Abstract
PURPOSE
Bladder cancer is the sixth most common cancer in the United States, but is exceedingly rare in young patients, leading to a lack of accepted standards for diagnosis, treatment, and surveillance. We review our institutional experience with bladder urothelial neoplasms in pediatric and young adult patients summarizing presentation, treatment, and outcomes.
METHODS
Surgical pathology records at our institution were searched for cases of urothelial neoplasms among patients ≤25 years of age treated between January 1997 and September 2016. Cases submitted exclusively for pathology review were excluded. Diagnoses were confirmed based on pathologic examination using the 2004 World Health Organization classification system.
RESULTS
Thirty-four patients were identified with a mean age of 21.1 years (range 8–25 years), and median follow-up was 25.1 months (1–187 months). The male to female ratio was 1.83:1. The most common presenting symptom was hematuria (n=26; 76%). Diagnoses were invasive urothelial carcinoma (n=3), noninvasive urothelial carcinoma (n=24), PUNLMP (n=6), and urothelial papilloma (n=1). Noninvasive lesions were resected by cystoscopy, after which 12% (n=4) experienced complications (grade II or greater). One patient with stage IV invasive disease at diagnosis died, and 2 patients developed recurrences. Of those with noninvasive carcinoma, 29% (n=7) required repeat cystoscopy soon after initial TURBT at outside institutions, and 17% (n=4) had tumors downgraded from high-grade to low-grade after pathology review.
CONCLUSION
Hematuria is the most common sign of bladder neoplasia in children and young adults and should be investigated by cystoscopy. The majority of urothelial neoplasms in these patients are noninvasive and can be successfully treated with transurethral resection.
Keywords: urothelial neoplasms, urothelial carcinoma, bladder neoplasia, pediatric, adolescent
1. INTRODUCTION
Bladder cancer is the sixth most common carcinoma in the United States. An estimated 79,030 new cases will be diagnosed in the United States in 2017 and there will be approximately 16,870 mortalities[1]. Bladder cancer usually occurs later in life, with a median patient age of 73 years at diagnosis; however, a small minority of cases occur in younger individuals, with approximately 0.5% in patients younger than 35 years of age [1]. Previous studies have demonstrated differing findings regarding genetic factors, clinical outcomes, and prognosis of urothelial cancers in young patients [2–8]. These studies are complicated by the fact that there is no established consensus on the definition of young patients, as some restrict analysis to patients under the age of 20, while others include patients up to age 45 [4, 6]. The definitions and diagnostic criteria of urothelial tumors have also been revised in the time since many of these studies have been published [4, 9]. In general, urothelial neoplasms in young patients appear to have distinct genetic, biologic, and clinical features compared to their counterparts in older patients. Urothelial neoplasms in young patients have greater genetic stability [6, 7], lower incidence of invasiveness [4, 10], and greater overall and disease-free survival compared to adult patients[4, 5, 8, 11, 12]. In this study, we review our institutional experience with urothelial neoplasms in patients aged 25 and younger. We present a series of 34 patients, comprising one of the largest single-institution analyses of pediatric and young adult patients with urothelial neoplasms.
2. METHODS
With institutional review board approval, we reviewed the medical records of 34 consecutive pediatric and young adult patients with urothelial neoplasms who were treated at our institution between January 1997 and September 2016. Inclusion criteria for the study cohort included an age at diagnosis of 25 years or younger and a histologically confirmed diagnosis of urothelial papilloma, papillary urothelial neoplasm of low malignant potential (PUNLMP), or urothelial carcinoma. The definition of these terms is per the 2004 World Health Organization/International Agency for Research on Cancer (WHO/IARC) Classification of Tumors of the Urinary System [13]. According to these guidelines, a urothelial papilloma is defined as a fibrovascular core covered by urothelium indistinguishable from that of the normal urothelium. A PUNLMP is defined as a papillary urothelial tumor that resembles the exophytic urothelial papilloma, but shows increased cellular proliferation exceeding the thickness of normal urothelium. A low-grade non-invasive papillary urothelial carcinoma includes papillary fronds that show an orderly appearance, but demonstrate easily recognizable variations in architecture and cytologic features. A high-grade non-invasive papillary urothelial carcinoma is similar to the low-grade lesion, except it demonstrates a predominant pattern of disorder with moderate-to-marked architectural and cytologic atypia. Finally, an invasive urothelial carcinoma is defined as a urothelial tumor that invades beyond the basement membrane.
