Abstract
Background
The prevalence of overweight and obese kidney transplant recipients (KTR) has risen in parallel to the obesity epidemic that has affected the general population over the last two decades. At present, there is an ongoing debate regarding the suitability for transplantation of obese patients.
Methods
Data were prospectively collected on consecutive single organ KTR transplanted between January 2014 and March 2016. The patients were stratified according to their body mass index (BMI) using the World Health Organization classification. As a measure of allograft function Modification of Diet in Renal Disease, estimated glomerular filtration rate was used at 3, 6, and 12 months posttransplant.
Results
We included 370 KTR: 126 of 370 women; median age, 52.7 years (range, 19-77 years), followed up for a median of 19.5 ± 8.6 months. In total, 155 (41.9%) KTR were underweight or of normal BMI at transplant, whereas 148 (40%) were overweight, and 67 (18.1%) were classified as obese (47 [12.7%] class 1, 11 [3%] class 2, 9 [2.4%] class 3). Overweight and obese KTR had a higher incidence of pretransplant diabetes (P = 0.021), but no difference was found in new-onset hyperglycemia posttransplant (P = 0.35). There was also no difference in posttransplant hospital length of stay (P = 0.386). Obese and overweight KTR had a significantly lower estimated glomerular filtration rate than underweight and normal BMI KTR at 3 and 6 months posttransplant, a finding that did not persist at 1 year follow-up. Overall, 23 patients lost their grafts, and 20 patients died during follow-up. Kaplan Meier analysis showed no difference in allograft loss between the different BMI groups (log rank P = 0.7).
Conclusions
In this single-center study, which used short-term data, overweight and obese patients were shown not to have inferior outcomes regarding renal function 1 year posttransplant.
Obesity has emerged as the largest pandemic of the 21st century, markedly increasing the risk of chronic kidney disease.1 According to country estimates for 2008, over 50% of both men and women in the World Health Organization European Region were overweight, and 23% of women and 20% of men were obese. Based on the latest estimates in European Union countries, being overweight affects 30% to 70% of adults, 10% to 30% of these adults being obese.2
Although obesity is assumed to be an unfavorable prognostic indicator in the general population, interestingly, it confers a survival advantage among the dialysis patients. Obese persons are better nourished and are thought to be better immune responders against devastating chronic infectious and other diseases, which are often a cause of death in the lower body mass index (BMI) dialysis population.3 A recent article from Yu et al4 addresses the historical unintended weight loss as an independent predictor of death. There is a J-shaped association with BMI and death, with the nadir of the curve for normal BMI patients.
The question of whether obese patients should receive kidney transplants or be obliged to lose weight before transplantation has been raised as an ethical conundrum. Many specialities have developed programs to encourage preoperative weight loss, arguing that reduced weight leads to better outcomes. This is particularly relevant in the context of the severe shortage of organs for transplantation.
Furthermore, analysis of the effect of recipient obesity after renal transplantation has demonstrated inferior outcomes among high BMI transplant recipients with multifactorial pathophysiologies mediating the risk of allograft failure and patient death.5 A number of factors6 are deemed responsible for this including an increased chance of metabolic syndrome7 and posttransplant diabetes mellitus,8 an increased risk of acute rejection,9,10 alteration in renal hemodynamics and hyperfiltration with proteinuria,10 exacerbated if a small organ went to a big recipient as what we could call a “small for size.”11,12
A key observation of Kwan et al13 is that the risks for adverse outcome of obesity are progressive with increasing BMI, and furthermore, preobese overweight recipients compared with normal weight recipients had increased risks of adverse outcomes related to kidney transplantation. In addition to this, a meta-analysis by Lafranca et al14 concluded that in high BMI candidates, weight loss before transplant should be emphasized because of poorer outcomes compared with normal BMI population; therefore, it is logical that some units use a BMI cutoff in transplant recipient selection.
