Abstract
Foot-sole somatosensation is critical for safe mobility in older adults. Somatosensation arises when afferent input activates a neural network that includes the primary somatosensory cortex. Transcranial direct current stimulation (tDCS), as a strategy to increase somatosensory cortical excitability, may therefore enhance foot-sole somatosensation. We hypothesized that a single session of tDCS would improve foot-sole somatosensation, and thus mobility, in older adults. Twenty healthy older adults completed this randomized, double-blinded, cross-over study consisting of two visits separated by one-week. On each visit, standing vibratory threshold (SVT) of each foot and the timed-up-and-go test (TUG) of mobility were assessed immediately before and after a 20-minute session of tDCS (2.0 mA) or sham stimulation with the anode placed over C3 (according to the 10/20 EEG placement system) and the cathode over the contralateral supraorbital margin. tDCS condition order was randomized. SVT was measured with a shoe insole system. This system automatically ramped up, or down, the amplitude of applied vibrations and the participant stated when they could or could no longer feel the vibration, such that lower SVT reflected better somatosensation. The SVTs of both foot soles were lower following tDCS as compared to sham and both pre-test conditions (F(1,76)>3.4, p<0.03). A trend towards better TUG performance following tDCS was also observed (F(1,76)=2.4, p=0.07). Greater improvement in SVT (averaged across feet) moderately correlated with greater improvement in TUG performance (r=0.48, p=0.03). These results suggest that tDCS may enhance lower-extremity somatosensory function, and potentially mobility, in healthy older adults.
Keywords: foot sole somatosensation, mobility, tDCS, standing vibratory threshold, timed-up and go test
Introduction
The foot soles are the only point of contact with the environment when standing and walking. Adequate foot sole somatosensation is thus critical to numerous aspects of mobility, including standing postural control, gait, postural transitions, turning and both planned and responsive motor adaptation to changing task constraints (Eils et al., 2002; Eils et al., 2004; Manchester et al., 1989; Lord et al., 1991). Previous studies have demonstrated that, for example, impaired ability to detect vibrations applied to the foot soles was correlated with worse standing balance (i.e., greater postural sway) and slower walking speed in healthy older adults and in those with multiple sclerosis or diabetic peripheral neuropathy (DPN) (Kristinsdottir et al., 2001; Citaker et al., 2011; Menz et al., 2004). Afferent inputs arising from mechanoreceptors in the feet are involved in the control of these behaviors via numerous spinal reflex arcs and supraspinal sensory integration. The conscious perception of somatosensory stimuli occurs when such afferent inputs activate a distributed cortical network that includes the primary somatosensory cortex (S1) (Gilbert & Sigman, 2007; Borich et al., 2015). It is now evident that in addition to peripheral nervous system degradation (Shaffer & Harrison, 2007), age-related somatosensory impairments arise in part from altered cortical activation in response to a given stimulus (Brodoehl et al., 2013; Malandraki et al., 2011). As such, therapeutic strategies designed to facilitate the excitability of somatosensory brain networks hold great potential to enhance foot sole somatosensation—and ultimately improve mobility—in older adults.
Transcranial direct current stimulation (tDCS) is a non-invasive technology that modulates cortical excitability by inducing low amplitude current flow between two or more electrodes placed on the scalp (Nitsche & Paulus, 2000). In healthy young adults with intact somatosensory function, 20 minutes of continuous tDCS with the anode electrode placed over the left sensorimotor cortex (i.e., C3 of the 10/20 EEG electrode placement system) and the cathode over the contralateral supraorbital margin appears to improve the ability to detect and discriminate vibrotactile stimuli applied to a finger of the right hand, when tested immediately following stimulation (Ragert et al., 2008; Mori et al., 2013). We therefore contend that this type of tDCS may also serve as a noninvasive strategy to enhance weight-bearing foot sole somatosensation in older adults. The aim of this study was to provide proof of this principle by examining the immediate effects of a single tDCS session on the ability to perceive vibrations applied to the foot soles when standing, as assessed by an instrumented shoe insole system. Our primary hypothesis was that tDCS, as compared to sham (i.e., control) stimulation, would decrease standing vibratory threshold (SVT) of the right foot sole; that is, improve somatosensation associated with the contralateral foot. We further hypothesized that tDCS-induced augmentation of foot sole SVT would correlate with improved performance in the timed up-and-go test of mobility.
Material and Methods
Participants
Twenty healthy older adults (age=61±4 years, height=1.58±0.04 m, body mass=61±8 kg) were recruited and provided written informed consent as approved by the Institutional Review Board of Peking University First Hospital, Beijing. All participants were right-handed as determined by the Edinburgh Handedness Inventory (Oldfield, 1971). Exclusion criteria included any self-reported musculoskeletal, neurological, or cardiovascular disorder; recent hospitalization, current use of any centrally-acting medication, and an inability to stand or walk without the use of an assistive device. We also excluded individuals who were unable to perceive vibrations applied to the foot soles, as delivered by an instrumented shoe insole at maximum output (See related section below for details).