Variables reviewed in this study were age at diagnosis, sex, symptoms at presentation, diagnostic methodology, tumor size, histology, surgical treatment, follow-up care, and clinical outcomes. Disease-free survival was calculated using Kaplan-Meier analysis. Statistical analyses were performed with R Statistical Software (version 3.3.3; R Project for Statistical Computing, Vienna, Austria).
3. RESULTS
3.1 Patient and tumor characteristics
A total of 34 patients (65% male, n=22; 82% Caucasian, n=28) with a mean age at diagnosis of 20.4 years (range 8–25) and a median follow-up of 25.1 months (range 1–187) were included in the analysis. Three patients (9%) in our series were 15 years old or younger at diagnosis. Histologic diagnoses were invasive urothelial carcinoma (n=3), noninvasive urothelial carcinoma (n=24; 22 low grade, 2 high grade), PUNLMP (n=6), and urothelial papilloma (n=1). The most common symptom at presentation was hematuria, present in 76% (n=26). Abdominal/pelvic pain was present in 26% (n=9). Notably, 2 of the 3 patients with invasive lesions presented with abdominal/pelvic pain. Cytology results at time of diagnosis were available for 29 patients. No patients with papilloma or PUNLMP had abnormal cytology, and 3 of 24 patients with noninvasive carcinoma had atypical cells on cytology. Both patients with invasive carcinoma and available cytology had malignant cells present in the urine. The tumor was unifocal in 88% (n=30) and multifocal in 12% (n=4). Of the 4 patients with multifocal disease, 2 had invasive carcinoma, one had PUNLMP and one had low-grade non-invasive carcinoma. The location of the tumor was specified in 82% (n=28) cases and varied significantly; the most common sites were the posterior wall of the bladder (n=8; 29%), the lateral walls of the bladder (n=6; 21%), and the trigone (n=5; 18%). Only one patient had disease in the proximal collecting system. This patient had invasive metastatic disease at diagnosis with a primary lesion in the proximal right ureter. Median tumor size was 0.9 cm for PUNLMP, 2.2 cm for noninvasive carcinoma, and 5.4 cm for invasive carcinoma.
3.2 Treatment
All patients were diagnosed by cystoscopic biopsy, with the exception of one patient with metastatic invasive disease at diagnosis who was diagnosed by CT scan and biopsy. This patient also did not undergo surgery. Of the 33 patients who underwent surgery, complete excision with negative margins was obtained in 91% (n=30). All three patients with positive margins had low grade noninvasive lesions, and upon repeat transurethral resection of the bladder tumor (TURBT) one of three patients showed no residual carcinoma. Thirty-one resections were performed using TURBT. Both patients with invasive disease who underwent surgery received a laparotomy and partial cystectomy. Three patients with low-grade noninvasive carcinoma received intravesicular chemotherapy at outside institutions: one received mitomycin alone, one received Bacillus Calmette-Guérin (BCG), and one received both drugs. The patient that received both BCG and mitomycin also received treatment with interferon.
3.3 Surveillance
Surveillance cystoscopy was documented in 22 of 33 patients. A median number of five surveillance cystoscopies were performed per patient (range 1–15). The interval between surveillance cystoscopies varied significantly between patients but was generally less than 6 months for the first 1–2 years and 6–12 months thereafter.
3.4 Complications
Of individuals with non-invasive carcinoma, 29% (n=7) required repeat cystoscopy soon after initial TURBT at outside institutions, and 17% (n=4) had tumors downgraded from high-grade to low-grade after pathology review. Four patients suffered complications from TURBT. Complications were graded according to the Clavien-Dindo system [14]. Two patients had significant hematuria and required continuous bladder irrigation (grade II), with one patient requiring reoperation to achieve hemostasis (grade IIIb). One patient had a bladder perforation during TURBT at an outside institution (grade IIIb). One patient had an aspiration event at the time of repeat TURBT resulting in an extended stay in the intensive care unit (grade IVa).
