Abstract
Purpose
The purpose of this report is to describe, for the first time, the pharmacokinetics of dacarbazine (DTIC) and its metabolites (5-[3-methyl-triazen-1-yl]-imidazole-4-carboxamide (MTIC), 5-[3-hydroxymethyl-3-methyl-triazen-1-yl]-imidazole-4-carboxamide (HMMTIC) and 5-aminoimidazole-4-carboxamide (AIC)) during pregnancy (n=2) and postpartum (n=1).
Methods
Non-compartmental DTIC, MTIC, HMMTIC and AIC pharmacokinetics (PK) were estimated in one case at 29 weeks gestation and 18 days postpartum and a second case at 32 weeks gestation, in women receiving DTIC in combination with doxorubicin, bleomycin and vinblastine for treatment of Hodgkin’s Lymphoma. Drug concentrations were measured by HPLC.
Results
In the subject who completed both pregnancy and postpartum study days, DTIC area under the concentration-time curve (AUC) was 27% higher and metabolite AUCs were lower by 27% for HMMTIC, 38% for MTIC and 83% of AIC during pregnancy compared to postpartum. At 7 and 9 years follow-up, both subjects were in remission of their Hodgkin’s Lymphoma.
Conclusions
Based on these two case reports, pregnancy appears to decrease the metabolism of the pro-drug dacarbazine, likely through inhibition of CYP1A2 activity. Lower concentrations of active metabolites and decreased efficacy may result, although both of these subjects experienced long-term remission of their Hodgkin’s Lymphoma.
Keywords: Dacarbazine, Metabolites, Pharmacokinetics, Pregnancy
Introduction
Dacarbazine (DTIC) has been used in combination with doxorubicin, bleomycin, and vinblastine for treatment of malignancies including Hodgkin’s lymphoma. Lymphoma occurs in approximately 1 in 1000 to 1 in 6000 pregnancies [1]. It is the fourth most common malignancy in pregnancy, with Hodgkin’s lymphoma being more common than non-Hodgkin’s lymphoma [2–5]. DTIC, an inactive pro-drug, is converted to 5-[3-hydroxy-methyl-3-methyl-triazen-1-yl]-imidazole-4-carboxamide (HMMTIC) primarily by CYP1A2 (and CYP1A1 in some tumors). CYP2E1 is also involved when CYP1A2 activity is very low. HMMTIC is chemically unstable and breaks down to 5-[3-methyl-triazen-1-yl]-imidazole-4-carboxamide (MTIC). Both HMMTIC and MTIC are reactive metabolites [6]. MTIC is relatively unstable and rapidly breaks down into 5-aminoimidazole-4-carboxamide (AIC), and diazomethane hydroxide, an active methylating compound. The exact mechanisms of action of dacarbazine’s active metabolites are unknown, but they appear to act through inhibition of DNA synthesis as purine analogs as well as alkylating agents. In non-pregnant patients, dacarbazine has been reported to have a 41–110 minute half-life, 1080 mL/min clearance, 350–700 mL/min renal clearance, minimal plasma protein binding and 40% excretion unchanged in the urine [7–11]. The pharmacokinetics of dacarbazine have not been previously studied during pregnancy. The purpose of this study is to report the pharmacokinetics of dacarbazine and its metabolites in two pregnant women.
Materials and Methods
The study was conducted at the University of Washington and Swedish Medical Center. The protocol was approved by the institutional review boards at each site and conducted in accordance with their guidelines. All subjects gave written informed consent.
Patients
Subjects were eligible to participate if they were pregnant, ≥ 18 years of age, hematocrit ≥ 28% and receiving dacarbazine for therapeutic purposes. Subjects received cycles of intravenous dacarbazine as part of combination chemotherapy during their clinical care (Table 1), with infusions lasting 25–42 minutes (375 mg/m2). Blood and urine samples were collected during one treatment episode per subject in late-pregnancy (n=2; at 29 and 32.3 weeks’ gestation) as well as postpartum (n=1; at 2.6 weeks).