Experimental protocol
Each participant completed two study visits at the same time of day separated by one week, during which SVT of each foot sole were assessed, along with mobility, immediately before and after a 20-minute session of tDCS. They received tDCS on one visit and sham stimulation on the other. The order of tDCS and sham stimulation was randomized, and both participants and study personnel were blinded to this order.
Determination of foot sole vibratory thresholds when standing
Foot sole vibratory thresholds were assessed with the participant standing. SVTs were measured by a vibratory insole system, which delivers vibrations varying randomly in frequency below 100 Hz (Lipsitz et al., 2015; Zhou et al., 2016; Miranda et al., 2016a; Miranda et al., 2016b). A full description of these vibratory insoles was previously reported (Lipsitz et al., 2015). Briefly, two urethane foam insoles, each containing two 2.5cm piezo-electric actuators (C-2 Actuators, Engineering Acoustics, Winter Park, FL, USA), were placed on the ground beneath the participant’s bare feet, which were placed shoulder-width apart (Lipsitz et al., 2015). Before the first assessment, the outlines of the foot placement were traced on paper and this outline was used for all subsequent trials to ensure consistency of foot placement. A custom-developed software program enabled the amplitude of vibrations to be automatically ramped up or down in 1% increments between 0% and 100% of maximum output (Lipsitz et al., 2015; Zhou et al., 2016; Miranda et al., 2016a; Miranda et al., 2016b). Each foot sole was tested separately and the testing order was randomized across participants, visits and trials. During each trial, participants were instructed to press a button when they could feel, or no longer feel, the vibrations. This procedure was repeated three times for each foot sole to determine the vibratory threshold. Lower percentage of the insole output at which the vibrations could be felt (i.e., lower SVTs) reflected better somatosensation.
Timed-up and go test (TUG)
The TUG test measures the time taken to stand from a chair, walk forward three meters, turn around, walk back and return to a seated position (Podsiadlo, & Richardson, 1991). This common clinical test was chosen because it correlates with general mobility (Podsiadlo, & Richardson, 1991) and is predictive of future falls (Shumway-Cook et al., 2000) in older adults. Participants completed two TUG trials (separated by one minute of rest), following determination of vibratory thresholds, both immediately before and after tDCS or sham stimulation. The averaged TUG time, where faster times reflect better mobility, was used for analysis.
tDCS
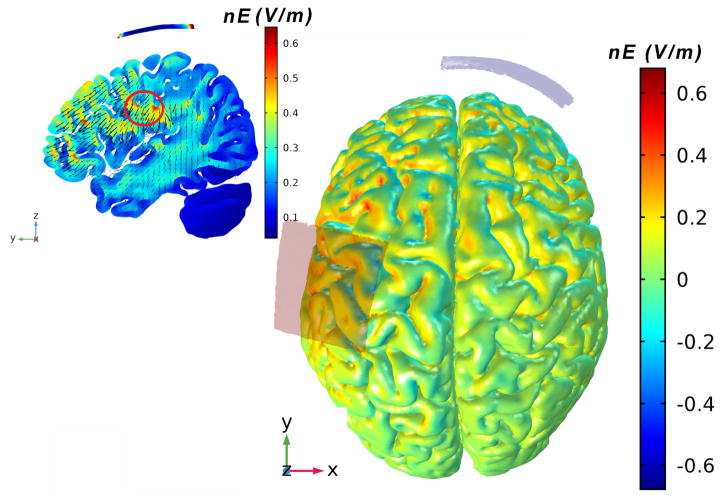
tDCS was administered by personnel uninvolved with any other study procedure. It was delivered with a battery-driven electrical stimulator (Chattanooga Ionto® Iontophoresis System) connected to a pair of saline-soaked 35 cm2 (7cm×5cm) synthetic surface sponges placed on the scalp. The anode was placed over left sensorimotor cortex, as defined by the C3 location of the 10/20 EEG electrode placement system (Boggio et al., 2008). The cathode was placed over the right supraorbital margin (Boggio et al., 2008) (Figure 1). The computational current flow model using standard head model indicated that the electrical field produced by this tDCS montage likely influenced the superficial and deep regions of the brain, including the foot regions of S1 (regions within the red circle in Figure 1). tDCS was administered for 20 continuous minutes at a target intensity of 2.0 mA. This dose of tDCS induces changes in cortical excitability lasting for at least 1.5 hours following stimulation (Nitsche & Paulus, 2001). Moreover, this intensity has been proven to be safe and well-tolerated by participants (Nitsche et al., 2008). At the beginning of stimulation, the investigator increased current manually from 0.1 to 2.0 mA in 0.1 mA increments over a 60 second period. Participants were instructed to notify the investigator when they felt any uncomfortable sensations induced by the stimulation. The ramp-up procedure was stopped at this point. tDCS was then delivered for the remainder of the session at an intensity of 0.1 mA below the highest level reached. Current was automatically ramped down to 0.0 mA over a 60-second period at the end of the session. The sham (i.e., control) condition utilized an “inactive” stimulation protocol (Davis et al., 2013). The same session duration, electrode montage and ramp-up procedures as tDCS were used; however, current was only delivered for 60 seconds following the initial ramp-up procedure. At this point, the current was automatically ramped back down to zero. This sham procedure has proven reliable as sensations arising from tDCS become negligible after the first 60 seconds of stimulation (Gandiga et al., 2006). At the end of each visit, participants completed a short questionnaire (Brunoni et al., 2011) to assess potential side effects. They were also asked to state if, in their opinion, they received tDCS or sham stimulation on that visit.
