Abstract
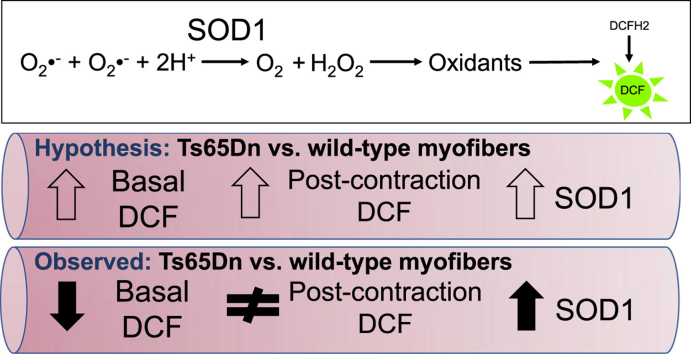
Down syndrome (DS) is a genetic condition caused by the triplication of chromosome 21. Persons with DS exhibit pronounced muscle weakness, which also occurs in the Ts65Dn mouse model of DS. Oxidative stress is thought to be an underlying factor in the development of DS-related pathologies including muscle dysfunction. High-levels of oxidative stress have been attributed to triplication and elevated expression of superoxide dismutase 1 (SOD1); a gene located on chromosome 21. The elevated expression of SOD1 is postulated to increase production of hydrogen peroxide and cause oxidative injury and cell death. However, it is unknown whether SOD1 protein expression is associated with greater oxidant production in skeletal muscle from Ts65Dn mice. Thus, our objective was to assess levels of SOD1 expression and oxidant production in skeletal myofibers from the flexor digitorum brevis obtained from Ts65Dn and control mice. Measurements of oxidant production were obtained from myofibers loaded with 2′,7′-dichlorodihydrofluorescein diacetate (DCFH2-DA) in the basal state and following 15 min of stimulated unloaded contraction. Ts65Dn myofibers exhibited a significant decrease in basal DCF emissions (p < 0.05) that was associated with an approximate 3-fold increase in SOD1 (p < 0.05). DCF emissions were not affected by stimulating contraction of Ts65Dn or wild-type myofibers (p > 0.05). Myofibers from Ts65Dn mice tended to be smaller and myonuclear domain was lower (p < 0.05). In summary, myofibers from Ts65Dn mice exhibited decreased basal DCF emissions that were coupled with elevated protein expression of SOD1. Stimulated contraction in isolated myofibers did not affect DCF emissions in either group. These findings suggest the skeletal muscle dysfunction in the adult Ts65Dn mouse is not associated with skeletal muscle oxidative stress.
Keywords: Segmental trisomy, Superoxide dismutase, Ts65Dn, Antioxidant
Graphical abstract
Highlights
-
•
Decreased basal oxidant levels corresponded with greater SOD1 in Ts65Dn myofibers.
-
•
Myofiber oxidant levels were similar between Ts65Dn and controls after contraction.
-
•
Myonuclear domain was smaller in Ts65Dn myofibers.
1. Introduction
Down syndrome (DS) is a genetic condition caused by triplication of chromosome 21. In addition to the numerous health conditions associated with DS, persons with DS exhibit severely reduced muscle strength that significantly limits their functional work capacity [1], [2], [3], [4], [5], [6]. Skeletal muscle weakness also occurs in the Ts65Dn mouse model of DS, thus making it a suitable model to help understand the muscle dysfunction occurring in the human condition [7], [8]. Persons with DS show evidence of oxidative stress that is thought to be a key factor in the development of muscle weakness and other DS-related pathologies such as Alzheimer's disease [9], [10].
Oxidative stress is an imbalance in the production of oxidants and their removal by antioxidants that leads to a disruption in redox signalling and/or cellular dysfunction and death [11]. The development of oxidative stress in DS has been attributed, at least in part, to the elevated expression of superoxide dismutase 1 (SOD1) [9], which is a gene encoded on chromosome 21 [12]. SOD1 is an essential antioxidant located in the cytosol and inter-membrane space of the mitochondria that functions to form hydrogen peroxide from the dismutation of the superoxide radical [11]. Hydrogen peroxide can undergo reaction with ferrous and cuprous complexes to produce the highly reactive hydroxyl radical. Thus, increased expression of SOD1 in DS could play a role in the development of muscle weakness by increasing the production of hydrogen peroxide and subsequent reactive oxygen species (ROS) leading to oxidative injury, dysregulation of redox signalling, and cell death [9], [13]. This concept is widely supported by findings from transgenic SOD1 over-expressing mice, which exhibit skeletal muscle oxidative stress and severe muscle atrophy and dysfunction [13], [14], [15], [16], [17].
