Abstract
Background
There is limited information on treatment strategies and monitoring strategies for late antibody-mediated rejection (ABMR) after kidney transplantation.
Methods
In this observational and nonrandomized study, we compared 78 patients diagnosed with late ABMR (>3 months after transplant) who were treated with standard of care steroids/IVIG (n = 38) ± rituximab (n = 40) at our center between March 1, 2013 and December 31, 2016. All patients had follow-up biopsy and donor-specific antibodies (DSA) monitoring within 3 to 12 weeks.
Results
Patients had biopsy 7.3 ± 7 years after transplant and were followed for 15.9 ± 9.6 months after ABMR was diagnosed. Both treatment strategies were associated with a significant decline in DSA, microvascular inflammation (peritubular capillaritis + glomerulitis), and C4d Banff scores. In univariate regression analyses, rituximab, estimated glomerular filtration rate (eGFR), Banff i, t, v, chronicity (interstitial fibrosis + tubular atrophy + fibrous intimal thickening + allograft glomerulopathy) scores on the first biopsy, and eGFR and Banff v score on follow-up biopsy were associated with graft loss. Multivariate analyses retained only rituximab (hazard ratio, 0.23; 95% confidence interval, 0.06-0.84; P = 0.03) and eGFR at follow-up biopsy (0.84; 95% confidence interval, 0.76-0.92; P < 0.001) as significant predictors of graft loss. Kaplan-Meier analyses demonstrated that the benefit associated with rituximab was apparent after 1 year (15% vs 32% graft loss, P = 0.02).
Conclusion
Treatment of late ABMR with steroids/IVIG ± rituximab was effective in reducing DSA and microcirculation inflammation. The addition of rituximab was associated with better graft survival. Follow-up biopsies could be considered in the management of acute rejection to monitor the effect of therapy. Randomized studies on the best therapeutic options for ABMR are needed.
Antibody-mediated rejection (ABMR) is an important barrier for long-term kidney allograft survival.1-3 The primary aim of treatment is to remove or stabilize antibodies and treat the reversible tissue injury; however, the management of ABMR is challenging. In early acute ABMR, plasmapheresis (PP) or immunoadsorption may be effective,1 but the management of late ABMR is more difficult owing to chronic tissue damage and suboptimal renal function.4 In the largest case series of patients with chronic active ABMR, 75% of patients lost their kidney allograft within 2 years after the diagnosis of rejection.5 In this study, treatment with steroids and intravenous immunoglobulin (IVIG) was associated with significant improvement in outcomes. However, the addition of thymoglobulin or anti–B-cell therapy did not confer further survival advantage, likely due to sample size limitations.5 Although therapies directly inhibiting B-cell immunity, including rituximab and IVIG, are frequently used for the treatment of ABMR,4,6 there is limited information on the effectiveness of these agents to reverse allograft injury and to reduce donor-specific antibodies (DSA) generation and predict graft survival.
We implemented a clinical protocol to include short-term follow-up biopsies and DSA monitoring to guide therapy in patients with late ABMR. Herein, we present preliminary findings on 78 kidney transplant recipients treated for late ABMR with steroids and IVIG (standard of care) ± rituximab who underwent prospective short-term follow-up DSA and biopsies to help with treatment guidance.
METHODS
Study Population and Design
We considered late rejection as rejection occurring after 3 months after transplant; therefore, all patients with late ABMR (>3 months after transplant) undergoing follow-up biopsy and DSA monitoring within 3 to 12 weeks were included in the study. The study covered the period of March 1, 2013 to December 31, 2016 and was approved by the Health Sciences Institutional Review Board at the University of Wisconsin. Biopsies were evaluated based on the most recent Banff diagnostic criteria3 and patients were treated with IVIG and steroid pulse (standard of care [SOC]) ± rituximab. In patients who had multiple biopsies and ABMR treatment during the study period, only the first treatment with follow-up biopsy were included. Per protocol, we use PP in early ABMR (<3 months after transplant) and exceptionally, in late ABMR. Seventy-eight cases of ABMR satisfied our criteria.
Baseline Characteristics
We analyzed data on age, sex, race, retransplant status, causes of end-stage renal disease, type of transplant, pretransplant DSA, crossmatch, induction immunosuppression, serum creatinine at time of the biopsy, estimated glomerular filtration rate (eGFR), urine protein to creatinine ratio (UPC), graft loss, and patient death between the groups. We also compared immunophenotypic changes including mean class I and class II DSA mean fluorescence intensity (MFIsum), immunodominant DSA (MFImax), microvascular injury (peritubular capillaritis [ptc] + glomerulitis [g]), C4d, tubulitis (t), mononuclear cell interstitial inflammation (i), intimal arteritis (v), and chronic changes including interstitial fibrosis (ci) + tubular atrophy (ct) + allograft glomerulopathy (cg) + fibrous intimal thickening (cv).
