Supplemental digital content is available in the text.
Abstract
Background
Antibody-mediated rejection in solid organ transplantation is an important immunological barrier to successful long-term graft survival. Next to complement activation, natural killer (NK) cells have been implicated in the process. Human cytomegalovirus (CMV), independently associated with decreased graft survival, has a strong imprint on the immune response. Here, we assessed the effect of CMV status on alloreactive NK cell reactivity.
Methods
We compared antibody-mediated NK cell cytolytic activity (CD107a expression) and IFNγ production between healthy CMV-seropositive (n = 8) and CMV-seronegative (n = 11) individuals, in cocultures of NK cells with anti-HLA class I or rituximab (control) antibody-coated Raji cells.
Results
First, we showed that within the NKG2C+ NK cells, it is specifically the NKG2C+/A− subset that is enriched in CMV+ individuals. We then observed that in particular the NK cell antibody-dependent cell mediated cytotoxicity (ADCC), but also non-ADCC alloreactivity toward HLA-positive target cells was increased in CMV+ individuals as compared to CMV− ones. This enhanced ADCC as well as non-ADCC NK cell reactivity in CMV+ individuals was particularly characterized by a significantly higher number of ILT2+ and NKG2C+ NK cells that possessed cytolytic activity and/or produced IFNγ in response to HLA-positive target cells.
Conclusions
With regard to organ transplantation, these data suggest that CMV infection enhances NK cell alloreactivity, which may pose an additional adverse effect on graft survival, especially in the presence of donor specific antibodies.
Solid-organ transplant rejection occurs when the graft is adversely affected by the recipient’s immune system. Despite the use of potent immunosuppressive drugs, the occurrence of chronic rejection, and consequently graft rejection is still a serious problem.
Several factors have been highlighted as risks for solid organ rejection; one being the occurrence of cytomegalovirus (CMV) infection. Infection with the human CMV is an important cause of morbidity and mortality in solid organ recipients and was implicated in the pathogenesis of allograft rejection.1-4 However, how CMV mediates this rejection is still unclear.
One of the key cells in the immune response to CMV infection is the natural killer (NK) cell. NK cells have been shown to proliferate and increase their reactivity in response to CMV viremia.5,6 Over time, CMV infection induces a stable imprint in the NK cell receptor repertoire, involving the activating lectin-like receptor NKG2C and killer immunoglobulin-like receptors (KIRs).7-9 The resultant CMV-specific NK cells have a differentiated mature phenotype exhibiting specialized antibody-dependent cell cytotoxicity (ADCC) and showing poor interferon gamma (IFNγ) production to cytokine stimulation.7,8,10,11
Antibody-mediated rejection (AMR) poses a significant risk for long-term graft survival of solid organ transplantation with hardly any effective immunosuppressive treatment.12-15 Compared with T cell–mediated rejection, AMR poses a greater risk to long-term graft survival.16,17 The antibodies involved are mostly directed against human leukocyte antigen (HLA) class I and II antigens. AMR can be mediated via the activation of the classical complement pathway or via complement independent ADCC.14,18 Although the complement pathway has been highlighted as the main cause of acute AMR, several studies have shown that NK cells have a significant role in complement-independent and chronic AMR.13,17,19,20 In kidney transplantation, ADCC pathways involving NK cells have been highlighted to be active during AMR and consistently suggest mediation of allograft injury in a complement independent manner.21,22
These observations led us to investigate in vitro the effect of CMV infection on NK cell antibody-mediated reactivity. We isolated NK cells from CMV+ and CMV− healthy individuals and tested them for in vitro reactivity to anti-HLA antibody-coated allogeneic target cells. Our results show that NK cells derived from CMV+ individuals have an increased reactivity to allogeneic target cells, both in the absence and presence of target cell-specific antibodies compared to NK cells from CMV− individuals.
MATERIALS AND METHODS
NK Cell Isolation and Enrichment
NK cells were isolated from buffy coats of 19 healthy blood donors purchased from Sanquin Blood Supply Foundation, region Southeast, Nijmegen, The Netherlands. Buffy coats were obtained upon written consent from the donor for scientific use, and according to Dutch law. Gradient centrifugation using Lymphoprep (Nycomed Pharma, Norway) was used to isolate peripheral blood mononuclear cells (PBMCs). NK cells were isolated using the MACS NK cell isolation kit according to the manufacturer’s instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). NK cell purity was measured by flow cytometry; NK cells were defined as CD56+/CD3− lymphocytes and purity ranged between 85% and 95%. NK cells were subsequently frozen at −80°C for later use.
CMV Testing
Of all voluntary blood donors, a serum aliquot was collected for CMV testing. Detection of anti-CMV IgG antibodies was performed using a commercially available ELISA immunoassay (Serion, Wurzburg, Germany), according to the manufacturer’s instructions.
