Abstract
Objectives
To demonstrate use of computer aided design and 3D printing in creating simulated costochondral cartilage to practice creation of auricular frameworks. To identify a material that most closely simulates cartilage.
Methods
The costal cartilage of a free floating rib with adjacent synchondrosis was digitally segmented from a CT scan using medical imaging software. The resulting 3D mesh model was exported to 3D modeling software. A negative model was constructed using mesh Boolean and 3D printed. The resulting mold was filled with vinyl polysiloxane or a mixture of silicone and cornstarch to produce the final model for framework carving. Microtia surgeons rated the models’ carving and suturing characteristics relative to human cartilage on a Likert scale.
Results
An accurate reproduction of patient specific costal cartilage was produced. The composition material produced a texture and firmness similar to human cartilage. Likert ratings are reported.
Discussion
This study demonstrates a low cost, readily reproducible tool to simulate auricular reconstruction. There may be benefit from use of a patient specific costal cartilage model.
Keywords: 3-dimensional printing, 3D printing, CAD/CAM, auricular reconstruction, computer-aided design, computer-aided manufacturing, craniofacial reconstruction, microtia, surgical simulation, surgical education
Introduction
Microtia, underdevelopment of the auricle, affects approximately 0.03% of live births1. Carving an auricular cartilage framework from autogenous cartilage, the most common technique for auricular reconstruction, is one of the most challenging skills for the reconstructive surgeon to learn. Given the potential morbidity associated with technical errors in framework carving, opportunities for acquisition of this skill are limited. It is critical for surgeons to be able to practice their carving skills. This presents an opportunity for surgical simulation.
Materials previously used for simulation of auricular framework carving include carrots, potatoes, porcine/bovine/human cadaveric costal cartilage, and dental impression material2,3. These materials poorly represent the geometry, texture, and size of the harvested costal cartilage presented to the reconstructive surgeon. There is a commercially available model (Medicon, Tuttlingen, Germany) that is based upon adult rib and is costly.
To better represent pediatric rib geometry and texture, techniques were developed to produce negative molds from harvested pediatric rib cartilage4,5. While these methods are an improvement on the simulation of shape and size, questions remained on the similarity of the material to costal cartilage.
In this report, we aim to use CAD and 3D printing to create a representative pediatric costal cartilage model for simulation of auricular framework reconstruction. Furthermore, by using CT scan data, the potential for patient-specific simulation is introduced, allowing for surgical planning.
Methods
Production of 3D model
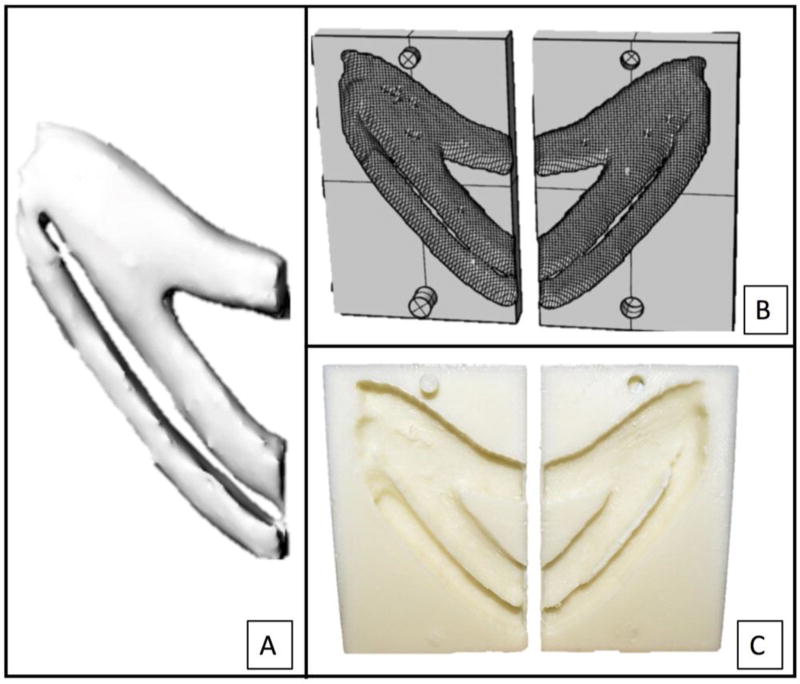
To create the costal cartilage model of a pediatric patient, a previously obtained high-resolution CT scan from an 8-year-old male was used (Seattle Children’s Hospital IRB approval #15913). The left costal cartilage of an attached floating rib with adjacent synchondrosis was cropped and segmented using a semi-automated threshold and watershed segmentation technique (3DSlicer, www.slicer.org). A 3D mesh model was exported to 3D modeling software (Rhinoceros, www.rhino3d.com) (Figure 1a). The negative model was constructed using mesh boolean of the original costal cartilage model (Figure 1b). The negative model was 3D printed using polylactic acid (Figure 1c).