Table 1.
Concomitant medications
| Subject | Chemotherapeutic Protocol | Other Medications |
|---|---|---|
| 1 (late-pregnancy) | ABVD (every 2 weeks) | Dexamethasone, ondansetron, prenatal vitamins, ferrous sulfate, folic acid |
| 1 (postpartum) | ABVD (every 2 weeks) | Dexamethasone, ondansetron, oxycodone, acetaminophen |
| 2 | ABVD (every 2 weeks) | Dexamethasone, palonosetron, ondansetron, diphenhydramine, prenatal vitamins, vitamin D, ferrous sulfate |
A = doxorubicin, B = bleomycin, V = vinblastine, D = dacarbazine
Sample collection and pharmacokinetic analysis
Following the initiation of dacarbazine infusion, serial venous blood samples were collected at 0, 0.3, 0.8, 1.5, 2, 3, 4, 6, 8, 10 and 12 hours. Urine was collected for 24 hours to determine creatinine clearance. Both subjects had their pregnancy studies on the fourth cycle of chemotherapy, after the first dose. MTIC, HMMTIC, and DTIC were measured in plasma using a reverse phase HPLC assay [12]. Briefly, plasma proteins were precipitated by the addition of 800 µL of ice-cold methanol to 400 µL of plasma. Samples were mixed for 30 seconds and placed in an ice bath for 5 minutes. Samples were centrifuged for 3 minutes at 14,000 RPM and 4° C. Fifty µL aliquots of supernatant were diluted 1:1 with 50 mM (NH4)2HPO4 buffer (pH 6.5) in autosampler vials and placed in a chilled autosampler (4°C) for analysis. Chromatographic separation was achieved with a Zorbax Stablebond-Cyano (150 × 4.6 mm i.d., 5 µm particle size) analytical column (Mac-Mod Analytical, Chadds Ford, PA) with the flow rate at 1 mL/min and UV absorbance monitored at 318 nm. The mobile phase consisted of 20% methanol, 80% 50 mM (NH4)2 HPO4 buffer, and 0.1% triethylamine (TEA). Plasma samples were stored at −80° C until analysis. The pharmacokinetic parameters were estimated utilizing standard non-compartmental techniques as previously described [13, 14].
Results
Two pregnant women with Hodgkin’s lymphoma were recruited for this study. One subject also completed a study 2.6 weeks postpartum. Their demographics included ages: 31 and 34 years, race: White, body weights: 74.5 and 81.3 kg (pregnancy) and 69 kg (postpartum), body surface areas: 1.8 and 1.9 m2, dacarbazine doses: 630 and 680 mg IV, creatinine clearances 178 and 265 mL/min (pregnancy) and 155 mL/min (postpartum).
The first subject was a 31-year-old gravida 2 para 1 woman who presented with enlarged supraclavicular nodes at 10 weeks gestation. After negative fine needle aspiration, excisional biopsy of the supraclavicular nodes led to a diagnosis of nodular sclerosing Hodgkin’s lymphoma. Neck and chest computerized tomography (CT) scan revealed supraclavicular, mediastinal, and paratracheal lymphadenopathy. Magnetic Resonance Imaging (MRI) studies showed no adenopathy below the diaphragm. A persistent cough and right lower lobe consolidation was unsuccessfully treated with amoxicillin clavulanate. Subsequent bronchoscopy, lung and bone marrow biopsies confirmed Stage IV disease. After oncology and maternal-fetal medicine consultations, doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) treatment were begun at 17 weeks gestation. Treatment proceeded at full dose for five cycles (chemotherapy on days 1 and 15 of each 28 days) during the remainder of pregnancy plus one cycle postpartum.