3.5 Outcomes
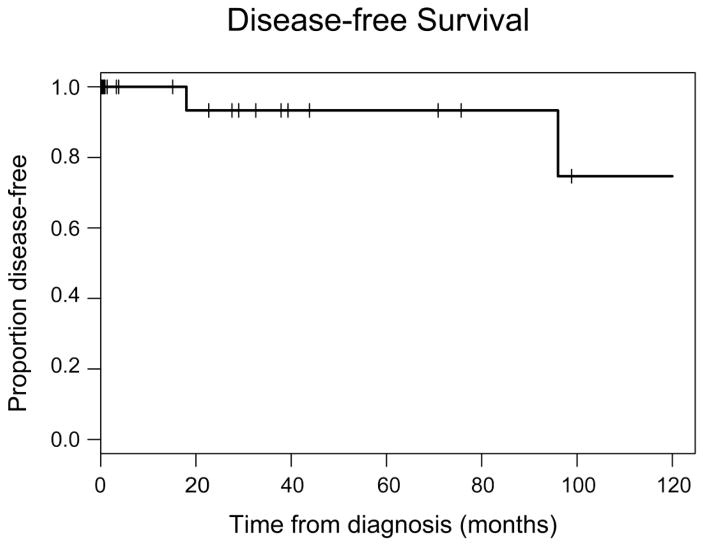
There was a single mortality, which occurred in the only patient with metastatic invasive disease at diagnosis. Two patients developed disease recurrence (figure 1); both had non-invasive disease, one high grade and one low grade. The patient with low grade disease had a recurrence diagnosed 18 months after initial resection, and developed an additional recurrence 5 years after the first. Interestingly, this patient was treated aggressively with mitomycin, BCG, and interferon during his initial treatment. The patient with high-grade disease developed recurrence 8 years after initial TURBT.
Figure 1.
The figure shows the Kaplan-Meier curve for disease free survival among patients with non-invasive urothelial carcinoma. There were no deaths among patients with noninvasive lesions. Events represent disease recurrence.
4. DISCUSSION
While relatively common in older adults, urothelial neoplasms are extremely rare in young patients. We report one of the largest patient series of these tumors. Our data demonstrate findings similar to those of previously published series with respect to age and sex of young patients with urothelial neoplasms. Even when limiting our series to patients under age 25, most cases occurred in individuals near the upper limit of this age group. This finding complements other series that describe cases occurring most frequently in this age group among teenagers and young adults [2, 4, 5, 8, 12, 15]. The youngest patient in our series was 8 years old and only 3 patients were 15 years old or younger. The youngest patient with a urothelial neoplasm reported in the literature was 4 years old at diagnosis [4, 16]. Urothelial neoplasms are also reported to be more common in young males, consistent with our patient cohort, 65% of whom were male. This is somewhat lower than proportions of 70–82% male patients reported in other series [4, 8, 10, 17].
Ninety-one percent (n=31) of the cases in our series were non-invasive lesions, supporting previous observations that urothelial neoplasms in the pediatric and young adult population are generally less aggressive than tumors common in older adults [4, 7, 11, 17]. Our series illustrates that within this rare group of tumors, low-grade non-invasive urothelial carcinoma is the most common tumor type. These lesions comprised 65% (n=22) of our 34 cases. Complete transurethral resection has consistently been described as the treatment of choice for noninvasive tumors, and was employed for all patients with these lesions in our study. Progression to invasive disease and cancer related mortality is rare for non-invasive tumors among both children and adults. In contrast, recurrence from noninvasive urothelial carcinoma is common among adult patients – reported in 48–71% of patients [18–21] – but rare in young patients. Eight percent (n=2) of patients in our series with noninvasive carcinoma experienced recurrences, which is similar to the 13% recurrence rate in the series presented by Fine and colleagues [4].
