to characteristics of the mother. Prenatal period: PFP = Positive Feelings about Pregnancy; AFP = Ambivalent Feelings about Pregnancy; NFP = Negative Feelings about Pregnancy; PS = Perceived Stress; RA = Reported Anxiety; PSA = Pregnancy-Specific Anxiety; RD = Reported Depression; TLE = Traumatic Life Events; NTLE = Non-traumatic Life Events; MSLE = Medical Self Life Events; RDH = Routine Daily Hassles; AOP = Ailments of Pregnancy; NAPA = Negative Affect About Pregnancy Ailments; ED = More than high school education; SMK = Maternal smoking during pregnancy. Childhood: WF = Worry about Finances; WM = Worry about Marriage; WE = Worry about Employment; WH = Worry about Health. Adolescence: Single parent = Single parent home.
Figure 2. Standardized means of each continuous variable by class.
Note. Prenatal period (Maternal) variables: MatAge = Maternal age at birth; BMI = Pre-pregnancy BMI; GA = Gestational Age; BWT = Birth weight. Childhood (Offspring variables): IntSxs = Childhood Internalizing symptoms; CondSxs = Childhood Conduct symptoms; PB-C = Childhood Peabody Score. Adolescent (Offspring variables): PB-A = Adolescent Peabody Score; FaSupp = Perceived paternal support; MaSupp = Perceived maternal support; MatDep = Maternal depression during offspring adolescence.
Sex-specific LCA
LCA statistics for females (n = 852) and males (n = 859) are displayed in Online Supplementary Table 2. For females, although the four-class model minimized the BIC, the smallest class in that model comprised only 3.2% of the sample, suggesting that the model was overfit to the sample. Statistical indices (i.e., lower BIC and significant BLRT) indicated that the three-class model for females was a better fit than the two-class model (see Online Supplementary Table 2); however, the three-class model added a class that seemed to track closely with the larger class from the two-class model, particularly among maternal pregnancy stress characteristics, and the decrease in BIC between the two- and three-class models was marginal. The three-class model had higher entropy compared to the two-class model (0.723 vs. 0.654), indicating better precision of individuals being placed into latent classes, but the posterior probabilities were similar for the two-class (range: 0.912 to 0.873) and three-class models (range: 0.931 to 0.843), indicating similar class separation in each. Compared to the three-class model, the two-class model produced not only substantively distinct but also conceptually meaningful classes; therefore this model was used.
As Figures 1 and 2 show, the two-class model for the females identified a low risk (n = 495) and a high risk (n = 357) group. The female high risk group was characterized by increased probability of the mother reporting ambivalent or negative feelings about the pregnancy, low probability of the mother reporting positive feelings about the pregnancy, high pre-pregnancy BMI, elevated offspring internalizing and conduct symptoms in childhood, low offspring Peabody scores in childhood and adolescence, low perceived maternal and paternal support as perceived by their offspring during adolescence; and a low probability of offspring being firstborn. Post hoc analyses indicated that high risk females had significantly higher levels of adolescent depressive symptom scores than the low risk females, X2(1) = 7.91, p = .005, Mhigh = 24.33, SEhigh = 0.33, Mlow = 23.018, SElow = 0.26, Cohen's d = 0.22. Further, females in the high risk group were 1.8 times more likely to have adolescent depressive symptom scores above the 75th percentile for the sample, X2(1) = 8.05, p = .005, OR = 1.81.
For males, the three-class model minimized the BIC and had a significant BLRT, indicating that the three-class model was the best statistical fit. Also, the three-class model had higher entropy compared to the two-class model (0.644 vs. 0.578), indicating better precision of individuals being placed into latent classes, and the posterior probabilities were similar for the two-class (range: 0.881 to 0.861) and three-class models (range: 0.869 to 0.819), indicating clearer class separation in the three-class model. However, the three-class model added a class that was qualitatively similar, particularly on pregnancy stress indicators, to the low risk group in the two-class model, and the decrease in BIC between the two- and three-class models was marginal. Because the two-class model produced classes that were substantively distinct and conceptually meaningful, this model was used for males in the sample.
The high risk male group (n = 424) was characterized by increased probability of the mother reporting ambivalent or negative feelings about the pregnancy; low probability of the mother reporting positive feelings about the pregnancy, high pre-pregnancy BMI, increased probability of the mother reporting stress, anxiety, and non-traumatic life events during pregnancy, elevated offspring internalizing symptoms in childhood, low offspring Peabody scores, particularly in adolescence, low maternal and paternal support as perceived by their offspring during adolescence, elevated probability of maternal depression during offspring adolescence, and a low probability of offspring being firstborn. Post hoc analyses indicated that high risk males had significantly higher levels of adolescent depressive symptom scores than the low risk (n = 435) males (X2(1) = 8.54, p = .003, Mhigh = 23.92, SEhigh = 0.33, Mlow = 22.49, SElow = 0.28, Cohen's d = 0.23). Further, males in the high risk group were 2.8 times more likely to have adolescent depressive symptom scores above the 75th percentile for the sample (X2(1) = 4.77, p = .031, OR = 2.81).
Auxiliary analyses showed that risk classes in the overall sample, in females, and in males did not significantly differ by interview version (i.e., whether or not specified probes were included). Risk classes differed on mean word count (natural log transformed; raw word count range: 1-287) of the maternal pregnancy narratives in the overall sample ((X2(1) = 69.08, p < .001, Cohen's d = 0.44).