Anti-HLA Antibody Screening by Luminex
Posttransplant DSA monitoring is performed at our center at 6 and 12 months, and thereafter annually in all kidney transplant patients. Patients with pretransplant DSA also had DSA checked at 6 weeks and 3 months after transplant. Patients with calculated panel reactive antibodies greater than zero received additional DSA monitoring at 3 weeks. Antibodies were screened using Luminex HLA class I and class II single-antigen beads (One Lambda). Antibodies with MFI values of 2000 or more were considered unacceptable and were used for panel reactive antibodies calculations, as this is an approximate level for a positive flow crossmatch. At our institution, we consider a virtual crossmatch to be positive if detectable DSA MFI is greater than 100. The presence of an HLA DSA at any MFI level was considered a positive virtual crossmatch. The same methods were used before and after transplantation.
ABMR Treatment
Antibody-mediated rejection treatment protocols at our institution are based on both the severity of rejection and the time after transplant at which ABMR was diagnosed. For early rejection (within 3 months after transplant), treatment includes dexamethasone, 100-mg bolus and taper; PP, 4 to 6 sessions; and IVIG, 100 mg/kg after each PP. Late rejection (>3 months after transplant) is treated with dexamethasone, 100 mg bolus and taper, and IVIG, 200 mg/kg every 2 weeks × 3. Baseline immunosuppression is also increased by approximately 25%. Rituximab, 375 mg/m2 single dose, is added based on clinical and immunophenotypic characteristics. Patients with younger age, better kidney function, higher DSA, diffuse C4d, greater microvascular inflammation, and lower chronicity score are more likely to receive rituximab. Our usual immunosuppression regimen is tacrolimus (12-hour trough goal of 5-7 ng/dL 6 months after transplant); mycophenolic acid, 720 mg twice a day; and prednisone, 5 mg daily, with doses adjusted based on adverse effects and immunological risk. Patient’s baseline immunosuppressive medication was increased by approximately 25% after the rejection. We implemented a clinical protocol to include short-term follow-up biopsies and DSA monitoring 3 to 12 weeks after initial ABMR to guide therapy in patients with late ABMR. In patients with persistent ABMR on follow-up biopsy, we recycled the treatment and used the higher dose of IVIG at 500 mg/kg per week for 4 weeks.
Statistical Analysis
Continuous data were compared using paired or unpaired Student t test or the Wilcoxon rank sum test, when appropriate, whereas categorical data were analyzed using the Fisher exact test or the χ2 test, when appropriate. Univariate and multivariate Cox regression analyses were performed to determine the risk factors associated with graft loss after the diagnosis of ABMR. P < 0.05 was considered statistically significant.
RESULTS
Baseline Characteristics, Kidney Function, and Immunopathology
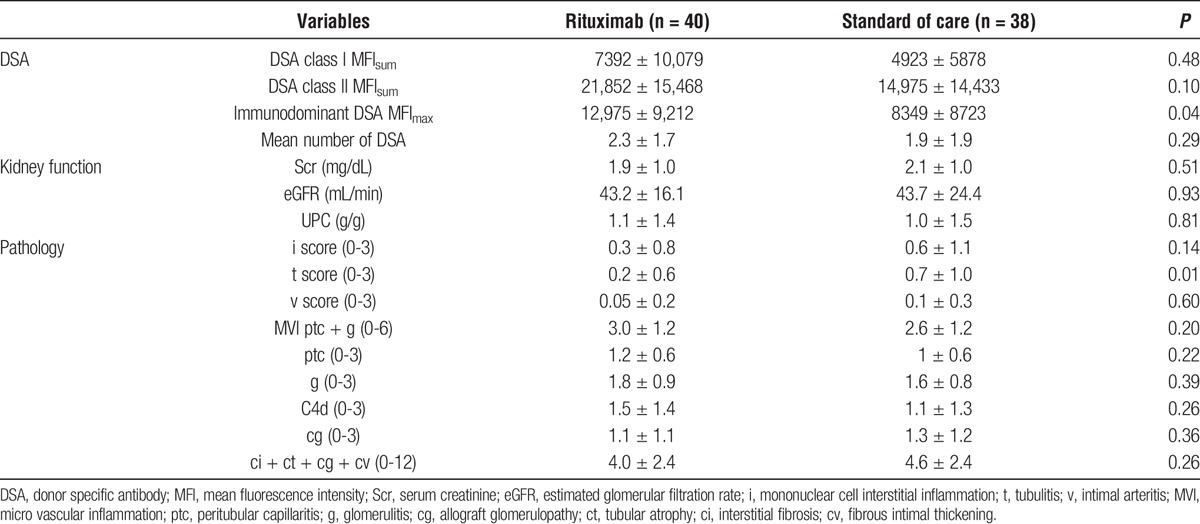
Late ABMR was diagnosed at 7.3 ± 7 years (minimum 3 months to maximum 27 years) after transplant. There were 40 patients in the rituximab group and 38 in the SOC group. Baseline demographics were similar between the 2 groups except that patients in the rituximab group were significantly younger (37.6 ± 15.5 vs 45.6 ± 15.8, P = 0.03; Table 1). Similarly, kidney function and immunopathology were not significantly different between the 2 groups except for lower t scores (0.2 ± 0.6 vs 0.7 ± 1.0, P = 0.01) and the higher immunodominant DSA MFImax (12,975 ± 9,212 vs 8349, P = 0.04) in the rituximab group (Table 2).
