INTRODUCTION
Injectable contraceptives are the most widely used method of contraception in sub-Saharan Africa among married or in-union women aged 15–44 [1]. Injectable contraceptive use grew more quickly than use of any other contraceptive method between 1994 and 2015: from 2% to 7% of the share of all contraceptive use (among married or in-union women) worldwide, and from 17% to 38% of the share of all contraceptive use in sub-Saharan Africa [1]. Injectables are quick to administer, highly effective, do not require daily user action, and can be used clandestinely [2]. Like all contraceptive methods, injectables can empower women and couples to achieve their reproductive goals, reduce unintended pregnancy, and prevent maternal morbidity and mortality [3].
Concerning observational data suggest that women who use the most common type of injectable contraception, depot medroxyprogesterone acetate (DMPA), may be at increased risk of HIV acquisition compared to women not using hormonal contraception [4]. In March 2017, the World Health Organization modified the Medical Eligibility Criteria for Contraceptive Use to indicate that women at high risk of HIV acquisition may use progestin-only contraceptive injectables (including intramuscular or subcutaneous DMPA, and norethisterone enanthate [NET-EN]), and should be advised about concerns that these methods may increase risk of HIV acquisition, the uncertainty over whether there is a causal relationship, and how to minimize the risk of acquiring HIV [5].
DMPA is most commonly administered intramuscularly at a dose of 150 mg every three months (hereafter, DMPA-IM). A subcutaneous formulation containing 31% less hormone (104 mg; hereafter DMPA-SC) and offering similar efficacy has more recently been developed [6–9]. While some side effects may depend on differences in dose and route of administration [10], whether these differences would have any impact on HIV-1 acquisition risk relative to DMPA-IM is unknown. A key question is whether the risk of HIV acquisition associated with DMPA-SC is likely to be lower compared to that associated with DMPA-IM. An ongoing trial (NCT02550067) assessing HIV acquisition in women randomized to DMPA-IM, copper IUD, and levonorgestrel sub-dermal implants will not evaluate DMPA-SC. Therefore, understanding the risk of HIV acquisition in DMPA-IM users relative to DMPA-SC users is critical, particularly as access to DMPA-SC is set to expand, given the recent rollout of Sayana® Press, a convenient DMPA-SC product which offers the potential for self-administration.
WHAT IS SAYANA® PRESS?
Sayana® Press is DMPA-SC packaged in a pre-filled, single-dose, non-reusable subcutaneous injection system called Uniject™. Uniject simplifies injection provision and requires minimal training, expanding possibilities for community-based distribution and self-injection. Thus, Sayana® Press could increase contraceptive access, including for underserved populations in hard-to-reach areas, and has been referred to as a potential “game-changer” in reducing unmet need for modern contraception [11].
The product has regulatory approval in the European Union and over 25 countries worldwide, and is currently available in over 15 countries. Between 2014 and 2016, PATH coordinated a pilot introduction initiative in Burkina Faso, Niger, Senegal, and Uganda [12]. They administered nearly 500,000 doses, 44% of which went to women under age 25 [12]. Women [13, 14] and health workers [15] have expressed preferences for DMPA-SC over DMPA-IM. Studies have documented interest in self-injection [16, 17] and feasibility has been assessed in Senegal, Uganda, Malawi, and elsewhere [12, 18–23]. Sayana® Press has been approved for self-injection in several countries [12].
DMPA-IM AND HIV RISK IN WOMEN
No epidemiological studies have assessed DMPA-SC with respect to risk of HIV acquisition. In contrast, 25 observational epidemiological studies provided information relevant to DMPA-IM (or non-disaggregated injectables) and risk of HIV acquisition in women, 13 of which were considered to be of higher quality [4]. The quality of observational studies on this issue has improved over time [4, 24]. Though inferring causality from observational data is challenging, a recent systematic review of available observational data concluded that new data amplifies existing concerns of a potential causal link between DMPA-IM use and increased HIV acquisition risk in women. Meta-analyses suggest that the hazard ratio would most likely be around 1.4, though such estimates should be interpreted with caution, given the potential for confounding in observational data [4, 25, 26].
Several biological mechanisms may underlie an association between DMPA and increased risk of HIV acquisition in women, including: structural changes and alterations in the permeability of the female genital tract, alterations of the frequency and activity of cellular targets for infection, modulation of levels of soluble mediators and defense molecules in the genital tract, alteration of innate and adaptive immune responses, and changes in the female genital tract microbiome [27, 28]. Research in this area has been stymied by lack of identified biomarkers that would predict significant risk of future HIV acquisition. Such biomarkers, if identified, could be used to link findings from biological and epidemiological studies on the issue of DMPA use and HIV risk. As discussed below, mounting evidence from human, animal, and ex vivo studies suggests that medroxyprogesterone acetate (MPA), at concentrations within the range of systemic MPA concentrations detected in DMPA-IM users, affects various biological mechanisms that may be integral to HIV acquisition risk, yet the clinical relevance remains unclear. Since epidemiological data directly comparing DMPA-IM and DMPA-SC for HIV outcomes are unavailable, below we explore the known similarities and differences between the two formulations to address potential differences in their impact on the acquisition of HIV.
DMPA-SC VS. DMPA-IM: SIMILAR OR DIFFERENT HIV ACQUISITION RISKS?