Discussion
The present study is the first (to our knowledge) to identify distinct profiles of individuals at increased risk for depression in adolescence, characterized by known developmental risk factors for depression, while including maternal stress indicators during pregnancy. LCA identified a high-risk group in the overall sample and within males and females that were characterized by factors that have been associated with increased risk for psychopathology, with more maternal negativity/less maternal positivity about the pregnancy (McNeil et al., 2009), more routine stressors during pregnancy (Fineberg et al., 2016), high pre-pregnancy BMI (Abenhaim et al., 2007), low probability of being firstborn (e.g., Gates et al., 1988), decreased offspring cognitive functioning during childhood and adolescence (Koenen et al., 2009), and elevated levels of maternal depression during adolescence (Murray et al., 2011) common to all high-risk groups. Offspring in both the male and female high-risk groups were characterized by elevated internalizing symptoms in childhood (Rosenbaum et al., 1993), although this difference did not manifest in the overall sample. High-risk profiles also showed different patterns by sex. The male high-risk group was additionally characterized by greater likelihood of the mother reporting perceived stress, anxiety, and non-traumatic life events during pregnancy, and decreased levels of perceived paternal and maternal support during offspring adolescence (Kaslow, Deering, & Racusin, 1994); while the female high-risk profile was additionally characterized by offspring with elevated conduct symptoms during childhood (Lahey et al., 2002). All high-risk groups had significantly higher levels of depressive symptoms during adolescence and a higher probability of having adolescent depressive scores above the 75th percentile, relative to the low-risk groups, with small effects for each. Our findings suggest that examining multiple indicators across domains and developmental periods may help to identify a subset of individuals who are at risk for future depression, and that while the profiles are similar for males and females, the predictive roles of some risk factors vary by sex. It is important to note that unlike previous studies (e.g., Betts et al., 2014), there were no main effects of maternal stress during pregnancy, evidenced by the lack of significant zero-order correlations between maternal stress during pregnancy and adolescent depression (see Online Supplementary Table 5), though one pregnancy theme appeared to be operating through childhood conduct problems to influence risk of depression in the overall sample (see Supplementary Figure 2). Rather, findings from the present study are likely linked to multiple development factors. As such, analyses such as a mediational model were not appropriate; however, future studies using alternative analytic techniques are necessary to help clarify the interlinked nature of developmental risk factors for adolescent depression.
To date, few studies have examined the association between prenatal maternal stress and offspring depression during adolescence in a longitudinal birth cohort (Betts et al., 2014; Brown et al., 2000; Watson et al., 1999). Many of these studies aimed to disentangle pre- and postnatal influences (e.g., Betts et al., 2014; Pearson et al., 2013) to determine which developmental periods were most influential in conferring risk of offspring psychopathology. We sought instead to characterize profiles of offspring development from the prenatal to adolescent period. One major contribution of our findings is an understanding of how different risk factors may operate in concert to predict adolescent depression, and how these risk factors may differ depending on sex. Linking these risk factors across development is the first step in developing strategies for early identification and prevention efforts. Although not directly tested, a dynamic cascade model of adolescent depression, in which earlier occurring events underpin a “cascade” of incremental factors contributing to larger effects over time, may help explain our findings. For example, some evidence suggests that stress during pregnancy may “prime” the fetus to experience more fearful temperaments and anxiety risk throughout development (Davis & Sandman, 2012; Sandman et al., 2013; Talge et al., 2007). Increased internalization symptoms throughout childhood may lead to poorer academic performance and cognitive functioning over time (Koenen et al. 2009; Slykerman et al., 2005; van den Bergh et al., 2006), potentially contributing to transactional processes between parent and child (less perceived parental support, increased maternal depression) that increase risk for adolescent depression (Murray et al. 2011; Stice et al. 2004). Further, research suggests that pre-pregnancy BMI is associated with a number of adverse outcomes during pregnancy, including higher levels of inflammation, which could be increased by increases in maternal stress (Abenhaim et al., 2007; Coussons-Read, Okun, Schmitt, & Giese, 2005; Madan et al., 2009). Maternal inflammation during pregnancy has been associated with a number of adverse outcomes in offspring (Fineberg & Ellman, 2013), suggesting that neurodevelopment could be disrupted early on by a number of pre- and perinatal risk factors that were either directly tested in the present study or associated with study variables.
Some of these processes appeared to be more subtle for males, as high-risk males exhibited fewer signs of difficulties during childhood, despite having similarly high risk for depression during adolescence. One possible explanation is that the high risk males may have had a higher genetic or familial loading for depression than the high risk females and/or that the prenatal period may be developmentally important for males, as previous reports indicate the males are more vulnerable to maternal stress during pregnancy. Specifically, mothers of high risk males reported more stress, anxiety, and non-traumatic life events during pregnancy compared to mothers of low risk males (a pattern not observed among females). Similarly, mothers of high risk males reported high levels of depression during adolescence coupled with adolescent reports of low perceived maternal support compared to low risk males. The possibility that the high risk developmental profile among males reflects potential genetic contributions and/or more sensitivity of males to maternal stress during the prenatal period should be followed up by future studies.
