Abstract
Inactivation of the androgen receptor (AR) pathway by androgen deprivation therapy (ADT) is the mainstay of (metastatic) prostate cancer therapy. Ultimately, the AR pathway will be re-activated despite castrate levels of circulating androgens. Thereby, maintaining its role even in castration resistant prostate cancer (CRPC). The recent STAMPEDE and CHAARTED trials showed that docetaxel in combination with ADT increased survival in hormone sensitive prostate cancer patients, suggesting cross-talk between AR signaling and chemotherapy efficacy. We hypothesized that a similar interaction may also apply for CRPC that is treated with cabazitaxel. We studied the impact of androgen status on the efficacy, pharmacodynamics and -kinetics of cabazitaxel in a unique and clinically relevant patient derived xenograft model of castration resistant disease. We found that cabazitaxel is highly effective in a castrate setting with strongly reduced AR activation, while tumor growth inhibition by cabazitaxel was completely abolished in the presence of high AR pathway activity. Moreover, additional experiments showed that intratumoral cabazitaxel levels were 3.5 times higher in tumors from castrated mice as compared to tumors from androgen-supplemented animals. We confirmed that cabazitaxel pharmacokinetics were not affected by testosterone, suggesting that androgen status might influence cabazitaxel tumor uptake directly. This study reveals the impact of androgen status on cabazitaxel efficacy and supports the potential of combination of taxane chemotherapeutics with AR axis targeting agents.
Keywords: Cabazitaxel, Androgen receptor, Testosterone, Castration resistant prostate cancer, Pharmacodynamics, Pharmacokinetics
Abbreviations: AR, Androgen receptor; ADT, Androgen deprivation therapy; CRPC, Castration resistant prostate cancer; IHC, Immunohistochemistry; TV, Tumor volume
Highlights
-
•
Cabazitaxel is highly effective in a castrate setting, while anti-tumor efficacy is abolished with high AR activity.
-
•
Intratumoral cabazitaxel levels were 3.5 times higher in tumors from castrate mice compared to androgen-supplemented mice.
We studied the efficacy of the chemotherapeutic cabazitaxel in castrate mice and mice receiving testosterone. While cabazitaxel was highly effective in castrate mice, it was ineffective in mice receiving testosterone. This was likely caused by higher cabazitaxel levels, as chemotherapeutic levels in tumors from castrate mice were about 3.5 fold higher. Our study shows that cabazitaxel efficacy might be impacted by androgens and cabazitaxel efficacy is improved in combination with androgen deprivation therapy and possibly androgen receptor targeted therapy.
1. Introduction
The treatment landscape of metastatic castration resistant prostate cancer (mCRPC) has expanded with the introduction of taxanes (docetaxel and cabazitaxel), second generation androgen receptor (AR) antagonists and the CYP17A inhibitor abiraterone. Despite substantial clinical impact, the absolute survival benefit in mCRPC remains limited, additionally cross-resistance between drugs may affect treatment efficacy (Mezynski et al., 2012). As a result, attention has been directed towards defining the optimal treatment sequence. Recent clinical trial results have shown that combining therapies rather than treatment sequence, may provide the most optimal approach for metastatic prostate cancer patients. The CHAARTED and STAMPEDE trials have shown robust overall survival benefits by combining androgen deprivation therapy (ADT) with docetaxel (James et al., 2016, Sweeney et al., 2015). Of note, preliminary results have shown that docetaxel without ADT is ineffective in castrate-naive patients (SPCG12, trial NCT00376792) (Ahlgren et al., 2016). This might suggest that reducing circulating androgen levels and androgen receptor activity affects taxane efficacy. Cabazitaxel is the second-line taxane, which is effective in patients with disease progression during or after docetaxel (de Bono et al., 2010). We hypothesized that, similarly to docetaxel, cabazitaxel efficacy could be affected by androgen-induced AR activation. To test our hypothesis, we evaluated the efficacy of cabazitaxel in a preclinical model of CRPC in the presence or absence of androgens. Since previous work from our group has shown that reduced intratumoral taxane concentrations affect treatment activity (de Morree et al., 2016a), we also studied the effect of hormone manipulation on cabazitaxel pharmacokinetics and intratumoral cabazitaxel concentrations.
