Abstract
Anxiety and depressive disorders are common in the pediatric primary care setting, and respond to both psychotherapeutic and antidepressant pharmacotherapy treatment. However, there are limited data regarding the optimal treatment duration. This paper systematically reviews guidelines and clinical trial data related to antidepressant treatment duration in pediatric depressive and anxiety disorders. The extant literature suggests 9–12 months of SSRI treatment for youth with major depressive disorder. For generalized, separation and social anxiety disorders, 6–9 months of SSRI treatment may be sufficient, though many clinicians extend treatment to 12 months based on extrapolation of data from adults with anxiety disorders. Such extended treatment periods may decrease the risk of long-term morbidity and recurrence; however, the goal of treatment is ultimately remission, rather than duration of antidepressant pharmacotherapy. Evidence-based guidelines represent a starting point; however, appropriate treatment duration varies and patient-specific response, psychological factors, and timing of discontinuation must be considered for individual pediatric patients.
Keywords: Selective Serotonin Reuptake Inhibitor (SSRI), Selective Norepinephrine Reuptake Inhibitor (SNRI), antidepressants, psychopharmacology, relapse, recurrence, treatment duration
INTRODUCTION
Anxiety and depressive disorders are common in the pediatric primary care setting and affect 10% of children and adolescents.1 Successful treatment of depressive and anxiety disorders in youth—whether psychotherapeutic or psychopharmacologic—should restore function, establish remission and decrease the likelihood of relapse and recurrence. With successful treatment, however, the following questions frequently arise in clinical care: How long should this patient be treated with an antidepressant? When can the antidepressant that I’ve started be discontinued? Will the treatment gains be sustained upon discontinuation of the patient’s antidepressant medication? If the patient cannot come off medication what is the next best solution?
To inform best practices for determining duration of antidepressant treatment, we systematically reviewed: (1) current practice guidelines for antidepressant treatment duration; (2) evidence for optimal duration of antidepressant treatment and (3) specific psychotherapeutic and psychopharmacologic strategies for preventing relapse.
BACKGROUND
Anxiety and depression are associated with substantial morbidity, including impaired social and academic functioning and increase the risk of suicide and suicide attempts2. Anxiety and depression both respond to psychotherapeutic and psychopharmacologic interventions. However, in some pediatric primary care settings these disorders may be under-recognized and untreated.3 Data have accumulated during the last several decades that support symptomatic and functional improvement with the use of psychotherapy4,5 and selective serotonin reuptake inhibitors (SSRIs),6–8 particularly fluoxetine9–11 for the treatment of depressive and anxiety disorders in youth.4,12–14 Despite the efficacy of these interventions in short-term treatment studies, the risk of relapse and recurrence remains high, with five-year recurrence rates of up to 70% and 50% in children and adolescents with major depressive disorder or anxiety disorders, respectively.15,16
In adults with affective and anxiety disorders, common strategies to prevent recurrence include: (1) continuation of acute treatment; (2) utilization of psychotherapy booster sessions to prolong response; and (3) implementation of uniquely tailored recurrence prevention interventions.17 However, the evidence for these interventions is limited in the pediatric population and these limitations are compounded by the short duration of many acute psychopharmacologic treatment studies, that often describe acute response over ≤12 weeks.4,9,13,14,18,19 These studies were primarily aimed at establishing the efficacy and determining the safety profile of antidepressants in the short-term; but, data regarding the impact on longer-term outcomes associated with these interventions are limited.
Major Depressive Disorder (MDD): Current Guidelines
Acute Treatment Guidelines for Pediatric MDD
Current treatment guidelines focus on the acute treatment of MDD in pediatric patients. Generally, for moderate to severe illness, these guidelines recommend treatment with a combination of an evidence-based psychotherapy and an SSRI.20–22 SSRIs have the most favorable risk-benefit profile as a first-line medication, with assessment of response every four weeks and dose titration when partial response is encountered. While symptom remission should occur in many studies by twelve weeks of psychopharmacologic treatment, there are conflicting recommendations for the duration of treatment following depression in pediatric patients. The GLAD-PC guidelines23 recommend “medications should be maintained for 6–12 months after the full resolution of depressive symptoms.” Similarly, the American Academy of Child & Adolescent Psychiatry (AACAP) Practice Parameters recommend continuation of treatment for 6–12 months “for all patients who have responded to the acute treatment.”24 Further, these parameters suggest that summer may be a preferred period for antidepressant discontinuation and suggest that longer treatment may be recommended for some individuals, particularly for those with risk factors such as multiple past episodes, comorbid disorders, or socio-environmental concerns.24
The Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Guidelines for MDD recommend that maintenance treatment for 6–12 months in youth without a history of MDD prior to the index episode and recommend >1 year of treatment for youth with a prior history of >2 depressive episodes or 1 “severe or chronic episode.”25 These guidelines note that the evidence for these recommendations is limited. Additionally, multiple studies have evaluated longer-term treatment since the publication of GLAD-PC23 and AACAP Practice Parameters.
