Abstract
Previous research has shown associations between brain structure and resting state high-frequency heart rate variability (HF-HRV). Age affects both brain structure and HF-HRV. Therefore we sought to examine the relationship between brain structure and HF-HRV as a function of age. Data from two independent studies were used for the present analysis. Study 1 included 19 older adults (10 males, age range 62–78 years) and 19 younger adults (12 males, age range 19–37). Study 2 included 23 older adults (12 males; age range 55–75) and 27 younger adults (17 males; age range 18–34). The root-mean-square of successive R-R-interval differences (RMSSD) from ECG recordings was used as time-domain measure of HF-HRV. MRI scans were performed on a 3.0-T Siemens Magnetom Trio scanner. Cortical reconstruction and volumetric segmentation were performed with the Freesurfer image analysis suite, including 12 regions as regions-of-interests (ROI). Zero-order and partial correlations were used to assess the correlation of RMSSD with cortical thickness in selected ROIs. Lateral orbitofrontal cortex (OFC) cortical thickness was significantly associated with RMSSD. Further, both studies, in line with previous research, showed correlations between RMSSD and anterior cingulate cortex (ACC) cortical thickness. Meta-analysis on adjusted correlation coefficients from individual studies confirmed an association of RMSSD with the left rostral ACC and the left lateral OFC. Future longitudinal studies are necessary to trace individual trajectories in the association of HRV and brain structure across aging.
Keywords: Heart Rate Variability, Vagal activity, Cortical Thickness, Age, Brain Structure
Introduction
The French physiologist Claude Bernard was the first to describe an intimate connection between the brain and the heart via the vagus nerve. His idea that the vagus serves as a structural and functional bidirectional link between the brain and the heart is now widely accepted (Thayer and Lane 2009). Parasympathetic modulation of the heart rate by the vagus is fast (timescale on the order of milliseconds) while sympathetic effects are much slower (Levy 1997). The resulting high-frequency heart rate variability (HF-HRV) and time-domain measures reflecting these fast changes (e.g., the time-domain root-mean-square of successive R-R-interval differences, RMSSD measure) provide a readily available, surrogate measure of cardiac vagal activity (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology 1996; Kuo et al. 2005).
Chronic reductions in vagal activity indexed by HRV are associated with poor physiological, emotional, cognitive, behavioral regulation and self-rated heath (Alvares et al. 2013; Beauchaine and Thayer 2015; Jarczok et al. 2015; Thayer and Lane 2000; Thayer et al. 2012; Thayer et al. 2009a; Thayer and Lane 2009), as well as psychopathology (Kemp et al. 2010; Koenig et al. 2015, 2016; Clamor et al. 2016). Functional neuroimaging studies and human lesion studies have shown several brain regions and networks involved in the neural control of HRV (Thayer et al. 2012). However, previously only two studies investigated the structural brain correlates of HRV. Winkelmann et al. (Winkelmann et al. 2016) studied 30 young participants (mean age = 22.53±3.89 years, 8 female) and found a significant correlation of resting state HF-HRV with the thickness of a cluster located within the cingulate cortex (CC). This area has been identified as the one most related to HRV in functional studies (Thayer et al. 2012). In addition, the thickness of several other frontal regions in the right hemisphere and the caudal anterior CC (ACC) were associated with HRV. Refining their analyses, the authors were able to localize the area of highest association with HRV to the anterior midcingulate cortex (MCC) and the rostral dorsal cingulate cortex (DCC). The other study (Woodward et al. 2008) found vagally mediated HRV positively associated with right but not left ACC volume in older (mean age = 49 years) combat veterans using a large region of interest (ROI) analysis of manually traced ACC volumes.
Importantly, HRV decreases with age (O’Brien et al. 1986; Zhang 2007). For example, research has shown that RMSSD declines approximately 3.6 milliseconds per decade (Antelmi et al. 2004). Similarly, there are substantial structural brain changes in aging (Peters 2006; Fjell et al. 2009; Fjell and Walhovd 2010). Interestingly, the more ventral regions of the brain including the ventromedial PFC and the anterior CC appear to be relatively preserved with age whereas more dorsal, lateral, and superior regions show greater decline in thickness with age (Fjell and Walhovd 2010; Mather 2016). Age-related differences in the cortical control of HRV have previously been investigated. A pharmacological blockade study found that adults aged 39–65 showed less inhibitory control of heart rate by the frontal cortex than younger subjects (13–27, and 28–38 years) (Thayer et al. 2009b). Therefore the systematic investigation of the effects of age on the relationship between cortical thickness and HRV is needed. Here we aimed to assess brain structural concomitants of HRV in two independent samples, investigating the effect of age on the association of cortical thickness and HRV. In addition, we also performed a meta-analysis of the results of these two samples to increase the reliability and aid the interpretation of the findings.
Materials and Methods Study 1
Procedures and Participants
The main results from Study 1 have been reported elsewhere (Sakaki et al. 2013), as have correlations between HRV and functional connectivity in BOLD fMRI activation (Sakaki et al., 2016). Participants included 21 older adults (10 males; age range = 61–78) and 20 younger adults (12 males; age range = 19–37). Prospective participants were screened and excluded for any medical, neurological, or psychiatric illness. Older adults were further screened for their cognitive function using a telephone protocol (Gatz et al., 1995) which includes 21 questions on cognitive function, such as short-term memory, general knowledge (e.g., current U.S. president), and attention (e.g., counting back by 3 s from 20). Data from one participant were excluded: one older adult who was considered neurologically abnormal by a neuroradiologist who reviewed all structural scans for incidental findings. In addition, one older and one younger adult were removed as their HRV measures were identified as outliers. The remaining participants included 19 older adults (10 males, age range=62–78 years) and 19 younger adults (12 males, age range 19–37 years). There were no statistical differences between groups in terms of intellectual level (Meducation: YA = 15.06 vs. OA = 16.17 years; MWechsler Test of Adult Reading: YA = 43.16 / 50 vs. OA = 43.63 / 50). They provided written informed consent approved by the University of Southern California Institutional Review Board and were paid for their participation.