A more detailed analysis of long-term outcomes showed mixed results: although recognizing obesity as a major prognostic factor, Gill et al15 found a survival benefit of transplantation from different donor sources versus dialysis treatment at 1 year. More in details, living donor transplantation was associated with a 66% risk reduction of death in all BMI groups, and standard criteria donor transplant was associated with a 48% reduction in risk of death in patients with a BMI greater than 40 kg/m2, compared with a 66% reduction in patients with BMI less than 40 kg/m2. Standard criteria donor was defined by age, younger than 60 years or older than 50 years, without at least two of the following: hypertension, cerebrovascular cause of brain death or terminal serum creatinine > 1.5 mg/dL.
Our article aims to build on the growing body of literature relating to renal transplantation in high BMI patients. It describes a cohort of patients treated with a steroid-sparing immunosuppressive protocol and its effect in obese kidney transplant recipients (KTR) comparing 1 year patient and graft survival between obese and nonobese recipients in a stratified manner.
MATERIALS AND METHODS
Data were prospectively collected on consecutive single KTR transplanted between January 2014 and March 2016. All the patients received a steroid sparing immunosuppressive regimen (7-day course of steroids) with alemtuzumab induction and tacrolimus monotherapy (trough level, 5-8 ng/mL) or IL2 induction with tacrolimus (trough level, 8-12 ng/mL) and mycophenolate mofetil. Steroids and mycophenolate mofetil, if not already on it, were only introduced to treat rejection. BMI was calculated using World Health Organization classification: weight (in kg) divided by height (in meters) squared. On the basis of BMI, patients were divided into 6 weight classes: underweight (BMI < 18.5), normal (18.5 < BMI < 25), overweight (25 < BMI < 30), obese class I (30 < BMI < 35), obese class II (35 < BMI < 40), obese class III (40 < BMI < 50). The patients were stratified in three groups for allograft survival analysis; underweight and normal (BMI < 25), overweight (25 < BMI < 30) and obese (BMI > 30).
As a measure of allograft function, Modification of Diet in Renal Disease estimated glomerular filtration rate (eGFR) was used at 3, 6, and 12 months posttransplant.16 DGF was defined as a need for dialysis within 1 week of transplantation with a perfused graft. Graft survival was measured by the composite endpoint of all-cause graft failure, including failure due to death.
Continuous variables are presented as mean ± standard deviation. Analysis of variance and t test were used to compare continuous variables between groups. For nominal or nonparametric variables, Pearson χ2 test was performed. Kaplan-Meier was applied for survival analysis. Confidence interval was set to 95%, and P was considered significant at less than 0.05. Analysis was performed using SPSS (IBM SPSS Statistics for Windows, Version 20.0; IBM Corp, Armonk, NY).
RESULTS
Three hundred seventy patients underwent kidney transplantation during the study period. The mean BMI of the overall cohort was 26.2. There were 148 (40%) preobese, 47 (12.7%) class I obese, 11 (3%) class II obese, and 9 (2.4%) class III obese patients. Table 1 demonstrates differences between the six weight classes in terms of baseline characteristics. Although there were statistically significant differences between the groups on all variables assessed, there were fewer variables demonstrating a linear trend from the normal group and moving through the progressively heavier groups, that is, male sex and younger age. Overweight and obese KTRs had a higher incidence of pretransplant diabetes (P = 0.021). No difference was found in new-onset hyperglycemia posttransplant (P = 0.35). Utilization of deceased cardiac death kidneys or the use of more marginal deceased donors, such as those from elderly donors did not appear to follow any particular trend with regard to weight class (Table 2). Immunosuppression induction IL-2 versus alemtuzumab did not differ as well according to BMI class (Table 1).
TABLE 1.