The second subject was a 34-year-old gravida 2 para 1 woman who sought medical attention for enlarged cervical nodes at 15 weeks gestation. An ultrasound examination demonstrated bilateral neck adenopathy and a biopsy provided a diagnosis of nodular sclerosing Hodgkin’s lymphoma. Chest x-ray showed paratracheal and hilar adenopathy and abdominal ultrasound was negative for lesions. MRI confirmed that the disease was confined to above the diaphragm. The subject consulted with an oncologist and maternal fetal medicine specialist before beginning ABVD chemotherapy at 20 weeks gestation for Stage IIA disease. The subject received five cycles of treatment during pregnancy.
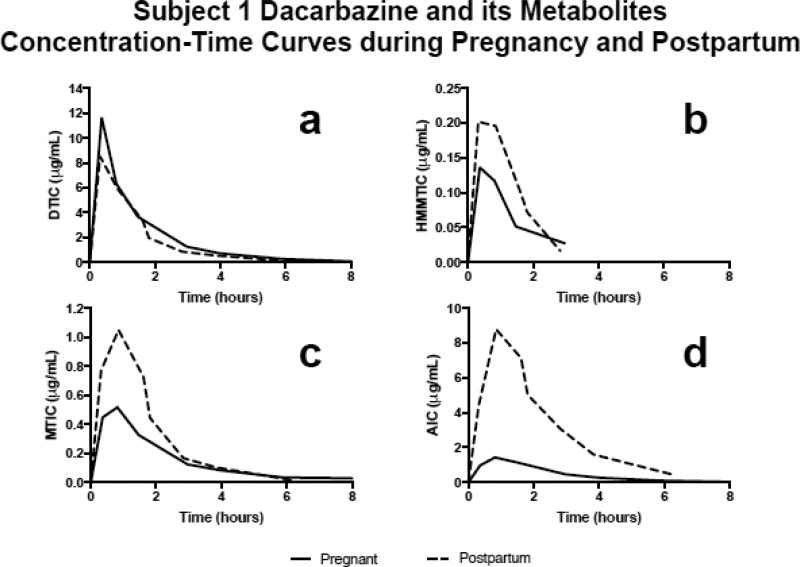
Figure 1 depicts subject 1’s concentration-time profiles for DTIC (Fig. 1a), HMMTIC (Fig. 1b), MTIC (Fig. 1c) and AIC (Fig. 1d) during pregnancy and postpartum. For subject 1, DTIC area under the concentration-time curve (AUC) was 27% higher (15.12 µg•hr/mL vs. 11.87 µg•hr/mL), peak concentration was 37% higher (11.65 µg/mL vs. 8.53 µg/mL), half-life was 22% longer (1.1 hours vs 0.9 hours) and renal clearance was 46% higher (402 mL/min vs 276 mL/min) during pregnancy compared to postpartum. In addition, for subject 1, DTIC clearance was 22% slower (694 mL/min vs 885 mL/min) and volume of distribution at steady state was 8% smaller (68 L vs 74 L) during pregnancy compared to postpartum. Subject 2 DTIC PK parameters were similar to the first subject’s parameters during pregnancy (half-life 1.3 hours, renal clearance 383 mL/min, total clearance 800 mL/min and volume of distribution at steady state 82 L).
Figure 1.
Graphically depicts Subject 1’s (a) dacarbazine (DTIC), (b) HMMTIC, (c) MTIC and (d) AIC plasma concentration-time curves during pregnancy (29 weeks gestation) and postpartum (18 days)
The first subject’s HMMTIC AUC was 27% lower (0.24 µg•hr/mL vs 0.33 µg•hr/mL), half-life was 102% longer (1.1 hours vs 0.5 hours) and HMMTIC/DTIC AUC metabolic ratio corrected for molecular weight was 44% lower (1.4% vs 2.5%) during pregnancy compared to postpartum. Subject 2’s HMMTIC PK parameters during pregnancy were: 0.13 µg•hr/mL, 0.66 hours and 0.9% for AUC, half-life and HMMTIC/DTIC AUC metabolic ratio, respectively.
