Abstract
Collagen IV is a major constituent of basement membranes, specialized form of extracellular matrix that provides a mechanical support for tissues, serves as a polyvalent ligand for cell adhesion receptors and as a scaffold for other proteins, and plays a key role in tissue genesis, differentiation, homeostasis, and remodeling. Collagen IV underlies the pathogenesis of several human disorders including Goodpasture’s disease, Alport’s syndrome, diabetic nephropathy, angiopathy, and porencephaly.
While the isolation of the collagen IV molecules from tissues is an ultimate prerequisite for structural and functional studies, it has been always hampered by the protein insolubility due to extensive intermolecular crosslinking and noncovalent associations with other components of basement membranes. In this chapter, we present methods for the isolation of collagen IV fragments from basement membranes or from extracellular matrix deposited by cultured cells, and the recombinant expression alternative. These methods are useful to address the fundamental questions on the role of collagen IV in tissue genesis under the normal and pathological conditions.
1 INTRODUCTION
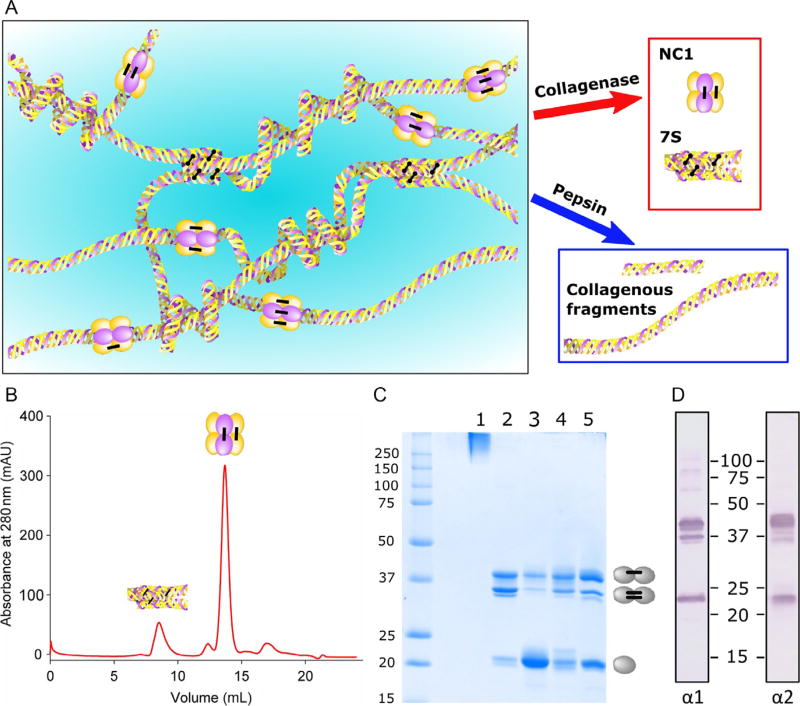
Basement membranes are specialized form of extracellular matrix that underlies epithelial, endothelial, muscle, fat, and nervous tissues and are essential for the development and maintenance of tissue architecture. They provide mechanical stability and regulate critical functions including cell growth, differentiation, morphogenesis, homeostasis, and regeneration. Collagen IV is a major constituent of basement membranes which forms a complex network and provides a tensile strength to underlying tissues and tethers diverse macromolecules, including laminins, proteoglycans, and growth factors and binds multiple cellular receptors including integrins. Defects in collagen IV network cause destabilization of basement membranes and impairment of tissue function (Fidler et al., 2014; Gupta, Graham, & Kramer, 1997; Poschl et al., 2004). Collagen IV is an ancient protein associated with the transition to multicellularity in animals (Fidler et al., 2017). In vertebrates, collagen IV molecule is a heterotrimer composed of limited combination of the six α-chains encoded by distinct genes, which are expressed in a tightly regulated temporal and tissue-specific manner. Intracellularly, three α-chains assembled together to form triple-helical protomer, a principal building block for supramolecular collagen IV networks in basement membrane. Upon secretion to the extracellular space, two collagen IV protomers associate via carboxyl terminal NC1 domains forming hexamer, while association of four hexamers through amino termini lead to the formation of 7S dodecamer. These associations are further stabilized by specific sulfilimine crosslinks between NC1 domains and disulfide and aldehyde crosslinks within 7S, which confer structural reinforcement to collagen IV networks (Anazco et al., 2016; Vanacore et al., 2009) (Fig. 1A).
FIG. 1.
Isolation of collagen IV domains from basement membrane. (A) Bacterial collagenase degrades triple-helical part of collagen IV network releasing 7S and NC1 domains, while limited pepsin digest releases collagenous domain. (B) Elution profile of collagenase digest of placental basement membrane (bPBM) from the Superdex S200 column. (C) SDS-PAGE of 7S (lane 1) and NC1 hexamers form bPBM (lane 2), bLBM (lane 3), bGBM (lane 4), and PFHR9 cells (lane 5). Dimers are stabilized by one or two sulfilimine crosslinks resulting in a two major bands on the gel. (D) Western blot of bPBM hexamer developed with α1NC1- and α2NC1-specific monoclonal antibody.