While our analyses targeted variables that have been primarily associated with prenatal stress, depression, and obstetric risk factors related to later psychopathology, we did not consider other potential risk factors for adolescent depression (e.g., peer factors, adolescent health behaviors) due to lack of available data. Information about paternal depression and/or genetic variations associated with depression was unavailable, but we attempted to account for some measure of genetic risk by including maternal depressive symptoms during offspring adolescence in our modeling. However, maternal depressive symptoms during offspring adolescence likely tap into both risk conferred by genetics and risk conferred by exposure to parental psychopathology, and, therefore, future studies should assess how genetic risk, combined with other developmental risk factors, contributes to the etiology of adolescent depression. Nevertheless, results from the present study may be a first step in developing algorithms to identify individuals at risk for depression prior to the onset of symptomatology, which can have major implications for early intervention and prevention strategies. For instance, King and colleagues (2008) emphasize that estimating overall risk across a range of possible factors plays an essential role in depression prevention efforts.
While we have interpreted our findings as identifying risk profiles for adolescent depression, the high comorbidity of depression and anxiety calls into question whether these risk profiles are specific to depression or whether they indicate risk for a broader category of disorders, potentially with shared behavioral phenotypes. For instance, Clauss and Blackford (2012) have found that childhood inhibition confers a sevenfold increased risk for developing social anxiety, and Davis & Sandman (2012) have found that prenatal stress increases risk for developing anxiety. Further, previous work has found that prenatal maternal stress increases risk for schizophrenia spectrum disorders among males but not females (Ellman et al., 2008; Fineberg et al., 2016), which was not assessed in the present study. Although there is a vast literature that characterizes risk factors and sex differences in rates and course of mood disorders, as well as a substantial body of work on the influences of prenatal maternal stress, the question of whether there are sex-dependent routes to different kinds of psychopathology has been underexplored and should be examined in future work. Of the studies that have examined maternal psychological distress during pregnancy in relation to adolescent depression (Betts et al., 2014; Pawlby et al., 2009; Pearson et al., 2013), few, if any, have specifically examined sex differences. In keeping with a growing literature on sex differences in the influence of maternal stress on offspring development (Sandman et al., 2013) and in the depression literature (Alloy & Abramson, 2007), we identified differences between the subgroups of males and females who may be particularly at risk for developing a depressive disorder and/or significant or potentially impairing symptoms. The high-risk groups do not necessarily represent a clinical sample, and the magnitudes of the differences in depressive symptom scores between the high- and low-risk groups appeared to be small. Nevertheless, results held for analyses that used 75th percentile cutoff scores for our depression scale, which has been validated against future diagnoses of depression, indicating that our findings may have clinical relevance. Our results are consistent with previous work indicating that decreased IQ commonly occurs in the premorbid period of depression (Koenen et al., 2009; Zammit et al., 2004). Our results also are consistent with an emerging body of evidence suggesting that prenatal stress may exert a subtle but persistent influence on development, influencing temperament, behavior, and brain development in ways that may predispose individuals to later affective problems (Sandman et al., 2013).
LCA results suggest that some stress-related themes appeared to be less influential indicators of class membership than others. For both males and females, these themes included traumatic life events, ailments of pregnancy, medical life events, and negative affect about pregnancy ailments, all of which occurred at low base rates in our sample (2.3-5.6%). Although the sample size was relatively large, traumatic life events may have been too rare to detect significant results, without access to population-level data (Abel et al., 2014). However, given the prevalence of the other stress themes, it seems unlikely that we were underpowered to detect results, and instead, the lack of findings may suggest that these stress-related themes may not have the same public health impact as other aspects of stress during pregnancy. Maternal reports of depressive symptoms during pregnancy also occurred at a low base rate in our sample (6.8%), which may stem from the wording of the interview questions related to recent worries, potentially diminishing spontaneous reports of depressive symptoms. Future research should continue to investigate the contribution of prenatal maternal depressive symptoms to risk for offspring depression in adolescence, as two recent studies have found support for an increased risk of adolescent depression among offspring of mothers who experienced depressive symptoms during pregnancy (Betts et al., 2014; Pearson et al., 2013). Although there is growing support for a link between pregnancy-specific anxiety and a variety of adverse developmental outcomes (reviewed in Buss et al., 2011), our results did not identify pregnancy-specific anxiety as an important indicator of high risk class membership or as predictive of offspring depressive symptoms. Although our coding of pregnancy-specific anxiety was based on a reliable, 10-item pregnancy-related anxiety scale developed for use in pregnancy research (Rini et al., 1999), it is possible that the full measure would more accurately capture women's experiences of pregnancy-specific anxiety. It should be noted that while the risk groupings did not differ by interview version (i.e., whether specified probes about stress, anxiety, etc., were included in the maternal pregnancy interview), the risk groups in the overall sample were differentiated by verbosity of the mother in the interview, as measured by word count. However, it stands to reason that mothers who endorsed pregnancy stress themes would have higher word counts.
Although maternal smoking during pregnancy did not differentiate subgroups, it is important to note that the frequency of smoking during pregnancy was higher in the 1960s when these pregnancies occurred (34.4% of mothers in the study sample smoked during some or all of pregnancy). Present day cohorts might detect an effect of prenatal maternal smoking on adolescent depression, as women who smoke during pregnancy despite health warnings may differ in important ways from women who smoked during pregnancy prior to these warnings. Another possibility is that maternal smoking during pregnancy may be more related to other types of offspring psychopathology, such as externalizing problems (Rodriguez & Bohlin, 2005).