Recording of Heart Rate Variability
The experimenters attached electrodes to monitor participants’ heart rates. Participants lay quietly during a pre-scan period (3 minutes), which provided a baseline measure of HRV. The electrocardiogram (ECG) was obtained by a BIOPAC MP150 data acquisition system at the University of Southern California Dana and David Dornsife Neuroimaging Center. A LEAD108 setup with EL508 MRI-compatible/radio translucent electrodes was used for recording. The raw signal was 0.05–35 Hz bandpass filtered and amplified using an ECG100C amplifier. The signal was continuously digitized at a sampling rate of 4,000 Hz. The recorded data were analyzed with Acqknowledge software (BIOPAC Systems Inc., USA) for noise reduction. Because the ECG was recorded in the MRI scanning setting, noise signal from the scanner can affect the ECG signal. Noise removal was performed through three steps. First, a comb band stop was applied to filter out the noise frequency from the scanner. In the next step, independent component analysis (ICA) was performed to separate the ECG signal, respiration and scanner signal. The last step was the application of a template-correlation function. The template functions performed a mathematical operation of the template waveform on the waveform to be compared, move one sample forward, and repeat the multiplication. The resulting data points replace the waveform to be correlated. HRV calculations were performed on the corrected data using Kubios version 2.2 (Tarvainen et al. 2014).
Inter-beat-intervals (RR intervals) were derived from the ECG signal, checked for artefacts, and corrected. The time domain analyses led to estimates of RMSSD in ms. We used RMSSD as an index of individual HRV. RMSSD is considered a reliable measure of vagal parasympathetic activity (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology 1996), highly related to HF power, and was used for the present analysis. In Study 1, RMSSD and HF-power were highly correlated (r = .890, p < .0001).
Assessment of Cortical Thickness
MRI scanning was performed on a 3.0-T Siemens MAGNETOM Trio scanner with a 12-channel matrix head coil at the University of Southern California Dana and David Dornsife Neuroimaging Center. Each participant’ T1 structural image was preprocessed using FMRIB's Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl), which includes skull stripping with BET. Cortical reconstruction and volumetric segmentation were performed with the Freesurfer image analysis suite version 5.2 (http://surfer.nmr.mgh.harvard.edu/). This method uses both intensity and continuity information from the entire three dimensional MR volume in segmentation and deformation procedures to produce representations of cortical thickness, calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex on the tessellated surface (Fischl and Dale 2000). The technical details of these procedures are described in prior publications (Dale and Sereno 1993; Fischl et al. 1999b; Dale et al. 1999; Fischl et al. 1999a; Fischl et al. 2001; Fischl et al. 2002; Fischl et al. 2004a; Fischl et al. 2004b; Ségonne et al. 2004; Jovicich et al. 2006; Han et al. 2006).
Based on prior findings (Winkelmann et al., 2016) and our hypothesis, we focused on the ACC, insula, and PFC in this study. Twelve regions were included (for each hemisphere) as our regions-of-interests (ROIs). These included two sub regions for ACC (caudal and rostral ACC), 9 sub regions for PFC (caudal middle frontal gyrus (MFG), rostral MFG, lateral OFC, medial OFC, superior frontal gyrus, pars opercularis, pars orbitalis, pars triangularis, and frontal pole), and insula. Thus, the cortical thicknesses of a total of 24 sub regions were estimated for both studies (12 in the right hemisphere and 12 in the left).
Materials and Methods Study 2
Procedures and Participants
The main fMRI findings from Study 2 are in preparation and will not be covered in this paper. Participants included 28 older adults (16 males; age range = 55–75) and 35 younger adults (21 males; age range = 18–34). All participants had normal or corrected-to-normal vision and hearing, and self reported no history of chronic illness or cognitive impairment. Data from 13 participants were excluded: 5 older adults and 6 younger adults due to failure to extract heart rate from noisy ECG and 2 younger adults who did not provide the scan on the session day. The remaining participants included 23 older adults (12 males; age range = 55–75) and 27 younger adults (17 males; age range = 18–34). There were no statistical differences between groups in terms of intellectual level (Meducation: YA = 16.30 vs. OA = 16.00 years; MWechsler Test of Adult Reading: YA = 43.96 / 50 vs. OA = 40.68 / 50). They provided written informed consent approved by the University of Southern California Institutional Review Board and were paid for their participation.
Recording of Heart Rate Variability
HRV was recorded and processed as described in Study 1, but the signal was digitized at a sampling rate of 10,000 Hz. In this study, participants performed 6 runs of task during scanning (about 30 minutes) which were then averaged to produce each participant’s HRV. The 6 runs of task were a fear-conditioning task and five runs of a spatial detection task. In Study 2, RMSSD and HF-power were correlated in the entire sample (r = .508, p < .0001).
Assessment of Cortical Thickness
Neuroimaging data was assessed with the same scanner at the University of Southern California Dana & David Dornsife Cognitive Neuroscience Imaging Center, and procedures described in Study 1. In Study 2, a 32-channel matrix head coil was used. Analyses were again performed with the Freesurfer image analysis suite version 5.2 as described in Study 1.
Statistical Analysis (Study 1 and 2)
Data from both studies were handled independently. Differences between participants of younger and older age on age, mean RR, and RMSSD were analyzed using t-tests for independent samples. First, zero-order Pearson correlations were calculated to investigate the relationship between resting HRV and cortical thickness in the left and right hemisphere for all 24 ROIs. Zero-order correlations were calculated each for the entire samples and the young/old samples separately. Second, partial correlations adjusting for age were calculated. Third, as adjustment of cortical thickness measures for global thickness is not universally recommended (FS Wiki 2016), partial correlations adjusted for cortical thickness in non-ROI regions in line with the approach presented by Harms et al. (2010) were calculated. Finally, we conducted fixed-effect meta-analysis on zero-order correlation coefficients and partial correlation coefficients adjusted for age, non-ROI cortical thickness, as well as non-ROI cortical thickness and age to investigate the correlation of RMSSD with cortical thickness in selected ROIs across studies. Pearson correlations were used as effect sizes in meta-analyses to obtain weighted mean correlations and their 95% confidence intervals. Fixed effect meta-analysis of correlations based either on Fisher’s z transformation of correlations or direct combination of correlations (see Cooper et al., 2009). Individual effect sizes were weighted by the inverse of their variance. The meta-analysis of correlation coefficients was performed in R (R Development Core Team, 2013) with the Meta package (Schwarzer, 2007), which implements the DerSimonian–Laird method to estimate the between-study variance (DerSimonian & Laird, 1986). All tests were two-tailed and were analyzed using a set level of significance of p < .05. All statistical tests were conducted using SPSS (ver. 22, IBM Chicago, IL, USA).
