Significance
Despite current treatments, lung cancers remain a major public health problem. Innovative ways are needed to treat or prevent these cancers. Centrosomes are critical for fidelity of mitosis. Abnormal centrosome numbers can cause aberrant mitosis and cell death. Polo-like kinase 4 (PLK4) regulates centriole duplication, and its deregulation alters centrosome number and mitosis. The potent PLK4 inhibitor CFI-400945 is reported here to exert marked antineoplastic effects against lung cancers. CDK2 inhibition also deregulates mitosis and was found to cooperate with PLK4 antagonism. CFI-400945 is now undergoing phase I clinical trial testing (NCT01954316). Taken together, targeting PLK4 for inhibition holds promise in lung cancer therapy either as a single agent or when combined with an agent that deregulates mitosis.
Keywords: PLK4 inhibitor, CFI-400945, lung cancer, centriole duplication, polyploidy
Abstract
Polo-like kinase 4 (PLK4) is a serine/threonine kinase regulating centriole duplication. CFI-400945 is a highly selective PLK4 inhibitor that deregulates centriole duplication, causing mitotic defects and death of aneuploid cancers. Prior work was substantially extended by showing CFI-400945 causes polyploidy, growth inhibition, and apoptotic death of murine and human lung cancer cells, despite expression of mutated KRAS or p53. Analysis of DNA content by propidium iodide (PI) staining revealed cells with >4N DNA content (polyploidy) markedly increased after CFI-400945 treatment. Centrosome numbers and mitotic spindles were scored. CFI-400945 treatment produced supernumerary centrosomes and mitotic defects in lung cancer cells. In vivo antineoplastic activity of CFI-400945 was established in mice with syngeneic lung cancer xenografts. Lung tumor growth was significantly inhibited at well-tolerated dosages. Phosphohistone H3 staining of resected lung cancers following CFI-400945 treatment confirmed the presence of aberrant mitosis. PLK4 expression profiles in human lung cancers were explored using The Cancer Genome Atlas (TCGA) and RNA in situ hybridization (RNA ISH) of microarrays containing normal and malignant lung tissues. PLK4 expression was significantly higher in the malignant versus normal lung and conferred an unfavorable survival (P < 0.05). Intriguingly, cyclin dependent kinase 2 (CDK2) antagonism cooperated with PLK4 inhibition. Taken together, PLK4 inhibition alone or as part of a combination regimen is a promising way to combat lung cancer.
Lung cancer is the leading cause of cancer-related mortality worldwide (1–4). Even with current treatments, the 5-y survival rate of lung cancer is only about 17% (1–4). Given this, there is a pressing need for innovative ways to address this major public health problem.
Polo-like kinase 4 (PLK4) is a polo-like kinase family member of the serine/threonine kinases that plays a critical role in regulating centriole duplication (5–8). PLK4 is overexpressed in breast cancers, and this is associated with poor clinical prognosis (9–11). A similar expression profile is found in brain tumors and certain pediatric tumors (12–14). PLK4 deregulation alters centriole duplication and causes aberrant numbers of centrosomes in cells (5–7). PLK4 depletion arrests centriole duplication; in contrast, PLK4 overexpression generates excessive centrioles and promotes centrosome amplification (5–7, 15, 16). These altered centrosome numbers can lead to asymmetric chromosome segregation at mitosis (17–20). While these actions could confer a proliferative advantage to some cell populations (19, 21–25), it often triggers cell death after chromosome missegregation and mitotic defects (26, 27). Indeed, depletion of PLK4 by RNAi hinders centriole duplication and causes death of breast cancer cells in vitro and inhibits breast cancer xenograft growth in vivo (28).
CFI-400945 is a potent and selective small-molecule inhibitor of PLK4 that is undergoing phase I clinical trial testing (NCT01954316) (28–30). The IC50 of CFI-400945 against PLK4 is 0.6 nM, which is a higher magnitude by at least two orders than those against other kinases, including other PLK family members (31). Notably, CFI-400945 has a bimodal effect on centriole number, based on its concentration. In the presence of low CFI-400945 concentrations, PLK4 activity is inhibited partially and PLK4 by this is not sufficiently autophosphorylated for its degradation (28). However, PLK4 retains the ability to phosphorylate its substrate (28). This results in increased PLK4 levels and centriole overduplication. At higher concentrations, CFI-400945 inhibits PLK4 activity fully and blocks centriole duplication (28). Both concentrations of CFI-400945 treatment cause aberrant centrosome numbers; this leads to mitotic catastrophe, cell-cycle arrest, as well as cell death (28).
Notably, CFI-400945 elicited antineoplastic effects in breast cancer cells through aberrant centriole duplication and abnormal mitosis (28). This marked antitumor activity was confirmed in vivo using breast cancer xenograft models, and treatment dosages were well-tolerated (28). CFI-400945 treatment reduced tumor growth and increased survival of studied human pancreatic cancer xenograft models (32).
These encouraging antineoplastic findings prompted us to explore the consequences of CFI-400945 treatment in lung cancer. In this study, effects of CFI-400945 on proliferation, apoptosis, DNA content, centrosome number, and mitosis were comprehensively interrogated using well-characterized murine and human lung cancer cells. In vivo antineoplastic effects of CFI-400945 were then systematically analyzed using a clinically relevant syngeneic murine lung cancer xenograft model. In addition, clinical consequences of PLK4 expression profiles were independently assessed in human lung cancers using The Cancer Genome Atlas (TCGA) and by RNA in situ hybridization (RNA ISH) of lung cancer arrays. Finally, the combination of CFI-400945 with CDK2 inhibition, which triggers multipolar anaphase catastrophe in lung cancer cells (33), was interrogated as a potential regimen to cooperatively reduce tumorigenicity. Taken together, the findings presented here indicate that PLK4 inhibition holds promise to combat lung cancers in the clinic.
Results
Effects of CFI-400945 in Lung Cancer Cells.