Figure 1. tDCS electrode placement and electrical current flow model.
A pair of saline-soaked 35 cm2 (7cmX5cm) synthetic surface sponges were used. The anode was placed over the C3 location of the 10/20 EEG electrode placement system, which covered the left S1 region. The cathode was placed over the right supraorbital margin. A computational model based upon a standard head model was generated via Stimweaver technology (Miranda et al., 2013). This computational model indicated that the produced electrical field likely influenced numerous superficial and deep regions of the brain, including the foot regions of the primary somatosensory cortex (regions within the red circle). Warmer and cooler colors depict the normal component of the electrical field (nE, V/m) produced by this stimulation.
Statistical analysis
Analyses were performed with JMP Pro 12 software (SAS Institute, Cary NC). The efficacy of tDCS blinding was examined using Fisher’s exact test to determine if participants’ guesses of tDCS condition were correct to a greater degree than that expected by chance. Dependent variables included the SVT of each foot sole and TUG time. Variable normality was examined with the Shapiro-Wilk W test and homogeneity of variance was determined with the Levene test. Two-way repeated-measures ANOVAs were then used to analyze the effects of tDCS on SVT of foot soles and TUG time. Model effects included time (pre-, post-tDCS), condition (tDCS, sham) and their interaction. Tukey’s post-hoc testing was used on statistically significant models to identify differences between variable means within each condition and time combination. Separate models were used for the SVT of each foot. Effect sizes were calculated using Cohen’s d.
To test the hypothesis that potential tDCS-induced increases in foot sole somatosensation would associate with an improvement in mobility, linear regression analyses were used to determine the relationship between the percent changes of SVT and the percent change in TUG time from pre- to post-tDCS. As both baseline foot sole SVT and the applied intensity of tDCS varied across participants, we further explored the potential influence of these two factors on observed tDCS-induced changes in foot sole somatosensation using linear regression analyses. Specifically, these tests were used to determine whether percent or absolute changes in SVT were dependent upon baseline vibratory thresholds, or applied tDCS intensity. Significance level was set to p<0.05 for all analyses.
Results
All 20 participants completed all study procedures. At baselines (i.e., prior to the administration of tDCS or sham stimulation), SVTs of the left and right foot soles were positively correlated with each other (r2>0.39, p<0.003). These data were normally distributed and exhibited homogeneity of variance.
The average current intensity of tDCS was 1.7±0.3 mA. Four participants received the maximum intensity of 2 mA. Stimulation was well-tolerated by all participants and no unexpected side effects or adverse events were reported. Participants were successfully blinded to stimulation condition. The percentage of correct “guesses” of condition were no more than that expected due of chance (30% correct rate after tDCS and 45% correct rate after sham, p = 0.51).
The effects of tDCS on standing foot sole vibratory sensation
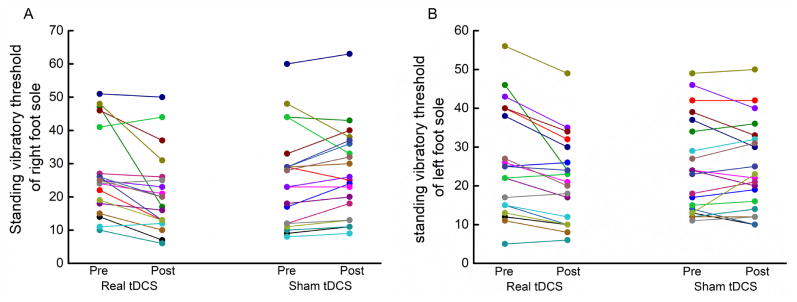
tDCS improved vibratory detection thresholds. In fact, fifteen of 20 participants exhibited lower SVTs in both the right and left foot soles following real tDCS (Figure 2). Significant interactions were observed between time (pre-, post-stimulation) and condition (tDCS, sham) for SVTs of both the right (F(1, 76) =3.4, p=0.03, Cohen’s d=0.72) and left (F(1, 76)=4.6, p=0.01, Cohen’s d=0.67) foot soles (Figure 3A). Post-hoc analyses revealed that for both feet, SVTs were lower (i.e., better vibratory somatosensation) following tDCS as compared to following sham stimulation, as well as compared to both baseline conditions.