Papillary urothelial neoplasm of low malignant potential (PUNLMP) was the second most common tumor type in our series. The relative prevalence of PUNLMP has varied significantly in previous analyses of young patients with urothelial neoplasms. Fine and colleagues reported a diagnosis of PUNLMP in 10 (43.5%) of 23 patients diagnosed under age 21 [4]. In contrast, Williamson and colleagues reported only one (6%) case of PUNLMP in a series of 17 patients diagnosed with urothelial neoplasms before age 30 [17]. PUNLMP was codified in the WHO/IARC classification of tumors for the first time in 2004 [13]. This change reclassified a subset of formerly grade 1 urothelial carcinomas as non-cancerous lesions (PUNLMPs), avoiding the psychological stress of a cancer diagnosis in these patients [4]. In our series, as in previous reports, patients with PUNLMP exhibit uniformly good outcomes.
Some authors have suggested that the prevalence of noninvasive lesions with favorable outcomes among young patients represents a unique tumor biology. This hypothesis is strengthened by observations by Williamson and colleagues who have demonstrated that urothelial neoplasms in young patients harbor very few mutations in FGFR3 or TP53, which are found in a majority of older patients with urothelial carcinoma [17, 22]. A number of studies have examined the role of microsatellite instability in the pathogenesis of urothelial neoplasms in young patients. These studies have reported conflicting results though, with some showing significant microsatellite instability [3, 23] and others showing minimal microsatellite instability [6, 7]. The collected findings of these studies suggest a role for additional research into the unique genetic mechanisms associated with urothelial neoplasms in young patients.
Our series and others provide evidence for the efficacy of TURBT alone in treating noninvasive urothelial neoplasms in pediatric patients. A careful review by pathology is critical to ensure that muscularis propria is included in the specimen and that a negative margin was obtained. If the specimen does not adequately demonstrate these findings, a repeat biopsy is mandatory to rule out muscular invasion. Based on the prevalence of pathologic downgrading upon expert review in our series, we recommend that a consultation from a pathologist with experience examining pediatric urothelial neoplasms be obtained for all young patients with a suspected diagnosis of urothelial neoplasm.
Invasive urothelial carcinoma is exceedingly rare in the pediatric and young adult population. Nine percent (n=3) of patients in our series had invasive tumors, which is significantly higher than the proportion of invasive cases reported in any other similar series. Indeed, many series of young patients with bladder neoplasms have not captured any patients with invasive tumors [4, 7, 24]. As our institution acts as a referral center for complex malignancies, the proportion of invasive carcinoma in our series is likely higher than in the general population of young patients with urothelial neoplasms. Case reports of invasive urothelial neoplasms in pediatric patients are present in the literature, but their rarity has prevented consensus regarding treatment [16, 25], and there is no molecular characterization of these tumors. In our series, the two cases of invasive carcinoma that were amenable to surgery were treated with partial cystectomy. One patient with a small component of invasive disease was not treated with chemotherapy; however, the two other patients with invasive tumors did receive chemotherapy.
Surveillance following treatment for urothelial neoplasms in young patients is not uniform in the literature. In an analysis of a multicenter patient cohort, Berrettini and colleagues noted significantly different follow-up regimens used by the three centers included [24]. Variables can include the timing of surveillance, as well as the use of ultrasound, cystoscopy, and cytology. At our institution, cystoscopy with cytology is the primary mode of surveillance and is typically performed at shorter intervals (3–6 months) immediately after treatment and at longer intervals (6–12 months) thereafter. In order to avoid the potential complications associated with anesthesia and cystoscopy, other authors have suggested a minimally invasive approach to surveillance that includes a single cystoscopy at 3 months after treatment and regular ultrasound examinations thereafter [26].
In conclusion, our series confirms the findings of previous studies that describe urothelial neoplasms in young patients as overwhelmingly low-grade, noninvasive lesions with favorable outcomes. Our study also confirms the rare but aggressive nature of invasive urothelial carcinoma in this age group. Any child or young adult with hematuria should be thoroughly evaluated to rule out these diagnoses.
Table 1.