Raji Cell Line
The Raji cell line was cultured in RPMI 1640 medium (Gibco, Life Technologies, Bleiswijk, Netherlands) supplemented with Gibco penicillin (100 U/mL), streptomycin (100 μg/mL), glutamax (2 mM) pyruvate (1 mM) and 10% Greiner Bio-one fetal calf serum (FCS) (Greiner Bio-one, Frickenhausen, Germany). Cultures were maintained at 37°C, 95% humidity and 5% CO2. DNA from Raji cells was isolated using a Qiagen DNA isolation kit (Qiagen Benelux B.V., Venlo, The Netherlands) according to the manufacturer’s instructions. HLA genotyping of the cell line was performed by PCR-SSOP, using Luminex technology (Sanbio, Uden, The Netherlands). HLA class I typing of the Raji cells used was confirmed as HLA: A*03, B*15, C*03, C*04.
NK Cell Antibody-Dependent Reactivity and Flow Cytometric Analysis
Raji cells were either left untouched or precoated with human anti-HLA-A3 IgG antibody, an anti–HLA-A3 human IgM antibody (as negative control)23 and the CD20 antibody rituximab (RTX) (Genentech, San Francisco, CA) (positive control). Rituximab and anti–HLA-A3 IgM coating of Raji cells was done at a concentration of 10 μg/mL, and the anti–HLA-A3 IgG antibody was used at 5 μg/mL. The coating was done in culture medium consisting of RPMI 1640 medium supplemented with Gibco penicillin (100 U/mL), streptomycin (100 μg/mL), glutamax (2 mM), pyruvate (1 mM), and 10% Greiner Bio-one fetal calf serum (FCS) at room temperature for 30 minutes, after which the cells were washed and co-cultured with NK cells. Approximately 2 × 105 NK cells were cocultured with the same number of Raji cells; 1:1 ET ratio in 200 μL culture medium. NK cells alone and NK cells cultured with uncoated Raji cells were used as additional controls. To measure NK cell cytolytic reactivity, the coculture was performed in 200 μL of culture medium in the presence of anti-CD107a BV510 (clone H4A3, Biolegend) antibody, 10 μg/mL brefeldin A and 10 μg/mL monensin. The cells were cultured for 4 hrs at 37 °C, 95% humidity and 5% CO2. After incubation, the cells were washed once and stained for NK cell surface markers with conjugated mAbs for 15 minutes: CD159c PE (NKG2C clone 134522) (R&D, Abingdon, United Kingdom), CD85j PC5.5 (clone HPF1.4), CD158b1b2/j PC7 (clone GL183), CD158e1e2 APC (clone Z27.3.7), CD158ah APC AF700 (EB6.B.3.1.1), CD3 APC AF750 (clone UCHT1), CD56 ECD (clone N901), and CD159a pacific blue(NKG2A)(clone Z199.1.10), all from Beckman Coulter (Woerden, The Netherlands). The cells were then washed and fixed with fixation/permeabilization buffer (BD Biosciences) and stained for IFNγ FITC (cat:554551, BD Pharmingen) for 30 minutes at 4 °C in 1× permeabilization buffer (eBioscience Vienna, Austria). Samples were measured on the Beckman Coulter Navios instrumentation, and the data were analyzed using the Beckman Coulter Kaluza v1.2 software. Marker analysis was done on the CD56+/CD3− NK cell populations gated from the FSc vs SSc plot (for gating strategy, see figures). NK cell marker gates were set based on isotype controls and/or positive/negative staining. We did not include a FSC-H/FSC-A doublet discrimination, which is a limitation to the experimental design.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism. Data are represented as sample median values. Statistical differences were calculated using the nonparametric Mann Whitney test for unpaired analysis, and the nonparametric Wilcoxon matched-pair signed rank test. Differences were considered significant at P < 0.05(*), P < 0.01(**) or P < 0.001(***).
RESULTS
Enhanced Reactivity of NK Cells From CMV+ Individuals
CD56dim NK cells from CMV+ (n = 8) and CMV− (n = 11) healthy individuals were evaluated for selected NK cell receptor expression, that is, NKG2C, NKG2A, ILT2, KIR2DL1/S1, KIR2DL2/L3/S2, and KIR3DL1/S, using flow cytometry (Figure 1A). Previously, selective expansion of NKG2C+ NK cells was highlighted as a hallmark of CMV infection.8,9,24-27 Also here, comparing CMV+ and CMV− individuals, we found higher percentages of this subset upon CMV infection (Figure 1B) (median: 19% versus 7%, respectively; P = 0.009). Notably, a more detailed analysis revealed that the increase in NKG2C-positive cells in the CMV+ individuals could be ascribed to a shift in the NKG2C+/NKG2A- NK cell subset (Figure 1C). Additional analysis of the NKG2C+ population in CMV+ individuals revealed that the NKG2C/ILT2 double positive subset was expanded compared to CMV− individuals (NKG2C+/ILT2+; median 12% vs 4%, P = 0.013) (Figure 1D). A similar analysis was done on CD56bright NK cells (Figure S1, SDC, http://links.lww.com/TXD/A55). This revealed that the NKG2C skew in CMV+ individuals was a particular characteristic of the CD56dim population. In addition, the CD56bright NK cell compartment in CMV+ individuals comprised more ILT2+ cells and less NKG2C–/A+ NK cells than found in CMV− individuals.