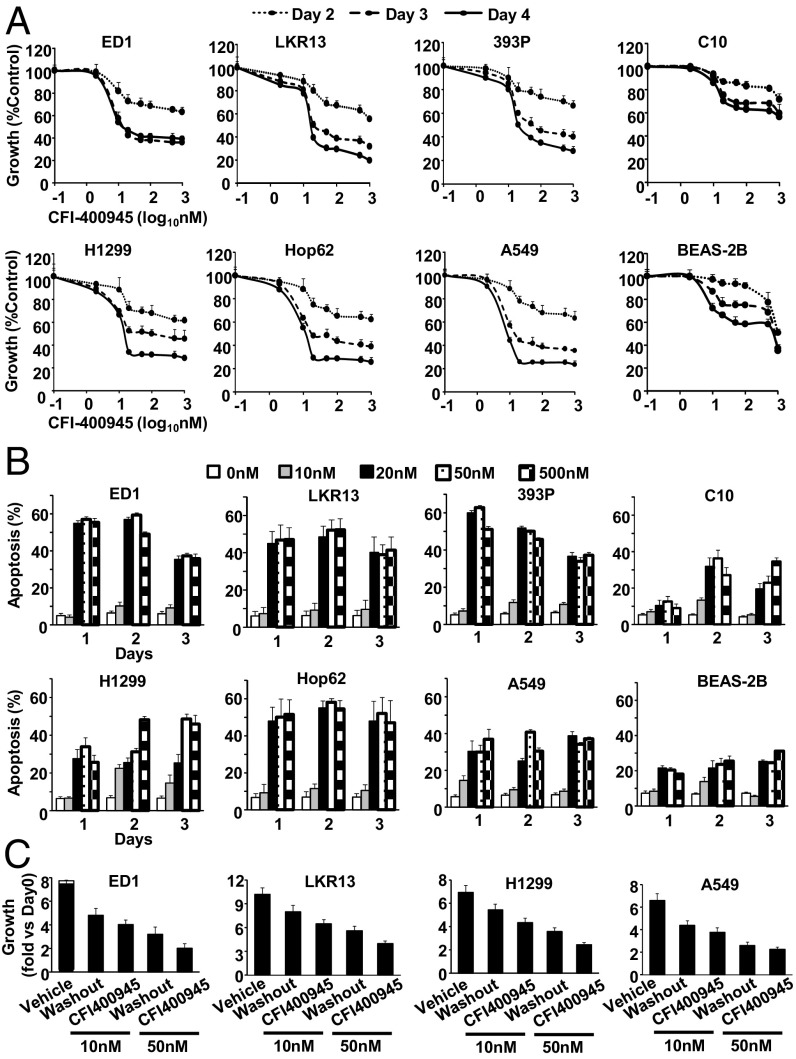
To study antineoplastic effects of CFI-400945 in lung cancer cells, cell proliferative responses to CFI-400945 treatments versus vehicle controls were first examined in genetically defined murine (ED1, LKR13, and 393P) and independently in human (H1299, Hop62, and A549) lung cancer cell lines. CFI-400945 markedly decreased cell proliferation in a dose- and time-dependent manner at nanomolar levels (Fig. 1A). Growth of C10 murine immortalized pulmonary epithelial cells and BEAS-2B human immortalized bronchial epithelial cells were substantially reduced by CFI-400945 treatments, but these effects were less evident than detected in lung cancer cells (Fig. 1A). Growth-inhibitory effects were independently confirmed in a panel of 20 lung cancer cell lines (SI Appendix, Fig. S1). No association was observed between the presence of common genetic alterations such as KRAS mutation and response to CFI-400945 treatment (SI Appendix, Fig. S2).
Fig. 1.
Antiproliferative effects and apoptosis induction by independent CFI-400945 treatments of murine and human lung cancer cells. (A) Dose–response consequences of CFI-400945 treatment in murine (ED1, LKR13, and 393P) and human (H1299, Hop62, and A549) lung cancer cells. Effects in murine immortalized pulmonary epithelial cells (C10) and human immortalized bronchial epithelial cells (BEAS-2B) are also shown. (B) Percentages of apoptotic cells after CFI-400945 treatment of murine (ED1, LKR13, and 393P) and human (H1299, Hop62, and A549) lung cancer cells. Effects on C10 and BEAS-2B cells are displayed. (C) Comparisons are presented for CFI-400945 treatments in lung cancer cells between vehicle controls, washout (CFI-400945 washout after 24 h treatment), and CFI-400945 continuously treated groups. Error bars are SD.
Apoptosis induction after CFI-400945 treatment was next examined in lung cancer cells. CFI-400945 readily induced apoptosis at concentrations of 20 nM or higher in murine (ED1, LKR13, and 393P) and human (H1299, Hop62, and A549) lung cancer cells (Fig. 1B). C10 murine immortalized pulmonary epithelial cells and BEAS-2B human immortalized bronchial epithelial cells were also affected but less so than were corresponding lung cancer cell lines (Fig. 1B).
Washout of CFI-400945 after treatment of lung cancer cells revealed growth inhibition by CFI-400945 was only partially reversed (Fig. 1C). This indicated an irreversible mechanism was engaged in these antineoplastic effects of CFI-400945 in lung cancer cells. Studies were next conducted to uncover the engaged mechanism.
Polyploidy and CFI-400945 Treatment.
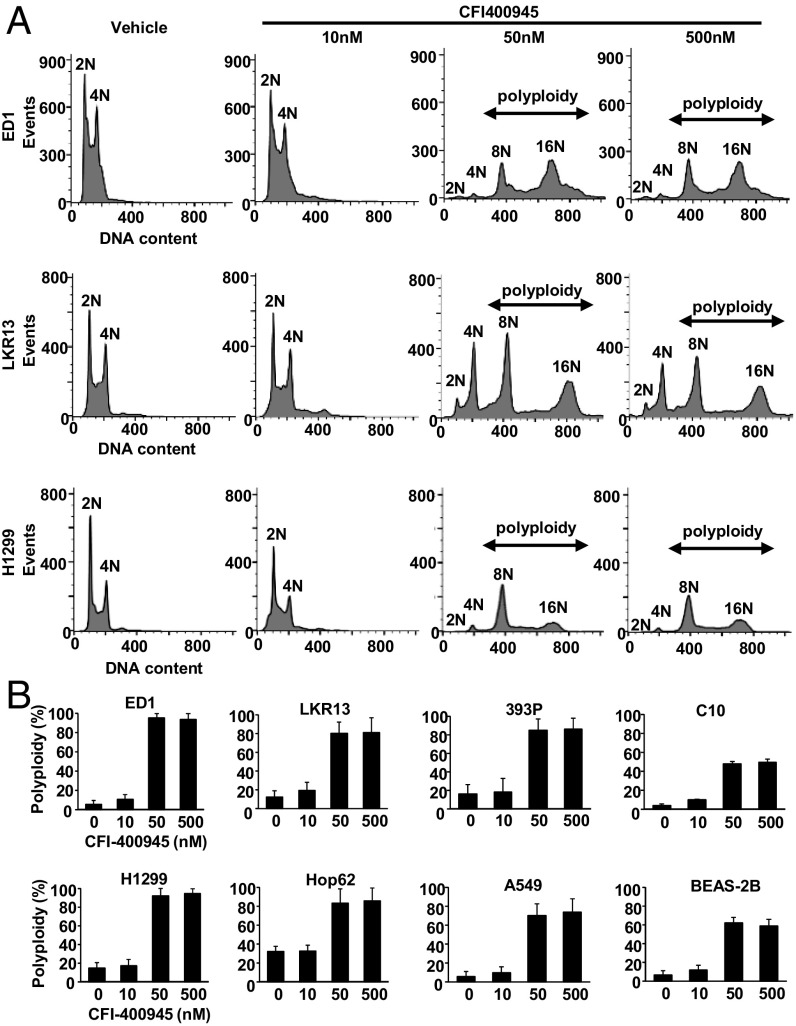
CFI-400945 effects on DNA content in lung cancer cells were determined by propidium iodide (PI) staining using flow cytometry. Representative histograms after flow cytometry analysis appear in Fig. 2A. CFI-400945 treatment increased cellular populations having >4N DNA content (polyploidy) in murine (ED1, LKR13, and 393P) and human (H1299, Hop62, and A549) lung cancer cells (Fig. 2B). This finding indicated cell mitotic defects and continued DNA replication occurred after CFI-400945 treatment of these cells. The increase in polyploid cells was less evident in C10 murine immortalized pulmonary epithelial cells and BEAS-2B human immortalized bronchial epithelial cells (Fig. 2B).