TABLE 1.
Baseline characteristics
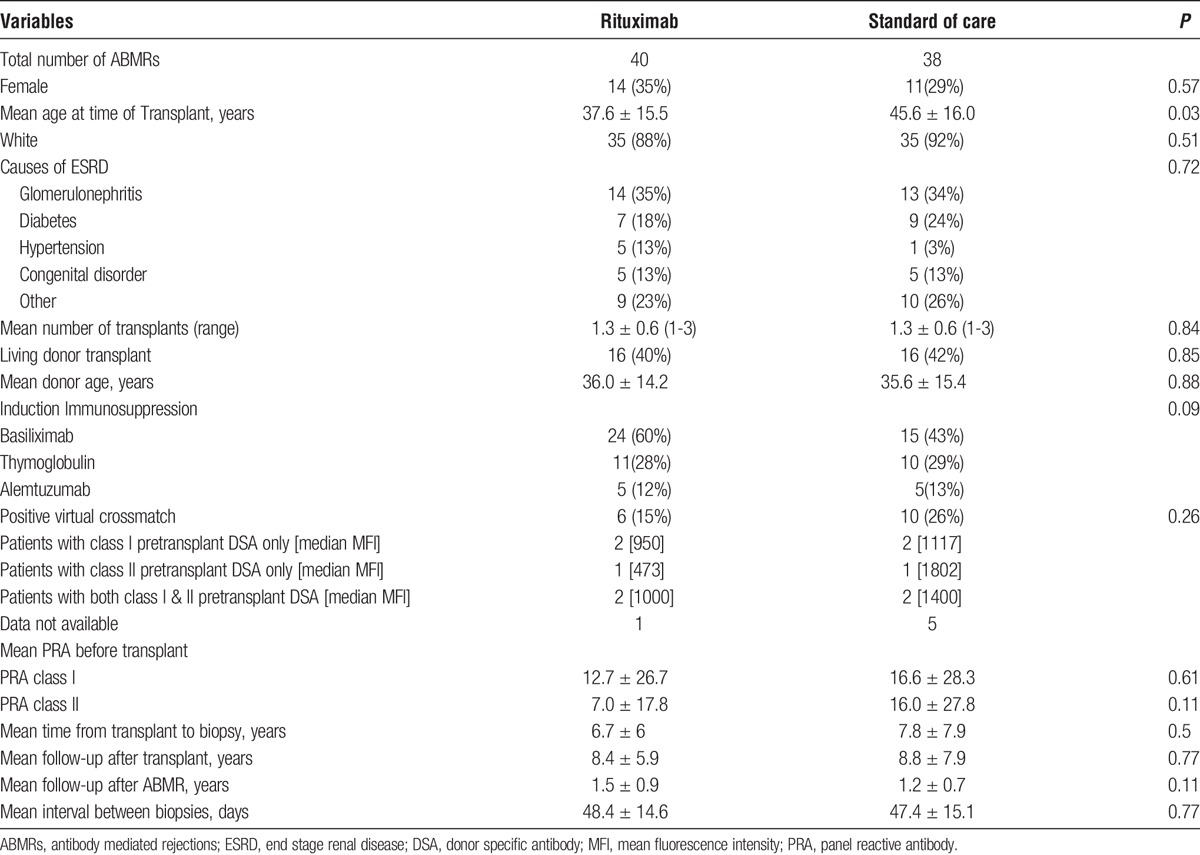
TABLE 2.
Baseline kidney function and immunopathology
There were a total of 16 patients with pretransplant DSA, but details were available only in 10 patients, 5 in each group. In the rituximab group, one each had pretransplant DSA against A25, B7, and DQ2, respectively, whereas the other 2 had pretransplant DSA against both class I and class II DSA (A11 + DQ6 and A11 + DR53). Similarly, in SOC group, one each had pretransplant DSA against DP04, B35, and Cw, respectively, whereas the other 2 had pretransplant DSA against both class I and class II DSA (B44 + DR53 and B49 + DQ6). Three of 16 patients with pretransplant DSA had graft loss at last follow-up, and all of these were in the SOC group.