Whether oxidant production is altered in Ts65Dn skeletal myofibers is currently unknown. This is an important issue to address as data signify the presence of skeletal muscle mitochondrial dysfunction in person with DS and Ts65Dn mice [8], [18], [19] that may be related to oxidative stress. Thus, our objective was to directly assess levels of oxidant production in myofibers from Ts65Dn and wild-type mice under basal conditions and following unloaded contraction. Experiments were performed on single myofibers of the flexor digitorum brevis using the 2′,7′-dichlorodihydrofluorescein (DCFH2) assay to assess oxidant production. We hypothesized that oxidant production of Ts65Dn myofibers would be greater than wild-type controls and would associate positively with SOD1 protein expression. We also hypothesized that oxidant production would increase with contraction in both wild-type and Ts65Dn myofibers, but the increase would be higher in Ts65Dn myofibers.
2. Material and methods
2.1. Experimental animals
Twelve-month-old male Ts65Dn (n = 4) mice and wild-type (WT; n = 5) colony controls were obtained from the Jackson Laboratory (Bar Harbor, ME). Ts65Dn mice weighed significantly less than control mice (Ts65Dn: 32.0 ± 0.2 g vs. WT: 42.6 ± 0.5 g; p < 0.05). Mice were housed in groups of 3–4 by genotype and maintained on a 12:12 h light-dark cycle with food and water provided ad libitum. The Syracuse University Institutional Animal Care and Use Committee approved the use of animals for these experiments, which complied with the Guide for the Care and Use of Laboratory Animals and the National Institutes of Health guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). Myofibers were obtained from the flexor digitorum brevis muscle of the foot. At the beginning of the procedure mice were deeply anesthetized by an injection of Fatal Plus (i.p., 120 mg/kg) (Vortech Pharmaceuticals, Ltd., Dearborn, MI) and the flexor digitorum brevis muscle was dissected from the left and right hindlimbs. At the end of the procedure the mice were sacrificed by performing a thoracotomy and removing the heart.
2.2. Isolation and culture of myofibers
Skeletal myofibers were isolated according to the method of Shefer and Yablonka-Reuveni [20] with minor modifications. Briefly, muscles were excised and incubated for 3 h at 37 °C in sterile conical tubes containing Dulbecco's modified eagle medium (DMEM) with L-glutamine and sodium pyruvate (MediaTech Inc., Manassas, VA), 0.4% (w/v) collagenase type I (Worthington Biomedical Corporation, Lakewood, NJ), and 25 μM HEPES. Myofibers were disassociated by gently pipetting the muscle with progressively smaller fire-polished Pasteur pipettes. Isolated myofibers were then plated on Matrigel (BD Biosciences, MD) coated 35 mm dishes and bathed in maintenance medium containing DMEM, 10% fetal bovine serum (Thermo Scientific Inc.), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cultured myofibers were maintained in 5% CO2 at 37 °C for at least 12 h before they were used in experiments.
2.3. Measurement of myofiber oxidant production
Culture dishes were removed from the incubator and the medium was replaced with Dulbecco's PBS (D-PBS) containing 20 μM 2′, 7′-dichlorodihydrofluorescein diacetate (DCFH2-DA; Invitrogen) in ethanol, and incubated in the dark for 45 min at room temperature. Following the incubation, myofibers were washed and incubated for an additional 20 min in D-PBS before imaging. Myofibers were imaged with a Nikon TE2000E inverted microscope (Nikon Instruments, Inc., Melville, NY) using a Y-2E/C filter (ex: 540–580 nm; em: 600–660 nm) to acquire the fluorescent 2′, 7′-dichlorofluorescein (DCF) emission signal.
Basal DCF emissions were measured over a single 15-min period for each myofiber. Measurements were obtained at time 0 and 15 min and data were expressed as a ratio (15 min/0 min). To examine the effect of contraction (i.e., unloaded shortening) on DCF emissions, a separate group of myofibers were stimulated to contract for 15 min using platinum electrodes according to published parameters that have been shown to increase DCF emissions in isolated skeletal myofibers (2 ms pulse, 500 ms train duration, 50 Hz, 30 V, repeated once every 5 s) [21]. The fluorescence intensity of the myofiber was converted to gray units using ImageJ software. The gray unit of a small area away from the myofiber was also measured for background correction. At the end of the experiment we induced DCFH2 oxidation by ~ 20 s of light exposure to ensure that the measured signals were not saturated.
The viability of each myofiber was assessed following the experiment in three ways: 1) morphology; 2) contractility; and 3) exclusion of trypan blue. The morphology of the myofibers was closely inspected for the presence of organized striations and absence of severe bending or twisting (See supplemental video 1). Maintenance of myofiber contractility was confirmed by stimulating the myofiber using platinum electrodes (See supplemental video 1). It was essential to test myofibers for contractility because myofibers that did not contract after the protocol exhibited significantly decreased DCF emissions compared to myofibers that contracted after the protocol (data not shown). Membrane damage was assessed using a solution of PBS containing 0.4% trypan blue. Myofibers with membrane damage take-up the dye and were considered non-viable. The myofiber was considered viable only if it met all of the above criteria.
Supplementary material related to this article can be found online at.
The following is the Supplementary material related to this article Video 1.
The viability of each myofiber was assessed before and after the experiment. This video shows examples of healthy and damaged myofibers.