Results Study 1
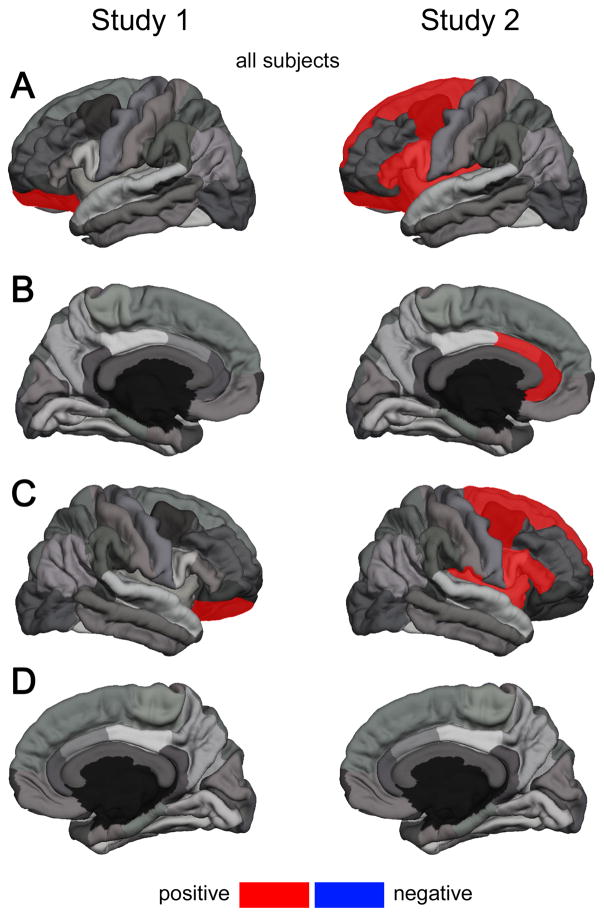
Table 1 shows the mean and standard deviation of age, RR, and RMSSD according to age group by study. Descriptive data on cortical thickness by age group are provided in the Supplementary Material Table S-1. In Study 1 groups significantly differed on age (t(36) = 25.58, p < .001), mean RR (t(36) = 2.53, p = .02) and RMSSD (t(36) = −2.80, p < .01). Correlation coefficients of cortical thickness across the entire cortical mantle with age, mean RR and RMSSD from Study 1 are provided in the Supplementary Material Table S-3. A summary of results from zero-order correlations in Study 1 is given in Table 2. Zero-order correlations in the entire sample revealed a significant correlation of RMSSD with the lateral OFC in the left (r(36) = .324, p = .047) and right hemisphere (r(36) = .335, p = .040). Findings are illustrated in Figure 1. Cortical thickness of the lateral OFC in the right hemisphere was correlated with RMSSD in the old (r(17) = .585, p = .008) but not the young sub-sample (r(17) = −.024, p = .922). Besides the lateral OFC, analyses showed significant association of RMSSD with thickness of the pars orbitalis in the right hemisphere in the old sample (r(17) = .517, p = .023) and the rostral ACC in the right hemisphere showed a trend towards significance in the young sample (r(17) = .451, p = .053; Table 2). No further associations were found for the left hemisphere (Table 2). A summary of results from partial correlations adjusting for age and non-ROI thickness are given in the Supplementary Material Table S-4 and Table S-6. Zero-order correlations showed no significant positive correlation of RR with cortical thickness. Only caudal ACC in the left hemisphere showed a significant negative correlation with mean RR (r(17) = −.349, p = .032; Supplementary Material Table S-8).
Table 1. Sample Characteristics according to Age Group and Study;
WTAR = Wechsler Test of Adult Reading test ; ms: milliseconds; p-values refer to group differences comparing subjects of younger and older age within the respective study; mean RR: mean inter-beat-interval between adjacent heart beats in ms; RMSSD: root mean square successive differences in ms
| Study 1 | Study 2 | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| older age | younger age | p | older age | younger age | p | |
|
|
|
|||||
| N (female) | 19 (9) | 19 (7) | 23 (11) | 27 (10) | ||
| Age, mean years (SD) | 68.11 (5.10) | 25.05 (5.28) | <.001 | 67.17 (5.49) | 23.63 (4.79) | <.001 |
| Education | 16.17 (2.33) | 15.21 (2.27) | .22 | 16.00 (2.28) | 16.30 (3.16) | .71 |
| WTAR | 43.63 (6.44) | 43.16 (3.70) | .78 | 40.68 (7.13) | 43.96 (5.69) | .11 |
| RR, mean ms (SD) | 942.77 (164.20) | 834.56(88.26) | .02 | 844.74 (212.08) | 862.21 (102.15) | .71 |
| RMSSD, mean ms (SD) | 18.43 (7.34) | 25.81 (8.85) | <.001 | 20.48 (6.28) | 24.88 (4.73) | .01 |
Table 2. Zero-Order Correlations of Cortical Thickness in Selected Regions of Interest (ROI) with Resting State Heart Rate Variability (Study 1);
values represent correlation coefficients (r) and their respective p-values; ACC: anterior cingulate cortex; MFG: middle frontal gyrus; OFC: orbital frontal cortex; * and bold: highlights a significant correlation on the p < .05 level; ** and bold: highlights a significant correlation on the p < .01 level
| Study 1 | Left Hemisphere | Right Hemisphere | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| all subjects | older age | younger age | all subjects | older age | younger age | |||||||
|
| ||||||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | |
| Caudal ACC | .059 | .723 | −.095 | .698 | −.100 | .683 | .110 | .509 | −.145 | .554 | .179 | .462 |
| Rostral ACC | .179 | .282 | −.096 | .697 | .137 | .575 | .206 | .215 | −.127 | .604 | .451 | .053 |
| Caudal MFG | .228 | .168 | .310 | .196 | −.059 | .812 | .116 | .489 | .086 | .726 | .125 | .610 |
| Rostral MFG | .094 | .574 | .318 | .184 | −.213 | .380 | .263 | .110 | .338 | .157 | .128 | .603 |
| Lateral OFC | .324* | .047 | .426 | .069 | .255 | .292 | .335* | .040 | .585** | .008 | −.024 | .922 |
| Medial OFC | .043 | .797 | .169 | .489 | −.225 | .354 | −.001 | .995 | −.077 | .754 | .003 | .989 |
| Pars opercularis | .039 | .817 | .244 | .313 | −.266 | .272 | .247 | .136 | .277 | .252 | .104 | .670 |
| Pars orbitalis | .263 | .110 | .206 | .397 | .209 | .389 | .259 | .116 | .517* | .023 | −.049 | .841 |
| Pars triangularis | .027 | .872 | −.131 | .593 | −.329 | .169 | .183 | .271 | .245 | .313 | −.107 | .662 |
| Superior frontal G. | .182 | .273 | −.110 | .655 | −.031 | .900 | .105 | .530 | −.033 | .893 | .050 | .838 |
| Frontal pole | .095 | .576 | .158 | .519 | .021 | .935 | .099 | .554 | .007 | .979 | −.012 | .962 |
| Insula | .240 | .147 | −.089 | .718 | .382 | .107 | .099 | .553 | .027 | .911 | .003 | .991 |
Figure 1. Significant Correlations of Cortical Thickness in Selected Regions of Interest (ROI) with Resting State Heart Rate Variability by Study;