Relapse and Remission in Youth with MDD
While the medical vernacular is replete with references to “relapse” and “remission,” there is considerable variability in its application to depression. Relapse is defined as a Diagnostic and Statistical Manual of Mental Disorders-defined depressive episode during a remission period, with remission being a period of no or few depressive symptoms for ≥2 weeks but <2 months.26 Recurrence represents the onset of a new depressive episode during a recovery period, with recovery being the absence of significant depressive symptoms for at least two months26 (Figure 1). Defining relapse and recurrence in adolescents is complicated by fluctuations in affective symptoms whether related to developmental factors or the underlying internalizing disorder.17,27
Figure 1. Definitions of disease course in children and adolescents with major depressive disorder.
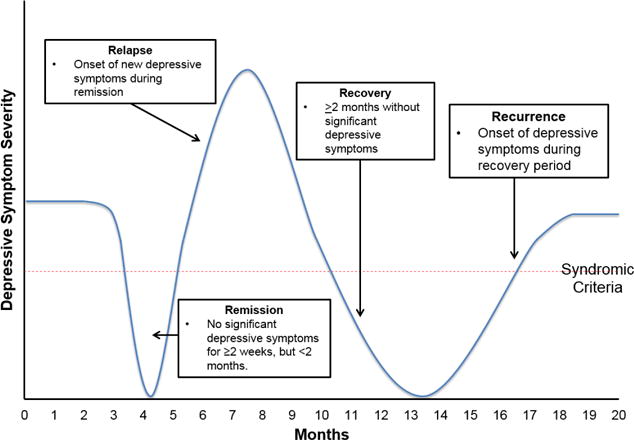
Definitions are operationalized from studies of the course of MDD in adults (Frank et al. 1991).
Controlled Medication Trials in Youth with MDD: Duration of Treatment Effects
Long-term Outcomes in the Treatment for Adolescents with Depression Study (TADS)
The Treatment for Adolescents with Depression Study (TADS) evaluated the acute efficacy of: (1) fluoxetine (monotherapy), (2) CBT (monotherapy), (3) fluoxetine + CBT, and (4) placebo in adolescents with MDD.11 During the initial 12-week treatment phase both fluoxetine monotherapy and fluoxetine+CBT resulted in higher response rates compared to CBT alone.28 During the extension phase, by week 18, response rates to CBT (65%) were comparable to fluoxetine (69%), but fluoxetine+CBT resulted in a response rate of 85%.11 In addition, combined treatment resulted in a more rapid effect, but by week 36 comparable response rates (81–86%) were observed.29 Taken together, the results of this study suggest a benefit to longer treatment duration, with remission rates for treated patients increasing from 23% at week 12 to 60% at week 36.29 The proportion of patients who experienced a sustained response (not necessarily remission) improved similarly, across treatment conditions but sustained response was more likely to be maintained through week 36 if the acute treatment included CBT.29
TADS11 included 1-year of naturalistic follow up, after the 36 week treatment period, with assessments at 3, 6, 9, and 12 months 30. Response rates and suicidality did not differ among the treatments at 12 months, although patients treated with CBT + fluoxetine “generally maintained numerical superiority relative to CBT and fluoxetine [monotherapies].”30 Symptomatic recurrence varied by acute treatment type but generally occurred in fewer than one third of participants during the 12 months following acute treatment.30 This is a lower rate than reported in many other relapse studies.16
Finally, the Survey of Outcomes Following Treatment for Adolescent Depression (SOFTAD) study31 evaluated nearly half (45%) of TADS participants at six-month intervals for the 3½ years following the initial 12 month acute/naturalistic treatment. During this period, 96% of patients recovered from their index major depressive episode. Recovery, by two years, was significantly more likely for those who responded to acute treatment. However, 47% of patients who recovered experienced a recurrence during the SOFTAD study period, with recurrence significantly more common in females (57% [females], versus 32.9% [males]). Last, recurrence rates did not differ among acute treatments in TADS.31
Long-Term Outcomes in the Treatment of SSRI-Resistant Depression in Adolescents (TORDIA) Study