2.4. Myofiber SOD1 expression by immunofluorescence
Following measurement of DCF emissions some of the myofibers were fixed in 2% formaldehyde in PBS at room temperature for 15 min, permeabilized with 0.2% Triton X-100 in PBS for 20 min, and blocked with a solution containing 10% normal goat serum and 1% bovine serum albumin for 30 min. The SOD1 primary antibody (1:100; BioVision, Inc., Mountainview, CA) was applied and incubated in a dark humid chamber at 4 °C for 24 h. Myofibers were washed with PBS then incubated with a Texas Red-labeled goat anti-rabbit secondary antibody (Invitrogen) for 1 h at room temperature. The myofibers were mounted in Vectashield with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA) and viewed with wide-field fluorescence microscopy using a B-2E filter (ex: 450–490 nm; em: 520–560 nm).
2.5. Calculation of myofiber volume and myonuclear domain
Myonuclei were stained with DAPI as detailed above. The total number of nuclei from each myofiber were counted. Myonuclear domain was calculated by dividing myofiber volume (myofiber length × π r2) by the total number of nuclei [22].
2.6. Statistical analysis
To determine whether the data were normally distributed the Shapiro-Francia test was used. In the event data were normally distributed (p > 0.05 on the Shapiro-Francia test) an independent sample t-tests for parametric data was used to determine if there were differences in the dependent variables between Ts65Dn and WT myofibers. If the assumption of normality was violated (p < 0.05 on the Shapiro-Francia test) then a two sample Kolmogorov-Smirnov test for non-parametric data was used to determine if there were differences in the dependent variables between Ts65Dn and WT myofibers. Normally distributed data included SOD1 protein expression, fiber length, fiber diameter, number of nuclei, and myonuclear domain. Non-normally distributed data included basal DCF emissions, contractile DCF emissions and fiber volume. Data are presented in the form mean ± SEM. The alpha was set a priori at p < 0.05. Stata 10.1 statistical package was used for data analysis.
3. Results
3.1. Myofiber oxidant production and SOD1 expression
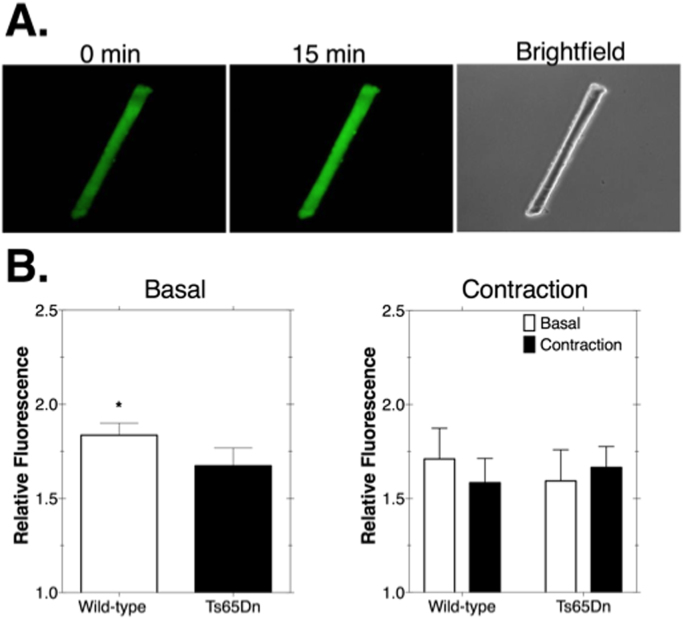
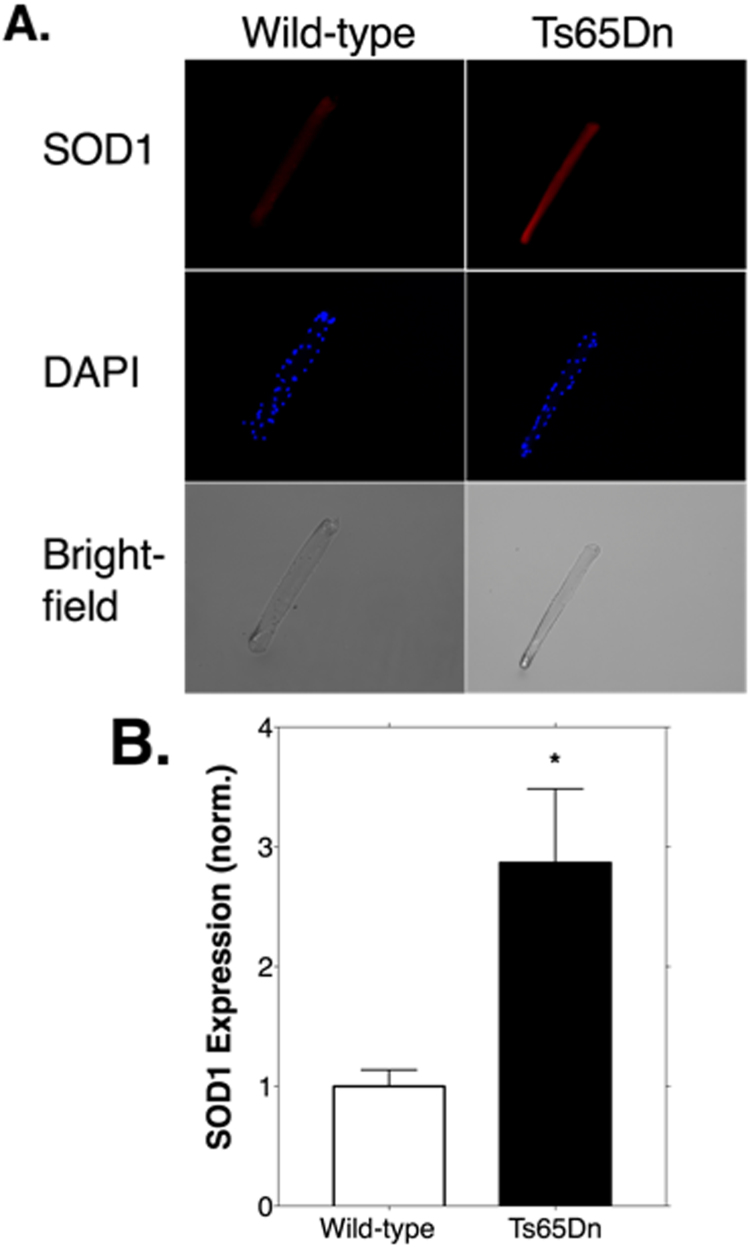
To test whether Ts65Dn myofibers exhibited a greater level of basal oxidant production we measured emissions of the highly fluorescent 2′ 7′-dichlorofluorscein (DCF) probe. As shown in Fig. 1, oxidant production levels were significantly lower in Ts65Dn myofibers (p < 0.05; Fig. 1B, left). Representative images of DCF emissions are shown in Fig. 1A. To test the effect of unloaded, stimulated contraction on oxidant production we monitored DCF emissions before and after 15 min of contractions. Contraction did not result in a significant effect on DCF emissions in Ts65Dn or WT myofibers (Fig. 1B, right). Fig. 2A shows a representative image of myofiber SOD1 protein expression. SOD1 levels were significantly greater in myofibers from Ts65Dn animals compared to WT myofibers by 2.9-fold (Fig. 2B; p < 0.05).