Patient, disease, and treatment characteristics
| Pathology | Sex | Median age at diagnosis (years) | Urine cytology | Median size in the greatest dimension (no. available) | Treatment | Median follow-up (months) |
|---|---|---|---|---|---|---|
| Urothelial papilloma (n=1) | 1 male | 21.4 | All negative | 1.2 cm (n = 1) |
|
5.6 |
| PUNLMP (n=6) | 5 males 1 female |
19.2 | All negative | 0.9 cm (n = 5) |
|
30.5 |
| Low-grade non-invasive urothelial carcinoma (n=22) | 14 males 8 females |
21.3 | 2 (10%) atypical cells | 2.2 cm (n = 16) |
|
28.3 |
| High-grade non-invasive urothelial carcinoma (n=2) | 1 male 1 female |
23.3 | 1 (50%) atypical cells | Not recorded |
|
78.8 |
| Invasive carcinoma (n=3) | 1 male 2 females |
18.5 | 2 (100%) malignant cells | 5.4 cm (n = 3) |
|
22.7 |
| All cases (n=34) | 22 males 12 females |
21.1 | 5 (17%) abnormal | 2.0 cm (n = 25) | NA | 25.1 |
BCG= Bacillus Calmette-Guerin; NA= not applicable; PUNLMP= papillary urothelial neoplasm of low malignant potential.
Acknowledgments
Funding: Research at Memorial Sloan Kettering is supported in part by a grant from the National Institutes of Health/National Cancer Institute (#P30 CA008748).
Footnotes
Conflict of Interest Disclosure: The authors declare that they have no financial conflicts of interest.
LEVEL OF EVIDENCE: Level IV (Retrospective study with no comparison group)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Surveillance, Epidemiology, and End Results Program. SEER Cancer Stat Facts: Bladder Cancer. Bethesda, MD: National Cancer Instititute/Division of Cancer Control and Population Sciences, National Cancer Institute; 2017. [Accessed May 25, 2017]. Available at: http://seer.cancer.gov/statfacts/html/urinb.html. [Google Scholar]
- 2.Benson RC, Tomera KM, Kelalis PP. Transitional cell carcinoma of the bladder in children and adolescents. J Urol. 1983;130:54–55. doi: 10.1016/s0022-5347(17)50950-7. [DOI] [PubMed] [Google Scholar]
- 3.Christensen M, Jensen MA, Wolf H, et al. Pronounced microsatellite instability in transitional cell carcinomas from young patients with bladder cancer. Int J Cancer. 1998;79:396–401. doi: 10.1002/(sici)1097-0215(19980821)79:4<396::aid-ijc15>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Fine SW, Humphrey PA, Dehner LP, et al. Urothelial neoplasms in patients 20 years or younger: a clinicopathological analysis using the world health organization 2004 bladder consensus classification. J Urol. 2005;174:1976–1980. doi: 10.1097/01.ju.0000176801.16827.82. [DOI] [PubMed] [Google Scholar]
- 5.Lerena J, Krauel L, Garcia-Aparicio L, et al. Transitional cell carcinoma of the bladder in children and adolescents: six-case series and review of the literature. J Pediatr Urol. 2010;6:481–485. doi: 10.1016/j.jpurol.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Mongiat-Artus P, Miquel C, van der Aa M, et al. Infrequent microsatellite instability in urothelial cell carcinoma of the bladder in young patients. Eur Urol. 2006;49:685–690. doi: 10.1016/j.eururo.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Wild PJ, Giedl J, Stoehr R, et al. Genomic aberrations are rare in urothelial neoplasms of patients 19 years or younger. J Pathol. 2007;211:18–25. doi: 10.1002/path.2075. [DOI] [PubMed] [Google Scholar]
- 8.Yossepowitch O, Dalbagni G. Transitional cell carcinoma of the bladder in young adults: presentation, natural history and outcome. J Urol. 2002;168:61–66. [PubMed] [Google Scholar]
- 9.Burger M, Oosterlinck W, Konety B, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63:36–44. doi: 10.1016/j.eururo.2012.08.061. [DOI] [PubMed] [Google Scholar]
- 10.Comperat E, Larre S, Roupret M, et al. Clinicopathological characteristics of urothelial bladder cancer in patients less than 40 years old. Virchows Arch. 2015;466:589–594. doi: 10.1007/s00428-015-1739-2. [DOI] [PubMed] [Google Scholar]