FIGURE 1.
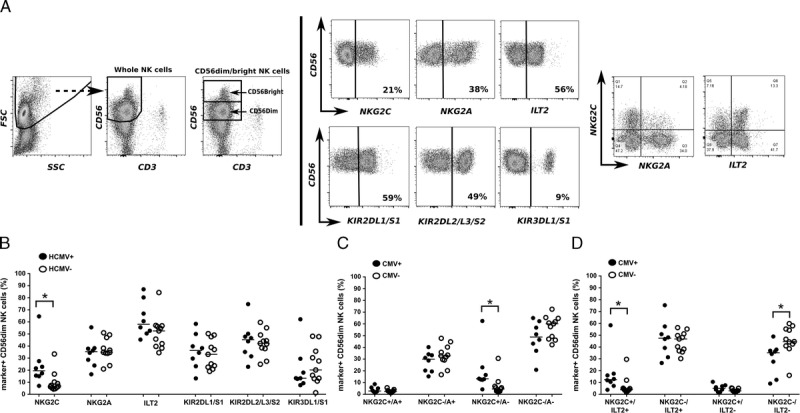
Flow cytometric analysis of NK receptor expression on isolated NK cells from CMV+ (n = 8) and CMV− (n = 11) individuals. A, Gating strategy and example dot plots from a single representative CMV+ individual. CD56+/CD3− cells NK cells gated from the FSc/SSc plot, were further subdivided into CD56dim and `CD56bright cells. Both subsets were further analyzed for the expression of selected markers (for data on CD56bright NK cells see Figure S1, SDC, http://links.lww.com/TXD/A55). B-C, Scatter plots showing cumulative data for the percentage of NK receptor-positive/negative cells (as indicated on the x-axis) within the CD56dim NK population in CMV+ and CMV− individuals; the solid lines indicate median value. Statistical differences were calculated using the nonparametric Mann-Whitney test and differences were considered significant at *P < 0.05, **P < 0.01.
Because the NKG2C+ NK cells resulting from CMV infection have been shown to exhibit a differentiated mature phenotype and enhanced antibody-dependent reactivity,7,8,10,11 we were interested to see how they behaved in the presence of a target cell coated with anti-HLA antibodies. We therefore evaluated the reactivity of NK cells from CMV+ and CMV− individuals to anti–HLA-A3 antibody-coated Raji cells. An IgM anti–HLA-A3 and RTX (anti-CD20 mAb) were used as negative and positive control, respectively. IFNγ production and CD107a expression were measured after stimulation (Figure 2). In CMV+ individuals, baseline (non-ADCC) reactivity against uncoated Raji cells or anti–HLA-A3 IgM-coated Raji cells was higher as compared with CMV− individuals (median %, CD107a-positive cells: CMV+, 29% (uncoated); 30% (IgM) vs CMV−, 17% (uncoated); 16% (IgM), P= 0.03; 0.02, respectively), and there was a trend toward higher IFNγ expression (median % positive cells: CMV+, 8%; 7% vs CMV−, 6%; 5%, P = 0.07; 0.06, respectively). The presence of anti–HLA-A3 IgG antibody, as well as RTX strongly increased the reactivity in both CMV+ and CMV− individuals, while still revealing a higher level of reactivity in the CMV+ group for both CD107a and IFNγ expression (median % CD107a for anti–HLA-A3 IgG, CMV+ vs CMV−, 45% vs 36%; P = 0.01; median % IFNγ CMV+ vs CMV−, 14% vs 10%; P = 0.02) (median % CD107a for RTX: CMV+ vs CMV−, 48% vs 36%, P = 0.05; median % IFNγ CMV+ vs CMV−, 14% vs 10%, P = 0.02) (Figure 2).
FIGURE 2.