Fig. 2.
Polyploidy in murine and human lung cancer cells after independent CFI-400945 treatments. (A) Representative histograms are shown of flow cytometry analysis with PI staining after CFI-400945 treatment of lung cancer cells. (B) Percentages of polyploid cells are displayed after CFI-400945 treatment of murine (ED1, LKR13, and 393P) and human (H1299, Hop62, and A549) lung cancer cells. Effects on C10 and BEAS-2B cells are also displayed. Error bars are SD.
Supernumerary Centrosomes and CFI-400945 Treatment.
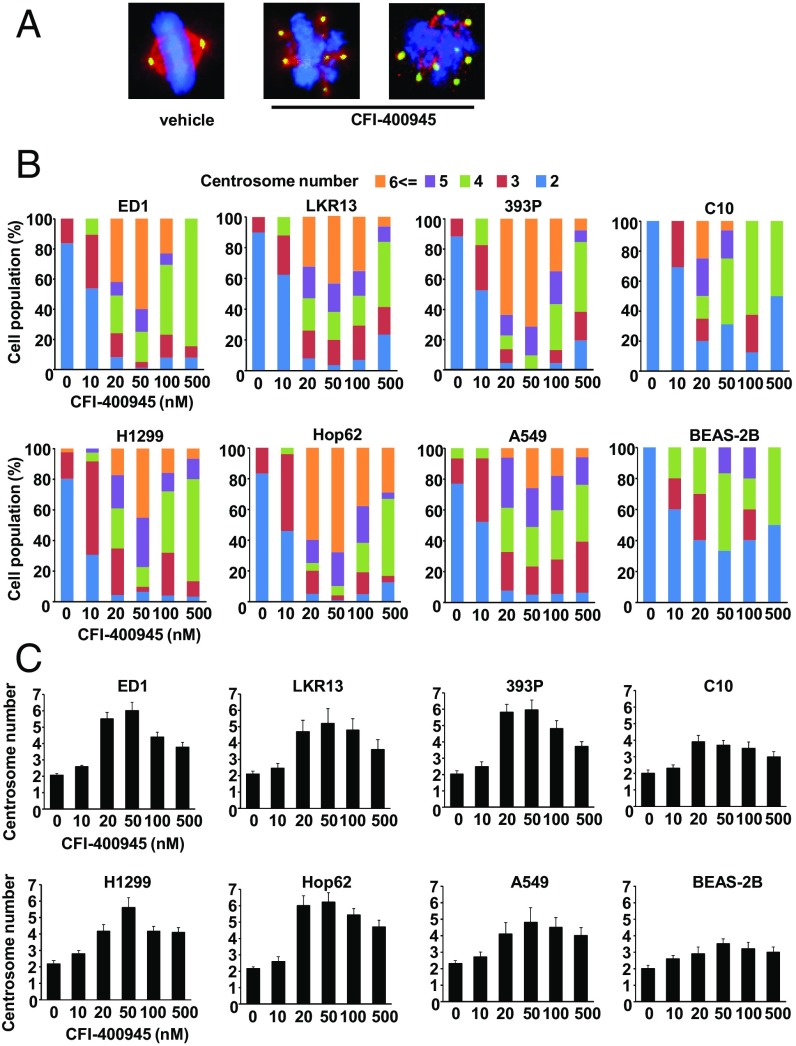
Centrosome number and mitotic status were scored after CFI-400945 or vehicle treatments by staining indicated cells with γ-tubulin, α-tubulin, or DAPI. Representative cell images are displayed in Fig. 3A. As expected, CFI-400945 treatment caused both aberrant centrosome number and altered mitosis. Cell populations with supernumerary centrosomes and multipolar spindles were increased in murine (ED1, LKR13, and 393P) and human (H1299, Hop62, and A549) lung cancer cells following CFI-400945 treatment (Fig. 3B). The average numbers of centrosomes per cell after treatments are shown in Fig. 3C. At concentrations higher than 50 nM, the ability of CFI-400945 to augment supernumerary centrosomes decreased (Fig. 3 B and C). This is likely due to a bimodal effect of CFI-400945 treatment on centrosome number where low CFI-400945 concentrations increased PLK4 activity through inhibition of PLK4 autophosphorylation that confers its degradation (28). This led to centriole overduplication. In contrast, high CFI-40945 concentrations inhibited PLK4 activity more fully and blocked centriole duplication.
Fig. 3.
CFI-400945 treatment affects centrosome number in murine and human lung cancer cells. (A) Representative cell images are shown for cells with two centrosomes or with supernumerary centrosomes and multipolar spindles before and after CFI-400945 treatment. The blue signal is DAPI staining, the red signal displays α-tubulin staining, and the green signal shows γ-tubulin staining. (Magnification: 60×.) (B) Percentages of cell populations are displayed according to centrosome number before or after CFI-400945 treatment of murine (ED1, LKR13, and 393P) and human (H1299, Hop62, and A549) lung cancer cells. Effects on C10 murine immortalized pulmonary epithelial cells and BEAS-2B human immortalized bronchial epithelial cells are shown. (C) Average centrosome number per cell after CFI-400945 treatment is shown for murine (ED1, LKR13, and 393P) and human (H1299, Hop62, and A549) lung cancer cells. Effects on C10 and BEAS-2B cells are displayed. Error bars are SD.