DMPA-IM and DMPA-SC contain the same progestogen but differ in dose and route of administration
Both DMPA-IM and DMPA-SC contain MPA as the active progestogenic contraceptive compound. Since MPA is virtually insoluble in water, both DMPA-IM and DMPA-SC are formulated as sterile aqueous suspensions of 150 mg/mL and 104 mg/0.65 mL, respectively [29]. Similar excipients, such as amino acids, salts, and agents to enhance viscosity are included in both formulations, although the quantities may differ [29].
Does administration of DMPA-IM vs. DMPA-SC result in a different systemic concentration of MPA?
Whether differences in dose and route of administration result in different systemic MPA concentrations (pharmacokinetics) and effects (pharmacodynamics) is likely relevant to possible effects on HIV acquisition. MPA concentrations above approximately 0.2 ng/mL are needed for high contraceptive efficacy and are maintained over at least 3 months for both regimens [30, 31]. Based on the 31% difference in MPA dose between formulations, an approximately 31% difference in systemic concentration might be assumed. Differences in serum concentrations could be due to different pharmacokinetic properties, metabolism, intrinsic individual factors, or different sites of injection (subcutaneous tissue of upper arm, thigh, or abdomen for DMPA-SC vs. muscle tissue of upper arm, hip, or buttock for DMPA-IM). DMPA-SC may have a lower peak serum concentration (Cmax) and a slower rate of absorption than DMPA-IM [10, 31]. To date, only one head-to-head study comparing women using DMPA-IM versus DMPA-SC has been published and identified no significant differences between the mean serum MPA trough levels (Cmin) [8]. Notably, this study collected limited pharmacokinetic data, with no Cmax data, and reported only serum Cmin concentrations of MPA 6, 12 and 24 months after initiating use.
Whether a true Cmax difference exists between DMPA-IM and DMPA-SC remains to be determined. Neither Cmax nor other pharmacokinetic properties of single or multi-dose studies have been reported for head-to-head evaluations. When evaluated separately (non-comparative studies), the reported Cmax values differ greatly between DMPA-SC and DMPA-IM. Three published primary studies evaluated pharmacokinetic parameters following a single injection of DMPA-SC with reported mean Cmax values of 1.56 ng/mL (4.0 nM) [31], 1.3 ng/mL (3.4 nM) [32] and 0.95 ng/mL (2.5 nM) [33], and all report peak serum MPA concentration occurring by median day 8–13. Cmax values reported for DMPA-IM from multiple studies are more variable, with average Cmax values ranging from 2.2–24 ng/mL (5.8–62 nM)a [29, 34–46] occurring 2–14 days post injection. Both large inter-individual and inter-study variations in Cmax concentrations have been reported for DMPA-IM [10, 47, 48], whereas reported Cmax values for DMPA-SC are less variable, though fewer studies exist.
Based on a comparison of non-parallel studies, the initial MPA serum concentration decline following administration of DMPA-IM appears steeper than that following DMPA-SC, particularly in the first month [10, 29, 31]. Thirty days after injection, serum MPA concentration has been reported to be 1 ng/mL for DMPA-IM [34, 49–51] and 0.8 ng/mL for DMPA-SC [31, 32]. Concentrations of about 0.4–1.2 ng/mL MPA measured at 90 days after administration do not appear to differ for DMPA-IM and DMPA-SC [8, 29, 31, 51]. Thus, serum concentrations of MPA from days 30 to 90 post administration may be similar between DMPA-SC and DMPA-IM. Although it appears likely that DMPA-SC and DMPA-IM have different Cmax and pharmacokinetic properties, differences from non-parallel studies may be confounded by differences in methodology, numbers of women investigated, and variations in individual intrinsic factors. Based on these non-parallel studies, DMPA-SC may have up to 85% lower Cmax as compared with DMPA-IM. To what extent such differences would exert an effect on MPA levels and pharmacokinetic parameters for DMPA-IM vs. DMPA-SC remains unclear [29, 30, 33]. Appropriately powered parallel studies of single and multi-dose regimens, randomized to minimize potential confounding by ethnicity, race, body mass index, weight, and other factors, are needed to address these questions.
How might different serum concentrations affect biological responses potentially relevant to HIV acquisition?
The intracellular mechanisms whereby MPA could influence biological effects are likely to involve the glucocorticoid receptor (GR) and/or the progesterone receptor (PR). MPA likely exerts contraceptive efficacy through high-affinity binding to and activation of the PR. However, MPA may act quite differently from other contraceptive progestins since MPA also exhibits significant binding to the GR [52–54]. The GR is ubiquitously expressed, regulates multiple biological processes, and is a well-established modulator of soluble mediators and innate and adaptive immune responses. Like all steroid receptors, the GR alters transcriptional activity in the cell and drives changes in expression levels of target genes once activated by steroid. Since steroid hormones regulate hundreds of genes and control multiple physiological functions, it is plausible that MPA may affect multiple biological processes, including those that are related to HIV acquisition.