Collagen IV plays an important role in the cells interaction with underlying basement membranes. Indeed, numerous studies have shown that collagen IV promotes the adhesion, activates migration, and stimulates proliferation of different cell types. Many of these responses are mediated by interaction of specific integrins, namely α1β1 and α2β1, with the central triple-helical domain of collagen IV protomers (Khoshnoodi, Pedchenko, & Hudson, 2008; Leitinger & Hohenester, 2007). However, the precise location and molecular structure of integrin binding sites is still unknown due to the significant heterogeneity of heterotrimeric collagen IV fragments that could be isolated from natural sources.
Early studies of the structure of collagen IV have relied extensively upon the use of proteolytic enzymes for the excision of individual domains from the insoluble matrix of authentic basement membranes (Fig. 1A). Bacterial collagenase destroys the triple-helical domain releasing NC1 and 7S domain with retention of their quaternary structures. In contrast, pepsin excises fragments of the collagenous domain with retention of triple-helical structure, but destroys the NC1 domain. More recently, this classical methodology was complemented with the expression of recombinant collagen IV fragments. These techniques have been useful for solving the crystal structure of collagen IV NC1 hexamers, discovery of the novel sulfilimine bond stabilizing its structure, and the principal role of the chloride ion for assembly of collagen IV networks (Cummings et al., 2016; Sundaramoorthy, Meiyappan, Todd, & Hudson, 2002; Vanacore et al., 2009).
2 ISOLATION OF 7S AND NC1 DOMAINS OF COLLAGEN IV FROM SOLID TISSUES
This section describes a basic method for the purification of collagen IV 7S and NC1 domains from bovine placental basement membrane (bPBM), which is based on selective proteolytic degradation of the triple-helical part of collagen IV networks by the purified bacterial collagenase. With minor modifications, it could be used for the isolation of these collagen IV fragments from bovine basement membranes of eye lens (bLBM), kidney glomeruli (bGBM), lung alveoli, seminiferous tubules of testis (bSTBM), and aorta (Gunwar et al., 1991; Kahsai et al., 1997; Langeveld et al., 1988; Reddy, Gunwar, Kalluri, Hudson, & Noelken, 1993). For example, purification of bGBM requires prior isolation of glomeruli from kidney cortex using differential sieving (Freytag, Ohno, & Hudson, 1978), while for the preparation of bLBM from the anterior lens capsule brief sonication is required (Langeveld et al., 1988). Noteworthy, NC1 hexamers from various basement membranes differ significantly by composition and degree of crosslinking. While NC1 hexamers from placenta consists from predominantly crosslinked α1 and α2 NC1 subunits forming dimers, bLBM hexamer is significantly enriched in NC1 monomers. In contrast, hexamers from bGBM and bSTBM are significantly enriched in crosslinked α3, α4, and α5 NC1 subunits. Moreover, using this methodology we have isolated NC1 hexamers from various invertebrate species including most primitive multicellular organisms from Cnidaria and Ctenophore phyla to study evolutionary aspects of collagen IV organization (Fidler et al., 2014, 2017).
Materials and Methods
Frozen bovine placenta (Pel-Freeze Biologicals). Bovine kidneys, lungs, testes, and eye lenses are available from the same source.
Polytron homogenizer (Brinkmann Instruments).
Sorvall RC5CPlus refrigerated centrifuge with SLA-1500 and SS34 rotors.
Eppendorf 5417R tabletop centrifuge.
Amicon 400 mL stirred cell with Ultracel 10 kDa membrane (EMD Millipore).
Amicon Ultra-4 centrifugal filters, 10,000 NMWL (Millipore).
NanoDrop 2000c spectrophotometer (Thermo Scientific).
ChemiDoc XRS + imaging system (BioRad).
Econo-Column, 2.5 × 50 cm2 (BioRad).
DEAE cellulose (Sigma-Aldrich).
Superdex 200 Increase 10/300 GL column on ÄKTA FPLC system (GE Healthcare).
Bacterial collagenase (CLSPA grade, 1000U/mg, Worthington Biochemical).
Store 1 mg/mL stock in TBS (50 mM Tris–HCl, pH 7.4, 150 mM NaCl) with 2 mM CaCl2 at −80°C.
DNase I from bovine pancreas, Type-IV, 2000U/mg (Sigma). Store 2 mg/mL stock in TBS at −80°C.
TRIZMA base, HEPES, ethylenediaminetetraacetic acid (EDTA), sodium azide, sodium deoxycholate, ε-amino caproic acid (EACA), phenylmethanesulfinyl fluoride (PMSF), N-ethylmaleimide (NEM), benzamidine hydrochloride (BAM), leupeptin, pepstatin A, aprotinin, 5-bromo-4-chloro-3-indolyl phosphate (BCIP), nitro blue tetrazolium (NBT), and dimethylformamide (all from Sigma-Aldrich). Nitrocellulose membrane, 0.45 µm (BioRad).
Rat monoclonal α1–α6 NC1 domain-specific antibodies H11, H22, H31, H43, H52, and H63 (kindly provided by Dr. Yoshikazu Sado, Shigei Medical Research Institute, Okayama, Japan).
Goat antirat IgG conjugated with alkaline phosphatase (Sigma).
Homogenization buffer: 50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 20 mM EDTA, 25 mM EACA, 5 mM BAM, and 5 mM NEM. Supplement with protease inhibitors (1 mM PMSF, 1 µg/mL each of leupeptin, pepstatin A, and aprotinin) immediately before use.