A major strength of our study is that the cohort includes continuous follow-up data on childhood and contextual factors throughout multiple periods of development, which is relatively rare, even in the prenatal stress literature. Further, data on different aspects of maternal stress during pregnancy were collected directly and prospectively from women during their pregnancies. Even so, unavailability of data on additional contextual and resilience factors meant that important variables that may have influenced our findings (e.g., detailed information on caregiving during childhood, etc.) could not be examined. Another important limitation to note is that much of the data used to characterize the risk profiles and the data used to characterize adolescent depressive symptoms were obtained via self-report (with some exceptions, e.g., cognitive testing, BMI, birth order, maternal age, gestational age, birth weight etc.). While self-reported data can be problematic (Gerald & George, 2010), we were able to corroborate adolescent self-reported depression with maternal report of the same construct (e.g., maternal report of adolescent happiness was significantly related to adolescent self-report of depressive symptoms; See Method). An additional complication in analyzing self-report data is created by missing values, which occurred most acutely in our data for childhood internalizing and conduct symptoms. If any of the underlying scale items were missing (most often because the mother answered “uncertain” to an item), that scale score was considered missing. While FIML is robust even at high levels of missingness, especially when auxiliary correlates are included in the analysis (Graham, 2009), any inferences to be drawn from our study about the roles of internalizing and conduct symptoms during childhood could be limited by the amount of missing data for those variables. Another potential limitation of the study is that qualitative coding of narrative themes carries the possibility of introducing subjectivity into the data. We attempted to safeguard against potential bias in coding by requiring coders to: follow a detailed coding manual, achieve high inter-rater reliability on representative narratives prior to coding study narratives, remain blind to offspring depressive symptoms, and maintain reliability throughout the coding process. Additionally, other studies have analyzed stress-related themes from open-ended narratives, providing support for the feasibility of our methods (Duggan, Albright, & Lequerica, 2008). A limitation of the LCA approach is that we are not able to draw conclusions about the relative contributions of pre- and postnatal factors to any association between high-risk subclasses and subsequent depressive symptoms; as such, this current study must be viewed as exploratory. Future studies should use a variable-centered analytic approach to determine whether specific variables and/or periods of development are particularly important in conferring risk for the development of depression in adolescence. Another limitation of the current study is that we could not determine exact timing of prenatal stress exposure; however, the majority of our interviews took place during second trimester of pregnancy, which previous work suggests is a key period for the influence of maternal stress on fetal development (Ellman et al., 2008).
The present study's measure of adolescent depressive symptoms had both strengths and limitations. The scale had good reliability and excellent predictive validity. Additionally, the scale taps into many of the themes associated with a depressogenic cognitive style (e.g., inadequacy, worthlessness, failure; Alloy et al., 1999). However, the scale is limited by a lack of items assessing other symptoms of depression (e.g., sleep, appetite). Other limitations of the study are shared by many longitudinal studies, including potential bias due to attrition (though the use of FIML somewhat reduces this concern) and cohort effects. Despite the continual follow up of nearly 2,000 birth cohort members of the CHDS through adolescence, an important caveat to consider when interpreting the generalizability of study results is that the adolescent sample represents only a portion of the original cohort and differed from that original cohort on some key variables (though the magnitudes of the differences were small). It is a limitation of the study that we could not compare our analytic sample to the entire cohort on all variables in the LCA, given that many in the overall cohort did not complete these assessments (see Online Supplementary Table 1, for available comparative demographics between the analytic sample and the remainder of the CHDS cohort). Further, our sample developed within a distinct sociocultural environment (the San Francisco Bay Area during the 1960s and 1970s), which may limit generalizability to present day cohorts with different demographic and cultural (e.g., parenting practices, mental health literacy) characteristics.
This study is among the first, to our knowledge, to examine maternal stress during pregnancy and postnatal influences in relation to adolescent depressive symptoms in a prospective, longitudinal, population-based design. Our findings characterize the developmental profiles of subgroups of adolescent males and females who exhibit increased depressive symptoms following exposure to prenatal maternal stress and other postnatal influences, and underscore the importance of considering pre- and postnatal contributors to offspring risk for psychopathology. Future research should continue to characterize developmental profiles from prenatal maternal stress to offspring psychopathology in an effort to better understand sex differences in risk factors and disorder onset and course. Our findings also highlight potential targets for early intervention that include both distal and proximal variables, which may allow clinicians to combine current and past information to target individuals who are most at risk during adolescence. Finally, the results from the present study support the necessity of taking a lifespan approach to studying risk for psychopathology, as multiple indicators across development were associated with risk for future depression.
Supplementary Material
Supplementary Figure 1. Study Timeline
Supplementary Figure 2. Path Analysis of Predictor Variables that were Significantly Correlated with Key Offspring Intermediary Variables and/or with Offspring Adolescent Depressive Symptoms
Supplementary Table 1. Comparative Demographic Characteristics for Pregnancy Interview Sample and CHDS Not in Analytic Sample (Total n = 19,044)
Supplementary Table 2. Examples of Stress Coding
Supplementary Table 3. Scales Assessing Offspring Childhood Internalizing and Conduct Symptoms; and during Ofspring Adolescence, Offspring and Maternal Depressive Symptoms and Offspring Perceived Parental Support
Supplementary Table 4. Class Models
Supplementary Table 5. Zero-order correlations among LCA indicator variables and depression variables.
Acknowledgments
The authors thank Barbara A. Cohn, Piera Cirillo, Lauren Zimmermann, and Nickilou Y. Krigbaum, our collaborators at the Child Health and Development Studies, for their thoughtful contributions to the manuscript.