Baseline patients characteristics
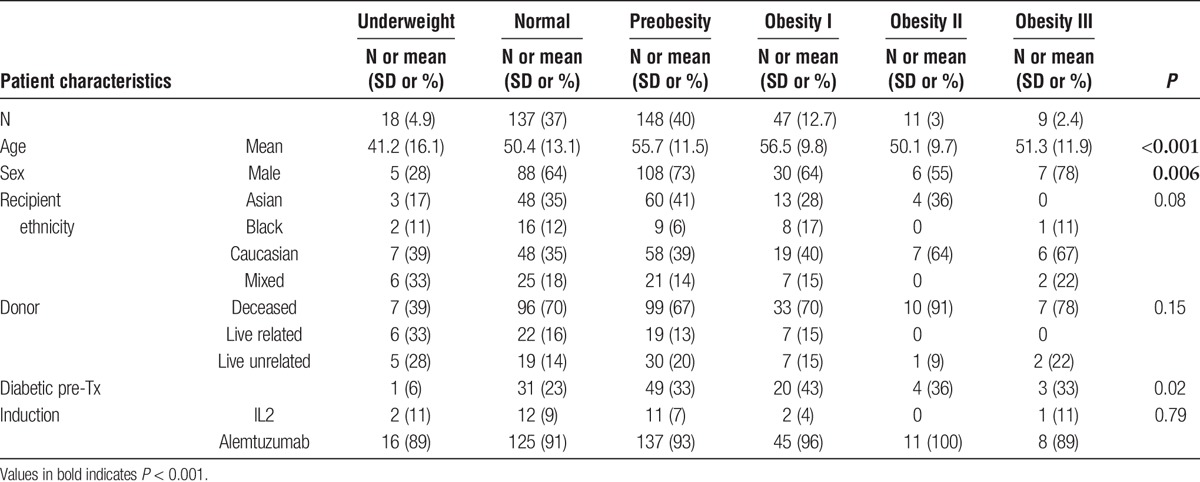
TABLE 2.
Post-trasplant results
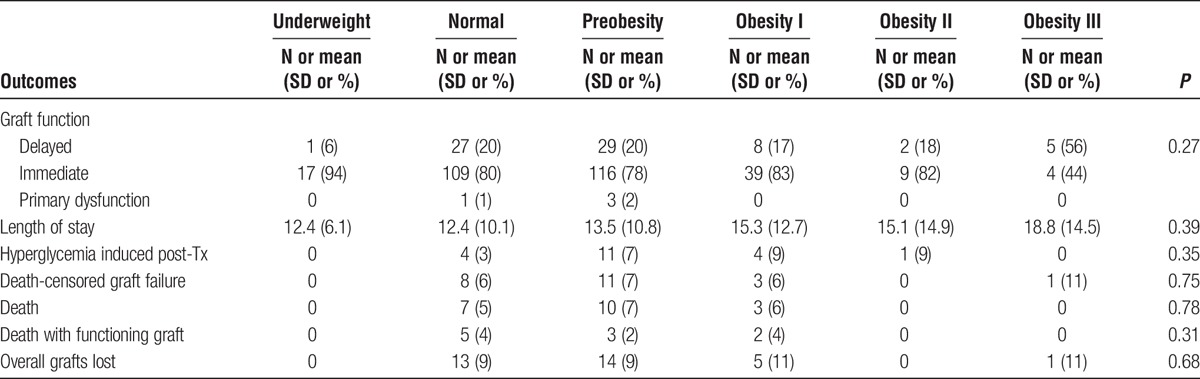
Obesity was a significant risk factor for lower eGFR at 3 and 6 months posttransplant, interestingly, this was a nonpersistant finding at 1 year follow-up (Figures 1-3). DGF was not statistically significant when comparing the different BMI classes. There was also no statistical difference significance in hospital length of stay between the nonobese and the obese groups (Table 2).
FIGURE 1.