Figure 2. Individual effects of tDCS and sham stimulation on standing vibratory thresholds of the left (A) and right (B) foot soles.
Real tDCS decreased standing vibratory thresholds of the left foot sole in 16 of 20 participants (A) and of right foot sole in 17 participants (B). In total, 15 participants exhibited lower thresholds of both foot soles following real tDCS. In contrast, only seven participants exhibited improvement in sensation of the left foot, and five for the right foot, following sham stimulation.
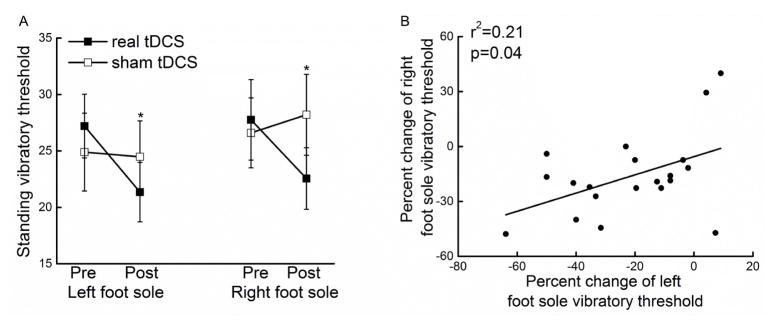
Figure 3. The effects of tDCS on standing foot sole somatosensation.
Real tDCS decreased standing vibratory thresholds of both the left and right foot soles (i.e., better somatosensation) as compared to sham stimulation and to both pre-tDCS conditions (F>3.4, p<0.03) (A). Observed enhancements in standing vibratory thresholds were similar between the left and right foot soles (r2 =0.21, p =0.04) (B). Errors bar reflect standard deviations and * indicates a significant tDCS condition by time interaction (p < 0.05).
The average absolute decrease in SVT induced by tDCS was 6±7 units for the right foot sole and 5±7 units for the left foot sole (percent decrease: 21±20% for the right and 16±20% for the left). The tDCS-induced changes in SVTs were moderately correlated across feet (absolute change: r2=0.34, p=0.007; percent change: r2 =0.21, p =0.04, Figure 3B).
Those with higher baseline SVTs, when averaged across feet (i.e., worse sensory function) exhibited greater absolute decreases in the SVT (i.e., greater improvement of vibratory somatosensation) following tDCS (r2=0.40, p=0.002). Neither averaged percent nor absolute change in SVT was associated with applied current intensity (p>0.16).
The effects of tDCS on mobility
The average time to complete the TUG test of mobility at baseline was 5.2±0.5 seconds, ranging from 4.3 to 6.4 seconds, suggesting that the current cohort of older adults had good-to-excellent mobility. This variable was also normally distributed and exhibited homogeneity of variance across participants. A trend towards a significant interaction between time and condition was observed for this variable (F(1, 76)=2.4, p=0.07, Cohen’s d=0.66). The average percent decrease in the time needed to complete the TUG test following tDCS was 6.1±6.0% (0.3±0.3 seconds), while the percent change following sham stimulation was negligible (0.5±7.1%, or 0.01±0.4 seconds).
Relationships between tDCS-induced changes in standing foot sole vibratory sensation and mobility
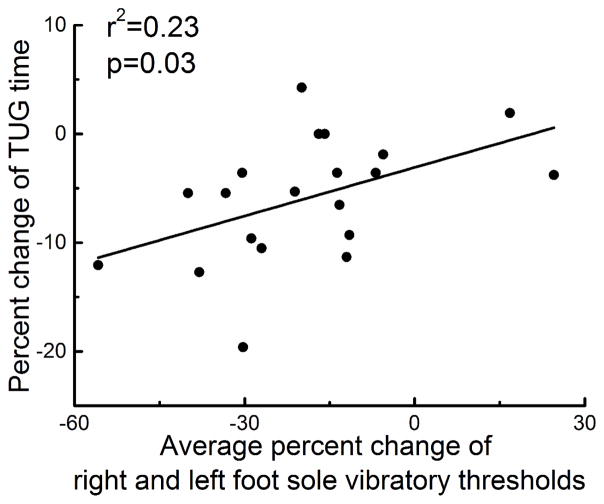
As mobility depends upon somatosensation arising from both feet and the percent change in SVTs following tDCS was correlated across feet as described above, we examined the relationship between tDCS-induced changes in somatosensation and mobility by using the average percent change (from pre-to post-tDCS) in SVT across both feet. A moderate but significant correlation between the changes in SVT and TUG time following tDCS was observed. Participants who exhibited greater percent decreases in foot sole SVT also exhibited greater percent decreases in TUG time (r2=0.23, p=0.03, Figure 4).