Collagenase buffer: 50 mM HEPES, pH 7.5, 10 mM CaCl2, 25 mM EACA, 5 mM BAM, and 1 mM PMSF.
Alkaline phosphatase substrate solution: mix 65 µL BCIP (26 mg/mL in dimethylformamide) and 65 µL of NBT (50 mg/mL in 70% dimethylformamide) with 10 mL 0.1 M Tris–HCl, pH 9.5, 0.1 M NaCl, and 5 mM MgCl2.
Procedure
Perform all steps at 4°C and use prechilled solutions unless otherwise specified.
Incubate bovine placenta on ice until just thawed. Remove large blood vessels and cut tissue into ~1 cm3 pieces. Homogenize on medium setting of Polytron homogenizer in 10 mL of homogenization buffer per 1 g tissue. Larger amounts of tissue could be grounded in electric meat grinder and further homogenized in Waring blender.
Stir homogenate using magnetic stirrer for 1 h at 4°C. Centrifuge at 6000 rpm for 15 min in SLA-1500 rotor.
Discard the supernatant, and resuspend the pellet in fresh homogenization buffer using Polytron. Centrifuge at 6000 rpm for 15 min. Repeat this step 2 × more.
Resuspend pellet in cold distilled water containing 0.05% sodium azide and 1 mM PMSF. Stir for 1 h and centrifuge as above.
Discard the supernatant. Resuspend the pellet in 1 M sodium chloride supplemented with 200 Kunitz units/mL of DNase I at 10 mL/g, stir for 2 h, and centrifuge as above.
Resuspend pellet in cold distilled water, gradually add 10% solution of sodium deoxycholate to final concentration 1%, and stir at room temperature for 2 h.
Centrifuge at 8000 rpm for 15 min and wash the pellet 2 × with cold distilled water discarding supernatants. At this stage, the pellet consists of essentially decellularized fragments of placental basement membrane (PBM).
Weigh and resuspend the pellet in collagenase buffer (2.5 mL/g), add 20U/mL of collagenase. For bLBM, resuspend five anterior lens capsules per 1 mL of collagenase buffer and add 150U/mL of collagenase. Perform collagenase digest for 24 h at 37°C with stirring. Centrifuge the suspension at 10,000 rpm for 20 min in SS34 rotor at 4°C.
Collect supernatant and stop the collagenase activity by adding 0.5 M EDTA to 20 mM. Dialyze for 24 h at 4°C against 2 L of 50 mM Tris–HCl, pH 7.5 with two buffer changes.
Pack an Econo-Column with 150 mL DEAE cellulose and equilibrate with two column volumes of 50 mM Tris–HCl, pH 7.5. Load dialyzed collagenase digest and rinse column with 200 mL of 50 mM Tris–HCl. Collect the unbound fraction and concentrate using Amicon stirred cell with 10kDa cut-off filter under the pressure of nitrogen to 4–6 mL.
Centrifuge concentrated sample at 14,000 rpm for 15 min in Eppendorf 5417R centrifuge.
Collect supernatant and load 250 µL aliquots on Superdex 200 increase size-exclusion column equilibrated in TBS. Elute at the flow rate 0.75 mL/min with TBS and collect 0.3 mL fractions in 96-well plate. The typical elution profile is shown in Fig. 1B. The 7S domain elutes in the first peak close to void volume followed by the major peak of NC1 hexamer.
Combine fractions corresponding to the 7S and NC1 domains from several runs and concentrate using Amicon ultra filters by centrifugation at 4000 rpm to 0.5–1.0 mL.
Determine the protein concertation by absorbance at 280 nm using NanoDrop spectrophotometer and extinction coefficients of 0.21 and 1.60 for 1 mg/mL of 7S and NC1 domain, respectively. Aliquoted proteins should be stored frozen at −80°C.
To analyze polypeptide composition by SDS-PAGE, mix collagen IV samples with an equal volume of 2 × loading buffer (0.125 M Tris–HCl, pH 6.8, 4% SDS, 20% glycerol, and 0.01% bromophenol blue), boil for 5 min and separate on 12% polyacrylamide gel (Laemmli, 1970). Under nonreducing conditions, NC1 hexamers dissociate into monomers and dimers (~23 and 45 kDa, respectively), while 7S dodecamer migrates as a high molecular weight band on the top of the gel (Fig. 1C). Relative amounts of NC1 monomers and dimers could be quantified by the densitometry of Coomassie Brilliant Blue stained gels using ChemiDoc imaging system with ImageLab software.
To analyze α-chain composition of NC1 hexamers by Western blotting, after separation by SDS-PAGE proteins are transferred to nitrocellulose membrane for 1 h at 90 V using standard Towbin buffer (25 mM Tris, 192 mM glycine, and 20% methanol). Block membrane with 5% dry milk (BioRad) in TBS for 1 h at room temperature followed by incubation with rat monoclonal antibodies specific for α1–α6 NC1 domain (1:1000 in 1% dry milk in TBS) for 2 h. After washing 3 × with TBS, 0.1% Tween-20 (TBST) incubate the membrane with antirat alkaline phosphatase conjugated antibody (1:2000) for 1 h. After washing with TBST add 10 mL of AP substrate solution and incubate with gentle shaking until intense purple bands developed (Fig. 1D). Stop reaction by rinsing membrane with distilled water, blot, and air dry the membrane. When amounts of NC1 hexamers are limited, enhanced chemiluminescent detection system (SuperSignal West Pico, Thermo Scientific) could be used as a more sensitive alternative to chromogenic protocol.