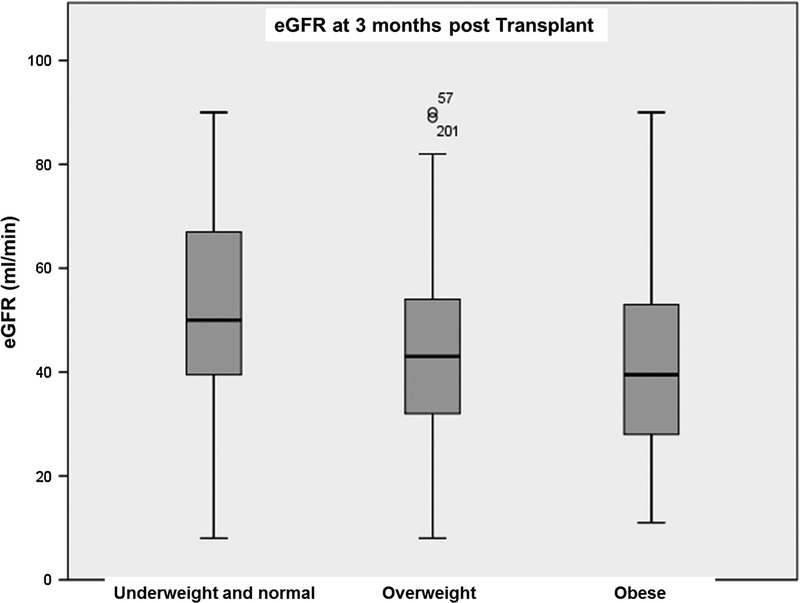
eGFR 3 months post kidney transplant according to patient BMI.
FIGURE 3.
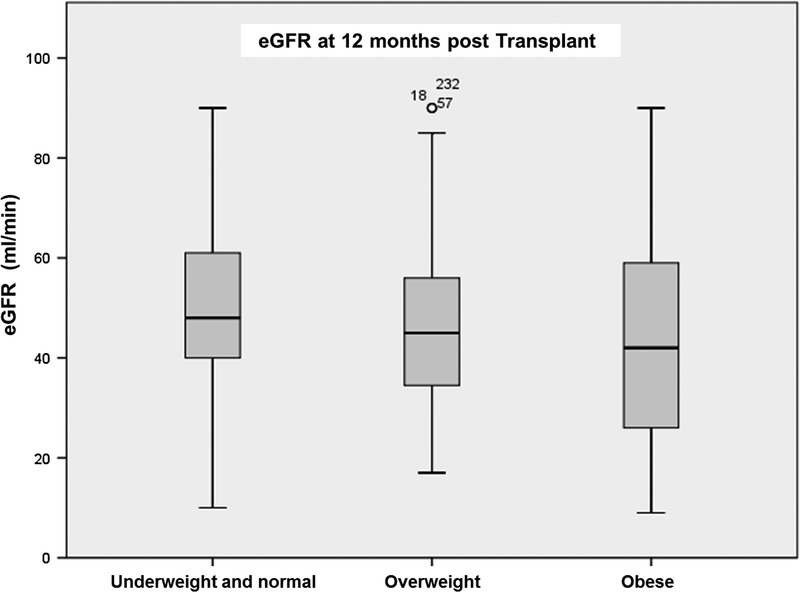
eGFR 12 months post kidney transplant according to patient BMI.
FIGURE 2.