The Treatment of SSRI-Resistant Depression in Adolescents (TORDIA) Study evaluated (1) switching to another SSRI, (2) switching to another SSRI and adding CBT, (3) switching to venlafaxine, or (4) switching to venlafaxine and adding CBT in adolescents (N=334) who failed to respond to an adequate initial SSRI treatment. Greater improvement was observed with the addition to CBT to either a new SSRI or SNRI.32 Following acute treatment (12 weeks), responders continued to receive their previously assigned treatment for an additional 12 weeks, while those who failed to respond to the medication switch or addition of CBT, were followed while receiving naturalistic (non-protocol directed) care (re-assessment at weeks 48 and 72). The cumulative remission rate at 24 weeks was 39% (62% of responders to acute 12-week treatment and 18.3% of non-responders). Nearly 20% of week 12 responders experienced a relapse by week 24. Moreover, approximately one quarter of week 24 responders experienced a relapse within 48 weeks. Remission rate improved to 61.1% by week 72, though many participants had residual symptoms. Continuation of antidepressant treatment for >48 weeks was associated with higher remission rates than for patients who discontinued treatment <48 weeks, although this difference was no longer statistically significant by approximately 18 months. Unlike in TADS, CBT was not associated with a longer term impact on remission or non-relapse33 but the “dose” of CBT treatment was relatively low and those not initially assigned to CBT were able to pursue this during the open treatment phase. This complicates interpretation, particularly given that subsequent studies have suggested a role of CBT for relapse prevention in depressed youth.34,35
Adolescent Depression and Psychotherapy Trial (ADAPT)
The Adolescent Depression and Psychotherapy Trial (ADAPT), pragmatic randomized controlled trial of combination therapy randomized adolescents (N=208) with moderate to severe MDD who failed to respond to a brief initial intervention (>2 sessions focused on an explanation of MDD and exploration of recent family and/or peer group conflict) to SSRI monotherapy (primarily fluoxetine) or SSRI+CBT.36 The proportion of participants who reported significant improvement increased from 43% at week 12 to 57% at week 28 and SSRI + CBT was not superior to SSRI monotherapy at 12 or 28 weeks of treatment. However, patients in the ADAPT trial may have had higher depressive symptom burdens that those in TADS which may subtend the differences in outcome. Interestingly, in a subgroup analysis of the most severely ill participants in TADS, superiority of combined treatment versus fluoxetine alone was not observed.36
Other Depression Randomized Controlled Trials with Long-Term Response Data
One study of adolescents with MDD who responded to fluoxetine (open-label, 12 weeks) randomized patients to continue fluoxetine or placebo for 6 months. Relapse was significantly more common in patients randomized to placebo than those randomized to fluoxetine (69% vs. 42%, N=102, p=0.009) and earlier relapse was observed in those patients treated with placebo (8–14 weeks vs. >24 weeks, p<0.006).37 An additional longer-term study of youth who had responded to acute treatment with sertraline (12 weeks) observed that during 24-weeks following the acute treatment phase, sertraline-treated patients had higher rates of sustained response compared to placebo (38% vs. 0%, p<0.05) during a 52-week double-blinded maintenance treatment period. This study, although well-designed, was limited by sample size and eo ipso power limitations (e.g., maintenance phase included only 22 participants).38 Finally, a 2012 Cochrane review of nine RCTs, evaluated relapse and recurrence in pediatric patients with MDD and noted that “antidepressant medication reduces the chance of relapse-recurrence in the future” and “limited evidence that continued medication is more effective than placebo in preventing the next episode of depression once a child or adolescent has initially responded to medication during acute phase treatment.” However, the optimal continuation therapy was not determined.
Major Depressive Disorder: Predictors of Recurrence
Recovery from an initial major depressive episode occurs within 1–2 years for most youth; however, recurrence is common and frequently occurs 6–12 months following the discontinuation of the acute treatment.39 Randomized controlled trials have recently suggested predictors of treatment response (e.g., low family conflict, higher socioeconomic status, lack of co-occurring anxiety) (Table 1). The evidence for longer-term predictors of relapse and recurrence are limited or, in some cases, conflicting (Table 1).
Table 1.
Factors associated with a lower likelihood of response and/or remission in the long-term treatment of MDD and anxiety disorders in children and adolescents.