Notably, cell populations with clustered centrosomes were not appreciably affected by CFI-400945 treatment (SI Appendix, Fig. S3). This indicated that supernumerary centrosomes after CFI-400945 treatment likely resulted from de novo centrosome production rather than from inhibition of clustering of supernumerary centrosomes that already existed. Engineered gain of expression of CP110, a centrosome protein engaged in centrosome clustering (34, 35), did not rescue CFI-400945 antiproliferative effects in examined lung cancer cells (SI Appendix, Fig. S4).
In Vivo CFI-400945 Treatment Effects.
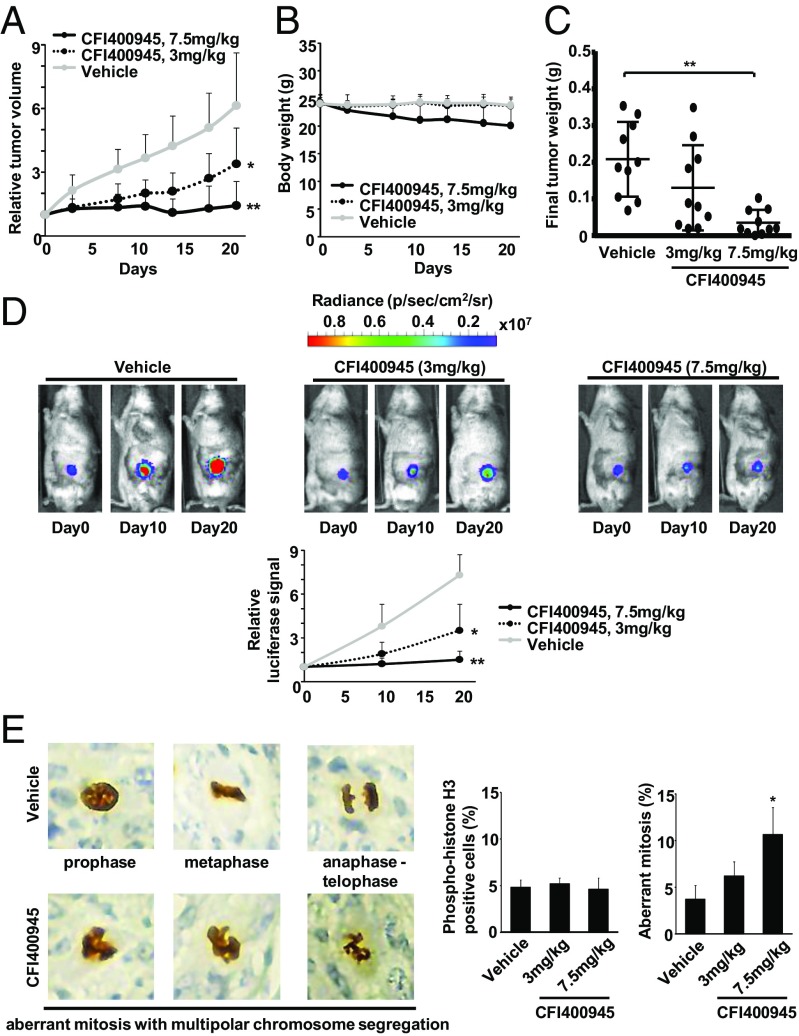
In vivo CFI-400945 effects on lung cancer growth were examined using a syngeneic murine lung cancer xenograft model. 393P KRAS mutant murine lung cancer cells were engineered to stably express luciferase. Cells were s.c. injected into immunocompetent syngeneic mice, which were then treated with vehicle or CFI-400945 (3 mg/kg or 7.5 mg/kg) by oral gavage once daily for 3 wk.
CFI-400945 treatments elicited in vivo antineoplastic effects in a dose-dependent manner with the greatest suppression of lung tumor growth at the highest treatment dose (7.5 mg/kg) (Fig. 4A). Some weight loss occurred at the high treatment dosage group (7.5 mg/kg), but this was clinically well-tolerated in mice (Fig. 4B). Size and weights of lung tumors excised after treatment were significantly (P < 0.01) smaller in the CFI-400945–treated than in the vehicle-treated groups (Fig. 4C and SI Appendix, Fig. S5). Tumor burdens were independently monitored using bioluminescent signals. Representative bioluminescent images of vehicle- versus CFI-400945–treated mice appear in Fig. 4D. Consistent with the results from tumor volume measurements (Fig. 4A), the observed increase in bioluminescence in vehicle-treated mice was inhibited in CFI-400945–treated mice in a dose-dependent manner (Fig. 4D).
Fig. 4.
In vivo antitumorigenic activity of CFI-400945 treatments. (A) Murine syngeneic 393P lung cancer cell line growth in mice treated with vehicle or CFI-400945 [3 mg/kg once per day (QD) and 7.5 mg/kg QD] is displayed. Day 0 is the treatment start date. (B) Mouse body weight changes are shown during vehicle or CFI-400945 (3 mg/kg QD and 7.5 mg/kg QD) treatments. Error bars are SD. (C) Comparison of tumor weights after treatments with vehicle or CFI-400945 (3 mg/kg QD and 7.5 mg/kg QD). Each symbol represents a single mouse. Bars represent mean value and SDs. (D) Comparison of bioluminescent signals of syngeneic lung cancers in mice treated with vehicle or CFI-400945 (3 mg/kg QD and 7.5 mg/kg QD). Representative bioluminescent images of these mice are shown over time in Upper. (E) Analysis of mitotic status by phosphohistone H3 Ser10 staining in in vivo murine lung cancers after CFI-400945 treatment. Representative phosphohistone H3 Ser10 immunostaining images are presented in Left. (Magnification: 40×.) Percentages of phosphohistone H3-positive cells and cells with aberrant mitosis in murine lung cancers after vehicle or CFI-400945 (3 mg/kg QD and 7.5 mg/kg QD) treatments appear in Right. Error bars are SD with *P < 0.05, **P < 0.01.
To examine in vivo effects on mitosis, lung tumor xenografts were stained with phosphohistone H3 10 d after CFI-400945 treatment. Representative immunostained images appear in Fig. 4E. There was no significant change in the overall population of the phosphohistone H3-positive cells (Fig. 4E). However, percentages of aberrant mitosis with multipolar segregation of chromosomes increased in CFI-400945–treated lung cancers compared with the vehicle-treated group (Fig. 4E). This confirmed and extended in vitro effects of CFI-400945 to the in vivo setting of treated lung cancers.
PLK4 Expression Profiles in Lung Cancers.