Funding: This study was supported by funding to LME (R01 MH096478, Temple University start-up award, Schizophrenia research fellowship – 5T32 MH018870-20) and AMF (National Science Foundation Graduate Research Fellowship awarded to AMF – DGE-1144462). This study was also supported by the Department of Health and Human Services Contract HHSN275201100020C from the Eunice Kennedy Shriver National Institute of Child Health and Development, National Institutes of Health. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the author (s) and do not necessarily reflect the views of the National Science Foundation.
Footnotes
Conflicts of Interest: The authors declare that they have no conflict of interest.
Ethical approval: “All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Informed consent: “Informed consent was obtained from all individual participants included in the study.”
References
- Abel KM, Heuvelman HP, Jorgensen L, Magnusson C, Wicks S, Susser E, et al. Dalman C. Severe bereavement stress during the prenatal and childhood periods and risk of psychosis in later life: Population based cohort study. BMJ. 2014;348(f7679) doi: 10.1136/bmj.f7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abenhaim HA, Kinch RA, Morin L, Benjamin A, Usher R. Effect of prepregnancy body mass index categories on obstetrical and neonatal outcomes. Archives of Gynecology and Obstetrics. 2007;275:39–43. doi: 10.1007/s00404-006-0219-y. [DOI] [PubMed] [Google Scholar]
- Achenbach TM. Integrative guide for the 1991 CBCL/4-18, YSR, and TRF profiles. Burlington, VT: Department of Psychiatry, University of Vermont; 1991. [Google Scholar]
- Agnafors S, Comasco E, Bladh M, Sydsjö G, DeKeyser L, Oreland L, Svedin CG. Effect of gene, environment and maternal depressive symptoms on pre-adolescence behavior problems: A longitudinal study. Child and Adolescent Psychiatry and Mental Health. 2013;7:1–9. doi: 10.1186/1753-2000-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnafors S, Sydsjö G, Comasco E, Bladh M, Oreland L, Svedin CG. Early predictors of behavioural problems in pre-schoolers: A longitudinal study of constitutional and environmental main and interaction effects. BMC Pediatrics. 2016;16:1–11. doi: 10.1186/s12887-016-0614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY. The adolescent surge in depression and emergence of gender differences: A biocognitive vulnerability-stress model in developmental context. D. In: Romer D, Walker EF, editors. Adolescent psychopathology and the developing brain: Integrating brain and prevention science. New York: Oxford University Press; 2007. pp. 284–312. [Google Scholar]
- Alloy LB, Abramson LY, Whitehouse WG, Hogan ME, Tashman NA, L Steinberg D, et al. Donovan P. Depressogenic cognitive styles: Predictive validity, information processing and personality characteristics, and developmental origins. Behaviour Research and Therapy. 1999;37:503–531. doi: 10.1016/S0005-7967(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Amato PR. The impact of family formation change on the cognitive, social, and emotional well-being of the next generation. The Future of Children / Center for the Future of Children, the David and Lucile Packard Foundation. 2005;15:75–96. doi: 10.1353/foc.2005.0012. [DOI] [PubMed] [Google Scholar]
- Betts KS, Williams GM, Najman JM, Alati R. Maternal depressive, anxious, and stress symptoms during pregnancy predict internalizing problems in adolescence. Depression and Anxiety. 2014;31:9–18. doi: 10.1002/da.22210. [DOI] [PubMed] [Google Scholar]
- Betts KS, Williams GM, Najman JM, Alati R. The relationship between maternal depressive, anxious, and stress symptoms during pregnancy and adult offspring behavioral and emotional problems. Depression and Anxiety. 2015;32:82–90. doi: 10.1002/da.22272. [DOI] [PubMed] [Google Scholar]
- Braithwaite EC, Murphy SE, Ramchandani PG. Prenatal risk factors for depression: A critical review of the evidence and potential mechanisms. Journal of Developmental Origins of Health and Disease. 2014;5:339–350. doi: 10.1017/S2040174414000324. [DOI] [PubMed] [Google Scholar]
- Brown AS, van Os J, Driessens C, Hoek HW, Susser ES. Further evidence of relation between prenatal famine and major affective disorder. The American Journal of Psychiatry. 2000;157:190–5. doi: 10.1176/appi.ajp.157.2.190. [DOI] [PubMed] [Google Scholar]
- Buss C, Davis EP, Hobel CJ, Sandman CA. Maternal pregnancy-specific anxiety is associated with child executive function at 6-9 years age. Stress (Amsterdam, Netherlands) 2011;14:665–76. doi: 10.3109/10253890.2011.623250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental psychopathology. Development and Psychopathology. 1996;8:597–600. [Google Scholar]
- Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: A meta-analytic study. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51:1066–1075. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary-Goldman J, Malone FD, Vidaver J, Ball RH, Nyberg Da, Comstock CH, et al. D'Alton M. Impact of maternal age on obstetric outcome. Obstetrics and Gynecology. 2005;105:983–990. doi: 10.1097/01.aog.0000158118.75532.51. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Cohen S, Kessler RC, Gordon LU. Measuring stress: A guide for health and social scientists. New York: Oxford University Press; 1997. [Google Scholar]
- Coussons-Read ME, Okun ML, Schmitt MP, Giese S. Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosomatic Medicine. 2005;67:625–631. doi: 10.1097/01.psy.0000170331.74960.ad. [DOI] [PubMed] [Google Scholar]