Fig. 1.
2′, 7′-dichlorofluorescein (DCF) emissions were lower in myofibers from Ts65Dn mice. A. Fluorescence images of DCF in a myofiber at the start, and after 15 min of incubation at room temperature. Gray values for each myofiber were calculated using Image J. The value at 15 min was expressed relative to the initial value. In this example the relative fluorescence value was 1.43. The brightfield image of the same myofiber is shown on the right. B. Left: Ts65Dn myofibers exhibit significantly reduced basal DCF emissions than myofibers from wild-type animals. N = 3–5 animals/group. 12 total myofibers from Ts65Dn and 27 from wild-type. * p < 0.05. Right: Fifteen minutes of contractile activity did not affect DCF emissions in wild-type or Ts65Dn myofibers compared to basal DCF emission levels. N = 2–3 animals/group. 11 total myofibers from Ts65Dn and 11 from wild-type. Bars are means ± standard error.
Fig. 2.
Protein expression of superoxide dismutase 1 (SOD1) is increased in myofibers from Ts65Dn mice. A. Immunofluorescence labelling of SOD1 from wild-type and Ts65Dn myofibers. Myofibers were labeled for SOD1 and 4′,6-diamidino-2-phenylindole (DAPI) to visualize SOD1 (left; pseudo color red) and nuclei (middle; pseudo color blue). B. Quantification of SOD1 expression from wild-type and Ts65Dn myofibers. Gray values for each myofiber were calculated using Image J. Values were normalized using the mean of the wild-type group. N = 4 animals/group. 14 total myofibers from Ts65Dn and 12 from wild-type. Bars are means ± standard error. * p < 0.05. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
3.2. Myofiber morphology
To examine morphology of myofibers we measured myofiber length and diameter, and quantified the number of DAPI positive nuclei in myofibers. We then derived myofiber volume and myonuclear domain from the above parameters. Myofiber length (p = 0.539), diameter (p = 0.068), volume (p = 0.051), and number of myonuclei (p = 0.311) were not significantly different between groups (Table 1). However, myonuclear domain was significantly lower in Ts65Dn myofibers compared to WT controls (p = 0.038) (Table 1).
Table 1.
Myofiber morphology.
| Variable | Wild-type | Ts65Dn | P-value |
|---|---|---|---|
| Number of myofibers | 8 | 12 | – |
| Myofiber length (μm) | 297.0 ± 10.2 | 285.4 ± 13.3 | 0.539 |
| Myofiber diameter (μm) | 31.8 ± 2.2 | 26.8 ± 1.5 | 0.068 |
| Myofiber volume (μm3) | 244,440 ± 34,137 | 169,212 ± 23,877 | 0.051 |
| Number of myonuclei | 44.5 ± 2.1 | 40.8 ± 2.6 | 0.311 |
| Myonuclear domain (μm3/nuclei) | 5370.8 ± 566.5 | 4009.0 ± 326.0* | 0.038 |
Values are mean ± SEM. *Ts65Dn less than Wild-type; p < 0.05.