3 ISOLATION OF 7S AND NC1 DOMAINS FROM ECM DEPOSITED BY CULTURED CELLS
Mouse embryonic carcinoma PFHR-9 is one of a few mammalian cell lines that produce significant amounts of collagen IV (Duncan, Fessler, Bachinger, & Fessler, 1983). It could be cultured under well-defined conditions providing a useful experimental system to study diverse mechanisms of assembly, secretion, deposition, and crosslinking of collagen IV in the extracellular matrix. Recent examples include discovery of two enzymes, peroxidasin and LOXL2 that catalyze crosslinking of NC1 and 7S domains, respectively (Anazco et al., 2016; Bhave et al., 2012), and delineating the critical role for chloride ions in extracellular assembly of collagen IV networks (Cummings et al., 2016).
Materials and Methods
CO2 incubator.
Class II biological safety cabinet.
S-250 Sonifier with 1/8″ microtip (Branson).
PFHR-9 cells (ATCC CRL-2423).
Hank’s balanced salt solution without calcium and magnesium (HBSS, Gibco).
Dulbecco’s modified eagle medium (DMEM, Gibco).
Fetal bovine serum (FBS, Gibco).
Penicillin–Streptomycin (Pen/Strep, Gibco).
GlutaMax supplement (200 mM, Gibco).
l-Ascorbic acid phosphate magnesium salt (Wako). Make 50 mg/mL stock solution in water, sterilize by filtering through 0.22 µm filter, store frozen in aliquots at −80°C.
Complete media: DMEM with 10% FBS, 1:100 (v/v) Pen/Strep, 2 mM GlutaMax. Add 50 µg/mL ascorbic acid immediately before use.
Lysis buffer: 10 mM Tris–HCl buffer, pH 7.5, 1% sodium deoxycholate, and 1 mM EDTA with protease inhibitors (0.5 mM PMSF, 1 µg/mL aprotinin, 1 µg/mL leupeptin, and 1 µg/mL pepstatin).
Procedure
Maintain PHFR-9 cells in complete medium in humidified incubator at 10% CO2 and 37°C. Split cells at 1:8–1:16 weekly by rinsing flasks with 2 mM EDTA in HBSS and incubating with trypsin in EDTA/HBSS for 5 min at 37°C. Add 10 mL of complete medium and collect cells by repeated pipetting and centrifugation at 400 rpm for 5 min. Resuspend the pellet in complete medium and count cells with hemocytometer.
Plate 2 × 106 cells per 150 mm tissue culture dish or T150 flask in 30 mL complete medium.
Grow cells to confluency for 2 days. Continue the culture for another 5–7 days with daily medium changes.
Remove medium and rinse cells once with cold TBS. Add 5 mL lysis buffer, collect cells with extracellular matrix with cell scraper and keep on ice.
Sonicate lysates for 3 × 15 s at 30% output to fragment genomic DNA. Centrifuge mix at 14,000 rpm for 20 min at 4°C. Discard the supernatant.
Resuspend the pellet by brief sonication in 5 mL 1 M NaCl, 50 mM Tris–HCl, pH 7.5. Incubate on ice for 30 min. Centrifuge as in step 5.
Resuspend the pellet in 5 mL 10 mM Tris–HCl, pH 7.5. Incubate on ice for 30 min. Centrifuge as in step 5.
Resuspend the pellet in 0.5 mL of collagenase buffer with 50U/mL collagenase and incubate for 5 h at 37°C with stirring.
For the purification of 7S and NC1 domains from collagenase digest follow steps 9–13 from Section 2.
For SDS-PAGE and Western blot analysis of NC1 hexamer follow steps 9–13 from Section 2 (see Fig. 1C, lane 5).
4 ISOLATION OF COLLAGENOUS DOMAIN BY LIMITED PEPSIN DIGESTION
The central triple-helical part of the collagen IV protomers mediates various aspects of cell-basement membrane interactions including adhesion, migration, proliferation, and signaling through the binding of the integrin receptors. It could be isolated based on the resistance to pepsin, which efficiently destroys NC1 domains releasing collagenous fragments of various lengths, and salt precipitation.
Procedure
Perform all steps at 4°C and use prechilled solutions:
Follow steps 1–7 outlined in Section 2 to isolate placental basement membrane.
Resuspend PBM in 0.5 M acetic acid using Polytron homogenizer at 10 mL/g.
Add 10 mg/mL pepsin (Worthington Biochemical) in water to tissue:pepsin ratio 1:100.
Incubate on magnetic stirrer for 24–36 h at 4°C. Centrifuge the suspension at 10,000 rpm for 20 min and collect supernatant.
Slowly add solid NaCl to 20% (w/v) under constant stirring and incubate for 1 h. Collect collagenous polypeptides by centrifugation at 25,000 × g for 1 h.
Dissolve the pellet in 0.5 M acetic acid and repeat NaCl precipitation as above.
Dissolve and dialyze the pellet against 0.5 M acetic acid, centrifuge for at 14,000 rpm for 20 min, lyophilize supernatant, and store at −20°C.