- 11.Ander H, Donmez MI, Yitgin Y, et al. Urothelial carcinoma of the urinary bladder in pediatric patients: a long-term follow-up. Int Urol Nephrol. 2015;47:771–774. doi: 10.1007/s11255-015-0950-z. [DOI] [PubMed] [Google Scholar]
- 12.Hoenig DM, McRae S, Chen SC, et al. Transitional cell carcinoma of the bladder in the pediatric patient. J Urol. 1996;156:203–205. [PubMed] [Google Scholar]
- 13.Sauter G, Algaba F, Amin M. Non-invasive urothelial tumours. In: Eble JN, et al., editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organ. Lyon, France: IARC Press; 2004. pp. 110–123. [Google Scholar]
- 14.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 15.Korrect GS, Minevich EA, Sivan B. High-grade transitional cell carcinoma of the pediatric bladder. J Pediatr Urol. 2012;8:e36–e38. doi: 10.1016/j.jpurol.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 16.Neogi S, Kariholu PL, Dhakre G, et al. Malignant urothelial carcinoma of urinary bladder in a young child: a rare case report. Urology. 2013;81:888–890. doi: 10.1016/j.urology.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Williamson SR, Wang M, Montironi R, et al. Molecular characteristics of urothelial neoplasms in children and young adults: a subset of tumors from young patients harbors chromosomal abnormalities but not FGFR3 or TP53 gene mutations. Mod Pathol. 2014;27:1540–1548. doi: 10.1038/modpathol.2014.48. [DOI] [PubMed] [Google Scholar]
- 18.Alsheikh A, Mohamedali Z, Jones E, et al. Comparison of the WHO/ISUP classification and cytokeratin 20 expression in predicting the behavior of low-grade papillary urothelial tumors. World/Health Organization/Internattional Society of Urologic Pathology. Mod Pathol. 2001;14:267–272. doi: 10.1038/modpathol.3880300. [DOI] [PubMed] [Google Scholar]
- 19.Holmang S, Andius P, Hedelin H, et al. Stage progression in Ta papillary urothelial tumors: relationship to grade, immunohistochemical expression of tumor markers, mitotic frequency and DNA ploidy. J Urol. 2001;165:1124–1130. [PubMed] [Google Scholar]
- 20.Holmang S, Johansson SL. Stage Ta-T1 bladder cancer: the relationship between findings at first followup cystoscopy and subsequent recurrence and progression. J Urol. 2002;167:1634–1637. [PubMed] [Google Scholar]
- 21.Herr HW, Donat SM, Reuter VE. Management of low grade papillary bladder tumors. J Urol. 2007;178:1201–1205. doi: 10.1016/j.juro.2007.05.148. [DOI] [PubMed] [Google Scholar]
- 22.van Rhijn BW, van der Kwast TH, Vis AN, et al. FGFR3 and P53 characterize alternative genetic pathways in the pathogenesis of urothelial cell carcinoma. Cancer Res. 2004;64:1911–1914. doi: 10.1158/0008-5472.can-03-2421. [DOI] [PubMed] [Google Scholar]
- 23.Migaldi M, Sartori G, Rossi G, et al. Prevalence and prognostic significance of microsatellite alterations in young patients with bladder cancer. Mod Pathol. 2005;18:1176–1186. doi: 10.1038/modpathol.3800399. [DOI] [PubMed] [Google Scholar]
- 24.Berrettini A, Castagnetti M, Salerno A, et al. Bladder urothelial neoplasms in pediatric age: experience at three tertiary centers. J Pediatr Urol. 2015;11:26, e1–e5. doi: 10.1016/j.jpurol.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Aykan S, Yuruk E, Tuken M, et al. Rare but lethal disease of childhood: metastatic, muscle invasive bladder cancer. Pediatr Rep. 2015;7:5928. doi: 10.4081/pr.2015.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Carlo D, Ferrari A, Perruccio K, et al. Management and follow-up of urothelial neoplasms of the bladder in children: a report from the TREP project. Pediatr Blood Cancer. 2015;62:1000–1003. doi: 10.1002/pbc.25380. [DOI] [PubMed] [Google Scholar]