Similarly, when comparing the earlier rejections (ie, within 6 months after transplant) and later rejection (ie, after 3 years after the transplant), there were 4 patients with ABMR within the first 6 months after the transplant and 50 patients 3 years after the transplant. Forty of 50 patients in the later ABMR group had chronic active ABMR (cABMR), whereas none in the earlier ABMR group had cABMR. In later ABMR, microvascular injury (ptc + g) scores were significantly higher than earlier ABMR (2.9 ± 1.3 vs 1.5 ± 0.6 [P = 0.005], respectively). Chronicity scores were also higher in later ABMR group compared to the earlier ABMR group (5.1 ± 2.3 vs 1.3 ± 1.9 [P = 0.02], respectively).
Treatment Was Associated with Improvement in ABMR
Treatment in all patients (Table 3A) was associated with a significant decline in class I DSA (6285 ± 8414 to 2958 ± 4464, P < 0.001), class II DSA (19,045 ± 15,287 to 15,224 ± 14,227, P < 0.001), and i, t, C4d, g, and ptc lesions (all <0.01). No significant treatment effect was noted on kidney function (serum creatinine, eGFR, or UPC), transplant glomerulopathy (cg), or sum chronicity score (ct + ci + cg + cv). Both treatment approaches were effective in reducing class I and class II DSA, g score, C4d staining, and microvascular injury (Tables 3B and C). The improvement in i, t, and ptc lesions was more significant in the SOC group (Table 3C). There was a significant decrement in the number of patients with C4d positive ABMR among both groups either with acute or acute or chronic ABMR (Figure 1).
TABLE 3.
Treatment was associated with a significant decline in DSA and improvement of acute ABMR
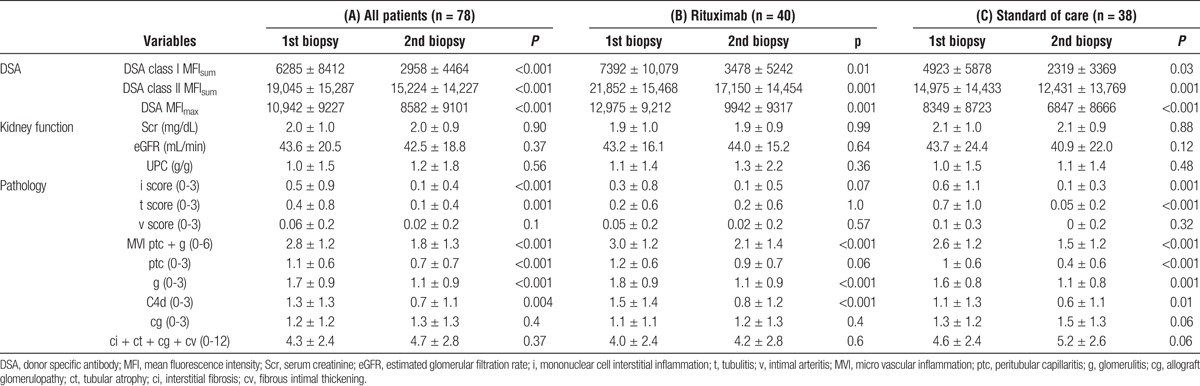
FIGURE 1.
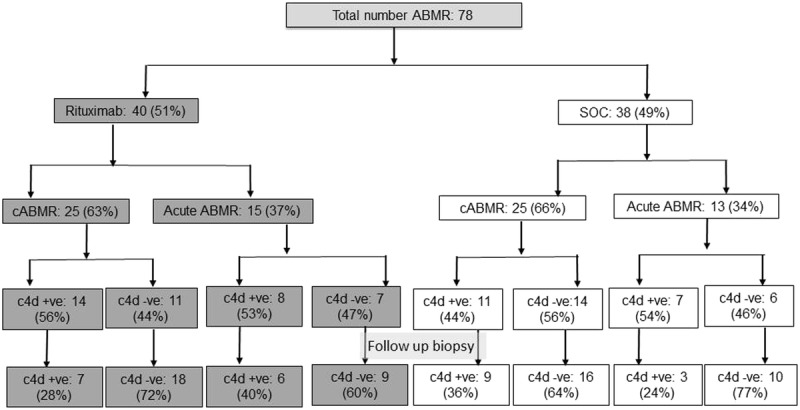
C4d positive ABMR decreased on follow-up biopsy in both groups.