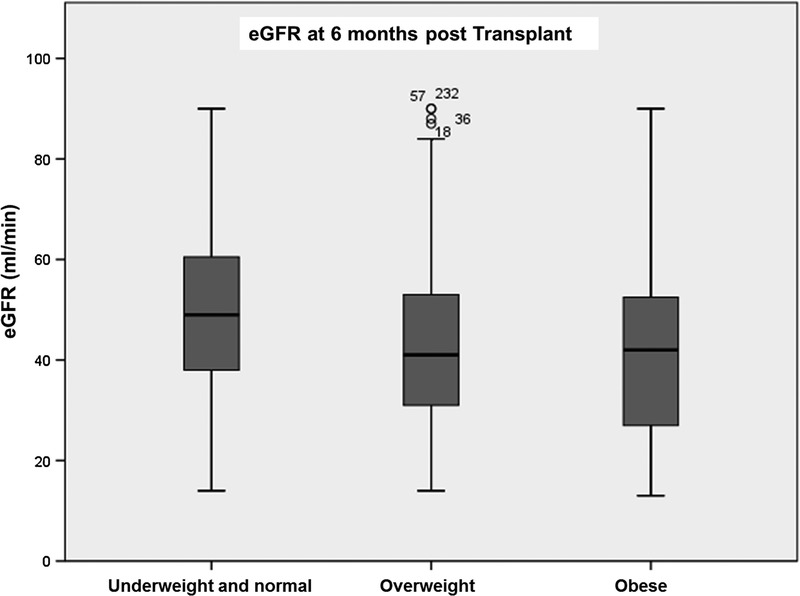
eGFR 6 months post kidney transplant according to patient BMI.
Overall, 23 patients lost their grafts, and 20 patients died during follow-up. Patients were stratified in three groups, underweight and normal (n = 164, 41.3%), overweight (n = 152, 38.3%), and obese (n = 72, 18.1%), for survival analysis. Kaplan-Meier analysis in Figure 4 showed no difference in all-cause allograft loss between the different BMI groups (log rank P = 0.7) (Figure 4) in a median follow-up of 19.5 months.
FIGURE 4.
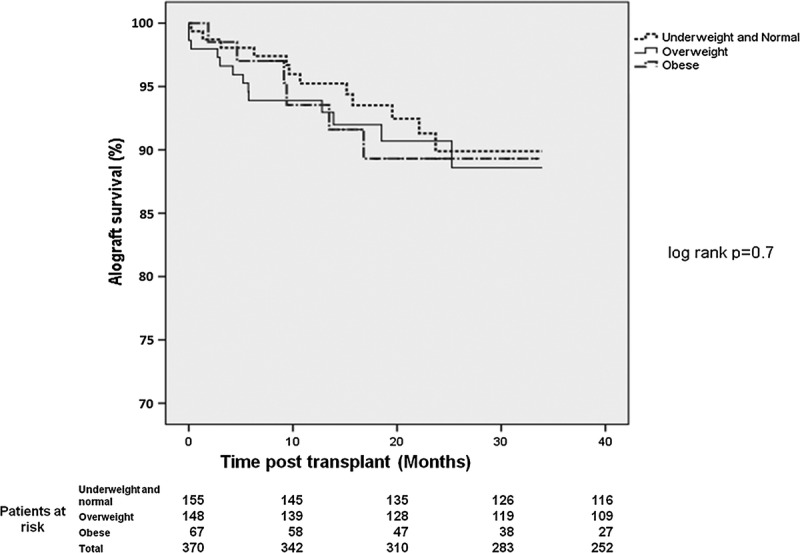
Allograft survival post kidney transplant according to patient BMI.
DISCUSSION
Many studies have underlined unfavorable effects of obesity both in the short and long term on various parameters after transplantation.17-20 Our study, interestingly, does not show inferior outcomes at 1 year for higher BMI classes in comparison to normal and lower BMI patients, reproducing what other articles have reported.21-23 We had a total of 67 obese patients transplanted, of whom 20 were class II or III. The sample size is limited, but a worth basis for considerations.
We hypothesize two responsible factors: the first is no statistical difference in donor type among the 6 classes, although in the short term, there is a lower eGFR in preobese and obese patients. The second and the most important we believe is that it is our steroid-sparing immunosuppressive regimen. In our center, we completely withdraw steroids after 1 week, leaving kidney transplant patients on a maintenance regimen usually aiming to run then on tacrolimus alone. A recent meta-analysis concluded that steroid avoidance does not suggest a difference in patient mortality or graft loss up to 5 years after transplantation,24 and in our cohort, chronic kidney disease risk factors associated to high BMI were not exacerbated by steroid-based immunosuppression.