2. Material and Methods
2.1. Cell Culture
The human prostate CRPC cell line PC346C-DCC-K was established from the PC346C cell line by long-term culturing in the absence of androgens (Marques et al., 2006, Marques et al., 2005). The AR expressing PC346C-DCC-K cell line can grow in the absence of androgens, but the AR pathway remains active, as indicated by prostate specific antigen (PSA) expression (Supplementary fig. 1a). Cell line authenticity was confirmed by short tandem repeat analysis with the Promega PowerPlex 16 kit. Cells were regularly tested for mycoplasma infection, and kept into culture for a maximum of 25 passages after initiating the castrate resistance phenotype.
2.2. Animal Welfare
All animal experiments were approved by the Animal experiment committee under the Dutch experiments on Animal Act. The current study is in compliance with the Arrive guidelines.
2.3. Effect of Testosterone on Cabazitaxel Efficacy
Twenty-four athymic male NMRI nude mice (NMRI-Foxn1nu; Taconic, Ry, Denmark) were subcutaneously inoculated with 5 million PC346-DCC-K cells, while being anesthetized with isoflurane/O2. Tumor volume (TV) was monitored twice weekly by digital calipers, animals were randomized based on TV at start to ensure homogeneous groups. Mice were surgical castrated once tumors surpassed a volume of 150 mm3, during the surgical castration mice were anesthetized with Ketamine/Medetomidine (75 mg/kg and 1 mg/kg) and analgesia was provided by Carprofen (5 mg/kg). Six to ten days after castration, mice were randomized to receive either a silastic implant (Freudenberg Medical) containing testosterone (40 mg), or an empty implant as a control. During implantation of the silastic pellets mice were anesthetized with isoflurane/O2. The next day, mice were randomized to receive either one intraperitoneal injection of cabazitaxel (33 mg/kg, Sanofi) or placebo (saline). Blood for PSA analysis was sampled (submandibular vein) at tumor formation and biweekly during the experiment until sacrifice. Plasma was isolated from whole blood samples by centrifugation at 6800 g for 10 min, and PSA was analyzed by an electrochemiluminescence immunoassay. Mice were kept on a 12 h dark/light cycle, food and water were provided ad libitum. Mice were sacrificed once the tumors surpassed a volume of 1500 mm3, or at 90 days after cabazitaxel. Statistical analysis was performed using either SPSS (IBM, version 21) or Graphpad prism (Graphpad software, version 5.01), sample size was calculated using G*power (Kiel University, version 3.1.9.2) and based on an in vivo pilot study (power of 90%, α = 0.01 and effect size of 0.85). Statistical analyses of tumor growth were performed using SPSS and Graphpad prism.
Tumor tissues were formalin fixed and paraffin embedded for immunohistochemistry (IHC) analysis of the AR and cell cycle marker Ki67. In short, 4 μm tissue sections were incubated with primary anti-AR (1:300, SP107 Cell Marque) or anti-Ki67 (1:100, MIB-1 Dako) and visualized with DAB/H2O2 (Dako EnVision kit). IHC staining's were blinded for treatment and scored by two readers, AR staining was scored by multiplying the percentage positive tumor cells with the staining intensity score (0–3). Ki67 staining was scored for percentage positive in the tumor cells.
2.4. Cabazitaxel Uptake
The experimental set-up and group size was similar to the cabazitaxel efficacy experiment, with the following exception; tumors were isolated 7 days after cabazitaxel injection. Tumors were snap frozen and stored in − 80 °C. Intratumoral cabazitaxel concentration was determined by a five times dilution of tissue in blank human lithium heparinized plasma (w/v), and homogenized by using a tissue homogenizer. Cabazitaxel concentrations were determined by a validated UPLC-MS/MS method and based on the method as described previously (de Morree et al., 2016a, de Bruijn et al., 2012), and corrected for tumor weight. The lower limit of quantitation (LLOQ) was established at 1.00 ng/mL for cabazitaxel in human lithium heparinized plasma. Peak area ratios of cabazitaxel versus the Internal Standard in human lithium heparinized plasma were a linear function of the concentration from 1.00 to 500 ng/mL. The within- and between-run precision at four tested concentrations, including the LLQ, were ≤ 10.1 and ≤ 10.9%, respectively, while the average accuracy ranged from 97.5 to 110%.