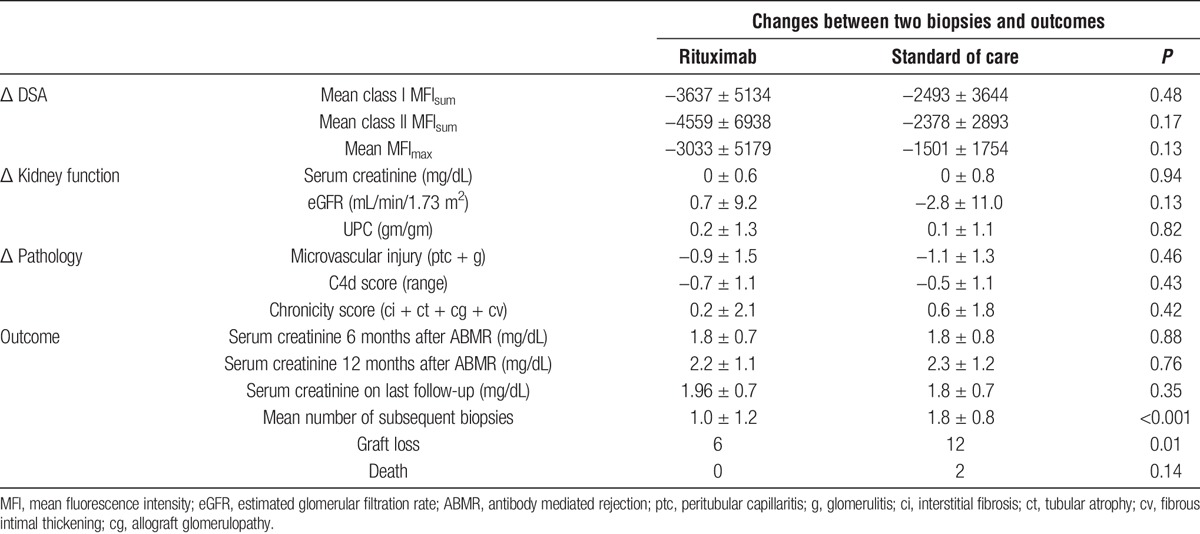
Changes Between Biopsies and Graft Survival
Treatment-associated changes in kidney function, DSA, and renal pathology did not reach statistical significance between the 2 biopsies. There was no difference between the 2 groups in renal function at 6 months and 12 months after ABMR. However, after more than 1 year of follow-up, there was a significant difference in graft loss between the SOC and rituximab groups (12 vs 6, respectively, P = 0.01), suggesting a delay in the therapeutic benefits of rituximab. This observation was confirmed in Kaplan-Meier analyses, demonstrating that the benefit associated with rituximab was apparent after 1 year (15% vs 32% graft loss, P = 0.02) (Figure 2). Patients in the SOC group were also more likely to have persistent rejection requiring multiple subsequent biopsies (Table 4).
FIGURE 2.
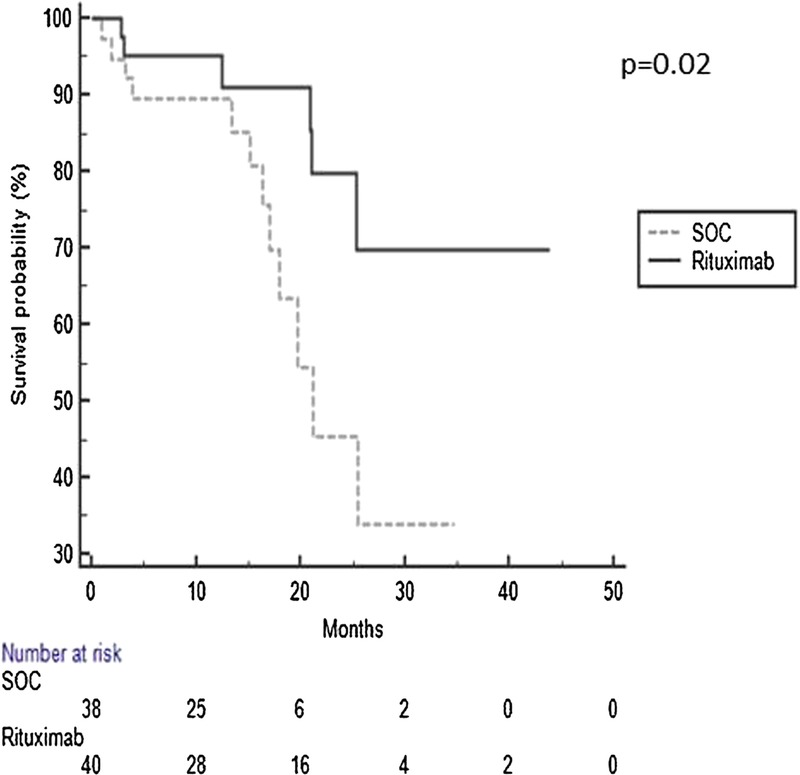
Rituximab was associated with improved graft survival.