Given that morbid obesity is not per se a reason to deny a kidney transplant, the critical question is can this statement be valid for all the stages of obesity? If no, what is the upper limit of BMI that allows safe kidney transplantation? Unfortunately, the answer is not clear. Literature regarding super-obese patients (BMI > 50) shows significant worse patient and graft outcomes as compared with any other BMI class in the early and late periods after transplantation.25 In our cohort, no super-obese patient was transplanted, so we could not compare results. However, because Bennet et al26 reported a survival advantage of transplantation over dialysis even in the morbidly obese population, our center policy does not define a contraindication for transplantation on the basis of BMI alone, particularly in case of a living donor option.
The real controversy stands for management of morbidly obese patients if they are on the waiting list for a deceased donor. Because there is a scarcity of donors, it has been argued that these limited organs should be offered to the persons who will benefit most. Thus, to guarantee the maximum benefit to the greatest number of patients, “only those patients, who achieve a target BMI should be transplanted,” which suggests that nonobese patients should take priority in the waiting lists.27 On the other hand, considering that obese patients also benefit from transplantation as compared with those in the waiting lists, this argument may give rise to many ethical concerns and puts responsibility on the shoulders of the nephrologists.
The observational nature of our study has some limitations: we have selected patients fit for transplantation and many have undergone intensive medical workup to optimize the cardiovascular risk factors. This could have biased in selection for transplantable patients, especially in the high BMI cohort. Also, the use of BMI as a measure for adiposity is imperfect because it does not differentiate between fat and lean body mass, as could do girth, for example, although most population variance in obesity is explained by BMI.28
In conclusion, we think that obese patients should have the same chance of their nonobese counterparts for deceased donor renal transplantation, trying to maximize all the adding risk factors to graft and patient loss, that is, steroid avoidance. A randomized trial could help in the future to better investigate what is the best management for end-stage renal disease obese population, although ethical dilemmas would still be questionable.
Footnotes
Published online 3 November, 2017.
The authors declare no funding or conflicts of interest.
M.I.B. participated in data collection, analysis, and article writing. K.K. conceptualized the study, data analysis, interpretation, and article writing. J.G. participated in the interpretation of data and intellectual content. P.H. conceptualized the study, data interpretation, intellectual content, and is the senior author.
REFERENCES
- 1.Camara NO, Iseki K, Kramer H, et al. Kidney disease and obesity: epidemiology, mechanisms and treatment. Nat Rev Nephrol. 2017;13:181–190. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Data and Statistics. http://www.euro.who.int/en/health-topics/noncommunicable-diseases/obesity/data-and-statistics. Accessed October 6, 2017.
- 3.Johansen KL, Young B, Kaysen GA, et al. Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr. 2004;80:324–332. [DOI] [PubMed] [Google Scholar]
- 4.Yu E, Ley SH, Manson JE, et al. Weight history and all-cause and cause-specific mortality in three prospective cohort studies. Ann Intern Med. 2017;166:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naik AS, Sakhuja A, Cibrik DM, et al. The impact of obesity on allograft failure after kidney transplantation: a competing risks analysis. Transplantation. 2016;100:1963–1969. [DOI] [PubMed] [Google Scholar]
- 6.D'Agati VD, Chagnac A, de Vries AP, et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12:453–471. [DOI] [PubMed] [Google Scholar]