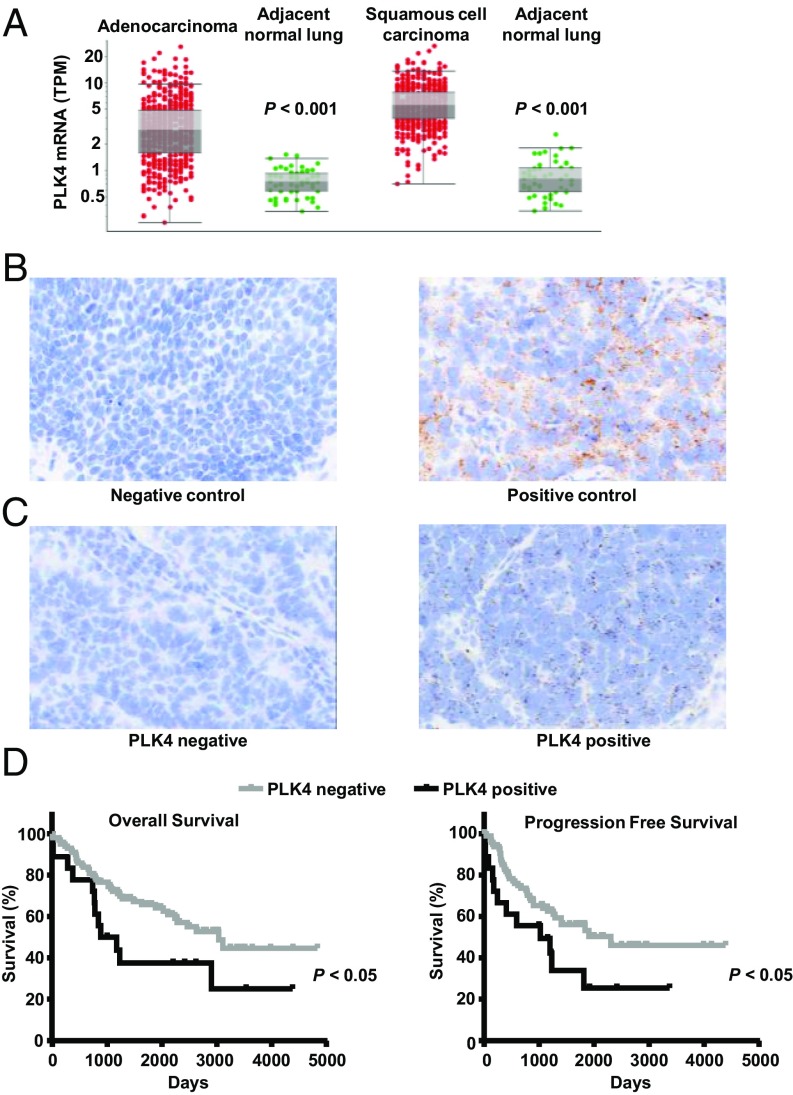
PLK4 mRNA expression profiles in lung cancers were examined using the TCGA database. PLK4 expression was significantly higher in lung adenocarcinomas and squamous cell carcinomas compared with adjacent normal lung tissues (Fig. 5A and SI Appendix, Fig. S6). These TCGA data established that PLK4 mRNA expression increased in diverse malignant compared with corresponding normal tissues (SI Appendix, Fig. S6).
Fig. 5.
PLK4 expression profiles in human lung cancer cases. (A) Comparisons of PLK4 mRNA expression of lung cancers (adenocarcinoma and squamous cell carcinoma) versus adjacent normal lung tissues were explored in the TCGA database. Each symbol represents a single case. (B) Positive and negative controls are displayed for RNA ISH assays. Probes targeting PPIB and DapB were used for positive and negative controls, respectively. (C) Representative images of PLK4 RNA ISH for human lung adenocarcinomas. (D) Kaplan–Meier analysis of overall survival and progression free survival in 235 human lung cancer cases stratified by the presence or absence of PLK4 mRNA expression. (Magnification: B and C, 40×.)
PLK4 mRNA expression was investigated in 235 human lung cancers using tissue microarrays (142 lung adenocarcinomas and 93 squamous cell lung carcinomas) and by RNA ISH. Specificity of hybridization was confirmed using the peptidylprolyl isomerase B (PPIB) probe (as a positive control) and the DapB probe (as a negative control) (Fig. 5B). Representative images for PLK4-negative and PLK4-positive lung adenocarcinomas appear in Fig. 5C. When comparing lung cancers versus adjacent normal lung tissues, PLK4 mRNA expression was substantially higher in lung cancers in agreement with TCGA mRNA data (Fig. 5A). Intriguingly, PLK4 mRNA expression was associated with unfavorable overall and progression-free survivals (P < 0.05) as shown in Fig. 5D. These findings established the translational relevance of examining PLK4 expression in human lung cancer. TCGA analysis independently revealed the association between increased PLK4 expression and unfavorable outcomes in other cancers (SI Appendix, Fig. S7).
Drug Combinations.
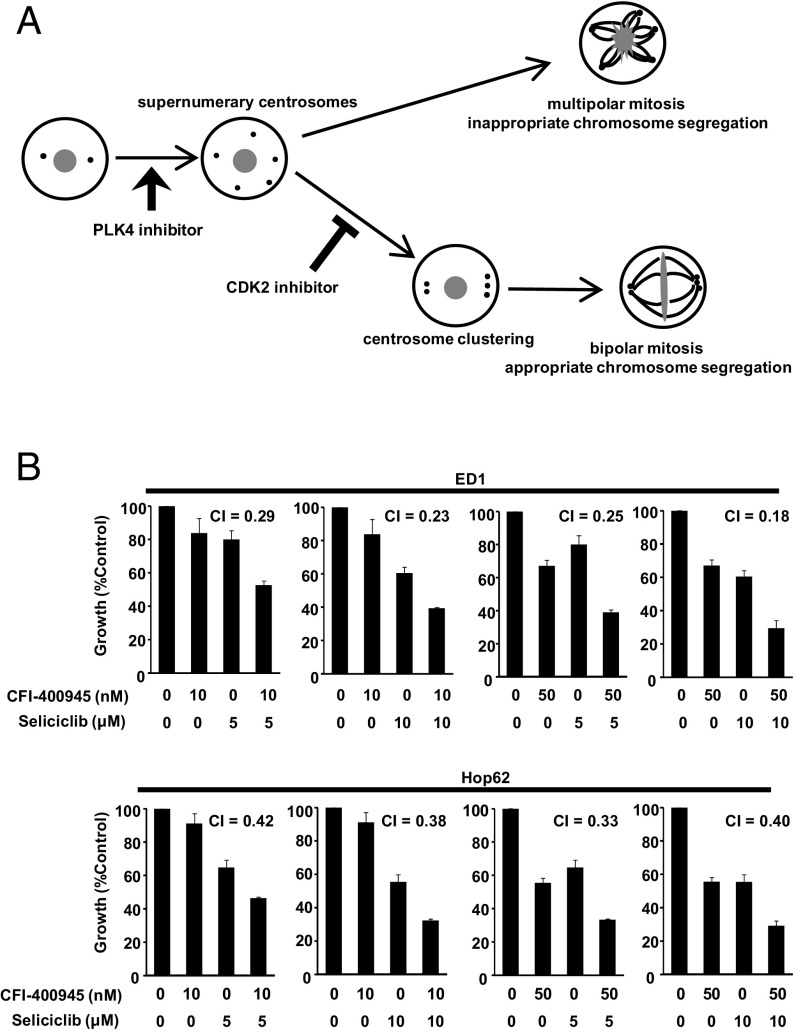
CDK2 inhibition was reported to confer multipolar anaphase catastrophe by inhibiting centrosome clustering (33, 36–38). As shown in Fig. 6A, the generation of supernumerary centrosomes by CFI-400945 and the inhibition of centrosome clustering by CDK2 inhibition each lead to multipolar mitotic defects. Hence, these drugs were hypothesized to elicit cooperative antineoplastic effects. Given this, it was not surprising that the combined treatment of CFI-400945 with seliciclib, a CDK2 inhibitor, caused synergistic effects [combination index (CI) < 1] on growth of ED1 murine and Hop62 human lung cancer cells (Fig. 6B).