Currently, limited and inconsistent data are available about how biological responses change with exposure to MPA in clinical studies, and no clinical data relate biological change to varying doses of MPA. DMPA-IM has been shown in some clinical studies to increase surface levels of CCR5 on peripheral and female genital tract (FGT) target T cells [55, 56], increase the frequency of CCR5+ T cells in the FGT mucosa [56], decrease the production of several immune modulators by immune cells [57, 58], increase FGT mucosal permeability and expression of barrier function proteins [59] and alter cervicovaginal levels of select secreted immunomodulators [57, 60–66]. Several of these studies are consistent with results from animal studies where MPA serum concentrations are similar to those of human DMPA-IM users [59, 67–71]. For example, in mice MPA at 4–7 nM decreases genital tract barrier function with an increased susceptibility to HSV-2 viral infection [59]. Ex vivo data are broadly consistent with results from animal studies; MPA appears to increase HIV-1 proliferation in human immune cells at about 0.1 nM [72], dampen the function of immune cells at 0.3 nM [73], increase transcytosis of HIV-1 across the FGT epithelial cells at 1 nM [74], and decrease expression of select immunomodulators by FGT epithelial cells at 1–20 nM [74, 75]. The relative roles of the GR and PR in these mechanisms is not established, although MPA has been shown in vitro to regulate expression of immunomodulators and the function of immune cells via the GR [54, 75–79].
A key pertinent question is: would a 31–85% reduction in the systemic concentration of MPA significantly affect biological processes critical for HIV susceptibility? As background, the dose-dependency for steroid responses varies depending on the specific response and cell-type, and cannot necessarily be predicted from the steroid affinity for a particular receptor. Further complicating this question is the presence of endogenous steroid hormones (progesterone and estradiol) in women using contraceptive progestins and their contributions to competitive target receptor binding and downstream impacts of receptor-mediated gene regulation. Many of these factors are also influenced by metabolic state, immune environment, and other physiological conditions. Thus, biological responses may be differentially affected by varying the progestin dose.
Plotting biological response (% response) versus steroid concentration (log10) typically results in a sigmoidal curve as hypothetically illustrated in Figure 1 [52, 54, 76, 78, 80–84]. The 50% effective concentration (EC50), or potency, is defined as the steroid concentration that imparts a response halfway between no response and the maximum possible response (Figure 1). The slope of the curve is steepest around the EC50; this is where a change of concentration causes the largest change in biological response. Consistent with published in vitro dose-response data for many steroid receptors [54, 76, 84, 85], lowering the systemic concentration of MPA by X% at concentrations around the EC50 would result in a <X% decrease in biological response (Figure 1). Dose-response curves reflecting in vivo data may differ in shape and/or slope and the actual impact on biological outcomes may be greater or less than those predicted here. Further studies are needed to examine both the true serum concentration differences between DMPA-IM and DMPA-SC and subsequent effects on individual biological responses.
Figure 1. Relationship between dose response and concentration of MPA.
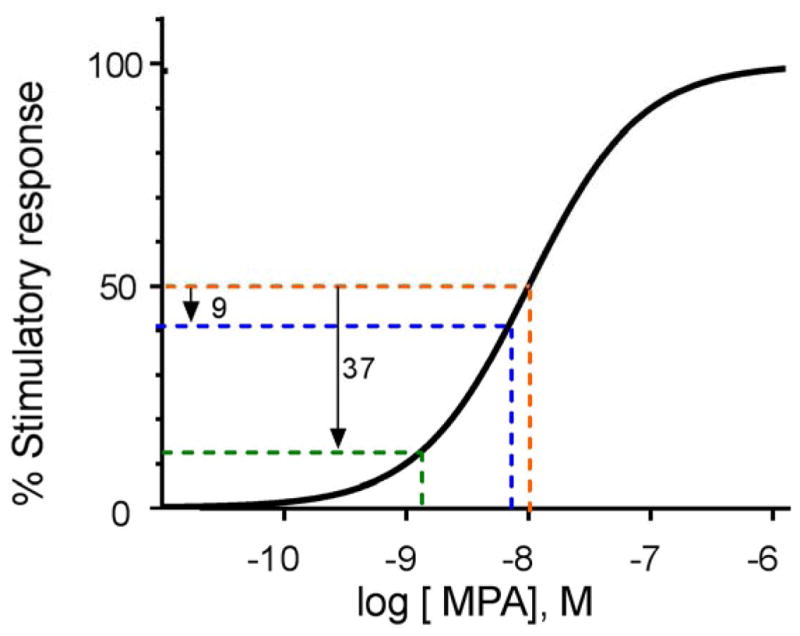
The orange line depicts a 50% increase in a given stimulatory response for a woman using DMPA-IM (versus a woman using no hormonal contraception), assuming that the EC50 value (potency) for that particular biological response occurs at 10 nM MPA (3.8 ng/mL; −8 on the log scale). If we assume that DMPA-SC reduces MPA intracellular concentrations by 31% (from 10 nM assumed with DMPA-IM to 6.9 nM assumed with DMPA-SC), that particular stimulatory response would theoretically be predicted to drop by 9 percentage points, to 41% (blue line) instead of 50% (orange line). If we assume that DMPA-SC reduces intracellular concentrations by 85% (from 10 nM assumed with DMPA-IM to 1.5 nM assumed with DMPA-SC), that particular stimulatory response would be theoretically predicted to drop by 37 percentage points, to 13% (green line) instead of 50% (orange line). Note that the actual EC50 for specific biological responses varies depending on the cell type and target gene, and cannot be predicted. The sigmoidal shaped curve with a Hill slope of one is based on theoretical predictions,[86] and supported by ex vivo data, as discussed in the text.