4. Discussion
Our data are the first to examine the relationship between SOD1 protein expression and oxidant production in myofibers from a well-characterized mouse model of DS. Notably, persons with DS and Ts65Dn mice exhibit muscle weakness [2], [3], [5], [7], [8]. Increased expression of SOD1 may induce oxidative stress and could play a role in muscle weakness by increasing the steady-state level of hydrogen peroxide leading to oxidative injury and cell death [9], [13]. Thus, we hypothesized that myofibers from Ts65Dn mice would exhibit increased oxidant production, which would be associated with increased SOD1. The major finding from this study is that in isolated Ts65Dn myofibers decreased basal oxidant production was associated with increased SOD1 expression.
We observed a ~ 3-fold increase in protein expression of SOD1 in Ts65Dn myofibers compared to WT myofibers. However, this was not associated with increased oxidant production as we had hypothesized. In fact, we detected the opposite; increased SOD1 expression in Ts65Dn myofibers was associated with decreased basal oxidant production. SOD1 is a particularly important enzyme because it converts a highly reactive oxidant, superoxide radical, to a non-radical (hydrogen peroxide) which needs to be further detoxified. It is not surprising then that both knockout of SOD1 (i.e., higher levels of superoxide radical) and over-expression of SOD1 (i.e., higher levels of hydrogen peroxide) can lead to skeletal muscle oxidative stress [13], [14], [15], [16], [17], [22], [23]. Thus, the effect of elevated SOD1 protein expression must depend on the balance between the beneficial effects of removing superoxide radical, and the potential adverse effects of elevated hydrogen production generation [13]. Therefore, increased SOD1 expression in Ts65Dn myofibers may have lowered oxidant production by decreasing levels of superoxide radical and derivatives of this molecule that are known to oxidize DCFH2 (e.g., peroxynitrite), but also did not result in a substantial increase in hydrogen peroxide production beyond the buffering capacity of other antioxidant enzyme systems including catalase, glutathione peroxidase, thioredoxin and peroxiredoxins. These peroxidases are highly efficient in removing excess hydrogen peroxide generated by increased SOD1. This redundancy in the hydrogen peroxide buffering capacity of the myofiber may be protective against small to moderate increases in SOD1 expression. Having knowledge of possible changes in the capacity and efficiency of these antioxidant enzyme systems would have improved our understanding of mechanisms of ROS detoxification in Ts65Dn myofibers.
Oxidant production is increased during periods of oxidative stress which can lead to the accumulation of oxidized proteins [11]. We previously reported that protein carbonyl formation is increased in Ts65Dn muscle, whereas lipid peroxidation product 4-hydroxynonenal was unaltered compared to controls [8]. In the current study, oxidant production was not increased in Ts65Dn myofibers which does not help to explain why protein carbonyl formation was increased in Ts65Dn soleus muscle. Notably, the disposal mechanisms for removing oxidized proteins is impaired in brain tissue from Ts65Dn mice [24], [25]. Thus, increased protein carbonyl formation is not likely an effect of increased oxidant production in Ts65Dn skeletal muscle but more likely due to a defect in the systems involved in degrading oxidized proteins.
We previously showed that isolated soleus muscles from Ts65Dn mice fail to recover from fatiguing contractions to the same degree as WT muscle [8]. We speculated that the post-exercise weakness in Ts65Dn muscles might be due to mitochondrial dysfunction. Contractile dysfunction could also result from oxidant-mediated protein damage to calcium handling or contractile proteins [26], [27], [28]. Thus, we employed a contractile protocol developed for use in single myofibers that was previously shown to increase oxidant production following 15 min of stimulated contraction [21], [29]. We hypothesized that contractile activity would significantly increase oxidant production in both groups, and that the increase would be higher in Ts65Dn myofibers. However, oxidant production was unaltered following 15 min of unloaded, stimulated contraction in both groups. A possible reason for the lack of an observed post-contraction elevation in oxidant production could be due to the design of our experiment. In the current study, we compared levels of basal oxidant production to post-contractile oxidant production between myofibers within the same animal. Previous studies made comparison within the same myofiber [21], [29]. Such a comparison may be more sensitive in detecting small changes in contraction-stimulated oxidant production. Also, since we utilized a general oxidant detector it was not possible to detect discrete changes in specific oxidant species.
A limitation of the DCFH2 assay is that it is a qualitative marker of cellular oxidative stress [30] and we did not specifically determine levels of superoxide radical or hydrogen peroxide. It is known that DCFH2 does not react directly with superoxide or hydrogen peroxide and thus does not provide information on specific oxidant activity [31], [32]. Oxidation of DCFH2 occurs in the presence of hydrogen peroxide but is dependent on the availability of redox active metals [30]. DCFH2 oxidation is also enhanced in the presence of heme-containing compounds including hematin, peroxidases and cytochrome c [30], [32]. The assay thus provides a global measure of oxidant production because it is well-known that a number of substances can also oxidize DCFH2 in the absence of hydrogen peroxide such as peroxynitrite and hypochlorous acid [30]. Although a more specific determination of selective oxidant levels would have been informative, the DCFH2 assay is considered a suitable method to determine the total oxidant generation in cells [33].