data from all subjects; (A): left hemisphere lateral view; (B): left hemisphere medial view; (C): right hemisphere lateral view; (D): right hemisphere medial view; ROIs highlighted in red show a significant positive zero-order correlation with RMSSD; ROIs highlighted in blue show a significant negative zero-order correlation with RMSSD; corresponding correlation coefficients are provided in Table 2 and Table 3 ; note: for clarity the superior frontal gyrus is only highlighted in the lateral view
Results Study 2
In Study 2, groups significantly differed on age (t(48) = 29.96, p < .001), and RMSSD (t(48) = −2.82, p <.01) but not mean RR (t(48) = −0.38, p = .71; Table 1). Descriptive data on cortical thickness by age group are provided in the Supplementary Material Table S-2. A summary of results from zero-order correlations in Study 2 is given in Table 3. Correlation coefficients of cortical thickness across the entire cortical mantle with age, mean RR and RMSSD from Study 2 are provided in the Supplementary Material Table S-3. Similar to findings from Study 1, zero-order correlations in the entire sample revealed a significant correlation of RMSSD with cortical thickness of the lateral OFC in the left (r(48) = .303, p = .032) but not the right hemisphere (r(48) = .134, p = .355). In the left hemisphere, the entire sample showed further correlations of RMSSD and cortical thickness of the caudal ACC (r(48) = .337, p = .017), the rostral ACC (r(48) = .409, p = .003), the caudal MFG (r(48) = .361, p = .010), the pars opercularis (r(48) = .370, p = .008), the pars triangularis (r(48) = .326, p = .021), the superior frontal gyrus (r(48) = .332, p = .019), and the insula (r(48) = .294, p = .038). In the right hemisphere, analyses revealed significant correlations of RMSSD with thickness of the caudal MFG (r(48) = .318, p = .024), the pars opercularis (r(48) = .304, p = .032), the pars triangularis (r(48) = .328, p = .020), the superior frontal G. (r(48) = .294, p = .038), and the insula (r(48) = .322, p = .023). Findings are illustrated in Figure 1. The old and young sub-sample showed no significant correlations. A summary of results from partial correlations adjusting for age and non-ROI thickness are given in the Supplementary Material Table S-5 and Table S-7. Zero-order correlations showed no significant correlation of RR with cortical thickness (results provided in the Supplementary Material Table S-9). > Insert Table 3 <
Table 3. Zero-Order Correlations of Cortical Thickness in Selected Regions of Interest (ROI) with Resting State Heart Rate Variability (Study 2);
values represent correlation coefficients (r) and their respective p-values; ACC: anterior cingulate cortex; MFG: middle frontal gyrus; OFC: orbital frontal cortex; * and bold: highlights a significant correlation on the p < .05 level; ** and bold: highlights a significant correlation on the p < .01 level
| Study 2 | Left Hemisphere | Right Hemisphere | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| all subjects | older age | younger age | all subjects | older age | younger age | ||||||||
|
| |||||||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | ||
| Caudal ACC | .337* | .017 | .356 | .096 | .165 | .410 | .160 | .268 | .116 | .598 | −.076 | .706 | |
| Rostral ACC | .409** | .003 | .288 | .182 | .325 | .098 | .152 | .291 | .018 | .935 | .078 | .699 | |
| Caudal MFG | .361** | .010 | .139 | .527 | .222 | .266 | .318* | .024 | .133 | .546 | .167 | .406 | |
| Rostral MFG | .274 | .054 | −.013 | .952 | .212 | .288 | .190 | .186 | −.146 | .506 | .205 | .304 | |
| Lateral OFC | .303* | .032 | .188 | .392 | .167 | .406 | .134 | .355 | .053 | .811 | −.013 | .949 | |
| Medial OFC | .215 | .133 | −.117 | .595 | .322 | .101 | .218 | .128 | .083 | .708 | .134 | .505 | |
| Pars opercularis | .370** | .008 | .112 | .611 | .308 | .118 | .304* | .032 | .178 | .416 | .037 | .854 | |
| Pars orbitalis | .165 | .251 | −.249 | .252 | .266 | .179 | .193 | .180 | −.117 | .594 | .204 | .308 | |
| Pars triangularis | .326* | .021 | .199 | .363 | .169 | .398 | .328* | .020 | .184 | .401 | .120 | .551 | |
| Superior frontal G. | .332* | .019 | .003 | .989 | .232 | .244 | .294* | .038 | .017 | .939 | .177 | .377 | |
| Frontal pole | .093 | .523 | −.277 | .201 | .238 | .232 | .073 | .615 | −.371 | .081 | .272 | .170 | |
| Insula | .294* | .038 | .110 | .617 | .027 | .895 | .322* | .023 | .247 | .257 | −.029 | .888 | |
Meta-Analyses
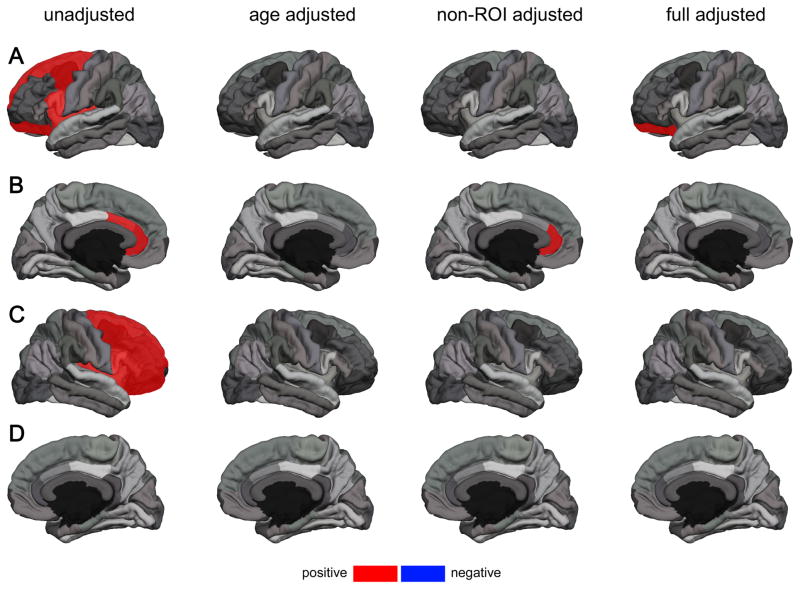
Meta-analysis across Study 1 and Study 2 using zero-order correlation coefficients, indicated correlations of RMSSD with cortical thickness across different ROIs in the left hemisphere, including the caudal (r = .222, p = .041) and rostral ACC (r = .315, p = .003), the caudal MFG (r = .306, p = .004), the lateral OFC (r = .312 p = .004), the pars opercularis (r = .235, p = .030), the superior frontal gyrus (r = .270, p = .012) and the insula (r = .271, p = .012). Within the right hemisphere meta-analysis showed a significant correlation of RMSSD with cortical thickness of the caudal MFG (r = .234, p = .031), the rostral MFG (r = .222, p = .041), the lateral OFC (r = .222 p = .041), the pars opercularis (r = .280, p = .009), the pars orbitalis (r = .221, p = .042), pars triangularis (r = .268, p = .013), superior