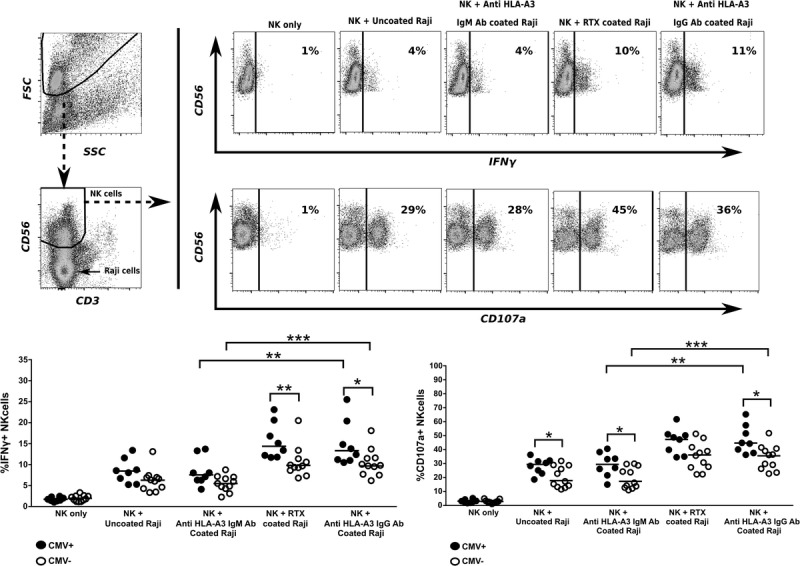
Percentage of IFNγ-producing and degranulating (CD107a expressing) NK cells in response to allogeneic Raji cells coated with (anti-HLA) antibodies in CMV+ (n = 8) and CMV− (n = 11) individuals. 2 × 105 NK cells from CMV+ and CMV− individuals were cultured for 4 hours, either alone, or with uncoated or anti–HLA-A3 IgG, anti–HLA-A3 IgM (negative control) or RTX (positive control) antibody-coated Raji cells. Coculture was done at a 1:1 ratio for 4 hours in 200-μL medium, in the presence of anti-CD107a BV510 antibody, 10 μg/mL brefeldin A and 10 μg/mL monensin. After incubation, the cells were additionally stained for IFNγ and selected NK cell markers and analysed using flow cytometry. The upper left panel shows the gating strategy, whereby NK cells were gated out based on the CD56/CD3 plot, using software settings that included the co-cultured Raji cells (indicated by arrow). The upper right panel shows a representative example of a CMV+ individual. The lower panel shows scatter plots of cumulative data on IFNγ expression (left panel) and CD107a expression (right panel); the solid line indicates median value. Statistical analysis was done using the nonparametric Mann-Whitney test for unpaired analysis, and the nonparametric Wilcoxon matched-pair signed rank test where appropriate and differences were considered significant at *P < 0.05, **P < 0.01, or ***P < 0.001.
We thus observed that NK cell reactivity toward Raji cells was augmented in CMV+ NK cells compared with CMV− NK cells, and this was particularly so for the antibody-dependent cell reactivity in the presence of target cell-specific antibodies, that is, both anti–HLA-A3 IgG as well as RTX.
NK Cell Receptor Expression on Reactive NK Cells From CMV+ and CMV—Individuals
Having observed an overall higher reactivity from NK cells derived from CMV+ individuals, and knowing that NK cell function is closely related to surface receptor repertoire,28,29 we set out to further characterize the reactive (CD107a+ or IFNγ+) NK cells for the expression of NKG2C, NKG2A, ILT2, KIR2DL1/S1, KIR2DL2/L3/S2, and KIR3DL1/S1 (Figure 3). In CMV+ individuals, regardless of the stimulus used, the distribution of the NK subsets in the reactive population was comparable, that is, the phenotype of NK cells reactive to uncoated Raji cells was similar to that of NK cells reacting to (control or test) antibody-coated Raji cells. Also within the CMV− individuals each of the stimuli tested resulted in a similar NK subset division. However, there were differences in subset distribution between CMV+ and CMV− individuals. NK cells from CMV+ individuals as compared with CMV− individuals revealed a higher percentage of NKG2C+ NK cells in the CD107a+ reactive cells (P < 0.05 for all conditions), and IFNγ+ cells (trend: P = 0.06 for the anti–HLA-A3 IgG condition) (Figure 3). Also, for the anti–HLA-A3 IgM and HLA-A3 IgG antibody-coated Raji cell conditions, the percentage of ILT2+ NK cells among the reactive IFNγ+ cells was higher (median, 92% vs 85%; P = 0.02; and median, 89% vs 75%; P = 0.01, respectively).
FIGURE 3.