CONCLUSIONS
Based on the available (notably, non-parallel) data, we hypothesize that DMPA-IM and DMPA-SC will not substantially differ in their effects on biological responses 30–90 days following injection since serum concentrations during this time are reportedly similar. Whether Cmax values actually differ remains to be determined and Cmax values in the first 30 days following DMPA administration may be substantially different and may exert differences on some biological responses. Clinically, the two regimens show therapeutic equivalence and similar effects on weight gain, bone mineral density, bleeding patterns, and frequency of serious adverse effects, which suggests limited differences, if any, in biological responses, despite the 31% dose difference [9]. Furthermore, both DMPA-IM and DMPA-SC result in similar degrees of hypoestrogenism, characterized by systemic estrogen concentrations of 10–100 pg/mL [31, 32, 56]. It is possible that within the initial 30 days following administration of DMPA-IM versus DMPA-SC, with notably different resulting serum concentrations of MPA, some biological responses critical to HIV susceptibility may be differentially impacted. This may occur despite no apparent effect on other side-effects, if these biological responses are more sensitive to changing serum concentrations of MPA (different EC50 values). Importantly, possible reduction of MPA Cmax concentrations for DMPA-SC versus DMPA-IM is not likely to ameliorate the impact of MPA on biological responses with EC50 values below 1 nM [66–68].
Given growing concerns about HIV acquisition risk with DMPA-IM use, and given the broad introduction of DMPA-SC in sub-Saharan Africa, direct parallel comparisons of DMPA-IM and DMPA-SC are critical, particularly since such comparisons will not be evaluated in the currently ongoing trial assessing HIV acquisition risk in women randomized to DMPA-IM, copper IUD, and levonorgestrel sub-dermal implants. Similarly, comparative data for injectable progestin contraceptives, including DMPA-IM and DMPA-SC, on putative biological markers of HIV acquisition are needed urgently to evaluate whether these methods are likely to have a differential impact on HIV acquisition risk. In order to effectively evaluate biomarker data, researchers will need to identify appropriate biomarkers that correlate with HIV acquisition risk, and align sampling schedules and analytical methods in order to yield meaningful and comparable results. Currently all injectable progestin-only contraceptives are categorized together in the Medical Eligibility Criteria for Contraceptive Use and there is concern for this entire class of highly effective and widely available injectable contraceptives if no progress is made differentiating these methods.
As indicated by World Health Organization guidance, women should be informed and counseled about the risks and benefits of their contraceptive options, including the potential effect of progestin-only injectables on HIV risk [5]. Furthermore, women and couples at high risk of HIV acquisition considering using progestin-only injectables should be informed about how to minimize their risk of HIV acquisition. Importantly, continued expansion of the hormonal and non-hormonal contraceptive method mix should remain a priority. Specific characteristics of injectable contraceptives, including the ability to use them discretely, make them popular and widely used in many populations. Development of new contraceptive injectable options with formulations that exhibit low immunosuppressive activity, potentially including alternative progestins to MPA, should also be considered. An understanding of whether such progestins could be delivered subcutaneously would be important, and could potentially benefit from the service delivery advantages provided by the Uniject system. Finally, DMPA-IM and DMPA-SC remain important options for many women, including women living with and at risk of HIV. Many women may benefit from access to a contraceptive with the potential for self-administration like Sayana® Press, particularly if it does not increase HIV acquisition risk.
Acknowledgments
FUNDING
Support for drafting this manuscript was provided by the Guttmacher Institute (C.B.P.), and National Institutes of Health (NIH) under award number R01 HD083026 (J.P.H., Z.H.) and R01 AI102835 (S.L.A.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Guttmacher Institute or the NIH.
Footnotes
These values are from all 14 published studies describing Cmax for DMPA IM and span ~40 years.[29, 34–46] Given that the study methods differ considerably, including times of measurement post injection, volume of injection, formulation, number and characteristics of participants, number of injections, and techniques used to measure MPA, all published studies have been included and contribute to this range in values. Reported Cmax values likely reflect the heterogeneity of the studies and there is no apparent trend in Cmax value that correlates with age of studies.
ROLE OF AUTHORS
CBP conceived of the idea for this manuscript, drafted the outline, and managed the overall writing process. All authors contributed equally to the writing and endorsed the final version.
CONFLICTS OF INTEREST
CBP, SLA, ZH, and JPH have no conflicts of interest to report.