2.5. Cabazitaxel PK
Fourteen non-tumor baring athymic male nude mice were castrated, and seven days later, randomized to receive either a silastic implant containing testosterone or an empty pellet as a control. Two days later mice received one bolus injection of cabazitaxel (33 mg/kg), and blood was sampled from the submandibular vein at the following time points: 30, 60, 120 and 180 min. Plasma was isolated from whole blood samples by centrifugation at 6800 g for 10 min. Cabazitaxel concentrations were determined by UPLC-MS/MS (de Morree et al., 2016a, de Bruijn et al., 2012). Sample size was calculated using Gpower and based on a previous study (de Morree et al., 2016a) (power of 80%, α = 0.05 and effect size of 0.4).
3. Results
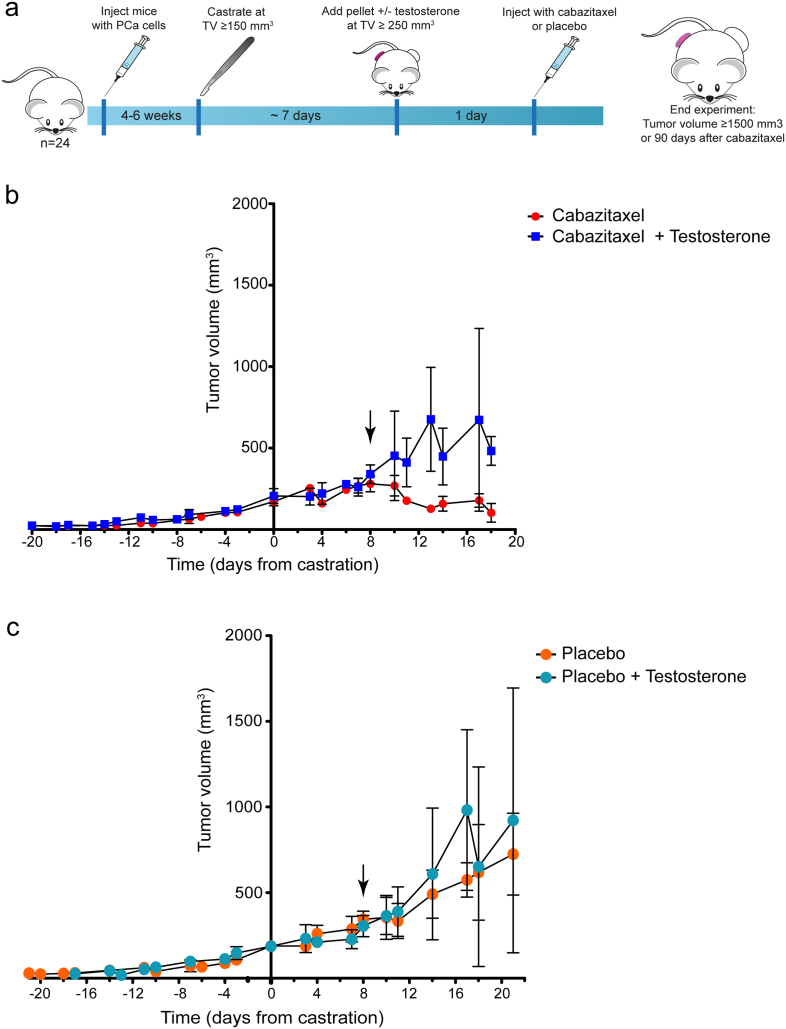
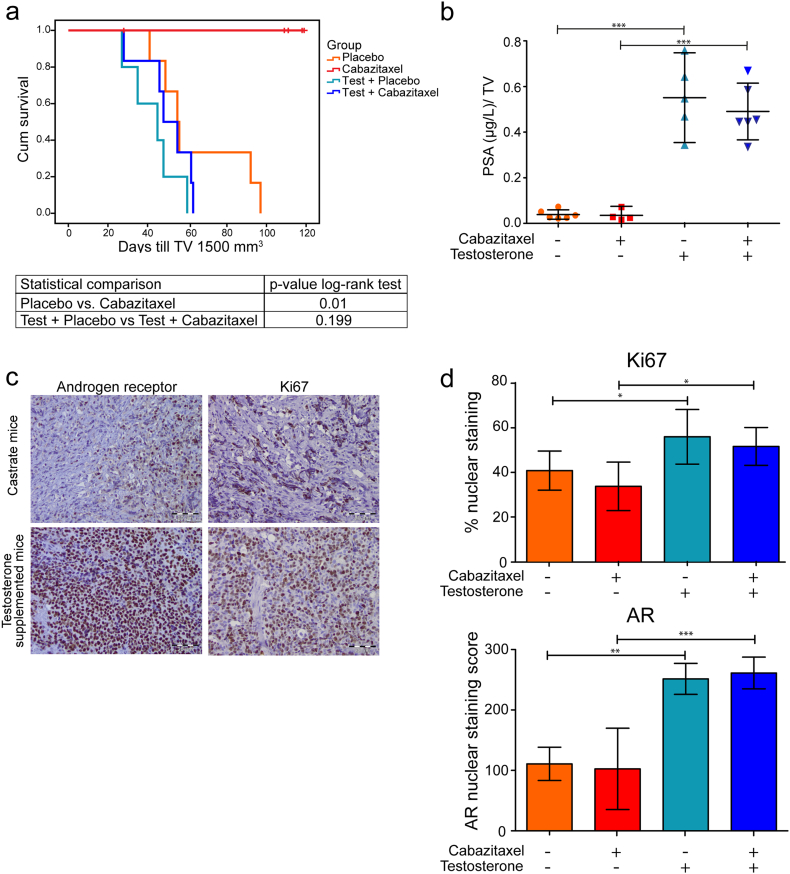
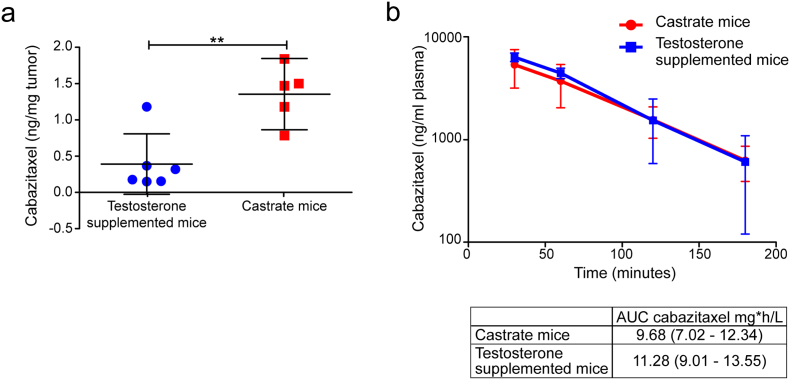
We subcutaneously implanted male immune-deficient mice with the AR wt and PSA secreting CRPC cell line PC346C-DCC-K (Fig. 1a) (Marques et al., 2006). The PC346-DCC-K model does not express AR variants (Supplementary fig. 1a) and shows reduced in vitro response to the anti-androgen enzalutamide compared to the parental model (van Soest et al., 2013) (Supplementary fig. 1b). We confirmed the CRPC phenotype of this model in vivo, as tumors continued to grow after surgical castration of the mice (Fig. 1b, c). Subsequent