Figure 1.
Precise reconstruction of pediatric costal cartilage with a 3-dimensional model from a computed tomography scan (A), negative model (B), and printed negative mold (C).
Materials
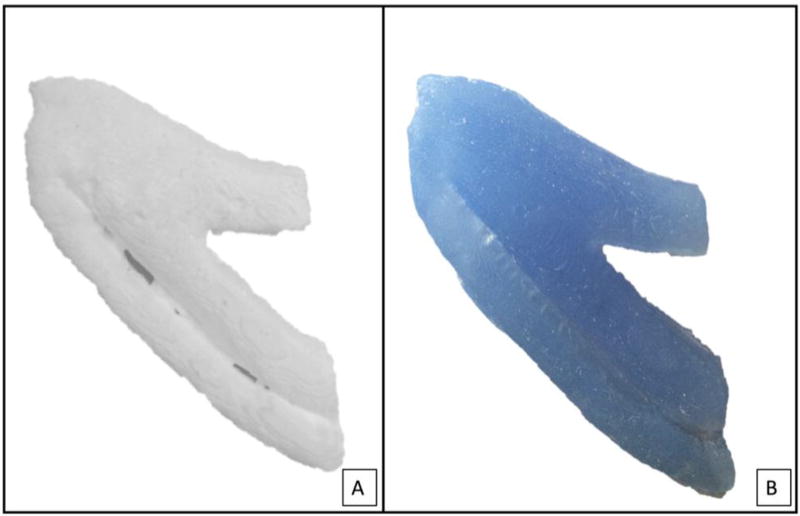
Industrial grade silicone (GE Silicone II White Window and Door Caulk) served as the base material and was combined with gradually higher proportions of starch (pure cornstarch). Weight-based silicone:starch composites were allowed to dry for 24 hours. Model I contains 6:5 silicone:starch, Model II contains 2:1 silicone:starch and Model III contains vinyl polysiloxane (Memosil 2, Hanau, Germany). Each material was used to fill the 3D printed negative mold (Figure 2a and 2b).
Figure 2.
Negative model was filled with starch:silicone composite material (A) and commercially available dental impression material (B) to produce costal cartilage model for carving.
Microtia surgeon comparison
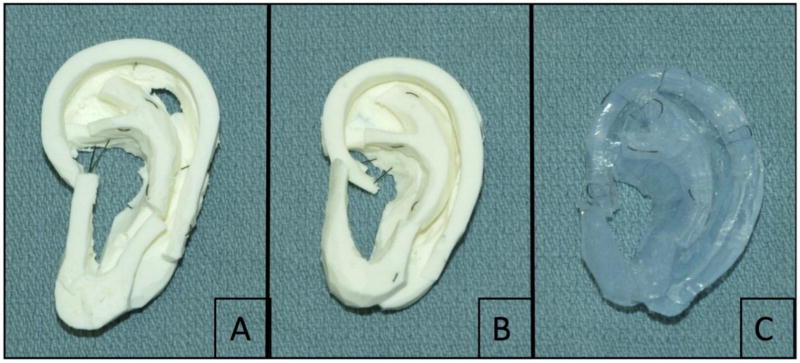
Three expert microtia surgeons, defined as having performed at least 50 microtia reconstructions, carved an auricular framework from each of the three materials. The raters carved and sutured each material with the same technique they use for microtia reconstruction (Figure 3). The surgeons were blinded to the nature of each material and were asked to rate each model on a Likert scale based on the similarity of the model to actual pediatric costal cartilage (Figure 4). Each surgeon performed the carving and rating independently from each other.