References
- 1.United Nations, Department of Economics and Social Affairs, Population Division. Trends in Contraceptive Use Worldwide 2015 (ST/ESA/SER.A/349) 2015. [Google Scholar]
- 2.Adetunji JA. Rising popularity of injectable contraceptives in sub-Saharan Africa. African Population Studies. 2011;25:587–604. [Google Scholar]
- 3.Ahmed S, Li Q, Liu L, Tsui AO. Maternal deaths averted by contraceptive use: an analysis of 172 countries. Lancet. 2012;380:111–25. doi: 10.1016/S0140-6736(12)60478-4. [DOI] [PubMed] [Google Scholar]
- 4.Polis CB, Curtis KM, Hannaford PC, et al. An updated systematic review of epidemiological evidence on hormonal contraceptive methods and HIV acquisition in women. AIDS. 2016;30:2665–83. doi: 10.1097/QAD.0000000000001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Guidance Statement: Recommendations concerning the use of hormonal contraceptive methods by women at high risk of HIV. Geneva, Switzerland: World Health Organization; 2017. Hormonal contraceptive eligibility for women at high risk of HIV. [Google Scholar]
- 6.Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception. 2004;70:269–75. doi: 10.1016/j.contraception.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Simon MA, Shulman LP. Subcutaneous versus intramuscular depot methoxyprogesterone acetate: a comparative review. Womens Health (Lond) 2006;2:191–7. doi: 10.2217/17455057.2.2.191. [DOI] [PubMed] [Google Scholar]
- 8.Kaunitz AM, Darney PD, Ross D, Wolter KD, Speroff L. Subcutaneous DMPA vs. intramuscular DMPA: a 2-year randomized study of contraceptive efficacy and bone mineral density. Contraception. 2009;80:7–17. doi: 10.1016/j.contraception.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Dragoman MV, Gaffield ME. The safety of subcutaneously administered depot medroxyprogesterone acetate (104mg/0. 65mL): A systematic review. Contraception. 2016;94:202–15. doi: 10.1016/j.contraception.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Shelton JD, Halpern V. Subcutaneous DMPA: a better lower dose approach. Contraception. 2014;89:341–3. doi: 10.1016/j.contraception.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Spieler J. Sayana® Press: can it be a “game changer” for reducing unmet need for family planning? Contraception. 2014;89:335–8. doi: 10.1016/j.contraception.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 12.PATH. Advocacy Pack for Subcutaneous DMPA. Washington, DC: PATH; 2017. [Accessed July 6, 2017]. [Google Scholar]
- 13.Burke HM, Mueller MP, Perry B, et al. Observational study of the acceptability of Sayana(R) Press among intramuscular DMPA users in Uganda and Senegal. Contraception. 2014;89:361–7. doi: 10.1016/j.contraception.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Polis CB, Nakigozi GF, Nakawooya H, et al. Preference for Sayana(R) Press versus intramuscular Depo-Provera among HIV-positive women in Rakai, Uganda: a randomized crossover trial. Contraception. 2014;89:385–95. doi: 10.1016/j.contraception.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Burke HM, Mueller MP, Packer C, et al. Provider acceptability of Sayana(R) Press: results from community health workers and clinic-based providers in Uganda and Senegal. Contraception. 2014;89:368–73. doi: 10.1016/j.contraception.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Keith B, Wood S, Chapman C, Alemu E. Perceptions of home and self-injection of Sayana(R) Press in Ethiopia: a qualitative study. Contraception. 2014;89:379–84. doi: 10.1016/j.contraception.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Upadhyay UD, Zlidar VM, Foster DG. Interest in self-administration of subcutaneous depot medroxyprogesterone acetate in the United States. Contraception. 2016;94:303–13. doi: 10.1016/j.contraception.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Prabhakaran S, Sweet A. Self-administration of subcutaneous depot medroxyprogesterone acetate for contraception: feasibility and acceptability. Contraception. 2012;85:453–7. doi: 10.1016/j.contraception.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Cameron ST, Glasier A, Johnstone A. Pilot study of home self-administration of subcutaneous depo-medroxyprogesterone acetate for contraception. Contraception. 2012;85:458–64. doi: 10.1016/j.contraception.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan I, Ross D, Hilton F, Morgenstern D, Wolter K. The role of training in effective simulated self-injection of subcutaneous depot medroxyprogesterone acetate: observations from a usability study. Contraception. 2016;94:314–20. doi: 10.1016/j.contraception.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Beasley A, White KO, Cremers S, Westhoff C. Randomized clinical trial of self versus clinical administration of subcutaneous depot medroxyprogesterone acetate. Contraception. 2014;89:352–6. doi: 10.1016/j.contraception.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams RL, Hensel DJ, Fortenberry JD. Self-administration of subcutaneous depot medroxyprogesterone acetate by adolescent women. Contraception. 2013;88:401–7. doi: 10.1016/j.contraception.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.FHI360. [accessed October 31, 2016];Sayana® Press study evaluating suitability for self-injection. 2014 Available from: https://www.fhi360.org/projects/sayana%C2%AE-press-study-evaluating-suitability-self-injection.