| Major Depressive Disorder | Anxiety Disorders |
|---|---|
| More prior depressive episodes (Kovacs et al.1984) Residual symptoms after treatment (in adults) Greater family levels of expressed emotion (Asarnow 1993) Perceived family conflict (Birmaher et al. 2000, Goodyer et al. 2002) Non-response to acute therapy (Survey of Outcomes Following Treatment for Adolescent Depression Study, unpublished) Female sex (Survey of Outcomes Following Treatment for Adolescent Depression Study, unpublished) |
Older age (Ginsburg et al.2011) Female sex (Ginsburg, et al. 2014) Minority status (Ginsburg et al. 2011) Baseline symptom severity Ginsburg et al. 2011 and 2014 Lower socioeconomic status (Ginsburg et al. 2014) Co-occurring internalizing disorder (Ginsburg et al. 2011 Social anxiety disorder(Ginsburg et al. 2011) Greater negative life events (Gibby et al. 2017) |
Anxiety Disorders: Current Acute and Long-Term Treatment Recommendations
Although more than a decade old, the AACAP Practice Parameters for the treatment of anxiety disorders20 suggest evidence-based psychotherapy (e.g., CBT) as the initial treatment for mild anxiety disorders in youth, while SSRIs are recommended for patients with (1) moderate to severe anxiety disorders; (2) impairments that limit their ability to engage in psychotherapy; and (3) partial response to psychotherapy.20 Following acute antidepressant treatment for anxious youth, only one recommendation has addressed the duration of treatment. This recommendation consists of an expert opinion paper, more than 15 years old,40 that recommends antidepressant discontinuation during a low-stress period, >1 year following reduction in anxiety or depressive symptoms. However, descriptions of relapse and remission, that have been operationalized in MDD, have—at times—been difficult to operationalize with pediatric anxiety disorders. Definitions for such terminology in anxiety disorders have adopted terminology specifically for MDD.26 Differences in assessment scales and definitions of treatment response and remission are frequently cited as a source of variation in findings across studies.15,41
Anxiety Disorders: Evidence for Antidepressant Treatment Duration
Child/Adolescent Anxiety Multimodal Study (CAMS)
The Child/Adolescent Anxiety Multimodal Study (CAMS) randomized youth with separation, generalized and/or social anxiety disorder (N=488) to: (1) sertraline monotherapy, (2) CBT monotherapy, (3) combined sertraline and CBT, and (4) pill placebo.42 After 12 weeks of acute treatment, all three treatments were superior to placebo, and combined therapy was associated with a response rate of approximately 80% and was superior to either monotherapy (CBT 60% vs. sertraline 55%, t=−1.32, p=0.19).4 Participants who had responded to any of the three treatment arms during the initial 12 week acute treatment period were followed for a 6-month maintenance period.43 Similar to TADS, the two monotherapy treatments continued to improve. Statistically significant differences for the monotherapies (i.e., sertraline or CBT) compared to combined therapy were no longer present by weeks 24 (combined treatment vs sertraline p=0.092; combined treatment vs CBT p=0.16; sertraline vs. CBT p=0.859) and 36 (combined treatment vs. sertraline p=0.176; combined treatment vs CBT p=0.144; sertraline vs. CBT p=0.931). However, when remission was defined as “no longer meeting diagnostic criteria for the anxiety disorders under study,” combined therapy maintained superiority at week 36 (combined treatment vs. sertraline p<0.005; combined treatment vs. CBT p<0.006).43
Child/Adolescent Anxiety Multimodal Extended Long-Term Study (CAMELS)
The Child/Adolescent Anxiety Multimodal Extended Long-term Study (CAMELS) followed a sub-set of CAMS participants for an average of 6.2 years (range: 3.7–9.9 years) following CAMS randomization (N=288).41 Nearly half (47%) of CAMELS participants were in remission, including 52% of those who responded to acute CAMS treatment and 37.6% of those who did not. However, the context of these remission rates is important. Participants left university-based care and were treated with community-based care. They were monitored at lower frequency, may have had shorter appointment times and may have had more conservative dosing and less aggressive management of co-morbid conditions.
Other Longer-Term Trials in Pediatric Anxiety Disorders
One follow up study of the Research Units on Pediatric Psychopharmacology Anxiety Study found that 94% of those who had previously responded to fluvoxamine during an acute 8-week treatment course (N=35) maintained remission status during a 6-month open treatment period.44 Another extension study of duloxetine showed continued treatment effects at 18 weeks, following acute treatment. In this study, improvement in anxiety severity was observed for both treatment groups (i.e., patients randomized to placebo followed by open-label duloxetine [PBO/DLX] as well as those randomized to duloxetine and then beginning open-label duloxetine [DLX/DLX]). The probability of achieving remission at Week 28 was identical (83%) for the DLX/DLX and PBO/DLX treatment groups.14
Anxiety Disorders: Predictors of Recurrence
As with MDD in youth, evidence for predictors of relapse and recurrence in pediatric anxiety disorders is limited45 and inconclusive. Longer-term studies suggest that children and adolescents who develop anxiety disorders are more likely to have persistent symptoms if the initial pathology is more severe or more functionally disabling, and even if the initial disorder remits, are more likely to develop new anxiety disorders or comorbidities. One study examined long-term (>2 years) outcomes (e.g., remission status, anxiety severity, global functioning) in anxious children and adolescents following treatment.41 This study involved 59% of CAMS participants; patients were followed for 6.2±1.4 years post-randomization). At follow-up, nearly half of the sample (47%) had achieved remission, which was defined as no longer meeting diagnostic criteria for social anxiety disorder, separation anxiety disorder or generalized anxiety disorder (GAD). Responders to acute treatment during the 12-week period CAMS were significantly more likely to be in remission than non-responders (OR=1.83, 95% CI: 1.08–3.09), and they had less severe anxiety (p=0.02) and improved global functioning scores (p=0.02). Other predictors of remission status included male sex (OR=1.69, 95% CI: 1.01–2.84), better family functioning, and lower baseline anxiety severity. Other variables, which were associated with positive outcomes, included higher socioeconomic status (lower anxiety severity, better functioning) and the absence of a comorbid externalizing disorder. The absence of attention-deficit/hyperactivity disorder (ADHD) predicted better functioning at follow-up. Predictors of long-term remission/response in pediatric anxiety disorders and depressive disorders are shown in Table 1.