frontal g. (r = .215, p = .048), and the insula (r = .230, p = .034) across studies. Meta-analysis across Study 1 and Study 2 using partial correlation coefficients adjusted for age showed no significant correlations. Meta-analysis using partial correlation coefficients adjusted for non-ROI thickness showed significant correlations of RMSSD and cortical thickness of the rostral ACC (r = .247, p = .024) and the lateral OFC in the left hemisphere showed a trend towards significance (r = .211, p = .055; Table 4). A further full adjusted Meta-analysis using partial correlation coefficients adjusted for age and non-ROI thickness, illustrated that when further adjusting for age, only the significant correlation of RMSSD and the lateral OFC in the left hemisphere remained (r = .222 p = .046; Table 4). Results from meta-analyses are provided in Table 4 for the left hemisphere and Table 5 for the right hemisphere and findings are illustrated in Figure 2.
Table 4. Meta-Analyses on Zero-Order, Age-Adjusted, Non-ROI Thickness-Adjusted Partial Correlation Coefficients of Cortical Thickness in Selected Regions of Interest (ROI) and Resting State Heart Rate Variability across Study 1 and Study 2 (Left Hemisphere);
Fixed effect meta-analyses; values represent correlation coefficients (r) and their 95% confidence interval; ACC: anterior cingulate cortex; MFG: middle frontal gyrus; OFC: orbital frontal cortex; * and bold: highlights a significant meta correlation on the p < .05 level; ** and bold: highlights a significant meta correlation on the p < .01 level
| Meta-analyses | Left Hemisphere | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| unadjusted | age adjusted | non-ROI thickness adjusted | Age & non-ROI thickness adjusted | |||||
|
| ||||||||
| meta r | 95%CI | meta r | 95%CI | meta r | 95%CI | meta r | 95%CI | |
| Caudal ACC | 0.222* | 0.010; 0.416 | 0.110 | −0.108; 0.319 | 0.157 | −0.061; 0.360 | 0.108 | −0.114; 0.318 |
| Rostral ACC | 0.315** | 0.109; 0.495 | 0.172 | −0.046; 0.374 | 0.247* | 0.033; 0.439 | 0.169 | −0.051; 0.374 |
| Caudal MFG | 0.306** | 0.099; 0.487 | 0.120 | −0.098; 0.327 | 0.207 | −0.009; 0.405 | 0.180 | −0.040; 0.383 |
| Rostral MFG | 0.199 | −0.015; 0.395 | 0.018 | −0.199; 0.232 | 0.033 | −0.184; 0.247 | 0.035 | −0.185; 0.251 |
| Lateral OFC | 0.312** | 0.106; 0.492 | 0.200 | −0.016; 0.399 | 0.211 | −0.005; 0.408 | 0.222* | 0.004; 0.420 |
| Medial OFC | 0.143 | −0.073; 0.345 | −0.020 | −0.234; 0.197 | 0.032 | −0.185; 0.246 | −0.016 | −0.234; 0.203 |
| Pars opercularis | 0.235* | 0.023; 0.427 | 0.049 | −0.168; 0.262 | 0.089 | −0.130; 0.299 | 0.096 | −0.125; 0.308 |
| Pars orbitalis | 0.207 | −0.006; 0.403 | 0.077 | −0.141; 0.288 | 0.053 | −0.164; 0.266 | 0.068 | −0.153; 0.282 |
| Pars triangularis | 0.203 | −0.011; 0.399 | −0.023 | −0.237; 0.194 | 0.045 | −0.173; 0.258 | 0.011 | −0.208; 0.228 |
| Superior frontal G. | 0.270* | 0.060; 0.456 | 0.018 | −0.198; 0.233 | 0.143 | −0.076; 0.348 | 0.037 | −0.183; 0.254 |
| Frontal pole | 0.094 | −0.122; 0.301 | 0.012 | −0.204; 0.227 | −0.047 | −0.260; 0.170 | 0.002 | −0.217; 0.220 |
| Insula | 0.271* | 0.062; 0.458 | 0.115 | −0.104; 0.322 | 0.151 | −0.067; 0.355 | 0.116 | −0.105; 0.326 |
Table 5. Meta-Analyses on Zero-Order, Age-Adjusted, Non-ROI thickness-Adjusted Partial Correlation Coefficients of Cortical Thickness in Selected Regions of Interest (ROI) and Resting State Heart Rate Variability across Study 1 and Study 2 (Right Hemisphere);
Fixed effect meta-analyses; values represent correlation coefficients (r) and their 95% confidence interval; ACC: anterior cingulate cortex; MFG: middle frontal gyrus; OFC: orbital frontal cortex; * and bold: highlights a significant meta correlation on the p < .05 level; ** and bold: highlights a significant meta correlation on the p < .01 level
| Meta-analyses | Right Hemisphere | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| unadjusted | age adjusted | non-ROI thickness adjusted | age & non-ROI thickness adjusted | |||||
|
| ||||||||
| meta r | 95%CI | meta r | 95%CI | meta r | 95%CI | meta r | 95%CI | |
| Caudal ACC | 0.139 | −0.077; 0.342 | 0.043 | −0.175; 0.256 | 0.056 | −0.162; 0.268 | 0.028 | −0.191; 0.245 |
| Rostral ACC | 0.175 | −0.039; 0.374 | 0.081 | −0.137; 0.292 | 0.069 | −0.149; 0.280 | 0.069 | −0.152; 0.283 |
| Caudal MFG | 0.234* | 0.022; 0.426 | 0.113 | −0.105; 0.321 | 0.094 | −0.124; 0.304 | 0.113 | −0.108; 0.324 |
| Rostral MFG | 0.222* | 0.009; 0.415 | 0.086 | −0.132; 0.297 | 0.062 | −0.156; 0.274 | 0.060 | −0.160; 0.275 |
| Lateral OFC | 0.222* | 0.010; 0.416 | 0.090 | −0.128; 0.300 | 0.055 | −0.163; 0.268 | 0.067 | −0.153; 0.282 |
| Medial OFC | 0.126 | −0.090; 0.330 | 0.040 | −0.178; 0.253 | 0.032 | −0.185; 0.246 | 0.027 | −0.193; 0.244 |
| Pars opercularis | 0.280** | 0.071; 0.465 | 0.117 | −0.101; 0.325 | 0.137 | −0.081; 0.343 | 0.105 | −0.116; 0.316 |
| Pars orbitalis | 0.221* | 0.009; 0.415 | 0.093 | −0.125; 0.303 | 0.067 | −0.151; 0.279 | 0.081 | −0.139; 0.295 |
| Pars triangularis | 0.268* | 0.058; 0.455 | 0.085 | −0.133; 0.295 | 0.143 | −0.075; 0.348 | 0.075 | −0.146; 0.289 |