- 7.Pedrollo EF, Corrêa C, Nicoletto BB, et al. Effects of metabolic syndrome on kidney transplantation outcomes: a systematic review and meta-analysis. Transpl Int. 2016;29:1059–1066. [DOI] [PubMed] [Google Scholar]
- 8.Kasiske BL, Snyder JJ, Gilbertson D, et al. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178–185. [DOI] [PubMed] [Google Scholar]
- 9.Gore JL, Pham PT, Danovitch GM, et al. Obesity and outcome following renal transplantation. Am J Transplant. 2006;6:357–363. [DOI] [PubMed] [Google Scholar]
- 10.Curran SP, Famure O, Li Y, et al. Increased recipient body mass index is associated with acute rejection and other adverse outcomes after kidney transplantation. Transplantation. 2014;97:64–70. [DOI] [PubMed] [Google Scholar]
- 11.Kasiske BL, Snyder JJ, Gilbertson D. Inadequate donor size in cadaver kidney transplantation. J Am Soc Nephrol. 2002;13:2152–2159. [DOI] [PubMed] [Google Scholar]
- 12.el-Agroudy AE, Hassan NA, Bakr MA, et al. Effect of donor/recipient body weight mismatch on patient and graft outcome in living-donor kidney transplantation. Am J Nephrol. 2003;23:294–299. [DOI] [PubMed] [Google Scholar]
- 13.Kwan JM, Hajjiri Z, Metwally A, et al. Effect of the Obesity Epidemic on Kidney Transplantation: Obesity Is Independent of Diabetes as a Risk Factor for Adverse Renal Transplant Outcomes. PLoS One. 2016;11:e0165712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lafranca JA, IJermans JN, Betjes MG, et al. Body mass index and outcome in renal transplant recipients: a systematic review and meta-analysis. BMC Med. 2015;13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill JS, Lan J, Dong J, et al. The survival benefit of kidney transplantation in obese patients. Am J Transplant. 2013;13:2083–2090. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 17.Pondrom S. The AJT report: news and issues that affect organ and tissue transplantation. A maze of information. Am J Transplant. 2012;12:799–800. [DOI] [PubMed] [Google Scholar]
- 18.Molnar MZ, Kovesdy CP, Mucsi I, et al. Higher recipient body mass index is associated with post-transplant delayed kidney graft function. Kidney Int. 2011;80:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aalten J, Christiaans MH, de Fijter H, et al. The influence of obesity on short- and long-term graft and patient survival after renal transplantation. Transpl Int. 2006;19:901–907. [DOI] [PubMed] [Google Scholar]
- 20.Lentine KL, Rocca-Rey LA, Bacchi G, et al. Obesity and cardiac risk after kidney transplantation: experience at one center and comprehensive literature review. Transplantation. 2008;86:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furriel F, Parada B, Campos L, et al. Pretransplantation overweight and obesity: does it really affect kidney transplantation outcomes? Transplant Proc. 2011;43:95–99. [DOI] [PubMed] [Google Scholar]
- 22.Howard RJ, Thai VB, Patton PR, et al. Obesity does not portend a bad outcome for kidney transplant recipients. Transplantation. 2002;73:53–55. [DOI] [PubMed] [Google Scholar]
- 23.Nicoletto BB, Fonseca NK, Manfro RC, et al. Effects of obesity on kidney transplantation outcomes: a systematic review and meta-analysis. Transplantation. 2014;98:167–176. [DOI] [PubMed] [Google Scholar]
- 24.Haller MC, Royuela A, Nagler EV, et al. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev. 2016:Cd005632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanthawar P, Mei X, Daily MF, et al. Kidney transplant outcomes in the super obese: a national study from the UNOS dataset. World J Surg. 2016;40:2808–2815. [DOI] [PubMed] [Google Scholar]
- 26.Bennett WM, McEvoy KM, Henell KR, et al. Kidney transplantation in the morbidly obese: complicated but still better than dialysis. Clin Transplant. 2011;25:401–405. [DOI] [PubMed] [Google Scholar]
- 27.Lentine KL. Pro: pretransplant weight loss: yes. Nephrol Dial Transplant. 2015;30:1798–1803. [DOI] [PubMed] [Google Scholar]
- 28.Janssen I, Heymsfield SB, Allison DB, et al. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr. 2002;75:683–688. [DOI] [PubMed] [Google Scholar]