DISCUSSION
While the evidence base that informs the optimal duration of treatment for pediatric patients with depressive and anxiety disorders significantly lags the data from adult studies, several studies that examine relapse as well as open-label continuation and some follow-up studies may enable clinicians to make informed recommendations regarding the duration of treatment. However, in discussing these studies the goal of treatment—remission—should be emphasized. For depressive disorders, current data suggest high and sustained remission rates associated with longer treatment durations (e.g., approximately 9–12 months).29,33,37 One study reported a lower recurrence rate with a 52-week maintenance phase38 compared to discontinuation prior to 12 months, the current data support acute antidepressant treatment for 6–12 months as an initial strategy for pediatric patients with MDD. By contrast, the anxiety literature suggests that the salutary effects of SSRI treatment—regardless of whether administered with CBT—are robust at 9 months while other open-label extension studies of antidepressants in anxious youth generally suggest similar findings at 6–7 months.14,44 However, based on a double-blinded antidepressant discontinuation study in adults with GAD that suggests that 12 months of treatment (relative to 6 months of treatment) halved the relapse rate,46 many clinicians treating anxious youth treat for 12 months rather than 6 months. Regardless of whether optimal treatment should be for 6–12 months, it is important for clinicians to bear in mind that there is no evidence suggesting harm from long-term SSRI treatment in the absence of problematic side effects. Though these approaches have not been systematically evaluated in the context of an adaptive design trial, clinicians would do well to tailor antidepressant treatment duration based on risk factors for poor prognosis (Table 1).
When antidepressants are to be discontinued, some data47 and expert opinion40 suggest that the timing of antidepressant discontinuation should be considered with regard to the adolescent’s psychosocial milieu. Shamseddeen and colleagues demonstrated that adolescents who end treatment during summer vacation were nearly twice as likely to demonstrate adequate treatment response.47 Thus, clinicians should consider planning discontinuation of antidepressant treatment during lower stress periods, recognizing the importance of incorporating non-disease state dependent factors (e.g., school, separation-related events) into the antidepressant discontinuation approach. However, clinicians should be reminded that for the anxious or depressed child some “low stress periods” for healthy youth (e.g., family vacations, summer camp) may be suffused with stress and anxiety that could be mistakenly attributed to medication discontinuation.
An important clinical consideration with regard to the duration of psychopharmacologic treatment in youth with both MDD and anxiety disorders is the type, frequency and duration of concurrent psychotherapeutic treatment. Long-term data in youth with MDD and anxiety disorders suggest more rapid improvement associated with combined therapy (i.e., SSRI + psychotherapy) relative to antidepressant treatment or psychotherapy alone.15,48 A patient who has successfully engaged in psychotherapy during the course of pharmacologic treatment or a patient with a more rapid response to acute treatment (more likely with combined treatment) might require a briefer psychopharmacologic intervention. However, no prospective, randomized controlled trials have evaluated this possibility.
While debate remains regarding the optimal duration of antidepressant treatment for youth with MDD or anxiety disorders, the overarching goal of treatment is obvious: remission. Any discussion regarding treatment discontinuation is predicated on the patient having achieved remission of depressive or anxiety symptoms; remission status indicates clinical relief is linked to functional recovery.33 If remission is not achieved with acute treatment with either antidepressant monotherapy or antidepressant treatment paired with psychotherapy, clinicians should consider changing antidepressants. Such a change should not be delayed excessively in hopes of a late response to the initial SSRI (unlikely after an initial 8 week treatment period.49 Moreover a switch in SSRI among non-responders has been associated with benefit.32,44 In fact, the results of the TORDIA study remind clinicians that, following a switch in antidepressant, “eventual remission is evident within the first 6 weeks in many, suggesting that earlier intervention among non-responders could be important.”39
In sum, 9–12 months of SSRI treatment is recommended for pediatric patients with MDD. For children and adolescents with generalized, separation and social anxiety disorders, 6–9 months of SSRI treatment may be sufficient. Many clinicians extend treatment to 12 months based on extrapolation of data from adults with anxiety disorders. Such extended treatment periods may decrease the risk of long-term morbidity and recurrence; however, the goal of treatment is ultimately remission, rather than duration of antidepressant pharmacotherapy.