Figure 3.
Completed auricular framework from starch:silicone composite and commercially available dental impression material by microtia surgeons: (A) starch:silicone, 6:5; (B) starch:silicone, 2:1; (C) MEMOSIL-2 Dental Impression, vinyl polysiloxane.
Results
Production and Cost of 3D model
The negative mold design allowed for repeated application of various materials and durable production of positive models. The material to produce silicone:starch composite Models I and II cost $0.55 and $0.60, respectively, per model. MEMOSIL-2 dental impression material cost $60 per model. This does not factor in the cost of 3D printing.
Grading by Microtia Surgeons
Results of microtia surgeons’ grading of the materials are shown in Table I.
Table 1.
Results of Likert Rating for Models 1–3 by 3 Independent Microtia Surgeons (Raters A–C).a
| Model 1
|
Model 2
|
Model 3
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | Avg | A | B | C | Avg | A | B | C | Avg | |
| Texture | 2 | 4 | 2 | 2.7 | 3 | 4 | 3 | 3.3 | 1 | 1 | 3 | 1.7 |
| Firmness | 3 | 3 | 2 | 2.7 | 4 | 4 | 3 | 3.7 | 2 | 2 | 4 | 2.7 |
| Carving | 3 | 3 | 3 | 3 | 4 | 4 | 3 | 3.7 | 2 | 2 | 2 | 2 |
| Suturing | 2 | 3 | 2 | 2.3 | 3 | 4 | 3 | 3.3 | 2 | 1 | 4 | 2.3 |
| Bending | 3 | 3 | 2 | 2.7 | 4 | 4 | 3 | 3.7 | 2 | 2 | 2 | 2 |
| Geometry | 4 | 4 | 3 | 3.7 | 4 | 4 | 3 | 3.7 | 1 | 2 | 3 | 2 |
| Overall | 3 | 3 | 2 | 2.7 | 4 | 5 | 3 | 4 | 2 | 1 | 2 | 1.7 |
| Recommend | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | N |
| Rank | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 3 | 3 | 3 | 3 |
| Total (possible 105) | 59 | 76 | 43 | |||||||||
Abbreviations: avg, average; N, no; Y, yes.
Model 1, starch:silicone, 6:5; model 2, starch:silicone, 2:1; model 3, vinyl polysiloxane.
Discussion
Auricular cartilage framework creation for microtia reconstruction is challenging to master. Surgical simulation with a highly realistic model may assist with skill attainment. A rib cartilage model can also help experienced surgeons refine their carving skills and prepare for complex cases.
In this report we outline the production of a cartilage carving model using CAD and 3D printing. We compare a novel silicone:starch composite with dental impression material previously reported4,5. The silicone:starch composite more accurately mimics the qualities of costal cartilage. The composite model with higher proportion starch was considered most similar to costal cartilage. The recommended model was similar to rib in geometry, carving characteristics and pliability, though less in suturing characteristics and texture. Additional benefit of the silicone:starch material is the low cost.
This is the first report of a patient specific model of costal cartilage for auricular framework rehearsal. This model surpasses the shortcomings of prior simulation models. Dental impression silicone was a poor match for costal cartilage characteristics. Silicone:starch composite closely resembles harvested cartilage4–6. Furthermore, these techniques can be applied to individual patient’s imaging, introducing the potential for patient-specific rehearsal.
A recent review of tissue engineering for auricular reconstruction7, highlights the advances made in ear scaffold design and implementation of 3D printing by the senior author’s group8. The use of 3D printing for the production of a printed ear framework for auricular reconstruction holds great promise and has been attempted in animal models but not yet in humans. We wanted to utilize 3D printing in an immediately implementable application, to train microtia surgeons.