- Da Costa D, Brender W, Larouche J. A prospective study of the impact of psychosocial and lifestyle variables on pregnancy complications. Journal of Psychosomatic Obstetrics & Gynecology. 1998;19:28–37. doi: 10.3109/01674829809044218. [DOI] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology. 2012;37:1224–33. doi: 10.1016/j.psyneuen.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan CH, Albright KJ, Lequerica A. Using the ICF to code and analyse women's disability narratives. Disability and Rehabilitation. 2008;30:978–90. doi: 10.1080/09638280701797549. [DOI] [PubMed] [Google Scholar]
- Ellman LM, Schetter CD, Hobel CJ, Chicz-Demet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: Effects on newborn physical and neuromuscular maturation. Developmental Psychobiology. 2008;50:232–41. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD. Prenatal stress, development, health and disease risk: A psychobiological perspective—2015 Curt Richter Award Paper. Psychoneuroendocrinology. 2015;62:366–375. doi: 10.1016/j.psyneuen.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg AM, Ellman LM. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biological Psychiatry. 2013;73:951–966. doi: 10.1016/j.biopsych.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg AM, Ellman LM, Maxwell SD, Chaudhury N, Cook AL, Schaefer CA, et al. Brown AS. Fetal exposure to maternal stress and risk for schizophrenia among offspring: Differential influences of fetal sex. Psychiatry Research. 2016;236:91–97. doi: 10.1016/j.psychres.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for Axis I DSM-IV disorders. New York: Biometrics Research; 1994. [Google Scholar]
- Fletcher JM. Adolescent depression: Diagnosis, treatment, and educational attainment. Health Economics. 2008;17:1215–1235. doi: 10.1002/hec.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale CR, Martyn CN. Birth weight and later risk of depression in a national birth cohort. The British Journal of Psychiatry: The Journal of Mental Science. 2004;184:28–33. doi: 10.1192/bjp.184.1.28. [DOI] [PubMed] [Google Scholar]
- Gates L, Lineberger MR, Crockett J, Hubbard J. Birth order and its relationship to depression, anxiety, and self-concept test scores in children. The Journal of Genetic Psychology. 1988;149:29–34. doi: 10.1080/00221325.1988.10532136. [DOI] [PubMed] [Google Scholar]
- Gerald JH, George SH. Self-report: Psychology's four-letter word. American Journal of Psychology. 2010;123:181–188. doi: 10.5406/amerjpsyc.123.2.0181. [DOI] [PubMed] [Google Scholar]
- Graham JW. Missing data analysis: Making it work in the real world. Annual Review of Psychology. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: An elaborated cognitive vulnerability-transactional stress theory. Psychological Bulletin. 2001;127:773–796. doi: 10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- Harrington R, Fudge H, Rutter M, Pickles A, Hill J. Adult outcomes of childhood and adolescent depression: I. Psychiatric status. Archives of General Psychiatry. 1990;47:465–473. doi: 10.1001/archpsyc.1990.01810170065010. [DOI] [PubMed] [Google Scholar]
- Haukka JK, Suvisaari J, Lönnqvist J. Family structure and risk factors for schizophrenia: Case-sibling study. BMC Psychiatry. 2004;4:1. doi: 10.1186/1471-244X-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman DB, Brown AS, Opler MG, Desai M, Malaspina D, Bresnahan M, et al. Susser ES. Does unwantedness of pregnancy predict schizophrenia in the offspring? Social Psychiatry and Psychiatric Epidemiology. 2006;41:605–610. doi: 10.1007/s00127-006-0078-7. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Robles De Medina PG, Mulder EJH, Visser GHA, Buitelaar JK. Psychological measures of prenatal stress as predictors of infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:1078–1085. doi: 10.1097/00004583-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Robles de Medina PG, Mulder EJH, Visser GHA, Buitelaar JK. Stress during pregnancy is associated with developmental outcome in infancy. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2003;44:810–8. doi: 10.1111/1469-7610.00166. [DOI] [PubMed] [Google Scholar]
- Kaslow N, Deering G, Racusin GR. Depressed children and their families. Clinical Psychology Review. 1994;14:39–59. [Google Scholar]
- Keyes KM, Keyes MA, March D, Susser E. Levels of risk: Maternal-, middle childhood-, and neighborhood-level predictors of adolescent disinhibitory behaviors from a longitudinal birth cohort in the United States. Mental Health and Substance Use. 2011;4:22–37. doi: 10.1080/17523281.2011.533445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, et al. Mortensen PB. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Archives of General Psychiatry. 2008;65:146–52. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R. Prior juvenile diagnoses in adults with mental disorder: Developmental follow-back of a prospective-longitudinal cohort. Archives of General Psychiatry. 2003;60:709–717. doi: 10.1001/archpsyc.60.7.709. [DOI] [PubMed] [Google Scholar]
- King M, Walker C, Levy G, Bottomley C, Royston P, Weich S, et al. Nazareth I. Development and validation of an international risk prediction algorithm for episodes of major depression in general practice attendees: The PredictD study. Archives of General Psychiatry. 2008;65:1368–1376. doi: 10.1001/archgenpsychiatry.2008.550. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Moffitt TE, Roberts AL, Martin LT, Kubzansky L, Harrington H, et al. Caspi A. Childhood IQ and adult mental disorders: A test of the cognitive reserve hypothesis. The American Journal of Psychiatry. 2009;166:50–7. doi: 10.1176/appi.ajp.2008.08030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N. Overcoming the absence of socioeconomic data in medical records: Validation and application of a census-based methodology. American Journal of Public Health. 1992;82:703–10. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Loeber R, Burke J, Rathouz PJ, McBurnett K. Waxing and waning in concert: Dynamic comorbidity of conduct disorder with other disruptive and emotional problems over 17 years among clinic-referred boys. Journal of Abnormal Psychology. 2002;111:556–567. doi: 10.1037//0021-843X.111.4.556. [DOI] [PubMed] [Google Scholar]