Subject 1’s MTIC AUC was 38% lower (1.3 µg•hr/mL vs 2.0 µg•hr/mL), half-life was 111% longer (1.7 hours vs 0.8 hours) and MTIC/DTIC AUC metabolic ratio corrected for molecular weight was 51% lower (9.0% vs 18.2%) during pregnancy compared to postpartum. Subject 2’s MTIC PK parameters during pregnancy were: 2.12 µg•hr/mL, 1.27 hours and 16.2%, for AUC, half-life and MTIC/DTIC AUC metabolic ratio, respectively.
Subject 1’s AIC AUC was 83% lower (3.65 µg•hr/mL vs 21.19 µg•hr/mL), AIC/DTIC AUC metabolic ratio corrected for molecular weight was 87% lower (35% vs 258%) and there was no change in half-life (1.2 hours vs 1.2 hours) during pregnancy compared to postpartum. Subject 2’s AIC PK parameters during pregnancy were: 3.56 µg•hr/mL, 1.56 hours and 36.3%, for AUC, half-life and AIC/DTIC AUC metabolic ratio, respectively.
Subject 1 tolerated dacarbazine treatment well. She did not experience chemotherapy-associated neutropenia, liver impairment, or urinary dysfunction (despite pre-existing left renal agenesis). Her pregnancy was complicated by mild anemia and vaginal bleeding which resolved spontaneously. The pregnancy implanted in the left horn of a bicornuate uterus but fetal growth proceeded normally. She entered spontaneous labor at 37 weeks and delivered a healthy female infant weighing 2580 grams by repeat cesarean delivery after an unsuccessful trial of labor. The baby’s Apgar scores were 7 and 9 at 1 and 5 minutes, respectively. Delivery was complicated by a postpartum hemorrhage treated with uterotonics and two units of packed red blood cells to replace the estimated 1500 mL of blood loss. Positron emission tomography (PET) scan on postpartum day one showed the subject was in remission and chemotherapy was resumed postpartum for one additional cycle. Her chest x-ray five months postpartum showed no masses and CT scans of the chest, abdomen, and pelvis 2 years postpartum were negative. However, PET and CT scans of the chest 2.5 years postpartum showed new mediastinal adenonpathy sarcoidosis, which was confirmed on biopsy and subsequently resolved. Nine years after delivery the subject remained in remission and the child was healthy.
Subject 2’s adverse effects from chemotherapy included anorexia, alopecia, and fatigue. Anemia was attributed to her pregnancy and treated with oral iron, but intravenous iron was required with the second chemotherapy cycle. After a dose of intravenous iron with cycle 3 she experienced fever, hypotension, and tachycardia, which was managed with intravenous fluids, intravenous steroids and overnight hospital observation. No further intravenous iron was given during pregnancy but she continued to have a similar reaction and treatment after subsequent chemotherapeutic doses. She experienced no chemotherapy associated neutropenia, liver impairment or dose delays. She developed spontaneous labor at 38 weeks gestation after the fifth cycle of chemotherapy and delivered a female infant vaginally, weighing 3864 grams. The baby’s Apgar scores were 8 and 9 at 1 and 5 minutes, respectively. The subject resumed chemotherapy two weeks postpartum for the sixth and final cycle. Subsequent PET and CT scanning showed no evidence of disease. She elected to receive consolidative radiation therapy after completion of chemotherapy. Seven years after delivery the subject remained in remission and the child was healthy.
Discussion
Treatment of Hodgkin’s lymphoma typically includes chemotherapy alone or chemotherapy followed by consolidation radiation therapy [15, 16]. In general, malignancies are treated during pregnancy if the mother’s condition will be compromised by delaying treatment until after delivery. Malignancy stage and location, patient’s condition and disease progression rate are taken into account when determining whether to proceed with chemotherapy during pregnancy. If chemotherapy is indicated, it is usually delayed until the second or third trimesters of pregnancy, if possible, to minimize the fetal risks. Optimization of the treatment approach for Hodgkin’s lymphoma during pregnancy has not been well studied. Published data are limited to case series and anecdotal reports. Combination ABVD chemotherapy is typically used for treatment of Hodgkin’s lymphoma during pregnancy and was utilized as described in the 2 cases reported here [17, 18]. The pharmacokinetics of most chemotherapeutic agents have not been studied during pregnancy. We have previously described the pharmacokinetics of doxorubicin during pregnancy [19]. This is the first report on the pharmacokinetics of dacarbazine and its metabolites during pregnancy.