Acknowledgments
DISCLOSURES
Dr. Walkup receives research support from the Tourette’s Association of America and the Hartwell Foundation. He also receives royalties from Guilford Press and Oxford Press for multi-author books published about Tourette syndrome. He is on advisory boards for the Anxiety and Depression Association of America, American Foundation of Suicide Prevention, and the TLC Foundation for Body-Focused Repetitive Behaviors. Dr. Strawn has received research support from the National Institutes of Health (NIMH/NIEHS), Edgemont, Forest Research Institute, Neuronetics, Shire and receives material support from Assurex/Genesight. He has received royalties from Springer Publishing and UpToDate.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Hathaway reports no biomedical conflicts of interest.
Contributor Information
Elizabeth E. Hathaway, Indiana University School of Medicine Indianapolis, Indiana 46202.
John T. Walkup, Weill Cornell Medical College and New York Presbyterian Hospital New York, New York.
Jeffrey R. Strawn, University of Cincinnati College of Medicine Cincinnati, Ohio 45267.
References
- 1.Merikangas KR, He J-P, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Husky MM, Olfson M, He J, Nock MK, Swanson SA, Merikangas KR. Twelve-month suicidal symptoms and use of services among adolescents: results from the National Comorbidity Survey. Psychiatr Serv. 2012;63(10):989–996. doi: 10.1176/appi.ps.201200058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chavira DA, Stein MB, Bailey K, Stein MT. Child anxiety in primary care: Prevalent but untreated. Depress Anxiety. 2004;20:155–164. doi: 10.1002/da.20039. [DOI] [PubMed] [Google Scholar]
- 4.Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359:2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner KD, KD W. Generalized anxiety disorder in children and adolescents. Psychiatr Clin North Am. 2001;24(1):139–153. doi: 10.1016/s0193-953x(05)70210-0. [DOI] [PubMed] [Google Scholar]
- 6.Emslie GJ, Ventura D, Korotzer A, Tourkodimitris S. Escitalopram in the treatment of adolescent depression: a randomized placebo-controlled multisite trial. J Am Acad Child Adolesc Psychiatry. 2009;48:721–729. doi: 10.1097/CHI.0b013e3181a2b304. [DOI] [PubMed] [Google Scholar]
- 7.Wagner KD, Ambrosini P, Rynn M, et al. Efficacy of sertraline in the treatment of children and adolescents with major depressive disorder: two randomized controlled trials. JAMA. 2003;290(8):1033–1041. doi: 10.1001/jama.290.8.1033. [DOI] [PubMed] [Google Scholar]
- 8.Wagner KD, Robb AS, Findling RL, Jin J, Gutierrez MM, Heydorn WE. A randomized, placebo-controlled trial of citalopram for the treatment of major depression in children and adolescents. Am J Psychiatry. 2004;161(6):1079–1083. doi: 10.1176/appi.ajp.161.6.1079. [DOI] [PubMed] [Google Scholar]
- 9.Emslie GJ, Heiligenstein JH, Wagner KD, et al. Fluoxetine for acute treatment of depression in children and adolescents: a placebo-controlled, randomized clinical trial. J Am Acad Child Adolesc Psychiatry. 2002;41(October):1205–1215. doi: 10.1097/00004583-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Emslie GJ, Rush J, Weinberg WA, et al. A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Arch Gen Psychiatry. 1997;54(11):1031–1037. doi: 10.1001/archpsyc.1997.01830230069010. [DOI] [PubMed] [Google Scholar]
- 11.March J, Silva S, Petrycki S, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA J Am Med Assoc. 2004;292(7):807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- 12.Pine DS, Walkup JT, Labellarte MJ, et al. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med. 2001;344:1279–1285. doi: 10.1056/NEJM200104263441703. [DOI] [PubMed] [Google Scholar]
- 13.Rynn MA, Siqueland L, Rickels K. Placebo-controlled trial of sertraline in the treatment of children with generalized anxiety disorder. Am J Psychiatry. 2001;158(12):2008–2014. doi: 10.1176/appi.ajp.158.12.2008. [DOI] [PubMed] [Google Scholar]