- Lanza ST, Tan X, Bray BC. Latent class analysis with distal outcomes: A flexible model-based approach. Structural Equation Modeling: A Multidisciplinary Journal. 2013;20:1–26. doi: 10.1080/10705511.2013.742377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. New York, NY: Oxford University Press; 2004. [Google Scholar]
- Loomans EM, van der Stelt O, van Eijsden M, Gemke RJBJ, Vrijkotte T, den Bergh BRHVan. Antenatal maternal anxiety is associated with problem behaviour at age five. Early Human Development. 2011;87:565–70. doi: 10.1016/j.earlhumdev.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Madan JC, Davis JM, Craig WY, Collins M, Allan W, Quinn R, Dammann O. Maternal obesity and markers of inflammation in pregnancy. Cytokine. 2009;47:61–64. doi: 10.1016/j.cyto.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Masyn KE, Henderson CE, Greenbaum PE. Exploring the latent structures of psychological constructs in social development using the dimensional-categorical spectrum. Social Development. 2010;19:470–493. doi: 10.1111/j.1467-9507.2009.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil TF, Schubert EW, Cantor-Graae E, Brossner M, Schubert P, Henriksson KM. Unwanted pregnancy as a risk factor for offspring schizophrenia-spectrum and affective disorders in adulthood: a prospective high-risk study. Psychological Medicine. 2009;39:957–65. doi: 10.1017/S0033291708004479. [DOI] [PubMed] [Google Scholar]
- Menezes AMB, Murray J, László M, Wehrmeister FC, Hallal PC, Gonçalves H, et al. Barros FC. Happiness and depression in adolescence after maternal smoking during pregnancy: Birth cohort study. PLoS ONE. 2013;8:e80370. doi: 10.1371/journal.pone.0080370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Arteche A, Fearon P, Halligan S, Goodyer I, Cooper P. Maternal postnatal depression and the development of depression in offspring up to 16 years of age. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:460–470. doi: 10.1016/j.jaac.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Muthén B, Muthén LK. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcoholism, Clinical and Experimental Research. 2000;24:882–891. doi: 10.1111/j.1530-0277.2000.tb02070.x. [DOI] [PubMed] [Google Scholar]
- Muthén BO, Muthén LK. Mplus Version 7: User's guide. Los Angeles, CA: Muthén & Muthén; 2012. [Google Scholar]
- Muthén B, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. 1999;55:463–469. doi: 10.1111/j.0006-341X.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Gender differences in depression. Current Directions in Psychological Science. 2001;10:173–176. [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Malaspina D. Diagnostic Interview for Genetic Studies. Archives of General Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Nylund K, Bellmore A, Nishina A, Graham S. Subtypes, severity, and structural stability of peer victimization: What does latent class analysis say? Child Development. 2007;78:1706–1722. doi: 10.1111/j.1467-8624.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling. 2007;14:535–569. doi: 10.1080/10705510701575396. [DOI] [Google Scholar]
- O'Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children's behavioural/emotional problems at 4 years: Report from the Avon Longitudinal Study of Parents and Children. The British Journal of Psychiatry. 2002;180:502–508. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- Parboosing R, Bao Y, Shen L, Schaefer CA, Brown AS. Gestational influenza and bipolar disorder in adult offspring. JAMA Psychiatry. 2013;70:677–685. doi: 10.1001/jamapsychiatry.2013.896. [DOI] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Olsson CA, Morley R. Prematurity at birth and adolescent depressive disorder. British Journal of Psychiatry. 2004;184:446–447. doi: 10.1192/bjp.184.5.446. [DOI] [PubMed] [Google Scholar]
- Pawlby S, Hay DF, Sharp D, Waters CS, O'Keane V. Antenatal depression predicts depression in adolescent offspring: Prospective longitudinal community-based study. Journal of Affective Disorders. 2009;113:236–243. doi: 10.1016/j.jad.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Pearson RM, Evans J, Kounali D, Lewis G, Heron J, Ramchandani PG, et al. Stein A. Maternal depression during pregnancy and the postnatal period. JAMA Psychiatry. 2013;70:1312. doi: 10.1001/jamapsychiatry.2013.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinelli M, Wilkinson G. Gender differences in depression. The British Journal of Psychiatry. 2000;177:486–492. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- Pritchard CW, Teo PYK. Preterm birth, low birthweight and the stressfulness of the household role for pregnant women. Social Science and Medicine. 1994;38:89–96. doi: 10.1016/0277-9536(94)90303-4. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rini CK, Dunkel-Schetter C, Wadhwa PD, Sandman CA. Psychological adaptation and birth outcomes: The role of personal resources, stress, and sociocultural context in pregnancy. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association. 1999;18:333–45. doi: 10.1037/0278-6133.18.4.333. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Bohlin G. Are maternal smoking and stress during pregnancy related to ADHD symptoms in children? Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2005;46:246–54. doi: 10.1111/j.1469-7610.2004.00359.x. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JF, Biederman J, Bolduc-Murphy EA, Faraone SV, Chaloff J, Hirshfeld DR, Kagan J. Behavioral inhibition in childhood: A risk factor for anxiety disorders. Harvard Review of Psychiatry. 1993;1:2–16. [Google Scholar]