Fig. 6.
Cooperative effects of CFI-400945 and seliciclib treatments. (A) Schematic of the functional relationship between supernumerary centrosomes and multipolar mitosis. (B) Effects of combination therapy of PLK4 antagonism (with CFI-400945) and CDK2 inhibition (with seliciclib) on growth of ED1 murine and Hop62 human lung cancer cells.
Discussion
This study reports that CFI-400945, a selective and orally active PLK4 inhibitor, has substantial antineoplastic activities against both murine and human lung cancers. Its activity was evident despite expression of the mutant KRAS oncoprotein that presents a major challenge to the management of lung cancer through onset of clinical resistance (39, 40). Expression of mutant p53 also did not affect the response to PLK4 antagonism.
CFI-400945 treatment readily caused supernumerary centrosomes and deregulated mitosis. This led to multipolar chromosome segregation in lung cancer cells. This triggered apoptotic death of the affected lung cancers, as was previously reported in breast cancer cells (28).
The extent of supernumerary centrosomes produced by CFI-400945 treatment was detected as highest at the 20–50-nM dosage. This gradually declined at even higher examined concentrations. This is likely because CFI-400945 exerts dual effects on centrosome number (28). At low concentrations, CFI-400945 preferentially inhibits autophosphorylation of PLK4 that would cause PLK4 degradation. This stabilization augments PLK4 activity and leads to centriole overduplication. In contrast, CFI-40945 treatment at high concentrations inhibits PLK4 phosphorylation fully. The consequence of this is inhibition of PLK4 activity, and this blocks centriole duplication (28). As expected, growth inhibition and apoptosis induction caused by CFI-400945 treatment of lung cancer cells peaked at concentrations of 20–50 nM. These effects were not enhanced at CFI-400945 treatment dosages higher than 50 nM.
The phenotype of multipolar mitosis induced by CFI-400945 treatment is similar to that of anaphase catastrophe that was previously described (33–38). In those prior studies, it was found that CDK2 inhibition can antagonize centrosome clustering, especially in aneuploid lung cancer cells (33, 37, 38). This confers death of daughter cells after multipolar cell divisions occur (33, 37, 38). The term anaphase catastrophe was chosen to underscore this point (36). Since anaphase catastrophe is caused by the inhibition of clustering of supernumerary centrosomes that already exist, it affects preferentially chromosomally unstable cancer cells with supernumerary centrosomes while relatively sparing cells with two centrosomes (33, 36, 38). Although the phenotype is similar to that of anaphase catastrophe, multipolar mitosis triggered by CFI-400945 treatment differs from that of CDK2 antagonism. It is caused by the distinct mechanisms described here. This is due to de novo generation of supernumerary centrosomes. This explains why control cells like C10 murine immortalized pulmonary epithelial cells and BEAS-2B human immortalized bronchial epithelial cells were still affected by CFI-400945 treatment. In contrast, these same cells were relatively spared from antineoplastic effects of anaphase catastrophe caused by CDK2 inhibition (33, 37, 38).
Cytotoxicity of CFI-400945 was particularly evident in lung cancer cells compared with immortalized pulmonary epithelial cells. This provides a basis for the hypothesized therapeutic window of CFI-400945 treatment in the cancer clinic. When used in vivo, CFI-400945 substantially reduced lung tumor growth at well-tolerated doses. The increased PLK4 RNA ISH expression was found to have an unfavorable outcome in lung cancer cases. PLK4 mRNA expression was examined using the TCGA database, and a statistically significant increase in PLK4 expression was found in the malignant compared with the normal lung. Unfavorable clinical survival was also observed in other cancers that exhibited elevated PLK4 expression profiles. These independent analyses confirmed the translational relevance of PLK4 expression in human lung cancer. The clinical importance of targeting PLK4 for repression should be explored in the cancer clinic.
Since the PLK4 inhibitor CFI-400945 has a distinct mechanism of antineoplastic action, combination therapies with other drugs that act on cooperating pathways are worth exploring. For example, in this study, it was found that combined treatments with CFI-400945 and seliciclib, a CDK2 inhibitor known to confer anaphase catastrophe (33), exert both additive and synergistic effects to reduce lung cancer cell growth. This is not a surprising consequence since both drugs increase multipolar cell division and subsequent cell death. However, these agents transmit their actions through different mechanisms. These include generation of supernumerary centrosomes (for PLK4 inhibition) and inhibition of centrosome clustering (for CDK2 antagonism). Combining these two mechanisms forces affected cancer cells into multipolar cell division even more readily than does each agent alone. Combining CFI-400945 treatment with other classes of drugs that would affect different cooperating pathways is a promising strategy to consider in future work. One example of this is to investigate PLK4 inhibition with a taxane that can target microtubule dynamics and promote death of chromosomally unstable cancer cells. Combination studies with immune checkpoint inhibitors are also interesting because after inducing polyploidy and mitotic defects, CFI-400945 would cause DNA damage that could increase neoantigens; this is proposed to augment immune responses against cancer (41). Notably, TCGA data showed that PLK4 expression in lung cancer is positively correlated with specific immune checkpoint markers (SI Appendix, Table S1).