| Superior frontal gyrus | 0.215* | 0.002; 0.410 | 0.034 | −0.183; 0.248 | 0.035 | −0.183; 0.248 | 0.016 | −0.203; 0.234 |
| Frontal pole | 0.084 | −0.131; 0.292 | −0.044 | −0.257; 0.174 | −0.080 | −0.291; 0.138 | −0.079 | −0.292; 0.142 |
| Insula | 0.230* | 0.017; 0.422 | 0.084 | −0.134; 0.294 | 0.068 | −0.150; 0.280 | 0.081 | −0.140; 0.294 |
Figure 2. Significant Correlations of Cortical Thickness in Selected Regions of Interest (ROI) with Resting State Heart Rate Variability according to Meta-Analyses across Studies;
results from fixed-effect meta-analyses on (1) zero-order correlation coefficients (unadjusted), (2) partial correlation coefficients adjusted for age (age adjusted), (3) partial correlation coefficients adjusted for non-ROI thickness (non-ROI adjusted), and (4) partial correlation coefficients adjusted for age and non-ROI thickness (full adjusted) (A): left hemisphere lateral view; (B): left hemisphere medial view; (C): right hemisphere lateral view; (D): right hemisphere medial view; ROIs highlighted in red show a significant positive correlation with RMSSD; ROIs highlighted in blue show a significant negative correlation with RMSSD; corresponding correlation coefficients are provided in Table 4 and 5 respectively; note: for clarity the superior frontal gyrus is only highlighted in the lateral view
Discussion
The present study aimed to investigate the association of vagally mediated resting state HRV with cortical thickness as a function of age in two independent samples. In both studies, when younger and older adults were included as one group, the lateral OFC’s cortical thickness was significantly associated with RMSSD. Study 1 revealed this association in the left and the right hemisphere, Study 2 showed this association only in the left hemisphere. In both studies, when age was included as a covariate in partial correlations, this OFC correlation was no longer significant. Meta-analyses on correlation coefficients adjusted for non-ROI cortical thickness and age highlight that cortical thickness of the lateral OFC of the left hemisphere is positively associated with HRV indexed by RMSSD across both study samples and age groups. The association of other regions of the PFC with vagally mediated resting-state HRV (Table 2 and Table 3) highlights the importance of the PFC in the neural control of HRV.
Besides the associations with PFC cortical thickness, in both studies, there were also correlations between vagally mediated HRV and ACC cortical thickness. In Study 1, the correlation of cortical thickness of the rostral ACC and RMSSD in the sub-sample of younger participants (right hemisphere) missed the set level of significance, whereas in Study 2 this association was significant for both the caudal and rostral ACC in the overall sample. Interestingly, results from meta-analysis on correlation coefficients adjusted for non-ROI cortical thickness, illustrate that the rostral ACC (left hemisphere) is associated with HRV indexed by RMSSD across studies only when not adjusting for age. Unlike findings for the lateral OFC–that remained after adjusting for non-ROI thickness and age–the association of HRV with cortical thickness of the ACC seems to change with age, as the correlation was weaker in models adjusting for age (Table 4).
The results of the meta-analyses contribute to the reliability and interpretation of the present findings. Research has shown that whereas the vmPFC, OFC and insula are relatively preserved with age, more superior, dorsal, and lateral regions decline in thickness significantly with age (Mather, 2016). In the analyses not adjusted for age, several regions such as the superior frontal gyrus, the caudal ACC, and the caudal MFG, known to decline in thickness with age, were identified to have significant correlations with RMSSD. However in analyses only adjusting for age, no ROI showed a significant correlation with RMSSD. When controlling for total thickness outside of the ROI (non-ROI thickness) only the left rostral ACC showed a significant correlation with RMSSD. In the present studies, non-ROI thickness of the left (Study 1: r = −.646, p < .0001; Study 2: r = −.598, p < .0001) and the right hemisphere (Study 1: r = −.636, p < .0001; Study 2: r = −.705, p < .0001) were inversely related to greater age. Only cortical thickness of the left lateral OFC survived further adjustment for age and non-ROI cortical thickness. The age-related decline in cortical thickness in the more superior regions may contribute to the age-related decline in HRV (Antelmi et al. 2004). Importantly, relatively independent of age, significant correlations with HRV were found in regions previously identified in functional MRI, positron emission tomography (PET), and lesion studies to be associated with HRV (Thayer et al. 2012). Given the cross-sectional nature of the present studies, however, it is not possible to say with certainty that the differences in cortical thickness with age are causally related to the age-related differences in HRV. Future longitudinal studies are therefore needed to more definitively examine the relationship between changes in cortical thickness and changes in HRV over time.