This preparation usually produces collagenous domain composed of several fragments varying in size (78 to >340 kDa) connected together by disulfide bonds (Butkowski, Brungardt, Grantham, & Hudson, 1981; West, Fox, Jodlowski, Freytag, & Hudson, 1980).
5 RECOMBINANT EXPRESSION OF COLLAGEN IV FRAGMENTS
NC1 domains of six human collagen IV α-chains were cloned and transfected by calcium phosphate precipitation in human embryonic kidney HEK-293 cells. Stable clones were selected in the presence of geneticin (G418) and expanded. Recombinant proteins fused with an amino terminal FLAG tag have been purified from the conditioned medium using affinity chromatography on anti-FLAG agarose and elution with the FLAG peptide. Application of this technology has led to the discovery of the target antigen for human autoantibodies in Goodpasture’s disease, mapping of pathogenic epitopes within the α3NC1 and α5NC1 domains and identification of critical residues for antibody binding (David, Borza, Leinonen, Belmont, & Hudson, 2001; Netzer et al., 1999; Pedchenko et al., 2010; Sado et al., 1998). Moreover, extending of NC1 domains with collagenous fragments provides a novel experimental strategy for engineering of recombinant heterotrimeric fragments to study molecular mechanism of collagen IV assembly (Cummings et al., 2016).
Human kidney cDNA (Clontech).
pRc-X expression vector (Netzer et al., 1999).
PCR thermocycler (Mastercycler, Eppendorf).
Phusion DNA polymerase; ApaI, NheI, and SacII restriction enzymes; T4 DNA ligase (New England BioLabs).
NucleoSpin gel extraction and NucleoSpin plasmid purification kits (Macherey-Nagel).
Econo-Column, 1 × 5 cm (BioRad).
Amicon Ultra-4 centrifugal filters, 10,000 NMWL (Millipore).
Human embryonic kidney HEK-293 cells (ATCC CRL-1573).
ProFection calcium phosphate mammalian transfection system (Promega).
Cloning disks, 3 mm (Fisher Scientific).
CO2 incubator.
Class II biological safety cabinet.
Penicillin–Streptomycin (Pen/Strep, Gibco).
Hank’s balanced salt solution without calcium and magnesium (HBSS, Gibco).
Trypsin 0.25% in HBSS/EDTA (Corning).
DMEM/F12 (1:1) medium with glutamine and 15 mM HEPES (Gibco).
Fetal bovine serum (FBS, Gibco).
G418 sulfate (Corning). Make 150 mg/mL stock solution in water, sterilize by filtering through 0.22 µm filter, and store frozen in aliquots at −20°C.
Anti-FLAG M2 agarose, monoclonal anti-FLAG M2-alkaline phosphatase conjugated antibody, FLAG peptide (Sigma).
Procedure
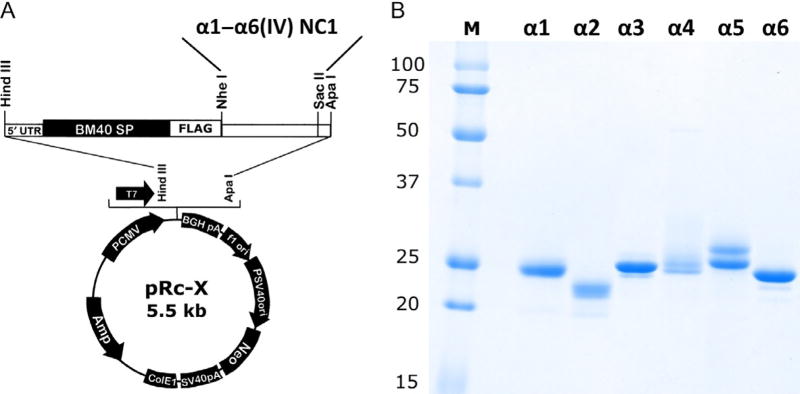
Amplify cDNA for human collagen IV α1–α6 NC1 domain from total kidney cDNA by PCR using specific primers (Table 1). Assemble 50 µL PCR reaction mixes containing 1 × Phusion buffer, 200 µM dNTPs, 0.5 µM each of two primers, 1 µL of human kidney cDNA, and 1 unit of Phusion DNA polymerase. Transfer tubes to thermocycler and heat for 30 s at 98°C followed by 30–35 cycles of denaturation (15 s, 98°C), annealing (30 s, 55°C), and elongation (45 s, 72°C). After final extension (5 min, 72°C) analyze products by 1% agarose gel electrophoresis in TAE buffer. Cut out and purify major 750 bp bands using NucleoSpin gel extraction kit.
Digest 1–2 µg of amplified α2–α6 NC1 cDNA with ApaI and NheI restriction enzymes. For α1 NC1 use SacII instead of ApaI due to the presence of internal restriction site. In parallel, digest 5–10 µg of pRc-X vector with the same enzyme pairs. Purify digested products by the agarose gel electrophoresis and NucleoSpin gel extraction kit. Determine DNA concentration by absorbance at 260 nm using NanoDrop spectrophotometer.
Perform ligation by mixing 35 ng of purified PCR products with 50 ng of vector in 1 × ligase buffer, add 1 µL of T4 DNA ligase, and incubate overnight at 16°C. Heat inactivate ligase and use 2 µL of the reaction to transform competent DH-5α Escherichia coli cells. Spread and grow bacteria overnight at 37°C on LB agar plates with 100 µg/mL ampicillin.