- 14.Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(4):283–293. doi: 10.1016/j.jaac.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Ginsburg GS, Kendall PC, Sakolsky D, et al. Remission after acute treatment in children and adolescents with anxiety disorders: Findings from the CAMS. J Consult Clin Psychol. 2011;79(6):806–813. doi: 10.1037/a0025933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emslie GJ, Kennard BD, Mayes TL, et al. Fluoxetine versus placebo in preventing relapse of major depression in children and adolescents. Am J Psychiatry. 2008;165(4):459–467. doi: 10.1176/appi.ajp.2007.07091453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simons AD, Rohde P, Kennard BD, Robins M. Relapse and recurrence prevention in the treatment for adolescents with depression study. Cogn Behav Pract. 2005;12(2):240–251. [Google Scholar]
- 18.Wagner KD, Berard R, Stein MB, et al. A multicenter, randomized, double-blind, placebo-controlled trial of paroxetine in children and adolescents with social anxiety disorder. Arch Gen Psychiatry. 2004;61(11):1153–1162. doi: 10.1001/archpsyc.61.11.1153. [DOI] [PubMed] [Google Scholar]
- 19.Rynn MA, Riddle MA, Yeung PP, Kunz NR. Efficacy and Safety of Extended-Release Venlafaxine in the Treatment of Generalized Anxiety Disorder in Children and Adolescents: Two Placebo-Controlled Trials. The American journal of psychiatry. 2007;164:290–300. doi: 10.1176/ajp.2007.164.2.290. [DOI] [PubMed] [Google Scholar]
- 20.Connolly SD, Bernstein GA. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267–283. doi: 10.1097/01.chi.0000246070.23695.06. [DOI] [PubMed] [Google Scholar]
- 21.Foy JM, Kelleher KJ, Laraque D. Enhancing pediatric mental health care: strategies for preparing a primary care practice. Pediatrics. 2010;125(Suppl (Supplement_3)):S87–S108. doi: 10.1542/peds.2010-0788E. [DOI] [PubMed] [Google Scholar]
- 22.Wehry AM, Beesdo-Baum K, Hennelly MM, Connolly SD, Strawn JR. Assessment and Treatment of Anxiety Disorders in Children and Adolescents. Curr Psychiatry Rep. 2015;17(52):99–110. doi: 10.1007/s11920-015-0591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung AH, Zuckerbrot Ra, Jensen PS, Ghalib K, Laraque D, Stein REK. Guidelines for adolescent depression in Primary Care (GLAD-PC): II. Treatment and ongoing management. Pediatrics. 2007;120(5):e1313–e1326. doi: 10.1542/peds.2006-1395. [DOI] [PubMed] [Google Scholar]
- 24.Birmaher B, Brent D, Bernet W, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1503–1526. doi: 10.1097/chi.0b013e318145ae1c. [DOI] [PubMed] [Google Scholar]
- 25.Milev RV, Giacobbe P, Kennedy SH, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 4. Neurostimulation treatments. Can J Psychiatry. 2016;61(9):561–575. doi: 10.1177/0706743716660033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank E, Prien RF, Jarrett RB, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48(9):851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 27.Rintelmann JW, Emslie GJ, Rush AJ, et al. The effects of extended evaluation on depressive symptoms in children and adolescents. J Affect Disord. 1996;41:149–156. doi: 10.1016/s0165-0327(96)00084-5. [DOI] [PubMed] [Google Scholar]
- 28.Kennard B, Silva S, Vitiello B, et al. Remission and Residual Symptoms After Short-Term Treatment in the Treatment of Adolescents with Depression Study (TADS) J Am Acad Child Adolesc Psychiatry. 2006;45(12):1404–1411. doi: 10.1097/01.chi.0000242228.75516.21. [DOI] [PubMed] [Google Scholar]
- 29.March JS, Silva S, Petrycki S, et al. The Treatment for Adolescents With Depression Study (TADS): long-term effectiveness and safety outcomes. Arch Gen Psychiatry. 2007;64(10):1132–1143. doi: 10.1001/archpsyc.64.10.1132. [DOI] [PubMed] [Google Scholar]
- 30.March J, Silva S, Curry J, et al. The Treatment for Adolescents With Depression Study (TADS): outcomes over 1 year of naturalistic follow-up. Am J Psychiatry. 2009;166(10):1141–1149. doi: 10.1176/appi.ajp.2009.08111620.. [DOI] [PubMed] [Google Scholar]