TABLE 4.
Rituximab was associated with improved graft survival
Infections After Treatment
There were 13 cases of BK virus and 10 cases of cytomegalovirus infection after the treatment of rejection. There was no difference in the rate of BK viremia between the groups; 5 (13%) in the rituximab group and 8 (21%) in the SOC group (P = 0.31). However, the SOC group had a higher incidence of cytomegalovirus infection compared to the rituximab group, 8 (21%) vs 2 (5%), respectively (P = 0.04). Similarly, 11 patients had other infections including pyelonephritis, cellulitis, and norovirus without significant difference between the groups (P = 0.41).
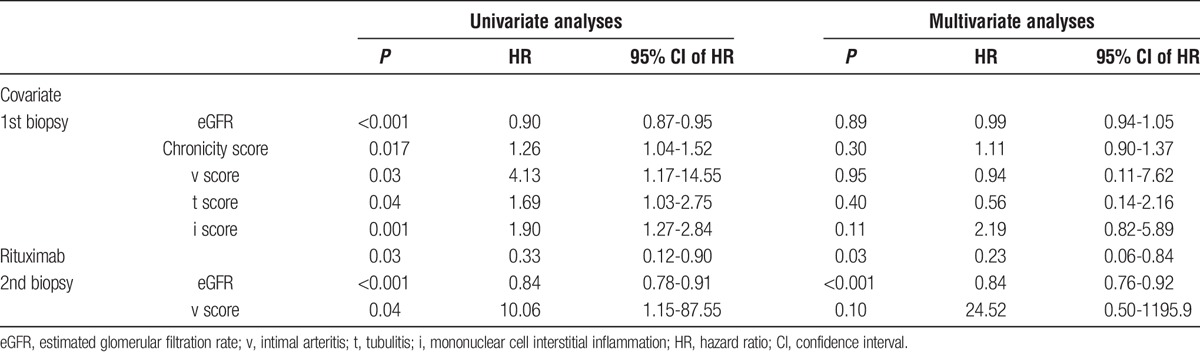
Risk Factors for Graft Loss After ABMR
We used univariate and multivariable regression analyses to examine the risk factors associated with graft loss after the diagnosis of ABMR (Table 5). In univariate regression analyses, rituximab, eGFR, Banff i, t, v, and chronicity (ci + ct + cv + cg) scores on the first biopsy, and eGFR and Banff v score on follow-up biopsy were associated with graft loss. Multivariate analyses retained only rituximab (hazard ratio, 0.23; 95% CI, 0.06-0.84; P = 0.03) and eGFR at follow-up biopsy (0.84; 95% CI, 0.76-0.92; P < 0.001) as significant predictors of graft loss, suggesting that renal function and anti–B-cell therapy are the most important variables while considering graft survival beyond 1 year in patients with late ABMR.
TABLE 5.
Variables Associated with Graft Loss after the Diagnosis of ABMR
DISCUSSION
There is little information about the immunophenotypic changes that occur after the treatment of late ABMR. In this large series of patients with late ABMR undergoing a follow-up biopsy within 6 weeks, we determined that treatment with steroid bolus/IVIG ± rituximab was effective in reducing class I and class II DSA, i, t, g, score, and microvascular injury. Importantly, rituximab and the recovery of kidney function on follow-up biopsy were the 2 most important variables predicting graft loss after 1 year.
The importance of renal function recovery after rejection was reported in a large Scientific Registry of Transplant Recipients cohort study, which demonstrated that patients with acute rejection whose serum creatinine return to baseline have similar graft outcomes to those who never experience rejection at all.7 Another study compared renal pathology in 1- and 2-year protocol biopsies of patients with acute rejection in the first year.8 Compared to patients without rejection, early acute rejection was associated with reduced graft survival (hazard ratio, 3.07; P <0.0001). However, only those rejection episodes followed by abnormal histology led to reduced graft survival.8
Effective treatment regimens are necessary to prolong graft and patient survival after ABMR. In our study, 18 of 78 grafts (23%) were lost within 15.9 ± 9.6 months after late ABMR. Treatment with steroid bolus/IVIG ± rituximab was effective in reducing both class I and class II DSA, along with most of the acute injury (i, t, ptc, g, and C4d staining), and there was no difference in renal function between the biopsies in either group. This might be because the histological findings may not correlate with graft function and outcome, the possibility that all DSAs are not detrimental, or perhaps the time course was too short for any meaningful changes. More importantly, there was no significant progression in the chronicity score parameters. Although short-term follow-up biopsies and DSA did not demonstrate significant differences between the SOC and rituximab groups, the therapeutic benefits of B-cell depletion are delayed with better graft survival. The role of rituximab for the treatment of ABMR remains unknown. Fehr et al found the combination of rituximab and IVIG improved kidney allograft function and DSA in a small group of 4 patients.9 Similarly, other case series have noted superior graft survival in patients treated with IVIG and rituximab for ABMR.10-13 However, Bachelet et al observed that the addition of rituximab and IVIG was associated with increased incidence of adverse effects without a significant change in the progression of late ABMR or transplant glomerulopathy.14 Morever, RITUX ERAH, a multicenter double-blind randomized placebo-controlled trial of patients receiving rituximab versus placebo noted no additional benefit of rituximab when compared at 1 year.15 However, because these studies are limited by sample size and/or follow-up time, there is no doubt that the therapeutic effects of B-cell depletion in late ABMR need further investigation. We adjusted for differences in baseline characteristics through regression analysis rather than propensity matching owing to the limited sample size.