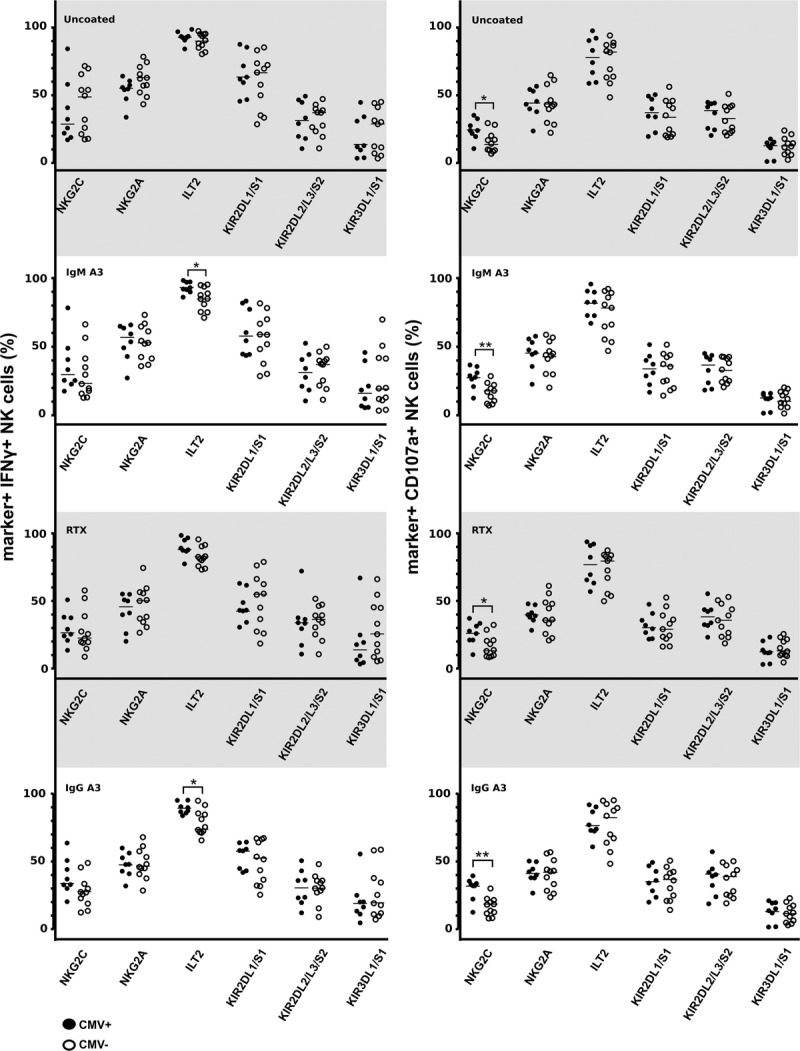
Phenotypic characteristics of the reactive NK cell population (CD107a+ or IFNγ+) in CMV+ (n = 8) and CMV− (n = 11) individuals upon coculture with uncoated or (allo) antibody-coated Raji cells (from the experiments in Figure 2). The expression of selected NK cells markers (NKG2C/A, ILT2, KIR2DL1/S1, KIR2DL2/L3/S2, and KIR3DL1/S1) was examined on IFNγ+ and CD107a+ cells. Scatter plots of cumulative data on % positive cells are shown; the solid line indicates median value. Statistical differences were calculated using the nonparametric Mann-Whitney test and differences were considered significant at *P < 0.05, **P < 0.01.
We further profiled NKG2C+ NK cells and their counterpart NKG2A+ NK cells for the expression of selected NK cell receptors (KIR2DL1/S1, KIR2DL2/L3/S2, KIR3DL1/S1, ILT2, NKG2C (for NKG2A+ cells only), and NKG2A (for NKG2C+ cells only) (Figure S2, SDC, http://links.lww.com/TXD/A55). In isolated NK cells, cultured for 4 hrs in medium alone, we observed an expansion of NKG2C+ cells expressing ILT2 in CMV+ as compared with CMV− individuals, and a relative loss of NKG2C+ cells that also express NKG2A+. This difference between CMV+ and CMV− individuals was not observed in the reactive population (IFNγ+ or CD107a+) upon coculture with anti–HLA-A3 IgG antibody-coated Raji cells. In CMV+ individuals, NKG2A+ cells expressed more NKG2C upon 4 hrs culture in medium alone, also observed in the reactive populations upon coculture with anti–HLA-A3 IgG antibody-coated Raji cells (trend in IFNγ-positive cells). In the latter condition, also a higher level of ILT2+ NKG2A+ cells was observed in the CMV+ compared with CMV− individuals.
Preferential Use of NKG2C+ and ILT2+ NK Cells in Reactive CMV+ NK Cells
Given the observed enhanced NK cell reactivity in CMV-positive individuals and the increased percentage of NKG2C+ and ILT2+ NK cells in the reactive population, we wished to determine the actual contribution of these 2 populations to the observed NK cell reactivity. Hereby, we focused our analysis on the experiments with HLA-A3 specific IgG-coated Raji cells. From a fixed number of NK cells, we calculated the proportions of reactive (IFNγ+ and/or CD107a+) NKG2C and ILT2-positive and -negative cells (Figure 4). The proportion of NKG2C+/IFNγ+ NK cells in CMV+ individuals was significantly larger than in CMV− individuals (median, 7% vs 3% of total NK cells, P = 0.005); this also held true for NKG2C+/CD107a+ cells (14% vs 5% of total NK cells, P = 0.001). A similar trend, albeit not significant, was observed for the NKG2C- cells (CMV+ vs CMV− median NKG2C-/IFNγ+ cells, 8% vs 6% of total NK cells P = 0.236; NKG2C−/CD107a+ cells: 32% vs 25% of total NK cells; P = 0.17). Interestingly, in both CMV+ and CMV− individuals, there were comparatively more reactive NKG2C− than NKG2C+ cells. For the ILT2+ population, the proportion of ILT2+/IFNγ+ NK cells was also significantly higher in CMV+ individuals as compared with CMV− individuals (11% vs 7% of total NK cells; P = 0.001). Also, the ILT2+/CD107a+ population was significantly higher in CMV+ individuals (38% vs 27% of total NK cells; P = 0.026). The reactive ILT2− population did not show a significant difference between CMV+ and CMV− individuals. Thus, CMV infection resulted in an increased proportion of both NKG2C+ and NKG2C− NK cells. Most reactive NK cells were ILT2-positive, particularly in CMV+ individuals.