- 24.Polis CB, Westreich D, Balkus JE, Heffron R Participants of the HCHIVOAM. Assessing the effect of hormonal contraception on HIV acquisition in observational data: challenges and recommended analytic approaches. AIDS. 2013;27(Suppl 1):S35–43. doi: 10.1097/QAD.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison CS, Chen PL, Kwok C, et al. Hormonal contraception and the risk of HIV acquisition: an individual participant data meta-analysis. PLoS Med. 2015;12:e1001778. doi: 10.1371/journal.pmed.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ralph LJ, McCoy SI, Shiu K, Padian NS. Hormonal contraceptive use and women’s risk of HIV acquisition: a meta-analysis of observational studies. Lancet Infect Dis. 2015;15:181–9. doi: 10.1016/S1473-3099(14)71052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hel Z, Stringer E, Mestecky J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocrine reviews. 2010;31:79–97. doi: 10.1210/er.2009-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy K, Irvin SC, Herold BC. Research Gaps in Defining the Biological Link between HIV Risk and Hormonal Contraception. American journal of reproductive immunology. 2014;72:228–35. doi: 10.1111/aji.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. Food and Drug Administration. Depo-SubQ Provera 104 (Medroxyprogesterone Acetate) Injectable Suspension New Drug Application No.: 021583. Center for Drug Evaluation and Research Clinical Pharmacology and Biopharmaceutics Review; [Google Scholar]
- 30.Nanda K, Callahan R, Taylor D, et al. Medroxyprogesterone acetate levels among Kenyan women using depot medroxyprogesterone acetate in the FEM-PrEP trial. Contraception. 2016;94:40–7. doi: 10.1016/j.contraception.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain J, Dutton C, Nicosia A, Wajszczuk C, Bode FR, Mishell DR., Jr Pharmacokinetics, ovulation suppression and return to ovulation following a lower dose subcutaneous formulation of Depo-Provera. Contraception. 2004;70:11–8. doi: 10.1016/j.contraception.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Toh YC, Jain J, Rahnny MH, Bode FR, Ross D. Suppression of ovulation by a new subcutaneous depot medroxyprogesterone acetate (104 mg/0. 65 mL) contraceptive formulation in Asian women. Clin Ther. 2004;26:1845–54. doi: 10.1016/j.clinthera.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Halpern V, Combes SL, Dorflinger LJ, Weiner DH, Archer DF. Contraception. Elsevier Inc; 2014. Pharmacokinetics of subcutaneous depot medroxyprogesterone acetate injected in the upper arm; pp. 31–5. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz A, Hirol M, Stanczyk FZ, Goebelsmann U, Mishell DR. Serum medroxyprogesterone acetate (MPA) concentrations and ovarian function following intramuscular injection of depo-MPA. J Clin Endocrinol Metab. 1977;44:32–8. doi: 10.1210/jcem-44-1-32. [DOI] [PubMed] [Google Scholar]
- 35.Jeppsson S, Gershagen S, Johansson ED, Rannevik G. Plasma levels of medroxyprogesterone acetate (MPA), sex-hormone binding globulin, gonadal steroids, gonadotrophins and prolactin in women during long-term use of depo-MPA (Depo-Provera) as a contraceptive agent. Acta Endocrinol (Copenh) 1982;99:339–43. doi: 10.1530/acta.0.0990339. [DOI] [PubMed] [Google Scholar]
- 36.Fotherby K, Saxena BN, Shrimanker K, et al. A preliminary pharmacokinetic and pharmacodynamic evaluation of depot-medroxyprogesterone acetate and norethisterone oenanthate. Fertil Steril. 1980;34:131–9. doi: 10.1016/s0015-0282(16)44895-8. [DOI] [PubMed] [Google Scholar]
- 37.Nanda K, Amaral E, Hays M, Viscola MA, Mehta N, Bahamondes L. Pharmacokinetic interactions between depot medroxyprogesterone acetate and combination antiretroviral therapy. Fertil Steril. 2008;90:965–71. doi: 10.1016/j.fertnstert.2007.07.1348. [DOI] [PubMed] [Google Scholar]
- 38.Bonny AE, Lange HL, Rogers LK, Gothard DM, Reed MD. A pilot study of depot medroxyprogesterone acetate pharmacokinetics and weight gain in adolescent females. Contraception. 2014;89:357–60. doi: 10.1016/j.contraception.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirton KT, Cornette JC. Return of ovulatory cyclicity following an intramuscular injection of medroxyprogesterone acetate (Provera) Contraception. 1974;10:39–45. doi: 10.1016/0010-7824(74)90130-9. [DOI] [PubMed] [Google Scholar]
- 40.Koetsawang S. Injected long--acting medroxyprogesterone acetate. Effect on human lactation and concentrations in milk. J Med Assoc Thai. 1977;60:57–60. [PubMed] [Google Scholar]
- 41.Shrimanker K, Saxena BN, Fotherby K. A radioimmunoassay for serum medroxyprogesterone acetate. J Steroid Biochem. 1978;9:359–63. doi: 10.1016/0022-4731(78)90631-3. [DOI] [PubMed] [Google Scholar]
- 42.Fotherby K, Koetsawang S, Mathrubutham M. Pharmacokinetic study of different doses of Depo Provera. Contraception. 1980;22:527–36. doi: 10.1016/0010-7824(80)90105-5. [DOI] [PubMed] [Google Scholar]
- 43.Fotherby K, Koetsawang S. Metabolism of injectable formulations of contraceptive steroids in obese and thin women. Contraception. 1982;26:51–8. doi: 10.1016/0010-7824(82)90171-8. [DOI] [PubMed] [Google Scholar]
- 44.Fang S, Sun D, Jiang H, Luo H. Concentration Changes of Medroxyprogesterone Acetate in Serum and Milk in Lactating Woman Who Used Depo Geston®. Journal of Reproduction & Contraception. 2004;15:157–62. [Google Scholar]
- 45.Virutamasen P, Leepipatpaiboon S, Kriengsinyot R, et al. Pharmacodynamic effects of depot-medroxyprogesterone acetate (DMPA) administered to lactating women on their male infants. Contraception. 1996;54:153–7. doi: 10.1016/s0010-7824(96)00170-9. [DOI] [PubMed] [Google Scholar]