- Sandman CA, Glynn LM, Davis EP. Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. Journal of Psychosomatic Research. 2013;75:327–35. doi: 10.1016/j.jpsychores.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. doi: 10.1037/1082-989X.7.2.147. [DOI] [PubMed] [Google Scholar]
- Schlotz W, Phillips DIW. Fetal origins of mental health: Evidence and mechanisms. Brain, Behavior, and Immunity. 2009;23:905–16. doi: 10.1016/j.bbi.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Serido J, Almeida DM, Wethington E. Chronic stressors and daily hassles: Unique and interactive relationships with psychological distress. Journal of Health and Social Behavior. 2004;45:17–33. doi: 10.1177/002214650404500102. [DOI] [PubMed] [Google Scholar]
- Shah PS, Balkhair T, Ohlsson A, Beyene J, Scott F, Frick C. Intention to become pregnant and low birth weight and preterm birth: A systematic review. Maternal and Child Health Journal. 2011;15:205–216. doi: 10.1007/s10995-009-0546-2. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Siegel LS. Reproductive, perinatal, and environmental factors as predictors of the cognitive and language development of preterm and full-term infants. Child Development. 1982;53:963–973. [PubMed] [Google Scholar]
- Slykerman RF, Thompson JMD, Pryor JE, Becroft DMO, Robinson E, Clark PM, et al. Mitchell EA. Maternal stress, social support and preschool children's intelligence. Early Human Development. 2005;81:815–21. doi: 10.1016/j.earlhumdev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Stice E, Ragan J, Randall P. Prospective relations between social support and depression: Differential direction of effects for parent and peer support? Journal of Abnormal Psychology. 2004;113:155–159. doi: 10.1037/0021-843X.113.1.155. [DOI] [PubMed] [Google Scholar]
- Stikkelbroek Y, Bodden DH, Deković M, van Baar AL. Effectiveness and cost effectiveness of cognitive behavioral therapy (CBT) in clinically depressed adolescents: Individual CBT versus treatment as usual (TAU) BMC Psychiatry. 2013;13:314. doi: 10.1186/1471-244X-13-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susser E, Buka S, Schaefer CA, Andrews H, Cirillo PM, Factor-Litvak P, et al. McKeague IW. The early determinants of adult health study. Journal of Developmental Origins of Health and Disease. 2011;2:311–321. doi: 10.1017/S2040174411000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talge NM, Neal C, Glover V the Early Stress, Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health. Antenatal maternal stress and long-term effects on child neurodevelopment: How and why? Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2007;48:245. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuddenham RD, Brooks J, Milkovich L. Mothers' reports of behavior of ten-year-olds: Relationships with sex, ethnicity, and mother's education. Developmental Psychology. 1974;10:959–995. doi: 10.1037/h0037260. [DOI] [Google Scholar]
- van den Berg BJ, Christianson RE, Oechsli FW. The California Child Health and Development Studies of the School of Public Health, University of California at Berkeley. Paediatric and Perinatal Epidemiology. 1988;2:265–282. doi: 10.1111/j.1365-3016.1988.tb00218.x. [DOI] [PubMed] [Google Scholar]
- van den Bergh BRH, Mennes M, Stevens V, van der Meere J, Börger N, Stiers P, et al. Lagae L. ADHD deficit as measured in adolescent boys with a continuous performance task is related to antenatal maternal anxiety. Pediatric Research. 2006;59:78–82. doi: 10.1203/01.pdr.0000191143.75673.52. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH, Van Calster B, Smits T, Van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: A prospective study on the fetal origins of depressed mood. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2008;33:536–45. doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- Watson JB, Mednick SA, Huttunen M, Wang X. Prenatal teratogens and the development of adult mental illness. Development and Psychopathology. 1999;11:457–66. doi: 10.1017/s0954579499002151. [DOI] [PubMed] [Google Scholar]
- Wickramaratne PJ, Greenwald S, Weissman MM. Psychiatric disorders in the relatives of probands with prepubertal-onset or adolescent-onset major depression. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:1396–1405. doi: 10.1097/00004583-200011000-00014. [DOI] [PubMed] [Google Scholar]
- Zammit S, Allebeck P, David AS, Dalman C, Hemmingsson T, Lundberg I, Lewis G. A longitudinal study of premorbid IQ Score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Archives of General Psychiatry. 2004;61:354–360. doi: 10.1001/archpsyc.61.4.354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Study Timeline
Supplementary Figure 2. Path Analysis of Predictor Variables that were Significantly Correlated with Key Offspring Intermediary Variables and/or with Offspring Adolescent Depressive Symptoms
Supplementary Table 1. Comparative Demographic Characteristics for Pregnancy Interview Sample and CHDS Not in Analytic Sample (Total n = 19,044)
Supplementary Table 2. Examples of Stress Coding
Supplementary Table 3. Scales Assessing Offspring Childhood Internalizing and Conduct Symptoms; and during Ofspring Adolescence, Offspring and Maternal Depressive Symptoms and Offspring Perceived Parental Support
Supplementary Table 4. Class Models
Supplementary Table 5. Zero-order correlations among LCA indicator variables and depression variables.