Conclusion
This study demonstrates a low cost, readily reproducible tool for simulation of auricular reconstruction. A silicone:starch composite closely resembled pediatric costochondral cartilage. There may be benefit for the trainee and more experienced microtia surgeon from use of a patient-specific representation of costal cartilage.
Acknowledgments
This work was supported in part by NIH T32 DC000018. We would like to thank the University of Washington BioRobotics Laboratory and Blake Hannaford, PhD for assistance with 3D printing. Additionally, we would like to thank P. Berens for her assistance in manuscript editing.
Footnotes
Presentation:
The work was presented as a podium by Angelique M. Berens, MD at the American Academy of Otolaryngology – Head and Neck Surgery Meeting September 28, 2015 in Dallas, Tx.
Contributor Information
Angelique Marie Berens, Department of Otolaryngology-Head and Neck Surgery, University of Washington, Seattle.
Sharon Newman, Department of Electrical Engineering, University of Washington, Seattle, WA.
Amit D. Bhrany, Department of Otolaryngology-Head and Neck Surgery, University of Washington, Seattle.
Craig Murakami, Division of Facial Plastic Surgery, Department of Otolaryngology-Head and Neck Surgery, Virginia Mason Medical Center, Seattle, Washington.
Kathleen C.Y. Sie, Department of Otolaryngology, Seattle Children's Hospital, Seattle, Washington.
David A. Zopf, Otolaryngology - Head and Neck Surgery, Pediatric Division, University of Michigan Health Systems, CS Mott Children’s Hospital.
References
- 1.Sie KY, Bhrany AD, Murakami CS. Cummings’ Otolaryngology Head and Neck Surgery. 6. Philadelphia, PA: Mosby/Elsevier; 2015. Microtia Reconstruction; pp. 2998–3005. [Google Scholar]
- 2.Agrawal K. Bovine Cartilage: A Near Perfect Training Tool for Carving Ear Cartilage Framework. The Cleft Palate-Craniofacial Journal. 2014 doi: 10.1597/14-079R. In-Press. [DOI] [PubMed] [Google Scholar]
- 3.Shin HS, Hong SC. A Porcine Rib Cartilage Model for Practicing Ear-Framework Fabrication. J Craniofac Surg. 2013;24:1756–1757. doi: 10.1097/SCS.0b013e3182902548. [DOI] [PubMed] [Google Scholar]
- 4.Yamada A, Imai K, Fujimoto T, Morimoto K, Niitsuma K, Matsumoto H. New Training Method of Creating Ear Framework by Using Precise Copy of Costal Cartilage. J Craniofac Surg. 2009;20:899–902. doi: 10.1097/scs.0b013e3181a2ef97. [DOI] [PubMed] [Google Scholar]
- 5.Sandeep T, Ladani P. A new method for training of ear framework creation by silicon dental impression material. Indian J Plast Surg. 2012;45:134–137. doi: 10.4103/0970-0358.96614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zung C, Chen P, Hung K, Lo L, Chen Y. Microtia Reconstruction with Adjuvant 3-Dimensional Template Model. Annals of Plastic Surgery. 2004;53:282–287. doi: 10.1097/01.sap.0000106434.69246.29. [DOI] [PubMed] [Google Scholar]
- 7.Otto IA, Melchels FP, Zhao X, et al. Auricular reconstruction using biofabrication-based tissue engineering strategies. Biofabrication. 2015;7 doi: 10.1088/1758-5090/7/3/032001. [DOI] [PubMed] [Google Scholar]
- 8.Zopf DA, Mitsak AG, Flanagan CL, et al. Computer aided-designed, 3-dimensionally printed porous tissue bioscaffolds for craniofacial soft tissue reconstruction. Otolaryngology Head Neck Surg. 2015;152:57–62. doi: 10.1177/0194599814552065. [DOI] [PMC free article] [PubMed] [Google Scholar]