Various histological factors may predict the graft survival in patients with ABMR. Patients with early ABMR in the setting of preexisting DSAs, also called type 1 ABMR, often present with severe v3-lesions and have a poor prognosis.16,17 The significance of v-lesions in the type 2 ABMR (ABMR occurring late and in the setting of de novo DSA) is unknown.18 In our study, there was no significant change in v-lesions with our treatment, and v-lesions at both initial biopsy and follow-up biopsy were predictors of graft loss in the univariate analysis. Isolated v-lesions received much attention at the Banf 2013 meeting, which will hopefully result in additional recommendations for the management of arteritis.2 A peritubular C4d deposit is another marker of the acute injury, and may or may not predict graft survival in ABMR.19,20 Severity of the g-lesions has been associated with poor outcomes, with g3 having the worst graft survival.21 Microvascular injury defined as microvascular inflammation (ptc and g) or microvascular deterioration (cg and ptc multilayering) is also an important predictor of graft loss in late ABMR independent of C4d staining.22,23 Similarly, the presence of cg on a biopsy is not favorable and is associated with significantly inferior graft survival.24 In our study, none of the histological findings were predictors of graft survival in the multivariate analysis; however, v scores in both initial and follow-up biopsy, t score, and i score were associated with graft survival in univariate analysis.
Our observations have the limitations inherent to this type of study. As a single center, observational, nonrandomized study, it may not be possible to generalize our results to other centers. As a result, and despite the use of regression analyses, there could be some selection bias on the use of treatment options. Additionally, although all patients had positive HLA DSA in our study, it is also possible that some had non-HLA antibodies, which are not routinely measured at our center. However, to our best knowledge, this is the largest reported series comparing various features of ABMR on follow-up biopsies, with 2 treatment strategies and more than 1 year of follow-up. In summary, treatment of late ABMR with steroids/IVIG + rituximab was effective in reducing DSA, microcirculation inflammation, and graft loss. Although short-term monitoring DSA and biopsies helped confirm the effectiveness of therapy, the advantage of graft survival was apparent after 1 year. Randomized clinical trials with long-term follow-up are needed to determine the role of B-cell depletion and DSA/biopsy monitoring in patients with ABMR.
ACKNOWLEDGMENTS
The authors are grateful to Ms Dana Clark, MA, for her editorial assistance.
Footnotes
Published online 27 October, 2017.
The authors declare no conflicts of interest.
This work was partially supported by a research grant from the Virginia Lee Cook Foundation (to D.A.M).
Author contribution: S.P. conceptualized and designed the study, performed data collection and analysis, and prepared the manuscript. D.A.M. designed the study, performed data analysis, prepared and edited the manuscript. B.M. conceptualized and designed the study, performed data analysis, and prepared and edited the manuscript. M.M., N.G., F.A., R.R.R., W.Z., and B.C.A. performed data analysis and edited the manuscript. A.D. provided the original idea, concept, and design; performed data analysis, and prepared and edited the manuscript.