Figure 4. Relationships between tDCS-induced changes in standing foot sole somatosensation and mobility.
Participants who exhibited greater average percent decrease in standing vibratory thresholds across the left and right foot soles (i.e., greater enhancement of foot sole somatosensation) following real tDCS also exhibited greater percent reduction in the time needed to complete the timed up-and-go test (i.e., greater improvement in mobility) (r2=0.23, p=0.03).
Discussion
This double-blinded and sham-controlled study provided proof-of-principle that in healthy older adults, a single 20-minute session of tDCS, as compared to sham stimulation, enhanced vibratory somatosensation of both the right and left foot soles, when tested immediately following stimulation. Moreover, those participants who exhibited greater tDCS-related enhancement of somatosensory function tended to exhibit greater performance improvements in the TUG test of mobility. These results provide preliminary evidence that non-invasive modulation of cortical excitability may be an effective strategy to enhance lower-extremity somatosensation, and thus improve functional outcomes, in older adults.
The perception of a somatosensory stimulus is dependent upon the degree to which it activates the somatosensory cortical network within the brain (Fregni & Pascual-Leone, 2007). The degree of cortical activation induced by a given stimulus is dependent upon the stimulus’s intensity, the integrity of the individual’s peripheral, spinal and subcortical neural circuitry, and the excitability of the involved cortical neurons (Adolphs et al., 2000; Maldjian et al., 1999; Goldberg et al., 2006). We previously demonstrated (Wang et al., 2015) that tDCS delivered with a similar montage as used in the current study increased activation of the left posterior paracentral lobule (including S1), as measured by fMRI, in response to relatively large and easily-perceivable pressure stimuli applied to the right foot sole that was designed to mimic those pressures experienced when walking. That result suggested that tDCS is capable of augmenting cortical activation in response to a relatively large, controlled stimulus. Ragert et al (2008) reported that 20 minutes of 1 mA tDCS with a similar montage increased spatial tactile acuity of the right hand index finger in healthy younger adults as measured by the grating orientation task. After tDCS intervention, participants were able to discriminate the orientation of gratings of much smaller width as compared to sham and pre-stimulation. Based upon this evidence and our current results, we contend that tDCS with the anode placed over C3 and the cathode over the contralateral supraorbital margin lowers the amplitude at which individuals are able to perceive vibrations beneath the feet when standing, presumably by augmenting somatosensory cortical activation in response to said stimuli.
In the current study, tDCS, which was delivered with the intent of increasing the excitability of the S1 foot region within the left hemisphere, resulted in improved vibratory perception of both the right and left foot soles. This result was somewhat unexpected, as activation of S1 within one hemisphere is primarily associated with somatosensation on the contralateral side of the body, since a majority of the axons ascending from the limbs to S1 via the corticospinal tract decussate in the medulla. Still, up to 20% of these axons do not cross and instead ascend into the ipsilateral S1. As such, modulation of cortical excitability within the left S1 may have directly enhanced somatosensation of both foot soles (Kuypers, 1981; Boubker et al., 2012; Martin 2005).
The observed bilateral effect of tDCS may be due to at least two additional mechanisms. First, electrical current was delivered through relatively large sponge electrode placed on the scalp. As can be observed in the computational current flow model (Figure 1), the electrical field induced by tDCS likely influenced numerous regions in addition to the left S1. Several of these regions (e.g., the right S1, the motor cortex, secondary somatosensory cortex and supplementary motor area) are involved in the processing of bilateral somatosensory information (Mori et al., 2013; Zhang et al., 2005; Bachmann et al., 2010; Legon et al., 2013). Second, S1 activation in one hemisphere influences S1 excitability in the opposite hemisphere via corticothalamic circuitry (Seyal et al., 1995; Blankenburg et al., 2008; Meehan et al., 2011). Blankenburg et al (2008), for example, demonstrated that transcranial magnetic stimulation (TMS) designed to facilitate right S1 excitability increased fMRI-measured activation of the left S1 in response to stimuli applied to the right wrist and enhanced participant ability to detection these stimuli. Future studies are therefore needed that utilize sophisticated neuro-imaging and navigation techniques, together with smaller multi-electrode arrays to deliver more focal current flow (Ruffini et al., 2014), to better understand the observed bilateral effects of tDCS as administered in the current study.