Several factors affect skeletal muscle oxidant production in vivo that are absent in ex vivo conditions. For example, in vivo, skeletal myofibers interact with inflammatory cells that can result in pro-oxidant producing inflammatory reactions [34]. Furthermore, lack of ferrous and cuprous protein complexes typically found in the blood are absent in ex-vivo conditions, which will in effect lower the potential for a pro-oxidant environment. Thus, increased oxidative stress in immune and red blood cells from Ts65Dn mice may affect the redox status of skeletal muscle in vivo [35], [36], [37]. While our data suggest elevated oxidant levels do not arise from isolated Ts65Dn myofibers the contribution of the aforementioned factors on in vivo oxidant production requires further investigation.
Analyses of myofiber morphology revealed a smaller myonuclear domain among Ts65Dn myofibers. Myofiber diameter and volume were lower in Ts65Dn myofibers which approached statistical significance (p = 0.068 and p = 0.051, respectively); however, there were a similar number of myonuclei between groups (p = 0.311). Thus, decreased myonuclear domain in Ts65Dn myofibers is driven more by the lower volume of the myofibers (31% lower in Ts65Dn myofiber volume compared to WT) than by the Ts65Dn myofibers having fewer myonuclei (only 8% fewer than WT). Our measurements did not allow us to infer a possible role that nitric oxide may have played in regulating satellite cells in Ts65Dn myofibers, which could have influenced the myonuclear domain. However, the smaller size of Ts65Dn myofibers is consistent with previous studies showing Ts65Dn mice exhibit early signs of muscle atrophy and aging-related changes in mitochondrial morphology [19]. Specifically, quadriceps muscle from Ts65Dn mice display muscle atrophy at 12 months of age which becomes more severe at 19 months [19]. In addition to the morphological data from the flexor digitorum brevis myofibers, from the same mice we also analysed the cross-sectional area of myofibers from frozen slices of whole soleus muscle, and found Ts65Dn myofibers were significantly smaller compared to WT (unpublished observation). Thus, our data along with others provide additional evidence that Ts65Dn mice show evidence of accelerated muscle aging.
5. Conclusions
Collectively, this study demonstrated that decreased basal oxidant levels were associated with increased SOD1 protein expression in Ts65Dn myofibers. Fifteen minutes of unloaded, stimulated contraction in isolated myofibers did not affect oxidant production. Ts65Dn myofibers were smaller with a decreased myonuclear domain that supports a previous observation of an accelerated-aging muscle phenotype (muscle atrophy and mitochondrial irregularities) in Ts65Dn mice at 12 months of age [19]. Contrary to other cell types in the Ts65Dn mouse [35], [36], [37], [38], [39], oxidant production is not increased in isolated skeletal myofibers; thus skeletal muscle oxidative stress does not appear to be the mechanism responsible for the muscle weakness previously reported.
Funding sources
This work was supported by an American Heart Association Post-Doctoral Fellowship [16POST30970031] to PMC, the National Institutes of Health [R15HD076379] to LRD, the Le Moyne College Research & Development Committee, and Syracuse University School of Education. Funding sources did not have a role in the study design, data collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Acknowledgements
We extend our sincere gratitude to Jesse Lloyd for assistance with cell culture aspects of this project.
References
- 1.Cowley P.M., Ploutz-Snyder L.L., Baynard T., Heffernan K., Jae S.Y., Hsu S., Lee M., Pitetti K.H., Reiman M.P., Fernhall B. Physical fitness predicts functional tasks in individuals with down syndrome. Med. Sci. Sports Exerc. 2010;42:388–393. doi: 10.1249/MSS.0b013e3181b07e7a. [DOI] [PubMed] [Google Scholar]
- 2.Pitetti K.H., Boneh S. Cardiovascular fitness as related to leg strength in adults with mental retardation. Med. Sci. Sports Exerc. 1995;27:423–428. [PubMed] [Google Scholar]
- 3.Carmeli E., Ayalon M., Barchad S., Sheklow S.L., Reznick A.Z. Isokinetic leg strength of institutionalized older adults with mental retardation with and without Down's syndrome. J. Strength Cond. Res. 2002;16:316–320. [PubMed] [Google Scholar]
- 4.Angelopoulou N., Matziari C., Tsimaras V., Sakadamis A., Souftas V., Mandroukas K. Bone mineral density and muscle strength in young men with mental retardation (with and without Down syndrome) Calcif. Tissue Int. 2000;66:176–180. doi: 10.1007/s002230010035. [DOI] [PubMed] [Google Scholar]