REFERENCES
- 1.Djamali A, Kaufman DB, Ellis TM, et al. Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am J Transplant. 2014;14:255–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–283. [DOI] [PubMed] [Google Scholar]
- 3.Loupy A, Haas M, Solez K, et al. The Banff 2015 Kidney meeting report: current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant. 2017;17:28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fehr T, Gaspert A. Antibody-mediated kidney allograft rejection: therapeutic options and their experimental rationale. Transplant Int. 2012;25:623–632. [DOI] [PubMed] [Google Scholar]
- 5.Redfield RR, Ellis TM, Zhong W, et al. Current outcomes of chronic active antibody mediated rejection—a large single center retrospective review using the updated BANFF 2013 criteria. Human Immunol. 2016;77:346–352. [DOI] [PubMed] [Google Scholar]
- 6.Jordan SC, Reinsmoen N, Peng A, et al. Advances in diagnosing and managing antibody-mediated rejection. Pediatric Nephrol. 2010;25:2035–2045; quiz 45–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meier-Kriesche HU, Schold JD, Srinivas TR, et al. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378–383. [DOI] [PubMed] [Google Scholar]
- 8.El Ters M, Grande JP, Keddis MT, et al. Kidney allograft survival after acute rejection, the value of follow-up biopsies. Am J Transplant. 2013;13:2334–2341. [DOI] [PubMed] [Google Scholar]
- 9.Fehr T, Rusi B, Fischer A, et al. Rituximab and intravenous immunoglobulin treatment of chronic antibody-mediated kidney allograft rejection. Transplantation. 2009;87:1837–1841. [DOI] [PubMed] [Google Scholar]
- 10.Vo AA, Sinha A, Haas M, et al. Factors predicting risk for antibody-mediated rejection and graft loss in highly human leukocyte antigen sensitized patients transplanted after desensitization. Transplantation. 2015;99:1423–1430. [DOI] [PubMed] [Google Scholar]
- 11.Smith RN, Malik F, Goes N, et al. Partial therapeutic response to Rituximab for the treatment of chronic alloantibody mediated rejection of kidney allografts. Transplant Immunol. 2012;27:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahwaji J, Najjar R, Kancherla D, et al. Histopathologic features of transplant glomerulopathy associated with response to therapy with intravenous immune globulin and rituximab. Clin Transplant. 2014;28:546–553. [DOI] [PubMed] [Google Scholar]
- 13.Lefaucheur C, Nochy D, Andrade J, et al. Comparison of combination plasmapheresis/IVIG/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant. 2009;9:1099–1107. [DOI] [PubMed] [Google Scholar]
- 14.Bachelet T, Nodimar C, Taupin JL, et al. Intravenous immunoglobulins and rituximab therapy for severe transplant glomerulopathy in chronic antibody-mediated rejection: a pilot study. Clin Transplant. 2015;29:439–446. [DOI] [PubMed] [Google Scholar]
- 15.Sautenet B, Blancho G, Buchler M, et al. One-year results of the effects of rituximab on acute antibody-mediated rejection in renal transplantation: RITUX ERAH, a multicenter double-blind randomized placebo-controlled trial. Transplantation. 2016;100:391–399. [DOI] [PubMed] [Google Scholar]
- 16.Sis B, Einecke G, Chang J, et al. Cluster analysis of lesions in nonselected kidney transplant biopsies: microcirculation changes, tubulointerstitial inflammation and scarring. Am J Transplant. 2010;10:421–430. [DOI] [PubMed] [Google Scholar]
- 17.Haas M. Pathologic features of antibody-mediated rejection in renal allografts: an expanding spectrum. Curr Opin Nephrol Hypertens. 2012;21:264–271. [DOI] [PubMed] [Google Scholar]
- 18.Brocker V, Hirzallah M, Gwinner W, et al. Histopathological and clinical findings in renal transplants with Banff type II and III acute cellular rejection without tubulointerstitial infiltrates. Virchows Arch. 2014;464:203–211. [DOI] [PubMed] [Google Scholar]
- 19.Herzenberg AM, Gill JS, Djurdjev O, et al. C4d deposition in acute rejection: an independent long-term prognostic factor. J Am Soc Nephrol. 2002;13:234–241. [DOI] [PubMed] [Google Scholar]
- 20.Lefaucheur C, Nochy D, Hill GS, et al. Determinants of poor graft outcome in patients with antibody-mediated acute rejection. Am J Transplant. 2007;7:832–841. [DOI] [PubMed] [Google Scholar]
- 21.Batal I, Lunz JG, 3rd, Aggarwal N, et al. A critical appraisal of methods to grade transplant glomerulitis in renal allograft biopsies. Am J Transplant. 2010;10:2442–2452. [DOI] [PubMed] [Google Scholar]
- 22.Hidalgo LG, Campbell PM, Sis B, et al. De novo donor-specific antibody at the time of kidney transplant biopsy associates with microvascular pathology and late graft failure. Am J Transplant. 2009;9:2532–2541. [DOI] [PubMed] [Google Scholar]
- 23.Einecke G, Sis B, Reeve J, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009;9:2520–2531. [DOI] [PubMed] [Google Scholar]
- 24.Issa N, Cosio FG, Gloor JM, et al. Transplant glomerulopathy: risk and prognosis related to anti-human leukocyte antigen class II antibody levels. Transplantation. 2008;86:681–685. [DOI] [PubMed] [Google Scholar]