Afferent feedback arising from mechanoreceptors in the foot soles is involved in both subcortical reflex loops and complex cortical circuity that are each critical to the control of walking, turning and postural transitions (Gilbert & Sigman, 2007; Borich et al., 2015; Shaffer & Harrison 2007; Scaglioni et al., 2002; Kavounoudias et al., 2001). Here, we observed that tDCS enhanced the conscious perception of vibratory tactile stimuli applied to the foot soles. This perception of foot sole stimuli is closely involved in the formation of the planned and responsive motor patterns necessary for completing motor tasks, especially maintaining one’s balance when standing or walking (Eils et al., 2002; Eils et al., 2004; Manchester et al., 1989; Lord et al., 1991). Biological aging from early adulthood into senescence is often associated with lower-extremity somatosensory decline that arises from alterations to both peripheral (e.g., decreased mechanoreceptor density (Bolton et al., 1966) and nerve conduction velocity) and central (e.g., altered excitability of the cortical network (Bordoehl et al., 2013; Malandraki, et al., 2011)) elements of the somatosensory system. Age-related diseases, such as DPN or other peripheral nerve deterioration, often accelerate such deficits in both peripheral and central components of the somatosensory system (Selvarajah et al., 2014; Tesfaye & Selvarajah, 2012), causing the loss of balance and high risk of falling in everyday life (Meyer et al., 2004; Richardson et al., 1992). Here, we observed preliminary evidence that participants who exhibited greater enhancement in the ability to perceive foot sole vibratory stimuli when standing tended to also exhibit greater improvement in mobility. As observed correlations were moderate, future studies should confirm these results, and ultimately, explore the effects of tDCS on the cortical integration and utilization of sensory feedback during standing, walking, and other weight-bearing volitional movements.
Participants in this study were heathy older adults and their baseline TUG performance was quite good. As such, the ability to capture tDCS-induced improvements in mobility may have been limited by a “floor effect” in this test. Future studies implementing other types of mobility tests, as well as more sophisticated gait and balance assessments, are thus needed to fully study the effects of tDCS on more subtle elements of the neuro-motor control of these behaviors.
As this study focused on standing vibratory sensation, the effects of tDCS on other forms of lower-extremity somatosensation involved to the control of balance and mobility (e.g., pressure detection, joint position sense, etc.) should also be explored. Moreover, this study focused on the acute effects of a single session of tDCS on foot sole vibratory sensation and mobility. Several previous studies have demonstrated that multi-session tDCS intervention induces longer-lasting effects on sensory function as compared to a single session (Mori et al., 2013; Reis et al., 2009; Alonzo et al., 2015; Boggio et al., 2007). Mori et al (2013) demonstrated that, for instance, in patients with multiple sclerosis, five consecutive daily sessions of this type of tDCS at a target current intensity of 2.0 mA improved spatial discrimination thresholds on the hypoesthetic hand for at least two weeks following the intervention. Additional work is thus warranted to explore the longer-term effects of multi-session tDCS on foot sole somatosensation and mobility in older adults.
Acknowledgments
We would like to thank Dr. Xiaohong Chen in Capital University of Physical Education and Sports for the help of participant recruitment. This study was supported by grants from the National Natural Science Foundation of China (grant number 11572003 and 11372013), and the National Institute on Aging (AG025037, AG041785, and 1-K01-AG044543–01A1). Dr. Lipsitz holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife. Dr. Junhong Zhou is supported by the Irma and Paul Milstein Program for Senior Health Fellowship Award from Milstein Medical Asian American Partnership (MMAAP) Foundation.
Footnotes
Competing Interests
All the authors disclose no conflict of interest.