In summary, CFI-400945, an orally active and selective inhibitor of PLK4, causes marked antitumorigenic effects against lung cancers through generation of supernumerary centrosomes. This causes death of cancer cells by triggering multipolar mitotic defects. CFI-400945 is now undergoing testing in a phase I clinical trial. Further clinical investigations of CFI-400945, including combination therapies with agents that affect different cooperating pathways, are warranted in lung and other cancers.
Materials and Methods
Chemicals and Cell Culture.
CFI-400945 was synthesized, as reported (29). Seliciclib was purchased (Selleck Chemical). The hydrochlorate and fumarate salt forms of CFI-400945 were used in that prior work. Murine lung cancer cell lines ED1, LKR13, and 393P cells were derived from lung cancers arising from wild-type cyclin E, KrasLA1/+, and KrasLA1/+/p53R172HΔG engineered mice, respectively, and were each previously authenticated (42–46). Human lung cancer cell lines (H1299, A549, and Hop62) as well as murine C10 pulmonary epithelial and human BEAS-2B immortalized bronchial epithelial cells were purchased and authenticated by the American Type Culture Collection. Cells were cultured in RPMI 1640 media with 10% FBS at 37 °C with 5% CO2 in a humidified incubator, as described (38).
In Vitro Assays.
Proliferation, apoptosis, and washout assays as well as measurements of cellular DNA contents and centrosome and mitotic analyses are described in SI Appendix.
In Vivo Experiments.
KRAS mutant murine lung cancer 393P cells (46) were infected with the luciferase lentivirus (Cellomics Technology) and selected with puromycin. 393P (1 × 106) stable transfectants were injected s.c. into 6–8-wk-old male immunocompetent 129S2/SVPasCrl mice (Charles River Laboratories). Mice with palpable tumors were treated with CFI-400945 (7.5 mg/kg or 3 mg/kg) or vehicle (water) daily for 3 wk by oral gavage following an Institutional Animal Care and Use-approved protocol (n = 10 mice per group). Body weights and tumor volumes were measured with tumor volume (V) calculated as V = (length × width2)/2. Bioluminescence imaging was by d-Luciferin (Gold Biotechnology) and IVIS Lumina (Xenogen) and Living Imaging software (Xenogen) under 2% isoflurane. Tumors were excised from killed mice and weighed. Independent replicate experiments were performed to confirm results.
In Vivo Mitotic Analyses.
Lung cancer xenografts were fixed with 10% formalin immediately after resection and embedded in paraffin. Paraffin-embedded cancers were sectioned and probed with phosphor-histone H3 (Ser10) antibody (9701; Cell Signaling Technology) and counterstained with hematoxylin. Stained cells were analyzed for mitosis using an Eclipse TE 2000-E microscope (Nikon).
RNA ISH.
All lung cancer tissues were evaluated and underwent surgical resection at The University of Texas MD Anderson Cancer Center. Informed consent was obtained from all patients under the protocol approved by the MD Anderson Institutional Review Boards (LAB07-0233). Detailed experimental methods are described in SI Appendix.
Statistical Analysis.
Differences between analyzed groups were assessed by the Student’s t test or the Mann–Whitney U test. For multiple comparisons, Tukey’s or Dunnett’s methods were applied. Kaplan–Meier survivals were by the log-rank test. Statistical analyses were with SPSS Statistics software (version 23, SPSS) and GraphPad Prism software (version 6; GraphPad Software). All statistical tests were two-sided, and a P value of less than 0.05 was considered significant. Potential additive or synergistic effects of drug combinations were analyzed by the Chou and Talalay (47) method. The CI was calculated using Calcusyn software (Biosoft) based on growth-inhibitory effects of single drugs or of drug combinations (47). CI was used to determine whether the drug combination had synergistic (CI < 1), additive (CI = 1), or antagonistic (CI > 1) effects.
Supplementary Material
Acknowledgments
The authors thank all members of the T.W.M. and E.D. laboratories for their helpful consultations and Dr. Adi Gazdar at the University of Texas Southwestern for generously providing us lung cancer cell lines. This work was supported by the National Institutes of Health (NIH), National Cancer Institute (NCI) Grants R01-CA087546 (to E.D.) and R01-CA190722 (to E.D.), a Samuel Waxman Cancer Research Foundation Award (to E.D.), University of Texas Science and Technology Acquisition and Retention Award (to E.D.), and an American Cancer Society Clinical Research Professorship (E.D.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1719760115/-/DCSupplemental.
References
- 1.Islami F, Torre LA, Jemal A. Global trends of lung cancer mortality and smoking prevalence. Transl Lung Cancer Res. 2015;4:327–338. doi: 10.3978/j.issn.2218-6751.2015.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends–An update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 4.Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. doi: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 5.Bettencourt-Dias M, et al. SAK/PLK4 is required for centriole duplication and flagella development. Curr Biol. 2005;15:2199–2207. doi: 10.1016/j.cub.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 6.Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA. The polo kinase Plk4 functions in centriole duplication. Nat Cell Biol. 2005;7:1140–1146. doi: 10.1038/ncb1320. [DOI] [PubMed] [Google Scholar]
- 7.Kleylein-Sohn J, et al. Plk4-induced centriole biogenesis in human cells. Dev Cell. 2007;13:190–202. doi: 10.1016/j.devcel.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Barr FA, Silljé HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–440. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- 9.Hu Z, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics. 2006;7:96. doi: 10.1186/1471-2164-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Vijver MJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 11.Miller LD, et al. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci USA. 2005;102:13550–13555. doi: 10.1073/pnas.0506230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pezuk JA, et al. PLK1-associated microRNAs are correlated with pediatric medulloblastoma prognosis. Childs Nerv Syst. 2017;33:609–615. doi: 10.1007/s00381-017-3366-5. [DOI] [PubMed] [Google Scholar]
- 13.Sredni ST, et al. A functional screening of the kinome identifies the polo-like kinase 4 as a potential therapeutic target for malignant rhabdoid tumors, and possibly, other embryonal tumors of the brain. Pediatr Blood Cancer. 2017;64 doi: 10.1002/pbc.26551. [DOI] [PubMed] [Google Scholar]
- 14.Sredni ST, Tomita T. The polo-like kinase 4 gene (PLK4) is overexpressed in pediatric medulloblastoma. Childs Nerv Syst. 2017;33:1031. doi: 10.1007/s00381-017-3452-8. [DOI] [PubMed] [Google Scholar]