There are several noteworthy similarities and differences between the present results and the previous report with a separate group of younger adults (Winkelmann et al. 2016). In the unadjusted meta-analysis, the caudal ACC (left hemisphere) which had previously been identified as having the strongest correlation with HRV, was again identified as was the superior frontal gyrus (bilateral), and the pars triangularis (right hemisphere). However, in the previous study whereas there were bilateral caudal ACC correlations with HRV with the right side being stronger, in the present study the left-sided association was stronger and the right-sided association did not reach the set level of significance. In addition several other right hemisphere regions such as the pars opercularis and the insula, not identified in the previous study, were identified in the present analyses. These differences may be due to the differences in age between the previous study, which primarily comprised a younger sample, and the present study. The combined sample size in the current study was more than twice as large as in the previous study and therefore random sampling differences cannot be ruled out. Additional studies with larger and more diverse samples are needed to help further clarify the brain regions associated with HRV.
The study has several limitations that need to be mentioned. The acquisition procedures of ECG data differed between studies. In Study 1 ECG data was obtained from a resting state pre-scan that was not available for Study 2. ECG data obtained in Study 2 was averaged across different in-scanner task conditions. While previous research has shown that RMSSD shows sufficient trait specificity (Bertsch et al. 2012)–in particular when averaged over multiple recordings–future studies should apply a common protocol in the measurement of HRV. The study draws on cross-sectional data to investigate age-related differences in the association of cortical thickness and HRV. In the future, large-scale longitudinal studies are warranted to investigate individual trajectories of aging in the neural control of HRV. Beyond these limitations, this is the third study that investigated brain structural concomitants of resting state HRV and the first that investigated the effects of age on the relationship between cortical thickness and HRV drawing on two independent samples.
We can only speculate on the exact mechanism and causal relationship between cortical thickness, HRV and age. While we suggest that the age-rerated decline of cortical thickness in certain frontal and cingulate regions is associated with the decline in HRV in aging due to the contributions of these regions to regulating HRV, different interpretations are possible. It is well known that HRV is a marker of poor peripheral cardiovascular health and cardiovascular disease (CVD) risk (Thayer et al. 2010). Poor peripheral cardiovascular health may lead (via bottom up mechanisms) to poor neurovascular health and loss of cortical tissue, as other CVD risk factors (e.g., poor cardiorespiratory fitness, atherosclerosis, hypertension) that have been shown to track with HRV (e.g. Virtanen et al. 2003) have also been shown to be associated with negative brain structural (morphological) outcomes (e.g. Alosco et al. 2014). Longitudinal studies are necessary to clarify the role of aging in the association of cortical thickness and HRV to address potential (1) top-down (i.e. decline in cortical thickness causing reduced HRV), (2) or bottom-up causality (i.e., decline in HRV causing reduced cortical thickness), or (3) a third factor in the association of HRV and cortical thickness, and the exact mechanisms behind these relationships (i.e., altered brain perfusion (Allen et al. 2015)). Finally, while the present study addressed the neural concomitants of resting state autonomic nervous system (ANS) function, focusing on HRV, previous reviews have summarized the existing evidence on neural structures involved in the regulation of ANS reactivity to different experimental tasks (Beissner et al. 2013; Oppenheimer & Cechetto 2016; Gianaros & Wager 2015). Studies are necessary to address differences and similarities in the neural control of ANS function and ANS reactivity.
In summary, the results of the present study and its meta-analyses suggest that there may be age-invariant associations between HRV and cortical thickness in more ventral brain regions such as the lateral OFC. These regions are less susceptible to age-related decreases in cortical thickness, which suggests that the regions may serve to preserve emotion-related functions as well as other functions associated with HRV and age (Mather 2016). In addition there may be more age-sensitive associations between HRV and cortical regions that can be recruited when available to support a wide range of tasks that are differentially expressed across the life span.
Supplementary Material
Acknowledgments
JK is supported by Physician-Scientist-Fellowship provided by the Medical School, University of Heidelberg, Germany. JK acknowledges the financial support through a Post-Doctoral Scholarship provided by the Daimler and Benz Foundation (Ladenburg, Germany) and the Thrasher Research Fund Early Career Award provided by the Thrasher Research Fund (Salt Lake City, UT, USA). The research studies were funded by NIA grants R01AG025340 and R01AG057184. Michiko Sakaki received a fellowship by the European Commision PCIG13-GA-2013-618600, JSPS 16H05959.
Footnotes
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest: The authors declare that they have no conflict of interest.
References
- Alvares GA, Quintana DS, Kemp AH, Van Zwieten A, Balleine BW, Hickie IB, Guastella AJ. Reduced heart rate variability in social anxiety disorder: associations with gender and symptom severity. PloS One. 2013;8:e70468. doi: 10.1371/journal.pone.0070468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Gunstad J, Xu X, Clark US, Labbe DR, Riskin-Jones HH, Terrero G, Schwarz NF, Walsh EG, Poppas A, Cohen RA, Sweet LH. The impact of hypertension on cerebral perfusion and cortical thickness in older adults. J Am Soc Hypertens. 2014;8(8):561–70. doi: 10.1016/j.jash.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen B, Jennings JR, Gianaros PJ, Thayer JF, Manuck SB. Resting high-frequency heart rate variability is related to resting brain perfusion. Psychophysiology. 2015;52(2):277–87. doi: 10.1111/psyp.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antelmi I, de Paula RS, Shinzato AR, Peres CA, Mansur AJ, Grupi CJ. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am J Cardiol. 2004;93:381–385. doi: 10.1016/j.amjcard.2003.09.065. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Thayer JF. Heart rate variability as a transdiagnostic biomarker of psychopathology. Int J Psychophysiol Off J Int Organ Psychophysiol. 2015;98:338–350. doi: 10.1016/j.ijpsycho.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bär KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013;33(25):10503–11. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch K, Hagemann D, Naumann E, Schächinger H, Schulz A. Stability of heart rate variability indices reflecting parasympathetic activity. Psychophysiology. 2012;49:672–682. doi: 10.1111/j.1469-8986.2011.01341.x. [DOI] [PubMed] [Google Scholar]
- Clamor A, Lincoln TM, Thayer JF, Koenig J. Resting vagal activity in schizophrenia: meta-analysis of heart rate variability as a potential endophenotype. Br J Psychiatry J Ment Sci. 2016;208:9–16. doi: 10.1192/bjp.bp.114.160762. [DOI] [PubMed] [Google Scholar]
- Cooper H, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta-analysis. Russell Sage Foundation; 2009. [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved Localizadon of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. J Cogn Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJW, Makris N, Ségonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004a;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999b;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex N Y N. 2004b;1991–14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21:187–221. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex N Y N. 2009;1991–19:2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Last access: 05.31.2017];FS Wiki, website. available at: http://www.freesurfer.net/fswiki/eTIV.