References
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. J Neurosci. 2000;20:2683–2690. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzo A, Brassil J, Taylor JL, Martin D, Loo CK. Daily transcranial direct current stimulation (tDCS) leads to greater increases in cortical excitability than second daily transcranial direct current stimulation. Brain Stimul. 2015;5:208–213. doi: 10.1016/j.brs.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Bachmann CG, Muschinsky S, Nitsche MA, et al. Transcranial direct current stimulation of the motor cortex induces distinct changes in thermal and mechanical sensory percepts. Clin Neurophysiol. 2010;121:2083–2089. doi: 10.1016/j.clinph.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Blankenburg F, Ruff CC, Bestmann S, Bjoertomt O, Eshel N, Josephs O, Weiskopf N, Driver J. Interhemispheric effect of parietal TMS on somatosensory response confirmed directly with concurrent TMS fMRI. J Neurosci. 2008;28:13202–13208. doi: 10.1523/JNEUROSCI.3043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25:123–129. [PubMed] [Google Scholar]
- Boggio PS, Rigonatti SP, Ribeiro RB, Myczkowski ML, Nitsche MA, Pascual-Leone A, Fregni F. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Neuropsychoph. 2008;11:249–254. doi: 10.1017/S1461145707007833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton CF, Winkelmann RK, Dyck PJ. A quantitative study of Meissner’s corpuscles in man. Neurology. 1966;16:1–9. doi: 10.1212/wnl.16.1.1. [DOI] [PubMed] [Google Scholar]
- Borich MR, Brodie SM, Gray WA, Ionta S, Boyd LA. Understanding the role of the primary somatosensory cortex: Opportunities for rehabilitation. Neuropsychologia. 2015;79:246–255. doi: 10.1016/j.neuropsychologia.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boubker Z, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain. 2012;135:2277–2289. doi: 10.1093/brain/aws115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodoehl S, Klingner C, Stieglitz K, Witte OW. Age-related changes in the somatosensory processing of tactile stimulation-An fMRI study. Behav Brain Res. 2013;238:259–264. doi: 10.1016/j.bbr.2012.10.038. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. Systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychoph. 2011;14:1133–1145. doi: 10.1017/S1461145710001690. [DOI] [PubMed] [Google Scholar]
- Citaker S, Gunduz AG, Guclu MB, Nazliel B, Irkec C, Kaya D. Relationship between foot sensation and standing balance in patients with multiple sclerosis. Gait Posture. 2011;34:275–278. doi: 10.1016/j.gaitpost.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Davis NJ, Gold E, Pascual-Leone A, Bracewell RM. Challenges of proper placebo control for non-invasive brain stimulation in clinical and experimental application. Eur J Neurosci. 2013;38:2973–2977. doi: 10.1111/ejn.12307. [DOI] [PubMed] [Google Scholar]
- Eils E, Behrens S, Mers O, Thorwesten L, Völker K, Rosenbaum D. Reduced plantar sensation causes a cautious walking pattern. Gait Posture. 2004;20:54–60. doi: 10.1016/S0966-6362(03)00095-X. [DOI] [PubMed] [Google Scholar]
- Eils E, Nolte S, Tewes M, Thorwesten L, Völker K, Rosenbaum D. Modified pressure distribution patterns in walking following reduction of plantar sensation. J Biomech. 2002;35:1307–1313. doi: 10.1016/s0021-9290(02)00168-9. [DOI] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology—perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neuro. 2007;3:383–393. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Goldberg RF, Perfetti CA, Schneider W. Perceptual knowledge retrieval activates sensory brain regions. J Neurosci. 2006;26:4917–4921. doi: 10.1523/JNEUROSCI.5389-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M. Brain states: top-down influences in sensory processing. Neuron. 2007;54:677–696. doi: 10.1016/j.neuron.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Kavounoudias A, Roll R, Roll JP. Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. J Physiol. 2001;532:869–878. doi: 10.1111/j.1469-7793.2001.0869e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristinsdottir EK, Fransson PA, Magnusson M. Changes in postural control in healthy elderly subjects are related to vibration sensation, vision and vestibular asymmetry. Acta Otolaryngol. 2001;121:700–706. doi: 10.1080/00016480152583647. [DOI] [PubMed] [Google Scholar]
- Kuypers HG. Anatomy of the descending pathways. Compr Physiol. 1981:597–666. [Google Scholar]
- Malandraki GA, Perlman AL, Karampinos DC, Sutton BP. Reduced somatosensory activations in swallowing with age. Hum Brain Mapp. 2011;32:730–743. doi: 10.1002/hbm.21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Gottschalk A, Patel RS, Pincus D, Detre JA, Alsop DC. Mapping of secondary somatosensory cortex activation induced by vibrational stimulation: an fMRI study. Brain Res. 1999;824:291–295. doi: 10.1016/s0006-8993(99)01126-9. [DOI] [PubMed] [Google Scholar]
- Manchester D, Woollacott M, Zederbauer-Hylton N, Marin O. Visual, vestibular and somatosensory contributions to balance control in the older adult. J Gerontol. 1989;44:M118–127. doi: 10.1093/geronj/44.4.m118. [DOI] [PubMed] [Google Scholar]