- 46.Jeppsson S, Johansson Medroxyprogesterone acetate, estradiol, FSH and LH in peripheral blood after intramuscular administration of Depo-ProveraR to women. Contraception. 1976;14:461–69. doi: 10.1016/s0010-7824(76)80060-1. [DOI] [PubMed] [Google Scholar]
- 47.Mathrubutham M, Fotherby K. Medroxyprogesterone acetate in human serum. Journal of steroid biochemistry. 1981;14:783–6. doi: 10.1016/0022-4731(81)90015-7. [DOI] [PubMed] [Google Scholar]
- 48.Smit J, Botha J, McFadyen L, Beksinska M. Serum medroxyprogesterone acetate levels in new and repeat users of depot medroxyprogesterone acetate at the end of the dosing interval. Contraception. 2004;69:3–7. doi: 10.1016/j.contraception.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Bassol S, Garza-Flores J, Cravioto MC, et al. Ovarian function following a single administration of depo-medroxyprogesterone acetate (DMPA) at different doses. Fertility and sterility. 1984;42:216–22. [PubMed] [Google Scholar]
- 50.Cohn SE, Park JG, Watts DH, et al. Depo-medroxyprogesterone in women on antiretroviral therapy: effective contraception and lack of clinically significant interactions. Clin Pharmacol Ther. 2007;81:222–7. doi: 10.1038/sj.clpt.6100040. [DOI] [PubMed] [Google Scholar]
- 51.Mishell DR., Jr Pharmacokinetics of depot medroxyprogesterone acetate contraception. J Reprod Med. 1996;41:381–90. [PubMed] [Google Scholar]
- 52.Africander D, Verhoog N, Hapgood JP. Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids. 2011;76:636–52. doi: 10.1016/j.steroids.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 53.Stanczyk FZ, Hapgood JP, Winer S, Mishell DR., Jr Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. 2013;34:171–208. doi: 10.1210/er.2012-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ronacher K, Hadley K, Avenant C, et al. Ligand-selective transactivation and transrepression via the glucocorticoid receptor: role of cofactor interaction. Mol Cell Endocrinol. 2009;299:219–31. doi: 10.1016/j.mce.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 55.Sciaranghella G, Wang C, Hu H, et al. CCR5 Expression Levels in HIV-Uninfected Women Receiving Hormonal Contraception. J Infect Dis. 2015;212:1397–401. doi: 10.1093/infdis/jiv233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Byrne EH, Anahtar MN, Cohen KE, et al. Association between injectable progestin-only contraceptives and HIV acquisition and HIV target cell frequency in the female genital tract in South African women: a prospective cohort study. The Lancet Infectious diseases. 2016;16:441–8. doi: 10.1016/S1473-3099(15)00429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michel KG, Huijbregts RP, Gleason JL, Richter HE, Hel Z. Effect of hormonal contraception on the function of plasmacytoid dendritic cells and distribution of immune cell populations in the female reproductive tract. J Acquir Immune Defic Syndr. 2015;68:511–8. doi: 10.1097/QAI.0000000000000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huijbregts RP, Helton ES, Michel KG, et al. Hormonal contraception and HIV-1 infection: medroxyprogesterone acetate suppresses innate and adaptive immune mechanisms. Endocrinology. 2013;154:1282–95. doi: 10.1210/en.2012-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quispe Calla NE, Vicetti Miguel RD, Boyaka PN, et al. Medroxyprogesterone acetate and levonorgestrel increase genital mucosal permeability and enhance susceptibility to genital herpes simplex virus type 2 infection. Mucosal immunology. 2016 doi: 10.1038/mi.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ngcapu S, Masson L, Sibeko S, et al. Lower concentrations of chemotactic cytokines and soluble innate factors in the lower female genital tract associated with the use of injectable hormonal contraceptive. J Reprod Immunol. 2015;110:14–21. doi: 10.1016/j.jri.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deese J, Masson L, Miller W, et al. Injectable Progestin-Only Contraception is Associated With Increased Levels of Pro-Inflammatory Cytokines in the Female Genital Tract. Am J Reprod Immunol. 2015;74:357–67. doi: 10.1111/aji.12415. [DOI] [PubMed] [Google Scholar]
- 62.Morrison C, Fichorova RN, Mauck C, et al. Cervical inflammation and immunity associated with hormonal contraception, pregnancy, and HIV-1 seroconversion. J Acquir Immune Defic Syndr. 2014;66:109–17. doi: 10.1097/QAI.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 63.Fichorova RN, Chen PL, Morrison CS, et al. The Contribution of Cervicovaginal Infections to the Immunomodulatory Effects of Hormonal Contraception. MBio. 2015;6:e00221–15. doi: 10.1128/mBio.00221-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goldfien GA, Barragan F, Chen J, et al. Progestin-Containing Contraceptives Alter Expression of Host Defense-Related Genes of the Endometrium and Cervix. Reprod Sci. 2015;22:814–28. doi: 10.1177/1933719114565035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li A, Felix JC, Yang W. Effect of mifepristone on the expression of endometrial secretory leukocyte protease inhibitor in new medroxyprogesterone acetate users. Fertility and sterility. 2008 doi: 10.1016/j.fertnstert.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 66.Fleming DC, King AE, Williams AR, Critchley HO, Kelly RW. Hormonal contraception can suppress natural antimicrobial gene transcription in human endometrium. Fertil Steril. 2003;79:856–63. doi: 10.1016/s0015-0282(02)04930-0. [DOI] [PubMed] [Google Scholar]