FIGURE 4.
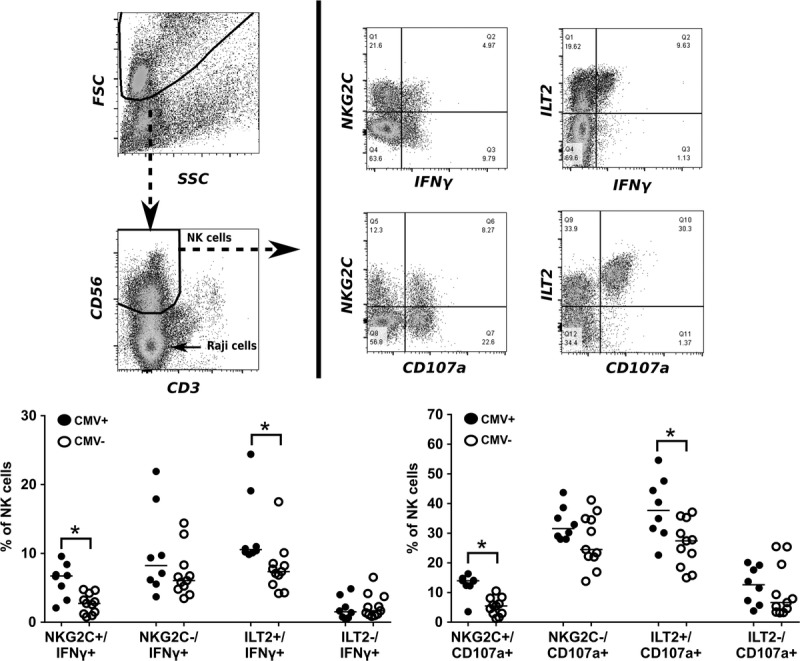
Contribution of the NKG2C+ and ILT2+ populations to overall NK cell reactivity. Data are derived from the experiments in Figure 2. From total NK cells, the proportions of reactive (IFNγ+ and/or CD107a+) NKG2C and ILT2-positive and -negative cells were calculated and compared between CMV+ and CMV− individuals. Statistical differences were calculated using the nonparametric Mann-Whitney test and differences were considered significant at *P < 0.05, **P < 0.01.
DISCUSSION
A role for CMV in the pathogenesis of solid organ allograft rejection has long been suggested.3,4,30,31 However, how CMV mediates this process is still unclear. In this study, we examined whether CMV infection and associated changes in NK cell phenotype and function might affect the antibody-dependent cellular reactivity of NK cells. This might be one of the ways in which CMV is involved in AMR of solid organs. AMR poses a risk for long-term graft survival in kidney transplantation.12-14,16 AMR can be mediated via the activation of the classical complementary pathway or via the complement independent ADCC.14 In kidney transplantation, the complement independent pathway involving NK cell ADCC activity and donor-specific antibodies has been highlighted as a major contributor to graft injury.13,19-22
CMV infection affects both phenotype and function of a subset of NK cells. Comparing NK cells from CMV+ and CMV− individuals, and in line with data from others, we show that the changes induced by CMV infection on NK cell phenotype results in a phenotypically more mature NK cell population, exhibiting a relative increase in NKG2C+ cells while maintaining a seemingly stable NKG2A, KIR and ILT2 expression.7,8,24,32 The increase in NKG2C+ NK cells varies between individuals,9,24,32 and is accompanied by functional changes in this cell subset, such as increased ADCC activity and a dampened response to exogenous cytokines.7,8,10,11 However, most studies so far compared the reactivity of NKG2C+ NK cells with that of either NKG2C− or NKG2A+ NK cells, from the same CMV+ individual. In contrast, using a different approach, we compared phenotype and function of the total NK cell population and subsets between CMV+ and CMV− individuals. Our results show that CMV infection leads to an increase in NK cell mediated direct (non-ADCC) reactivity toward allogeneic target cells. This reactivity was further augmented in the presence of target cell-specific antibodies, and was as such also observed in the presence of anti-HLA antibodies. Significantly higher proportions of reactive NKG2C+ and ILT2+ NK cells were associated with the enhanced cytotoxicity and IFNγ production by NK cells found in CMV+ individuals. Indeed, in all stimulatory conditions both the ADCC or non-ADCC alloreactive NK cells (CD107a+) from CMV+ individuals contained a higher percentage of NKG2C+ cells as compared with CMV− individuals, in line with the percentage of NK cells initially expressing NKG2C (Figure 1). A similar phenomenon was observed for ILT2 expression on IFNγ-producing NK cells, that is, higher percentages of ILT2+ reactive NK cells in CMV+ individuals. Notably, the percentage of ILT2-positive cells in the reactive population was much higher than in the starting population in both CMV+ and CMV− individuals. ILT2 is an inhibitory receptor expressed by NK cells and targeted by UL18, a CMV homolog for the MHC class I molecules.33 CMV infected cells down regulate MHC class I molecules to evade T cells and upregulate HLA-E and UL-18 to inhibit NK cells via NKG2A and ILT2 receptors, respectively.34-36 In CMV infection, ILT2 expression on lymphocytes was found to be significantly increased.32,37 The mechanism driving this increased expression is not clear but it might be that expansion of ILT2+ cells is driven by an upregulated UL18 expression or, alternatively, this may serve as a protective mechanism to suppress autoimmunity in the face of chronic antigen stimulation.34 However, our study shows that the ILT2+ NK cells are in fact potent effector cells. The high proportions of reactive ILT2+ NK cells suggest that this receptor plays a major role in the response to the allogeneic Raji cells. NK cells derived from CMV+ individuals were more reactive than those of CMV− individuals, which was observed both for NKG2C+ cells, and to a lesser extent, NKG2C− NK cells (trend). This is in line with the study by Bèziat et al,7 who showed that CMV+ individuals that lacked the NKG2C expanded population exhibited similar functional characteristics in other NK cell phenotypes.