treatment with cabazitaxel induced a near-complete tumor response with none of the castrate mice reaching the end-point (TV 1500 mm3) (Fig. 2a). In contrast, tumors in castrate mice that received testosterone supplementation failed to show a response to cabazitaxel treatment, with time till TV 1500 mm3 not significantly different from placebo-treated mice (log-rank P = 0.199). Testosterone supplemented mice had, even after correcting for tumor volume, increased levels of PSA compared to castrate mice, indicating an active AR pathway in these tumors (Fig. 2b) (Marques et al., 2010). IHC analysis of cabazitaxel-treated tumors in testosterone-supplemented mice showed high levels of AR positive cells with strong nuclear staining (Fig. 2c). Moreover, in tumors from cabazitaxel-treated castrate mice, fewer AR positive cells with less intense nuclear staining were observed, as well as reduced Ki67 staining (Fig. 2d). To determine a potential mechanism of interaction we measured intratumoral cabazitaxel levels; drug concentrations were 3.5 times higher in tumors from castrate mice compared to testosterone supplemented mice (Fig. 3a; 1.36 ng cabazitaxel/mg tumor tissue vs. 0.39 ng cabazitaxel/mg tumor tissue, respectively). Pharmacokinetic analysis of cabazitaxel serum levels showed that the systemic exposure to cabazitaxel was not affected by testosterone supplementation (Fig. 3b).