- 31.Curry J, Silva S, Rohde P, et al. Recovery and recurrence following treatment for adolescent major depression. Arch Gen Psychiatry. 2011;68(3):263–269. doi: 10.1001/archgenpsychiatry.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299(8):901–913. doi: 10.1001/jama.299.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vitiello B, Emslie G, Clarke G, et al. Long-term outcome of adolescent depression initially resistant to selective serotonin reuptake inhibitor treatment: A follow-up study of the TORDIA sample. J Clin Psychiatry. 2011;72(3):388–396. doi: 10.4088/JCP.09m05885blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennard BD, Stewart SM, Hughes JL, Jarrett RB, Emslie GJ. Developing Cognitive Behavioral Therapy to Prevent Depressive Relapse in Youth. Cogn Behav Pract. 2008;15(4):387–399. doi: 10.1016/j.cbpra.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emslie GJ, Kennard BD, Mayes TL, et al. Continued Effectiveness of Relapse Prevention Cognitive-Behavioral Therapy Following Fluoxetine Treatment in Youth with Major Depressive Disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(12):991–998. doi: 10.1016/j.jaac.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodyer I, Dubicka B, Wilkinson P, et al. Selective serotonin reuptake inhibitors (SSRIs) and routine specialist care with and without cognitive behaviour therapy in adolescents with major depression: randomised controlled trial. BMJ. 2007;335(7611):142. doi: 10.1136/bmj.39224.494340.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emslie GJ, Kennard BD, Mayes TL, et al. Fluoxetine versus placebo in preventing relapse of major depression in children and adolescents. Am J Psychiatry. 2008;165(4):459–467. doi: 10.1176/appi.ajp.2007.07091453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheung A, Kusumakar V, Kutcher S, et al. Maintenance study for adolescent depression. J Child Adolesc Psychopharmacol. 2008;18(4):389–394. doi: 10.1089/cap.2008.0001. [DOI] [PubMed] [Google Scholar]
- 39.Emslie GJ, Mayes T, Porta G, et al. Treatment of Resistant Depression in Adolescents (TORDIA): Week 24 outcomes. Am J Psychiatry. 2010;167(7):782–791. doi: 10.1176/appi.ajp.2010.09040552.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pine DS. Treating children and adolescents with selective serotonin reuptake inhibitors: how long is appropriate? J Child Adolesc Psychopharmacol. 2002;12(3):189–203. doi: 10.1089/104454602760386888. [DOI] [PubMed] [Google Scholar]
- 41.Ginsburg GS, Becker EM, Keeton CP, et al. Naturalistic follow-up of youths treated for pediatric anxiety disorders. JAMA psychiatry. 2014;71(3):310–318. doi: 10.1001/jamapsychiatry.2013.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Compton SN, Walkup JT, Albano AM, et al. Child/Adolescent Anxiety Multimodal Study (CAMS): rationale, design, and methods. Child Adolesc Psychiatry Ment Health. 2010;4:1. doi: 10.1186/1753-2000-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piacentini J, Bennett S, Compton SN, et al. 24- and 36-week outcomes for the Child/Adolescent Anxiety Multimodal Study (CAMS) J Am Acad Child Adolesc Psychiatry. 2014;53(3):297–310. doi: 10.1016/j.jaac.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walkup J, Labellarte M, Riddle MA, et al. Treatment of pediatric anxiety disorders: an open-label extension of the research units on pediatric psychopharmacology anxiety study. J Child Adolesc Psychopharmacol. 2002;12(3):175–188. doi: 10.1089/104454602760386879. [DOI] [PubMed] [Google Scholar]
- 45.Wehry AM, Beesdo-Baum K, Hennelly MM, Connolly SD, Strawn JR. Assessment and treatment of anxiety disorders in children and adolescents. Curr Psychiatry Rep. 2015;17(7):591. doi: 10.1007/s11920-015-0591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rickels K, Etemad B, Khalid-Khan S, Lohoff FW, Rynn MA, Gallop RJ. Time to relapse after 6 and 12 months’ treatment of generalized anxiety disorder with venlafaxine extended release. Arch Gen Psychiatry. 2010;67(12):1274–1281. doi: 10.1001/archgenpsychiatry.2010.170. [DOI] [PubMed] [Google Scholar]
- 47.Shamseddeen W, Clarke G, Wagner KD, et al. Treatment-resistant depressed youth show a higher response rate if treatment ends during summer school break. J Am Acad Child Adolesc Psychiatry. 2011;50(11):1140–1148. doi: 10.1016/j.jaac.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kratochvil C, Emslie G, Silva S, et al. Acute time to response in the Treatment for Adolescents with Depression Study (TADS) J Am Acad Child Adolesc Psychiatry. 2006;45(12):1412–1418. doi: 10.1097/01.chi.0000237710.73755.14. [DOI] [PubMed] [Google Scholar]
- 49.Strawn JR, Dobson ET, Mills JA, et al. Placebo Response in Pediatric Anxiety Disorders: Results from the Child/Adolescent Anxiety Multimodal Study. J Child Adolesc Psychopharmacol. 2017 doi: 10.1089/cap.2016.0198. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]