- 67.Butler K, Ritter JM, Ellis S, et al. A Depot Medroxyprogesterone Acetate Dose That Models Human Use and Its Effect on Vaginal SHIV Acquisition Risk. J Acquir Immune Defic Syndr. 2016;72:363–71. doi: 10.1097/QAI.0000000000000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kleynhans L, Du Plessis N, Allie N, et al. The contraceptive depot medroxyprogesterone acetate impairs mycobacterial control and inhibits cytokine secretion in mice infected with Mycobacterium tuberculosis. Infect Immun. 2013;81:1234–44. doi: 10.1128/IAI.01189-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quispe Calla NE, Vicetti Miguel RD, Mei A, Fan S, Gilmore JR, Cherpes TL. Dendritic cell function and pathogen-specific T cell immunity are inhibited in mice administered levonorgestrel prior to intranasal Chlamydia trachomatis infection. Sci Rep. 2016;6:37723. doi: 10.1038/srep37723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gillgrass AE, Ashkar AA, Rosenthal KL, Kaushic C. Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. J Virol. 2003;77:9845–51. doi: 10.1128/JVI.77.18.9845-9851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vicetti Miguel RD, Hendricks RL, Aguirre AJ, et al. Dendritic cell activation and memory cell development are impaired among mice administered medroxyprogesterone acetate prior to mucosal herpes simplex virus type 1 infection. J Immunol. 2012;189:3449–61. doi: 10.4049/jimmunol.1103054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sampah ME, Laird GM, Blankson JN, Siliciano RF, Coleman JS. Medroxyprogesterone acetate increases HIV-1 infection of unstimulated peripheral blood mononuclear cells in vitro. AIDS. 2015;29:1137–46. doi: 10.1097/QAD.0000000000000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quispe Calla NE, Ghonime MG, Cherpes TL, Vicetti Miguel RD. Medroxyprogesterone acetate impairs human dendritic cell activation and function. Hum Reprod. 2015;30:1169–77. doi: 10.1093/humrep/dev035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferreira VH, Dizzell S, Nazli A, et al. Medroxyprogesterone Acetate Regulates HIV-1 Uptake and Transcytosis but Not Replication in Primary Genital Epithelial Cells, Resulting in Enhanced T-Cell Infection. J Infect Dis. 2015;211:1745–56. doi: 10.1093/infdis/jiu832. [DOI] [PubMed] [Google Scholar]
- 75.Govender Y, Avenant C, Verhoog NJ, et al. The injectable-only contraceptive medroxyprogesterone acetate, unlike norethisterone acetate and progesterone, regulates inflammatory genes in endocervical cells via the glucocorticoid receptor. PLoS One. 2014;9:e96497. doi: 10.1371/journal.pone.0096497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Louw-du Toit R, Hapgood JP, Africander D. Medroxyprogesterone acetate differentially regulates interleukin (IL)-12 and IL-10 in a human ectocervical epithelial cell line in a glucocorticoid receptor (GR)-dependent manner. J Biol Chem. 2014;289:31136–49. doi: 10.1074/jbc.M114.587311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tomasicchio M, Avenant C, Du Toit A, Ray RM, Hapgood JP. The progestin-only contraceptive medroxyprogesterone acetate, but not norethisterone acetate, enhances HIV-1 Vpr-mediated apoptosis in human CD4+ T cells through the glucocorticoid receptor. PLoS One. 2013;8:e62895. doi: 10.1371/journal.pone.0062895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koubovec D, Ronacher K, Stubsrud E, Louw A, Hapgood JP. Synthetic progestins used in HRT have different glucocorticoid agonist properties. Mol Cell Endocrinol. 2005;242:23–32. doi: 10.1016/j.mce.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 79.Koubovec D, Vanden Berghe W, Vermeulen L, Haegeman G, Hapgood JP. Medroxyprogesterone acetate downregulates cytokine gene expression in mouse fibroblast cells. Mol Cell Endocrinol. 2004;221:75–85. doi: 10.1016/j.mce.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 80.Africander D, Louw R, Hapgood JP. Investigating the anti-mineralocorticoid properties of synthetic progestins used in hormone therapy. Biochemical and biophysical research communications. 2013;433:305–10. doi: 10.1016/j.bbrc.2013.02.086. [DOI] [PubMed] [Google Scholar]
- 81.Africander DJ, Storbeck KH, Hapgood JP. A comparative study of the androgenic properties of progesterone and the progestins, medroxyprogesterone acetate (MPA) and norethisterone acetate (NET-A) J Steroid Biochem Mol Biol. 2014;143:404–15. doi: 10.1016/j.jsbmb.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 82.Giannoukos G, Szapary D, Smith CL, Meeker JE, Simons SS., Jr New antiprogestins with partial agonist activity: potential selective progesterone receptor modulators (SPRMs) and probes for receptor- and coregulator-induced changes in progesterone receptor induction properties. Molecular endocrinology. 2001;15:255–70. doi: 10.1210/mend.15.2.0596. [DOI] [PubMed] [Google Scholar]
- 83.Sasagawa S, Shimizu Y, Kami H, et al. Dienogest is a selective progesterone receptor agonist in transactivation analysis with potent oral endometrial activity due to its efficient pharmacokinetic profile. Steroids. 2008:222–31. doi: 10.1016/j.steroids.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 84.Kleynhans L, Du Plessis N, Black GF, et al. PLoS ONE. Public Library of Science; 2011. Medroxyprogesterone acetate alters mycobacterium bovis BCG-induced cytokine production in peripheral blood mononuclear cells of contraceptive users; p. e24639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Engler JB, Kursawe N, Solano ME, et al. Glucocorticoid receptor in T cells mediates protection from autoimmunity in pregnancy. Proc Natl Acad Sci U S A. 2017;114:E181–E90. doi: 10.1073/pnas.1617115114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Motulsky HJ. [Accessed 5 March 2016];GraphPad Curve Fitting Guide. http://www.graphpad.com/guides/prism/7/curve-fitting/index.htm.