- 5.Mercer V.S., Lewis C.L. Hip abductor and knee extensor muscle strength of children with and without down syndrome. Pediatr. Phys. Ther. 2001;13:18–26. [PubMed] [Google Scholar]
- 6.Carmeli E., Barchad S., Lenger R., Coleman R. Muscle power, locomotor performance and flexibility in aging mentally-retarded adults with and without Down's syndrome. J. Musculoskelet. Neuron. Interact. 2002;2:457–462. [PubMed] [Google Scholar]
- 7.Costa A.C., Walsh K., Davisson M.T. Motor dysfunction in a mouse model for Down syndrome. Physiol. Behav. 1999;68:211–220. doi: 10.1016/s0031-9384(99)00178-x. [DOI] [PubMed] [Google Scholar]
- 8.Cowley P.M., Keslacy S., Middleton F.A., DeRuisseau L.R., Fernhall B., Kanaley J.A., DeRuisseau K.C. Functional and biochemical characterization of soleus muscle in down syndrome mice: insight into the muscle dysfunction seen in the human condition. AJP Regul. Integr. Comp. Physiol. 2012:1251–1260. doi: 10.1152/ajpregu.00312.2012. [DOI] [PubMed] [Google Scholar]
- 9.Busciglio J., Yankner B. Apoptosis and increased generation of reactive oxygen species in Down's syndrome neurons in vitro. Nature. 1995;378:776–779. doi: 10.1038/378776a0. [DOI] [PubMed] [Google Scholar]
- 10.Zana M., Janka Z., Kalman J. Oxidative stress: a bridge between Down's syndrome and Alzheimer's disease. Neurobiol. Aging. 2007;28:648–676. doi: 10.1016/j.neurobiolaging.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Powers S.K., Jackson M.J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zelko I.N., Mariani T.J., Folz R.J. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 13.Xing J., Yu Y., Rando T.A. The modulation of cellular susceptibility to oxidative stress: protective and destructive actions of Cu,Zn-superoxide dismutase. Neurobiol. Dis. 2002;10:234–246. doi: 10.1006/nbdi.2002.0504. [DOI] [PubMed] [Google Scholar]
- 14.Peled-Kamar M., Lotem J., Wirguin I., Weiner L., Hermalin A., Groner Y. Oxidative stress mediates impairment of muscle function in transgenic mice with elevated level of wild-type Cu/Zn superoxide dismutase. Proc. Natl. Acad. Sci. USA. 1997;94:3883–3887. doi: 10.1073/pnas.94.8.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rando T.A., Crowley R.S., Carlson E.J., Epstein C.J., Mohapatra P.K. Overexpression of copper/zinc superoxide dismutase: a novel cause of murine muscular dystrophy. Ann. Neurol. 1998;44:381–386. doi: 10.1002/ana.410440315. [DOI] [PubMed] [Google Scholar]
- 16.Yarom R., Sapoznikov D., Havivi Y., Avraham K.B., Schickler M., Groner Y. Premature aging changes in neuromuscular junctions of transgenic mice with an extra human CuZnSOD gene: a model for tongue pathology in Down's syndrome. J. Neurol. Sci. 1988;88:41–53. doi: 10.1016/0022-510x(88)90204-3. [DOI] [PubMed] [Google Scholar]
- 17.Avraham K.B., Schickler M., Sapoznikov D., Yarom R., Groner Y. Down's syndrome: abnormal neuromuscular junction in tongue of transgenic mice with elevated levels of human Cu/Zn-superoxide dismutase. Cell. 1988;54:823–829. doi: 10.1016/s0092-8674(88)91153-1. [DOI] [PubMed] [Google Scholar]
- 18.Phillips A.C., Sleigh A., McAllister C.J., Brage S., Carpenter T.A., Kemp G.J., Holland A.J. Defective mitochondrial function in vivo in skeletal muscle in adults with Down's syndrome: a 31P-MRS study. PLoS One. 2013;8:e84031. doi: 10.1371/journal.pone.0084031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cisterna B., Costanzo M., Scherini E., Zancanaro C., Malatesta M. Ultrastructural features of skeletal muscle in adult and aging Ts65Dn mice, a murine model of Down syndrome. Muscles Ligaments Tendons J. 2013;3:287–294. [PMC free article] [PubMed] [Google Scholar]
- 20.Shefer G., Yablonka-Reuveni Z. Isolation and culture of skeletal muscle myofibers as a means to analyze satellite cells. Methods Mol. Biol. 2005;290:281–304. doi: 10.1385/1-59259-838-2:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palomero J., Pye D., Kabayo T., Spiller D.G., Jackson M.J. In situ detection and measurement of intracellular reactive oxygen species in single isolated mature skeletal muscle fibers by real time fluorescence microscopy. Antioxid. Redox Signal. 2008;10:1463–1474. doi: 10.1089/ars.2007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang Y.C., Lustgarten M.S., Liu Y., Muller F.L., Bhattacharya A., Liang H., Salmon A.B., Brooks S.V., Larkin L., Hayworth C.R., Richardson A., Van Remmen H. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010;24:1376–1390. doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller F.L., Song W., Liu Y., Chaudhuri A., Pieke-Dahl S., Strong R., Huang T.T., Epstein C.J., Roberts L.J., Csete M., Faulkner J.A., Van Remmen H. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic. Biol. Med. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 24.Necchi D., Lomoio S., Scherini E. Dysfunction of the ubiquitin-proteasome system in the cerebellum of aging Ts65Dn mice. Exp. Neurol. 2011;232:114–118. doi: 10.1016/j.expneurol.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Tramutola A., Lanzillotta C., Arena A., Barone E., Perluigi M., Di Domenico F. Increased mammalian target of rapamycin signaling contributes to the accumulation of protein oxidative damage in a mouse model of down's syndrome. Neurodegener. Dis. 2016;16:62–68. doi: 10.1159/000441419. [DOI] [PubMed] [Google Scholar]