- Gatz M, Reynolds C, Nikolic J, Lowe B, Karel M, Pedersen N. An empirical test of telephone screening to identify potential dementia cases. International Psychogeriatrics. 1995;7:429–438. doi: 10.1017/s1041610295002171. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Wager TD. Brain-Body Pathways Linking Psychological Stress and Physical Health. Curr Dir Psychol Sci. 2015;24(4):313–321. doi: 10.1177/0963721415581476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MP, Wang L, Campanella C, Aldridge K, Moffitt AJ, Kuelper J, Ratnanather JT, Miller MI, Barch DM, Csernansky JG. Correspondence. The British Journal of Psychiatry. 2010;196(5):414–415. doi: 10.1192/bjp.bp.109.067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Jarczok MN, Kleber ME, Koenig J, Loerbroks A, Herr RM, Hoffmann K, Fischer JE, Benyamini Y, Thayer JF. Investigating the associations of self-rated health: heart rate variability is more strongly associated than inflammatory and other frequently used biomarkers in a cross sectional occupational sample. PloS One. 2015;10:e0117196. doi: 10.1371/journal.pone.0117196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J, Fischl B, Dale A. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. NeuroImage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. 2010;67:1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Koenig J, Kemp AH, Beauchaine TP, Thayer JF, Kaess M. Depression and resting state heart rate variability in children and adolescents - A systematic review and meta-analysis. Clin Psychol Rev. 2016;46:136–150. doi: 10.1016/j.cpr.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Koenig J, Kemp AH, Feeling NR, Thayer JF, Kaess M. Resting state vagal tone in borderline personality disorder: A meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2015;64:18–26. doi: 10.1016/j.pnpbp.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Kuo TBJ, Lai CJ, Huang Y-T, Yang CCH. Regression analysis between heart rate variability and baroreflex-related vagus nerve activity in rats. J Cardiovasc Electrophysiol. 2005;16:864–869. doi: 10.1111/j.1540-8167.2005.40656.x. [DOI] [PubMed] [Google Scholar]
- Levy MN. Neural control of cardiac function. Baillierès Clin Neurol. 1997;6:227–244. [PubMed] [Google Scholar]
- Mather M. The Affective Neuroscience of Aging. Annu Rev Psychol. 2016;67:213–238. doi: 10.1146/annurev-psych-122414-033540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien IA, O’Hare P, Corrall RJ. Heart rate variability in healthy subjects: effect of age and the derivation of normal ranges for tests of autonomic function. Br Heart J. 1986;55:348–354. doi: 10.1136/hrt.55.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer S, Cechetto D. The Insular Cortex and the Regulation of Cardiac Function. Compr Physiol. 2016;6(2):1081–133. doi: 10.1002/cphy.c140076. [DOI] [PubMed] [Google Scholar]
- Peters R. Ageing and the brain. Postgrad Med J. 2006;82:84–88. doi: 10.1136/pgmj.2005.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. [Google Scholar]
- Sakaki M, Nga L, Mather M. Amygdala Functional Connectivity with Medial Prefrontal Cortex at Rest Predicts the Positivity Effect in Older Adults’ Memory. J Cogn Neurosci. 2013;25:1206–1224. doi: 10.1162/jocn_a_00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Yoo HJ, Nga L, Lee T-H, Thayer JF, Mather M. Heart rate variability is associated with amygdala functional connectivity with MPFC across younger and older adults. NeuroImage. 2016;139:44–52. doi: 10.1016/j.neuroimage.2016.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer G. Meta: An R package for meta-analysis. R news. 2007;7(3):40–45. [Google Scholar]
- Ségonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Tarvainen MP, Niskanen J-P, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV--heart rate variability analysis software. Comput Methods Programs Biomed. 2014;113:210–220. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med Publ Soc Behav Med. 2009a;37:141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Sollers JJ, Labiner DM, Weinand M, Herring AM, Lane RD, Ahern GL. Age-related differences in prefrontal control of heart rate in humans: a pharmacological blockade study. Int J Psychophysiol Off J Int Organ Psychophysiol. 2009b;72:81–88. doi: 10.1016/j.ijpsycho.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141(2):122–31. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- Virtanen R, Jula A, Kuusela T, Helenius H, Voipio-Pulkki LM. Reduced heart rate variability in hypertension: associations with lifestyle factors and plasma renin activity. J Hum Hypertens. 2003;17(3):171–9. doi: 10.1038/sj.jhh.1001529. [DOI] [PubMed] [Google Scholar]
- Winkelmann T, Thayer JF, Pohlack S, Nees F, Grimm O, Flor H. Structural brain correlates of heart rate variability in a healthy young adult population. Brain Struct Funct. 2016 doi: 10.1007/s00429-016-1185-1. [DOI] [PubMed] [Google Scholar]
- Woodward SH, Kaloupek DG, Schaer M, Martinez C, Eliez S. Right anterior cingulate cortical volume covaries with respiratory sinus arrhythmia magnitude in combat veterans. J Rehabil Res Dev. 2008;45:451–463. doi: 10.1682/jrrd.2007.06.0082. [DOI] [PubMed] [Google Scholar]
- Zhang J. Effect of age and sex on heart rate variability in healthy subjects. J Manipulative Physiol Ther. 2007;30:374–379. doi: 10.1016/j.jmpt.2007.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.