Fig. 1.
Tumor growth dynamics of the PC346-DCC-K tumor model, confirms CRPC phenotype. (a) Schematic overview of the experimental set-up; mice were inoculated with PC346-DCC-K cells and castrated at TV > 150 mm3. The next week, mice were randomized to receive either a testosterone pellet or treatment control, and either cabazitaxel or placebo. (b, c) Average tumor growth of the cabazitaxel (b) and placebo (c) treated tumors (± SEM). The PC346-DCC-K tumors (n = 5–6) continued to grow after surgical castration of the mice (day 0), underlining the CRPC phenotype of this model. Arrow indicates time of cabazitaxel treatment (b) or placebo control (c).
Fig. 2.
The efficacy of cabazitaxel is abolished by testosterone supplementation while remaining high in castrate mice. (a) Kaplan Meier survival curve showing the time till TV 1500 mm3 for the four treatment groups (n = 5–6), survival was compared by a log-rank test. One mice in the cabazitaxel group was excluded due to weight loss. (b) Normalized PSA (μg/L) levels ± SD. PSA was measured in plasma collected at the end of the experiment and normalized for TV. One cabazitaxel treated castrate mice had undetectable PSA levels (< 0.1 μg/L). PSA values were compared by a one-way Anova with a Bonferroni post-test, *** indicates a p-value < 0.001. (c) Representative images of the IHC staining of the AR and Ki67 in PC346-DCC-K tumors from cabazitaxel treated castrate or testosterone supplemented mice. (d) Scoring of IHC staining; the percentage positive Ki67 cells ± SEM and the AR nuclear staining score ± SEM. The percentage Ki67 positive cells was compared by a one-way Anova with a Bonferroni post-test, * indicates a p-value < 0.05. The AR nuclear staining score was compared by a Kruskal-Wallis test with a Dunn's post-test, ** indicates a p-value of < 0.01 and *** indicate a p value of < 0.001.
Fig. 3.
Testosterone supplementation decreases the intratumoral cabazitaxel concentration, but does not alter the cabazitaxel pharmacokinetics. (a), Intratumoral cabazitaxel concentrations were measured in PC346-DCC-K tumors from castrate and testosterone supplemented mice, tumors were excised seven days after cabazitaxel treatment (33 mg/kg). Mean intratumoral cabazitaxel concentrations (ng/mg tumor) ± SD are plotted, t-test was used to compare the concentrations, ** indicates a P-value of 0.0031. (b), Castrate or testosterone supplemented mice received one bolus injection of cabazitaxel, blood samples were drawn at four time-points (30, 60, 120 and 180 min). Cabazitaxel concentrations were measured in plasma fraction, mean cabazitaxel concentrations ± SEM (n = 7) are shown.