Inoculate single bacterial colonies into LB medium, 100 µg/mL ampicillin, and grow overnight in shaking incubator at 250 rpm, 37°C. Isolate plasmid using NucleoSpin purification kit and verify insert by restriction digest and sequencing using T7 and SP6 primers.
Human embryonic kidney 293 cells are maintained in DMEM/F12 medium supplemented with 10% FBS and Penicillin–Streptomycin mix (1:100). One day before transfection trypsinize and plate 1 × 106 cells per 60 mm culture plate.
Replace culture medium 3 h before transfection.
In a tissue culture hood, mix 5 µg of expression vector DNA with 25 µL 2 M CaCl2 and add sterile deionized water to 300 µL. Add mix dropwise to 300 µL of 2 × HBS solution (ProFection, Promega) with gentle vortexing and incubate at room temperature for 30 min to form DNA–Ca phosphate complexes.
Mix and add solution dropwise to cells. Return plates to CO2 incubator for 48 h.
Remove medium, trypsinize, and plate cells in two 150 mm culture plates in selection medium (DMEM/F12, 10% FBS, 500 µg/mL G418). Continue cell culture changing selection medium weekly for 18–21 days until distinct colonies are formed.
Remove culture medium and rinse plates briefly with 2 mM EDTA in HBSS. Transfer individual colonies using cloning disks soaked in trypsin–EDTA–HBSS solution with sterilized forceps to the 48-well tissue culture plates (Falcon).
When cells are confluent, screen 25 µL aliquots of conditioned medium for NC1 expression by SDS-PAGE/Western blotting with M2 anti-FLAG antibodies. We found that commercially available M2-alkaline phosphatase conjugate is a most convenient tool for one-step detection.
Trypsinize cells and expand clones with the highest expression level into 12-well plates, and subsequently into T25, T75, and T225 large culture flasks.
Collect conditioned medium from 5 to 10 large flasks (35–40 mL/flask) over 2–4 weeks changing medium twice a week. Supplement collected medium with 0.2 M NaCl, 5 mM EDTA, 0.5 mM PMSF, and 0.02% sodium azide, and centrifuge at 10,000 rpm for 15 min. Store supernatants frozen at −20°C.
Pack a small Econo-Column with 3 mL of M2 agarose and equilibrate with five volumes of TBS. Pass 1–1.5 L of conditioned medium through the column under low hydrostatic pressure overnight at 4°C.
Wash the column with 50 mL TBS and elute bound protein with 10 mL FLAG peptide (100 µg/mL in TBS). Concentrate recombinant protein using Amicon filters to 0.5 mL by centrifugation at 5000 rpm for 10 min. Dilute with 10 mL TBS and repeat concentration step 2 × to remove the FLAG peptide.
Determine the protein concertation using NanoDrop spectrophotometer (1 mg/mL of NC1 domain corresponds to A280 = 1.60). Analyze recombinant NC1s by SDS-PAGE (Fig. 2B) and Western blot as outlined in Section 2. Store protein aliquots frozen at −80°C.
Table 1.
Primers for PCR Amplification of Human Collagen IV NC1 Domains (Restriction Sites for ApaI, NheI, and SacII Are Underlined)
| Amplicon | Primer Sequences |
|---|---|
|
| |
| α1(IV)NC1 | TAATGCTAGCATCTGTTGATCACGGCTTCC |
| ATATCCGCGGTAGCTGAGTCAGGCTTCATTATG | |
| α2(IV)NC1 | TAATGCTAGCCGTCAGCATCGGCTACCTCC |
| ATATGGGCCCTGGCACGCGCCGGCTCACAGG | |
| α3(IV)NC1 | TAATGCTAGCAGGTTTGAAAGGAAAACGTGGAGACAGTGG |
| TATAGGGCCCTCAGTGTCTTTTCTTCATGCAC | |
| α4(IV)NC1 | ATAAGCTAGCCGGGCCCAAAGGGTTTGGCCCTGGATACCTCGG |
| ATAAGGGCCCCTAGCTATACTTCACGCAG | |
| α5(IV)NC1 | TAATGCTAGCAGGTCCCCCTGGTCCAGATGGATTGC |
| TATAGGGCCCATGTTATGTCCTCTTCATGCACAC | |
| α6(IV)NC1 | TAATGCTAGCAGGGATGCCTGGAATGCCTGGCCAGAGC |
| ATATGGGCCCGTGGCAGGTGCCACCCTACAGGC | |
FIG. 2.
Expression of recombinant α1–α6 NC1 domains. (A) Map of the pRc-X expression vector showing positions of BM40 signal peptide, FLAG tag and NheI, ApaI/SacII sites used for cloning human NC1 domains. (B) SDS-PAGE of purified α1–α6 NC1 proteins (2 µg/lane). Small variations of MW are due to the presence of short additional Gly-X-Y repeats from collagenous domain in the α1, α3, α4, and α5 NC1s.