- Martin JH. The corticospinal system: from development to motor control. Neuroscientist. 2005;11:161–173. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- Meehan SK, Linsdell MA, Handy TC, Boyd LA. Interhemispheric enhancement of somatosensory cortical excitability through contralateral repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2011;122:1637–1644. doi: 10.1016/j.clinph.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Menz HB, Lord SR, St George R, Fitzpatrick RC. Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch Phys Med Rehabil. 2004;85:245–52. doi: 10.1016/j.apmr.2003.06.015. [DOI] [PubMed] [Google Scholar]
- Meyer PF, Oddsson LI, De Luca CJ. The role of plantar cutaneous sensation in unperturbed stance. Exp Brain Res. 2004;156:505–512. doi: 10.1007/s00221-003-1804-y. [DOI] [PubMed] [Google Scholar]
- Miranda DL, Hsu WH, Gravelle DC, Petersen K, Ryzman R, Niemi J, Lesniewski-Laas N. Sensory enhancing insoles improve athletic performance during a hexagonal agility task. J Biomech. 2016;49:1058–1063. doi: 10.1016/j.jbiomech.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Miranda DL, Hsu WH, Petersen K, Fitzgibbons S, Niemi J, Lesniewski-Laas N, Walsh CJ. Sensory enhancing insoles modify gait during inclined treadmill walking with load. Med Sci Sport Exer. 2016;48:860–868. doi: 10.1249/MSS.0000000000000831. [DOI] [PubMed] [Google Scholar]
- Miranda PC, Mekonnen A, Salvador R, Ruffini G. The electric field in the cortex during transcranial current stimulation. Neuroimage. 2013;15:48–58. doi: 10.1016/j.neuroimage.2012.12.034. [DOI] [PubMed] [Google Scholar]
- Mori F, Nicoletti CG, Kusayanagi H, Foti C, Restivo DA, Marciani MG, Centonze D. Transcranial direct current stimulation ameliorates tactile sensory deficit in multiple sclerosis. Brain Stimul. 2013;6:654–659. doi: 10.1016/j.brs.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Legon W, Dionne JK, Staines WR. Continuous theta burst stimulation of the supplementary motor area: effect upon perception and somatosensory and motor evoked potentials. Brain Stimul. 2013;6:877–883. doi: 10.1016/j.brs.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Lough M, Niemi J, Travison T, Howlett H, Manor B. A shoe insole delivering subsensory vibratory noise improves balance and gait in healthy elderly people. Arch Phys Med Rehab. 2015;96:432–439. doi: 10.1016/j.apmr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord SR, Clark RD, Webster IW. Postural stability and associated physiological factors in a population of aged persons. J Gerontol. 1991;46:M69–76. doi: 10.1093/geronj/46.3.m69. [DOI] [PubMed] [Google Scholar]
- Ng SS, Hui-Chan CW. The timed “up & go” test: its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch Phys Med Rehab. 2005;86:1641–1647. doi: 10.1016/j.apmr.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Podsiadlo D, Richardson S. The timed Up & Go: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- Ragert P, Vandermeeren Y, Camus M, Cohen LG. Improvement of spatial tactile acuity by transcranial direct current stimulation. Clin Neurophysiol. 2008;119:805–811. doi: 10.1016/j.clinph.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. P Nat Acad Sci USA. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JK, Ching C, Hurvitz EA. The Relationship between Electromyographically Documented Peripheral Neuropatny and Falls. J Am Geriatr Soc. 1992;40:1008–1012. doi: 10.1111/j.1532-5415.1992.tb04477.x. [DOI] [PubMed] [Google Scholar]
- Ruffini G, Fox MD, Ripolles O, Miranda PC, Pascual-Leone A. Optimization of multifocal transcranial current stimulation for weighted cortical pattern targeting from realistic modeling of electric fields. Neuroimage. 2014;89:216–225. doi: 10.1016/j.neuroimage.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaglioni G, Ferri A, Minetti AE, et al. Plantar flexor activation capacity and H reflex in older adults: adaptations to strength training. J Appl Physiol. 2002;92:2292–2302. doi: 10.1152/japplphysiol.00367.2001. [DOI] [PubMed] [Google Scholar]
- Selvarajah D, Wilkinson ID, Maxwell M, et al. Magnetic resonance neuroimaging study of brain structural differences in diabetic peripheral neuropathy. Diabetes Care. 2014;37:1681–1688. doi: 10.2337/dc13-2610. [DOI] [PubMed] [Google Scholar]
- Seyal M, Ro T, Rafal R. Increased sensitivity to ipsilateral cutaneous stimuli following transcranial magnetic stimulation of the parietal lobe. Ann Neurol. 1995;38:264–267. doi: 10.1002/ana.410380221. [DOI] [PubMed] [Google Scholar]
- Shaffer SW, Harrison AL. Aging of the somatosensory system: a translational perspective. Phys Ther. 2007;87:193–207. doi: 10.2522/ptj.20060083. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80:896–903. [PubMed] [Google Scholar]
- Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2012;28:8–14. doi: 10.1002/dmrr.2239. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hao Y, Zhou J, et al. Direct current stimulation over the human sensorimotor cortex modulates the brain’s hemodynamic response to tactile stimulation. Eur J Neurosci. 2015;42:1933–1940. doi: 10.1111/ejn.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Mariola E, Stilla R, et al. Tactile discrimination of grating orientation: fMRI activation patterns. Hum Brain Mapp. 2005;25:370–377. doi: 10.1002/hbm.20107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Lipsitz L, Habtemariam D, Manor B. Sub-sensory vibratory noise augments the physiologic complexity of postural control in older adults. J Neuroeng Rehabil. 2016;13:44. doi: 10.1186/s12984-016-0152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]