Next to the antibody-dependent cellular reactivity induced by the anti-HLA antibody, the baseline cytotoxic reactivity against the Raji cells was also higher in CMV+ individuals compared to CMV− individuals. The exact nature of this reactivity could not be defined, but interactions of ligands on the target cell with either NK cell inhibitory receptors as is the case in a missing-self alloresponse, and/or NK cell activating receptors may play a role. Brodin et al29 showed that NK cell reactivity can be quantitatively fine-tuned by external NK cell ligands, the so-called rheostat model of NK cell education.29 Considering that CMV induces a mature NK cell phenotype, which theoretically could have undergone extensive functional education,8 this may have resulted in different activation threshold levels.
In summary, CMV results in a higher overall NK cell reactivity, which augments antibody-dependent reactivity, including reactivity to anti–HLA-specific antibodies. These data suggest that patients who have donor-specific antibodies may be at an additional risk to develop AMR when they are CMV-positive.
Supplementary Material
Footnotes
Published online 20 November, 2017.
C.M.M. was funded by the Dutch Kidney Foundation consortium grant CP09.04 (AlloVir).
The authors declare no conflicts of interest.
C.M.M. participated in the study conception and design, data acquisition, data analysis and interpretation, drafting of the article, study/article critical revision. B.v.C. participated in the study conception and design of the work, data analysis and interpretation, and study/article critical revision. P.T. participated in the data acquisition, data analysis and interpretation, study/article critical revision. F.H.J.C. participated in the study conception and design of the work, data analysis and interpretation, study/article revision. A.v.d.M. participated in the study conception and design, data analysis and interpretation, drafting of the article, study/article critical revision. I.J. participated in the study conception and design, data analysis and interpretation, drafting of article, study/article critical revision.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.De Keyzer K, Van Laecke S, Peeters P, et al. Human cytomegalovirus and kidney transplantation: a clinician’s update. Am J Kidney Dis. 2011;58:118–126. [DOI] [PubMed] [Google Scholar]
- 2.Taherimahmoudi M, Ahmadi H, Baradaran N, et al. Cytomegalovirus infection and disease following renal transplantation: preliminary report of incidence and potential risk factors. Transplant Proc. 2009;41:2841–2844. [DOI] [PubMed] [Google Scholar]
- 3.Dickenmann MJ, Cathomas G, Steiger J, et al. Cytomegalovirus infection and graft rejection in renal transplantation. Transplantation. 2001;71:764–767. [DOI] [PubMed] [Google Scholar]
- 4.Sagedal S, Nordal KP, Hartmann A, et al. The impact of cytomegalovirus infection and disease on rejection episodes in renal allograft recipients. Am J Transplant. 2002;2:850–856. [DOI] [PubMed] [Google Scholar]
- 5.Kuijpers TW, Baars PA, Dantin C, et al. Human NK cells can control CMV infection in the absence of T cells. Blood. 2008;112:914–915. [DOI] [PubMed] [Google Scholar]
- 6.Venema H, van den Berg AP, van Zanten C, et al. Natural killer cell responses in renal transplant patients with cytomegalovirus infection. J Med Virol. 1994;42:188–192. [DOI] [PubMed] [Google Scholar]
- 7.Béziat V, Liu L, Malmberg J-A, et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013;121:2678–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Béziat V, Dalgard O, Asselah T, et al. CMV drives clonal expansion of NKG2C+ NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur J Immunol. 2012;42:447–457. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Vergès S, Milush JM, Schwartz BS, et al. Expansion of a unique CD57 + NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011;108:14725–14732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Z, Sinzger C, Frascaroli G, et al. Human cytomegalovirus-induced NKG2C(hi) CD57(hi) natural killer cells are effectors dependent on humoral antiviral immunity. J Virol. 2013;87:7717–7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa-Garcia M, Vera A, Moraru M, et al. Antibody-mediated response of NKG2Cbright NK cells against human cytomegalovirus. J Immunol. 2015;194:2715–2724. [DOI] [PubMed] [Google Scholar]
- 12.Sellars J, De Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399. [DOI] [PubMed] [Google Scholar]