4. Discussion
In conclusion, this preclinical study supports recent clinical data of improved taxane efficacy when combined with ADT. Additionally it provides a potential mechanism of action by greater intratumoral accumulation of taxanes during ADT. This preclinical study emphasizes the permanent role of the AR pathway in CRPC and demonstrates its impact on the antitumor activity of cabazitaxel. We show that response to cabazitaxel in an AR-positive CRPC model can be successfully prolonged and resistance delayed by concomitant androgen depletion, thereby reducing AR-activation. As reduced cabazitaxel accumulation seems not to be a consequence of a testosterone-induced change in cabazitaxel exposure, alternatively, testosterone may have an impact on cabazitaxel accumulation. We have previously shown that docetaxel and cabazitaxel uptake is reduced by knockdown of SLCO1B3 (de Morree et al., 2016b). Interestingly, OATP drug transporters (encoded by the SLCO family) are also known to function as steroid transporters (Green et al., 2017). Further studies are underway to unravel the molecular mechanisms that link the testosterone to the cellular cytotoxic effect of cabazitaxel. The current study was performed in a unique CRPC tumor models that retained its AR expression upon castration (or testosterone-free culture conditions), which allows us to study the role of the AR in cabazitaxel efficacy. Most hormone sensitive prostate cancer cell lines cultured in the absence of androgens lose their AR expression, and are therefore unfit to examine the interplay between the AR and taxane efficacy. Our data provides a rationale for the combined treatment of cabazitaxel with novel antiandrogen treatments in CRPC patients with an active AR pathway, in order to maximize efficacy of the taxane treatment (Sternberg, 2016). Additional studies are warranted to test whether novel antiandrogen treatment, such as abiraterone and enzalutamide, indeed increase taxane efficacy. Such studies may provide guidance to define the optimal window of opportunity for clinical use of combined treatment of taxanes and antiandrogen agents not only in castrate-naïve, but also in metastatic CRPC patients.
Conflicts of Interest
This study was financially supported by an unrestricted grant by Sanofi (CW214392). Sanofi was not involved in either design of the study, data collection, data analysis, interpretation or writing of the report.
Author Contributions
Concept and design: LM, WvW, ML, RdW and RM.
Acquisition of data: LM.
Analysis and interpretation of data: LM, ML and WvW.
Drafting the manuscript: LM.
Critical revision of the manuscript: ML, WvW and RdW.
Statistical analysis: LM.
Administrative, technical and methodical support: CdR, DS and EV.
Supervision: WvW, ML and RdW.
Footnotes
Notes: This study was presented in part at the 108th AACR Annual Meeting, Washington, DC, April 1–5, 2017, #1099.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2017.12.024.
Appendix A. Supplementary Data
Supplementary material and methods
References
- Ahlgren G., Flodgren P., Tammela T.L.J., Kellokumpu-Lehtinen P., Borre M., Angelsen A., Iversen J.R., Sverrisdottir A., Jonsson E., Sengelov L., GROUP, S. P. C A randomized phase III trial between adjuvant docetaxel andsurveillance after radical prostatectomy for high risk prostate cancer: Results of SPCG12. ASCO J. Clin. Oncol. 2016;34:5001-5001. [Google Scholar]