- 26.Viner R.I., Williams T.D., Schoneich C. Nitric oxide-dependent modification of the sarcoplasmic reticulum Ca-ATPase: localization of cysteine target sites. Free Radic. Biol. Med. 2000;29:489–496. doi: 10.1016/s0891-5849(00)00325-7. [DOI] [PubMed] [Google Scholar]
- 27.DalleDonne I., Milzani A., Colombo R. H2O2-treated actin: assembly and polymer interactions with cross-linking proteins. Biophys. J. 1995;69:2710–2719. doi: 10.1016/S0006-3495(95)80142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun J., Xu L., Eu J.P., Stamler J.S., Meissner G. Classes of thiols that influence the activity of the skeletal muscle calcium release channel. J. Biol. Chem. 2001;276:15625–15630. doi: 10.1074/jbc.M100083200. [DOI] [PubMed] [Google Scholar]
- 29.Palomero J., Vasilaki A., Pye D., McArdle A., Jackson M.J. Aging increases the oxidation of dichlorohydrofluorescein in single isolated skeletal muscle fibers at rest, but not during contractions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305 doi: 10.1152/ajpregu.00530.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarpey M.M., Wink D.A., Grisham M.B. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R431–R444. doi: 10.1152/ajpregu.00361.2003. [DOI] [PubMed] [Google Scholar]
- 31.Gutteridge J., Halliwell B. Fourth, Oxford University Press, Inc.; United States: 2007. Free Radicals in Biology and Medicine. [Google Scholar]
- 32.Forman H.J., Augusto O., Brigelius-Flohe R., Dennery P.A., Kalyanaraman B., Ischiropoulos H., Mann G.E., Radi R., Roberts L.J., 2nd, Vina J., Davies K.J.A. Even free radicals should follow some rules: a guide to free radical research terminology and methodology. Free Radic. Biol. Med. 2015;78:233–235. doi: 10.1016/j.freeradbiomed.2014.10.504. [DOI] [PubMed] [Google Scholar]
- 33.Soh N. Recent advances in fluorescent probes for the detection of reactive oxygen species. Anal. Bioanal. Chem. 2006;386:532–543. doi: 10.1007/s00216-006-0366-9. [DOI] [PubMed] [Google Scholar]
- 34.Li X., Moody M.R., Engel D., Walker S., Clubb F.J.J., Sivasubramanian N., Mann D.L., Reid M.B. Cardiac-specific overexpression of tumor necrosis factor-alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation. 2000;102:1690–1696. doi: 10.1161/01.cir.102.14.1690. [DOI] [PubMed] [Google Scholar]
- 35.Paz-Miguel J.E., Flores R., Sanchez-Velasco P., Ocejo-Vinyals G., Escribano de Diego J., Lopez de Rego J., Leyva-Cobian F. Reactive oxygen intermediates during programmed cell death induced in the thymus of the Ts(1716)65Dn mouse, a murine model for human Down's syndrome. J. Immunol. 1999;163:5399–5410. [PubMed] [Google Scholar]
- 36.Lorenzo L.P.E., Chen H., Shatynski K.E., Clark S., Yuan R., Harrison D.E., Yarowsky P.J., Williams M.S. Defective hematopoietic stem cell and lymphoid progenitor development in the Ts65Dn mouse model of Down syndrome: potential role of oxidative stress. Antioxid. Redox Signal. 2011;15:2083–2094. doi: 10.1089/ars.2010.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorenzo L.P.E., Shatynski K.E., Clark S., Yarowsky P.J., Williams M.S. Defective thymic progenitor development and mature T-cell responses in a mouse model for Down syndrome. Immunology. 2013;139:447–458. doi: 10.1111/imm.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shichiri M., Yoshida Y., Ishida N., Hagihara Y., Iwahashi H., Tamai H., Niki E. alpha-Tocopherol suppresses lipid peroxidation and behavioral and cognitive impairments in the Ts65Dn mouse model of downsyndrome. Free Radic. Biol. Med. 2011;50:1801–1811. doi: 10.1016/j.freeradbiomed.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 39.Parisotto E.B., Vidal V., Garcia-Cerro S., Lantigua S., Wilhelm Filho D., Sanchez-Barcelo E.J., Martinez-Cue C., Rueda N. Chronic melatonin administration reduced oxidative damage and cellular senescence in the hippocampus of a mouse model of down syndrome. Neurochem. Res. 2016;41:2904–2913. doi: 10.1007/s11064-016-2008-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The viability of each myofiber was assessed before and after the experiment. This video shows examples of healthy and damaged myofibers.