- 13.Smith RN, Colvin RB. Chronic alloantibody mediated rejection. Semin Immunol. 2012;24:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puttarajappa C, Shapiro R, Tan HP. Antibody-mediated rejection in kidney transplantation: a review. J Transplant. 2012;2012:193724, doi: 10.1155/2012/193724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria—an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–714. [DOI] [PubMed] [Google Scholar]
- 16.Halloran PF, Chang J, Famulski K, et al. Disappearance of T cell-mediated rejection despite continued antibody-mediated rejection in late kidney transplant recipients. J Am Soc Nephrol. 2014:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hidalgo LG, Sis B, Sellares J, et al. NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: evidence for NK cell involvement in antibody-mediated rejection. Am J Transplant. 2010;10:1812–1822. [DOI] [PubMed] [Google Scholar]
- 18.Akiyoshi T, Hirohashi T, Alessandrini A, et al. Role of complement and NK cells in antibody mediated rejection. Hum Immunol. 2012;73:1226–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed EF. Mechanisms of action and effects of antibodies on the cells of the allograft. Hum Immunol. 2012;73:1211–1212. [DOI] [PubMed] [Google Scholar]
- 20.Sis B. Endothelial molecules decipher the mechanisms and functional pathways in antibody-mediated rejection. Hum Immunol. 2012;73:1218–1225. [DOI] [PubMed] [Google Scholar]
- 21.Crespo M, Yelamos J, Redondo D, et al. Circulating NK-cell subsets in renal allograft recipients with anti-HLA donor-specific antibodies. Am J Transplant. 2015;15:806–814. [DOI] [PubMed] [Google Scholar]
- 22.Suviolahti E, Ge S, Nast CC, et al. Genes associated with antibody-dependent cell activation are overexpressed in renal biopsies from patients with antibody-mediated rejection. Transpl Immunol. 2015;32:9–17. [DOI] [PubMed] [Google Scholar]
- 23.Mulder A, Kardol M, Regan J, et al. Reactivity of twenty-two cytotoxic human monoclonal HLA antibodies towards soluble HLA class I in an enzyme-linked immunosorbent assay (PRA-STAT). Hum Immunol. 1997;56:106–113. [DOI] [PubMed] [Google Scholar]
- 24.Gumá M, Angulo A, Vilches C, et al. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–3671. [DOI] [PubMed] [Google Scholar]
- 25.Petersen L, Roug AS, Skovbo A, et al. The CD94/NKG2C-expressing NK cell subset is augmented in chronic lymphocytic leukemia patients with positive human cytomegalovirus serostatus. Viral Immunol. 2009;22:333–337. [DOI] [PubMed] [Google Scholar]
- 26.Gumá M, Cabrera C, Erkizia I, et al. Human cytomegalovirus infection is associated with increased proportions of NK cells that express the CD94/NKG2C receptor in aviremic HIV-1-positive patients. J Infect Dis. 2006;194:38–41. [DOI] [PubMed] [Google Scholar]
- 27.Brunetta E, Fogli M, Varchetta S, et al. Chronic HIV-1 viremia reverses NKG2A/NKG2C ratio on natural killer cells in patients with human cytomegalovirus co-infection. AIDS. 2010;24:27–34. [DOI] [PubMed] [Google Scholar]
- 28.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. [DOI] [PubMed] [Google Scholar]
- 29.Brodin P, Kärre K, Höglund P. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol. 2009;30:143–149. [DOI] [PubMed] [Google Scholar]
- 30.Cordero E, Casasola C, Ecarma R, et al. Cytomegalovirus disease in kidney transplant recipients: incidence, clinical profile, and risk factors. Transplant Proc. 2012;44:694–700. [DOI] [PubMed] [Google Scholar]
- 31.Sagedal S, Hartmann A, Nordal KP, et al. Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney Int. 2004;66:329–337. [DOI] [PubMed] [Google Scholar]
- 32.Gumá M, Budt M, Sáez A, et al. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107:3624–3631. [DOI] [PubMed] [Google Scholar]
- 33.Valés-Gómez M, Shiroishi M, Maenaka K, et al. Genetic variability of the major histocompatibility complex class I homologue encoded by human cytomegalovirus leads to differential binding to the inhibitory receptor ILT2. J Virol. 2005;79:2251–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prod’homme V, Griffin C, Aicheler RJ, et al. The human cytomegalovirus MHC class I homolog UL18 inhibits LIR-1+ but activates LIR-1− NK cells. J Immunol. 2007;178:4473–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim Y, Park B, Cho S, et al. Human cytomegalovirus UL18 utilizes US6 for evading the NK and T-cell responses. Jung JU, ed. PLoS Pathog. : 2008;4:e1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bryceson YT, March ME, Ljunggren H, et al. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berg L, Riise GC, Cosman D, et al. LIR-1 expression on lymphocytes, and cytomegalovirus disease in lung-transplant recipients. Lancet (London, England). 2003;361:1099–1101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