- 15.Peel N, Stevens NR, Basto R, Raff JW. Overexpressing centriole-replication proteins in vivo induces centriole overduplication and de novo formation. Curr Biol. 2007;17:834–843. doi: 10.1016/j.cub.2007.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holland AJ, Lan W, Niessen S, Hoover H, Cleveland DW. Polo-like kinase 4 kinase activity limits centrosome overduplication by autoregulating its own stability. J Cell Biol. 2010;188:191–198. doi: 10.1083/jcb.200911102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macmillan JC, Hudson JW, Bull S, Dennis JW, Swallow CJ. Comparative expression of the mitotic regulators SAK and PLK in colorectal cancer. Ann Surg Oncol. 2001;8:729–740. doi: 10.1007/s10434-001-0729-6. [DOI] [PubMed] [Google Scholar]
- 18.Basto R, et al. Centrosome amplification can initiate tumorigenesis in flies. Cell. 2008;133:1032–1042. doi: 10.1016/j.cell.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko MA, et al. Plk4 haploinsufficiency causes mitotic infidelity and carcinogenesis. Nat Genet. 2005;37:883–888. doi: 10.1038/ng1605. [DOI] [PubMed] [Google Scholar]
- 21.Lingle WL, et al. Centrosome amplification drives chromosomal instability in breast tumor development. Proc Natl Acad Sci USA. 2002;99:1978–1983. doi: 10.1073/pnas.032479999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajagopalan H, Lengauer C. Aneuploidy and cancer. Nature. 2004;432:338–341. doi: 10.1038/nature03099. [DOI] [PubMed] [Google Scholar]
- 23.Gao C, et al. Chromosome instability, chromosome transcriptome, and clonal evolution of tumor cell populations. Proc Natl Acad Sci USA. 2007;104:8995–9000. doi: 10.1073/pnas.0700631104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silkworth WT, Nardi IK, Scholl LM, Cimini D. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS One. 2009;4:e6564. doi: 10.1371/journal.pone.0006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beach RR, et al. Aneuploidy causes non-genetic individuality. Cell. 2017;169:229–242.e21. doi: 10.1016/j.cell.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nigg EA. Centrosome aberrations: Cause or consequence of cancer progression? Nat Rev Cancer. 2002;2:815–825. doi: 10.1038/nrc924. [DOI] [PubMed] [Google Scholar]
- 27.Kwon M, et al. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev. 2008;22:2189–2203. doi: 10.1101/gad.1700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason JM, et al. Functional characterization of CFI-400945, a polo-like kinase 4 inhibitor, as a potential anticancer agent. Cancer Cell. 2014;26:163–176. doi: 10.1016/j.ccr.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Sampson PB, et al. The discovery of polo-like kinase 4 inhibitors: Identification of (1R,2S).2-(3-((E).4-(((cis).2,6-dimethylmorpholino)methyl)styryl). 1H.indazol-6-yl)-5′-methoxyspiro[cyclopropane-1,3′-indolin]-2′-one (CFI-400945) as a potent, orally active antitumor agent. J Med Chem. 2015;58:147–169. [PubMed] [Google Scholar]
- 30.Yu B, Yu Z, Qi PP, Yu DQ, Liu HM. Discovery of orally active anticancer candidate CFI-400945 derived from biologically promising spirooxindoles: Success and challenges. Eur J Med Chem. 2015;95:35–40. doi: 10.1016/j.ejmech.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 31.Liu X. Targeting polo-like kinases: A promising therapeutic approach for cancer treatment. Transl Oncol. 2015;8:185–195. doi: 10.1016/j.tranon.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lohse I, et al. Activity of the novel polo-like kinase 4 inhibitor CFI-400945 in pancreatic cancer patient-derived xenografts. Oncotarget. 2017;8:3064–3071. doi: 10.18632/oncotarget.13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galimberti F, et al. Targeting the cyclin E-Cdk-2 complex represses lung cancer growth by triggering anaphase catastrophe. Clin Cancer Res. 2010;16:109–120. doi: 10.1158/1078-0432.CCR-09-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu S, et al. CDK2 inhibition causes anaphase catastrophe in lung cancer through the centrosomal protein CP110. Cancer Res. 2015;75:2029–2038. doi: 10.1158/0008-5472.CAN-14-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu S, et al. Specific CP110 phosphorylation sites mediate anaphase catastrophe after CDK2 inhibition: Evidence for cooperation with USP33 knockdown. Mol Cancer Ther. 2015;14:2576–2585. doi: 10.1158/1535-7163.MCT-15-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galimberti F, Thompson SL, Ravi S, Compton DA, Dmitrovsky E. Anaphase catastrophe is a target for cancer therapy. Clin Cancer Res. 2011;17:1218–1222. doi: 10.1158/1078-0432.CCR-10-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danilov AV, et al. Dinaciclib induces anaphase catastrophe in lung cancer cells via inhibition of cyclin dependent kinases 1 and 2. Mol Cancer Ther. 2016;15:2758–2766. doi: 10.1158/1535-7163.MCT-16-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawakami M, et al. Next-generation CDK2/9 inhibitors and anaphase catastrophe in lung cancer. J Natl Cancer Inst. 2017;109:djw297. doi: 10.1093/jnci/djw297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts PJ, Stinchcombe TE. KRAS mutation: Should we test for it, and does it matter? J Clin Oncol. 2013;31:1112–1121. doi: 10.1200/JCO.2012.43.0454. [DOI] [PubMed] [Google Scholar]
- 40.Aviel-Ronen S, Blackhall FH, Shepherd FA, Tsao MS. K-ras mutations in non-small-cell lung carcinoma: A review. Clin Lung Cancer. 2006;8:30–38. doi: 10.3816/CLC.2006.n.030. [DOI] [PubMed] [Google Scholar]
- 41.Germano G, et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature. 2017;552:116–120. doi: 10.1038/nature24673. [DOI] [PubMed] [Google Scholar]
- 42.Ma Y, et al. Transgenic cyclin E triggers dysplasia and multiple pulmonary adenocarcinomas. Proc Natl Acad Sci USA. 2007;104:4089–4094. doi: 10.1073/pnas.0606537104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freemantle SJ, Dmitrovsky E. Cyclin E transgenic mice: Discovery tools for lung cancer biology, therapy, and prevention. Cancer Prev Res (Phila) 2010;3:1513–1518. doi: 10.1158/1940-6207.CAPR-10-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu X, et al. MicroRNA-31 functions as an oncogenic microRNA in mouse and human lung cancer cells by repressing specific tumor suppressors. J Clin Invest. 2010;120:1298–1309. doi: 10.1172/JCI39566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wislez M, et al. High expression of ligands for chemokine receptor CXCR2 in alveolar epithelial neoplasia induced by oncogenic kras. Cancer Res. 2006;66:4198–4207. doi: 10.1158/0008-5472.CAN-05-3842. [DOI] [PubMed] [Google Scholar]
- 46.Gibbons DL, et al. Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression. Genes Dev. 2009;23:2140–2151. doi: 10.1101/gad.1820209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.