6 DISSOCIATION AND ASSEMBLY OF COLLAGEN IV NC1 HEXAMERS
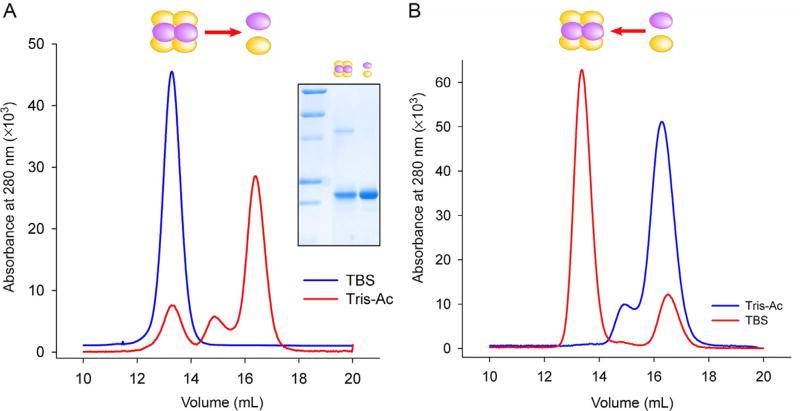
NC1 domains play a key role in the initiation of collagen IV assembly determining the stoichiometry and spatial arrangement (register) of α-chains. However, the molecular mechanism of collagen IV protomer and network formation until recently remained largely unknown. Recombinant NC1 domains described in previous section were instrumental in addressing this problem. Alternatively, NC1 monomers could be isolated from native sources, such as lens basement membrane hexamers, which are composed of predominantly uncrosslinked α1 and α2 NC1s (Fig. 1C), and could be reversibly dissociated into monomers in the chloride-free environment and assembled into hexamers in the presence of chloride.
Procedure
Dialyze bLBM NC1 hexamers, 1 mg/mL in TBS against 1 L of 50 mM Trisacetate buffer, pH 7.4 with one buffer change for 24 h at 4°C. Centrifuge at 14,000 rpm for 15 min.
Inject the supernatant on Superdex 200 Increase column equilibrated in 25 mM Tris-acetate, 0.1 M sodium acetated buffer, pH 7.4. Elute the column with the same buffer at 0.75 mL/min, and collect 0.3 mL fractions in 96-well plates (Fig. 3A).
Combine fractions containing dissociated NC1 monomers and concentrate using Amicon filters to 0.1 mL by centrifugation at 5000 rpm for 10 min. Change the buffer to 50 mM Tris-acetate, pH 7.4 by diluting and repeating the concentration step. Final protein concertation determined using NanoDrop spectrophotometer should be above 2 mg/mL.
Prepare assembly reaction by mixing of purified NC1 monomers with various concentrations of sodium chloride or other salts in 25µL final volume. Incubate samples at 37°C. After brief centrifugation at 14,000 rpm for 10 min load samples onto the Superdex column equilibrated in TBS. Standard assembly conditions include 2 mg/mL NC1 monomers, 150 mM NaCl for 24 h (Fig. 3B). Assembly is quantified by the peak integration and expressing the hexamer area as a percentage of the total peak area.
FIG. 3.
Dissociation and assembly of collagen IV NC1 hexamer. (A) Dissociation of purified bLBM NC1 hexamers after dialysis in Tris-acetate buffer illustrated by the elution profile form Superdex S200 column. NC1 monomers (16.4 mL peak) were concentrated and used for assembly. Inset shows SDS-PAGE of NC1 hexamer and monomer peaks. (B) Chloride-mediated assembly of NC1 hexamer from purified LBM monomers. Incubation in the presence of NaCl (150 mM, 37°C, 24 h) induces formation of hexamer (peak at 13.2 mL) from NC1 monomers (peak at 16.4 mL).
7 CONCLUSION
Collagen IV networks play a pivotal role in the organization, signaling, and architecture of the overall basement membrane. The network functionality resides within the three distinct domains, triple-helical collagenous, 7S, and NC1. They could be purified from animal tissues, ECM deposited by the cultured mammalian cells or recombinantly expressed to study composition, molecular structure, mechanisms of assembly, and diverse biological functions of collagen IV. Here we have presented methodology for purification, expression, and analysis of collagen IV domains. These protocols can be used on various tissues of animal models to address critical questions in the biology and pathobiology of collagen IV.
Acknowledgments
The technical assistance of Parvin Todd and Mohamed Rafi is appreciated. This work was supported by National Institutes of Health Grant R01 DK18381 to B.G.H.