- de Bono J.S., Oudard S., Ozguroglu M., Hansen S., Machiels J.P., Kocak I., Gravis G., Bodrogi I., Mackenzie M.J., Shen L., Roessner M., Gupta S., Sartor A.O., Investigators T. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- de Bruijn P., de Graan A.J., Nieuweboer A., Mathijssen R.H., Lam M.H., de Wit R., Wiemer E.A., Loos W.J. Quantification of cabazitaxel in human plasma by liquid chromatography/triple-quadrupole mass spectrometry: a practical solution for non-specific binding. J. Pharm. Biomed. Anal. 2012;59:117–122. doi: 10.1016/j.jpba.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Green S.M., Kaipainen A., Bullock K., Zhang A., Lucas J.M., Matson C., Banks W.A., Mostaghel E.A. Role of OATP transporters in steroid uptake by prostate cancer cells in vivo. Prostate Cancer Prostatic Dis. 2017;20:20–27. doi: 10.1038/pcan.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James N.D., Sydes M.R., Clarke N.W., Mason M.D., Dearnaley D.P., Spears M.R., Ritchie A.W., Parker C.C., Russell J.M., Attard G., de Bono J., Cross W., Jones R.J., Thalmann G., Amos C., Matheson D., Millman R., Alzouebi M., Beesley S., Birtle A.J., Brock S., Cathomas R., Chakraborti P., Chowdhury S., Cook A., Elliott T., Gale J., Gibbs S., Graham J.D., Hetherington J., Hughes R., Laing R., Mckinna F., Mclaren D.B., O'sullivan J.M., Parikh O., Peedell C., Protheroe A., Robinson A.J., Srihari N., Srinivasan R., Staffurth J., Sundar S., Tolan S., Tsang D., Wagstaff J., Parmar M.K., Investigators S. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques R.B., Erkens-Schulze S., de Ridder C.M., Hermans K.G., Waltering K., Visakorpi T., Trapman J., Romijn J.C., van Weerden W.M., Jenster G. Androgen receptor modifications in prostate cancer cells upon long-termandrogen ablation and antiandrogen treatment. Int. J. Cancer. 2005;117:221–229. doi: 10.1002/ijc.21201. [DOI] [PubMed] [Google Scholar]
- Marques R.B., van Weerden W.M., Erkens-Schulze S., de Ridder C.M., Bangma C.H., Trapman J., Jenster G. The human PC346 xenograft and cell line panel: a model system for prostate cancer progression. Eur. Urol. 2006;49:245–257. doi: 10.1016/j.eururo.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Marques R.B., Dits N.F., Erkens-Schulze S., van Weerden W.M., Jenster G. Bypass mechanisms of the androgen receptor pathway in therapy-resistant prostate cancer cell models. PLoS One. 2010;5:e13500. doi: 10.1371/journal.pone.0013500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezynski J., Pezaro C., Bianchini D., Zivi A., Sandhu S., Thompson E., Hunt J., Sheridan E., Baikady B., Sarvadikar A., Maier G., Reid A.H., Mulick Cassidy A., Olmos D., Attard G., de Bono J. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Ann. Oncol. 2012;23:2943–2947. doi: 10.1093/annonc/mds119. [DOI] [PubMed] [Google Scholar]
- de Morree E., van Soest R., Aghai A., de Ridder C., de Bruijn P., Ghobadi Moghaddam-Helmantel I., Burger H., Mathijssen R., Wiemer E., de Wit R., van Weerden W. Understanding taxanes in prostate cancer; importance of intratumoral drug accumulation. Prostate. 2016;76:927–936. doi: 10.1002/pros.23182. [DOI] [PubMed] [Google Scholar]
- de Morree E.S., Bottcher R., van Soest R.J., Aghai A., de Ridder C.M., Gibson A.A., Mathijssen R.H., Burger H., Wiemer E.A., Sparreboom A., de Wit R., van Weerden W.M. Loss of SLCO1B3 drives taxane resistance in prostate cancer. Br. J. Cancer. 2016;115:674–681. doi: 10.1038/bjc.2016.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Soest R.J., van Royen M.E., de Morree E.S., Moll J.M., Teubel W., Wiemer E.A., Mathijssen R.H., de Wit R., van Weerden W.M. Cross-resistance between taxanes and new hormonal agents abiraterone and enzalutamide may affect drug sequence choices in metastatic castration-resistant prostate cancer. Eur. J. Cancer. 2013;49:3821–3830. doi: 10.1016/j.ejca.2013.09.026. [DOI] [PubMed] [Google Scholar]
- Sternberg C.N. Improving survival for metastatic castrate-resistant prostate cancer: will combination therapy help us to move forward? Eur. Urol. 2016;70:722–723. doi: 10.1016/j.eururo.2016.05.030. [DOI] [PubMed] [Google Scholar]
- Sweeney C.J., Chen Y.H., Carducci M., Liu G., Jarrard D.F., Eisenberger M., Wong Y.N., Hahn N., Kohli M., Cooney M.M., Dreicer R., Vogelzang N.J., Picus J., Shevrin D., Hussain M., Garcia J.A., Dipaola R.S. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material and methods