References
- Anazco C, Lopez-Jimenez AJ, Rafi M, Vega-Montoto L, Zhang MZ, Hudson BG, et al. Lysyl oxidase-like-2 cross-links collagen IV of glomerular basement membrane. The Journal of Biological Chemistry. 2016;291:25999–26012. doi: 10.1074/jbc.M116.738856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Cummings CF, Vanacore RM, Kumagai-Cresse C, Ero-Tolliver IA, Rafi M, et al. Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nature Chemical Biology. 2012;8:784–790. doi: 10.1038/nchembio.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butkowski RJ, Brungardt GS, Grantham JJ, Hudson BG. Characterization of the collagenous domain of tubular basement membrane. The Journal of Biological Chemistry. 1981;256:7603–7609. [PubMed] [Google Scholar]
- Cummings CF, Pedchenko V, Brown KL, Colon S, Rafi M, Jones-Paris C, et al. Extracellular chloride signals collagen IV network assembly during basement membrane formation. The Journal of Cell Biology. 2016;213:479–494. doi: 10.1083/jcb.201510065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M, Borza DB, Leinonen A, Belmont JM, Hudson BG. Hydrophobic amino acid residues are critical for the immunodominant epitope of the Goodpasture autoantigen. A molecular basis for the cryptic nature of the epitope. The Journal of Biological Chemistry. 2001;276:6370–6377. doi: 10.1074/jbc.M008956200. [DOI] [PubMed] [Google Scholar]
- Duncan KG, Fessler LI, Bachinger HP, Fessler JH. Procollagen IV. Association to tetramers. The Journal of Biological Chemistry. 1983;258:5869–5877. [PubMed] [Google Scholar]
- Fidler AL, Darris CE, Chetyrkin SV, Pedchenko VK, Boudko SP, Brown KL, et al. Collagen IV and basement membrane at the evolutionary dawn of metazoan tissues. eLife. 2017;6:e24176. doi: 10.7554/eLife.24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler AL, Vanacore RM, Chetyrkin SV, Pedchenko VK, Bhave G, Yin VP, et al. A unique covalent bond in basement membrane is a primordial innovation for tissue evolution. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:331–336. doi: 10.1073/pnas.1318499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freytag JW, Ohno M, Hudson BG. Large scale preparation of bovine renal glomerular basement membrane in the presence of protease inhibitors. Preparative Biochemistry. 1978;8:215–224. doi: 10.1080/00327487808069061. [DOI] [PubMed] [Google Scholar]
- Gunwar S, Bejarano PA, Kalluri R, Langeveld JP, Wisdom BJ, Jr, Noelken ME, et al. Alveolar basement membrane: Molecular properties of the noncollagenous domain (hexamer) of collagen IV and its reactivity with Goodpasture autoantibodies. American Journal of Respiratory Cell and Molecular Biology. 1991;5:107–112. doi: 10.1165/ajrcmb/5.2.107. [DOI] [PubMed] [Google Scholar]
- Gupta MC, Graham PL, Kramer JM. Characterization of alpha1(IV) collagen mutations in Caenorhabditis elegans and the effects of alpha1 and alpha2(IV) mutations on type IV collagen distribution. The Journal of Cell Biology. 1997;137:1185–1196. doi: 10.1083/jcb.137.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahsai TZ, Enders GC, Gunwar S, Brunmark C, Wieslander J, Kalluri R, et al. Seminiferous tubule basement membrane. Composition and organization of type IV collagen chains, and the linkage of alpha3(IV) and alpha5(IV) chains. The Journal of Biological Chemistry. 1997;272:17023–17032. doi: 10.1074/jbc.272.27.17023. [DOI] [PubMed] [Google Scholar]
- Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microscopy Research and Technique. 2008;71:357–370. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langeveld JP, Wieslander J, Timoneda J, McKinney P, Butkowski RJ, Wisdom BJ., Jr Structural heterogeneity of the noncollagenous domain of basement membrane collagen. The Journal of Biological Chemistry. 1988;263:10481–10488. [PubMed] [Google Scholar]
- Leitinger B, Hohenester E. Mammalian collagen receptors. Matrix Biology: Journal of the International Society for Matrix Biology. 2007;26:146–155. doi: 10.1016/j.matbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Netzer KO, Leinonen A, Boutaud A, Borza DB, Todd P, Gunwar S, et al. The Goodpasture autoantigen. Mapping the major conformational epitope(s) of alpha3(IV) collagen to residues 17–31 and 127–141 of the NC1 domain. The Journal of Biological Chemistry. 1999;274:11267–11274. doi: 10.1074/jbc.274.16.11267. [DOI] [PubMed] [Google Scholar]
- Pedchenko V, Bondar O, Fogo AB, Vanacore R, Voziyan P, Kitching AR, et al. Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. The New England Journal of Medicine. 2010;363:343–354. doi: 10.1056/NEJMoa0910500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- Reddy GK, Gunwar S, Kalluri R, Hudson BG, Noelken ME. Structure and composition of type IV collagen of bovine aorta. Biochimica et Biophysica Acta. 1993;1157:241–251. doi: 10.1016/0304-4165(93)90106-i. [DOI] [PubMed] [Google Scholar]
- Sado Y, Boutaud A, Kagawa M, Naito I, Ninomiya Y, Hudson BG. Induction of anti-GBM nephritis in rats by recombinant a3(IV)NC1 and a4(IV)NC1 of type IV collagen. Kidney International. 1998;53:664–671. doi: 10.1046/j.1523-1755.1998.00795.x. [DOI] [PubMed] [Google Scholar]
- Sundaramoorthy M, Meiyappan M, Todd P, Hudson BG. Crystal structure of NC1 domains. Structural basis for type IV collagen assembly in basement membranes. The Journal of Biological Chemistry. 2002;277:31142–31153. doi: 10.1074/jbc.M201740200. [DOI] [PubMed] [Google Scholar]
- Vanacore R, Ham AJ, Voehler M, Sanders CR, Conrads TP, Veenstra TD, et al. A sulfilimine bond identified in collagen IV. Science. 2009;325:1230–1234. doi: 10.1126/science.1176811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West TW, Fox JW, Jodlowski M, Freytag JW, Hudson BG. Bovine glomerular basement membrane. Properties of the collagenous domain. The Journal of Biological Chemistry. 1980;255:10451–10459. [PubMed] [Google Scholar]