DTIC is metabolized to HMMTIC, primarily by CYP1A2, with CYP2E1 and CYP1A1 being the secondary pathways. Pregnancy decreases the activity of CYP1A2 and increases the activity of CYP2E1 [20, 21]. CYP1A2 activity is reported to decrease by 33%, 48%, and 65% in early-, mid- and late pregnancy, respectively. Given that CYP1A2 is the primary pathway for DTIC metabolism, it is not surprising that pregnancy decreased the metabolism of DTIC [6], and thereby increased its AUC by 27% (Fig. 1a). This inhibition of CYP1A2 activity during pregnancy also resulted in lower exposure to DTIC’s metabolites, those being HMMTIC (27% lower), MTIC (38% lower) and AIC (83% lower) (Fig. 1 b–d). HMMTIC is chemically unstable. With the elimination of formaldehyde, HMMTIC becomes MTIC. MTIC is also chemically unstable and breaks down into AIC and diazomethane hydroxide [6]. AIC and diazomethane hydroxide are renally eliminated. Renal filtration increases on average by approximately 50–60% over baseline during pregnancy [22, 23]. This is consistent with the 71% increase in creatinine clearance seen in the first subject reported here. With lower concentrations of MTIC during pregnancy and increased renal filtration, AIC concentrations were lower and diazomethane hydroxide concentrations would also be expected to be lower during pregnancy. Neither of the subjects were receiving inducers or inhibitors of CYP1A2.
DTIC is a pro-drug, which lacks chemotherapeutic activity [6, 24, 25]. Therefore, the higher DTIC concentrations during pregnancy are not expected to increase the chemotherapeutic effect. HMMTIC and MTIC are reactive metabolites with some chemotherapeutic activity [6]. The lower concentrations of these metabolites during pregnancy might lower the therapeutic efficacy of DTIC. AIC does not have chemotherapeutic activity, so its lower concentrations during pregnancy will not affect efficacy. Diazomethane hydroxide separates into molecular nitrogen and a methyl cation, which binds to DNA and is the main DNA methylating metabolite of DTIC [6, 24, 25]. Diazomethane hydroxide concentrations were not quantified in this study, but as described above were likely lower during pregnancy and potentially decreased DTIC efficacy.
Despite the lower concentrations of the active metabolites of DTIC during pregnancy, both subjects in this study successfully achieved long-term remission of their Hodgkin’s lymphoma (9 and 7 years follow-up) following treatment with combination ABVD chemotherapy.
Higher concentrations of DTIC during pregnancy, although not expected to enhance efficacy, could potentially increase other maternal and fetal adverse effects. Subject 2 did experience adverse reactions to her chemotherapy, but it is unclear whether this was related to her DTIC, since she also was receiving doxorubicin, bleomycin and vinblastine. Some of the effects could also be attributed to her pregnancy. Our previous work suggested that doxorubicin clearance was deceased during pregnancy, leading to higher concentrations and potentially increased risk of side effects [19]. We are unable to locate any published reports on bleomycin or vinblastine pharmacokinetics during pregnancy.
In summary, we report for the first time the pharmacokinetics of dacarbazine and its metabolites during pregnancy (n=2) and in the early postpartum period (n=1). Based on these case reports, it appears as though pregnancy increases the concentrations of dacarbazine and decreases the concentrations of its metabolites. Although the active metabolite concentrations were lower during pregnancy, both women experience long-term remission of their Hodgkin’s lymphoma.
Acknowledgments
This work was supported in part by grant number U10HD047892 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and UL1TR000423 from the National Institutes of Health, National Center for Advancing Translational Sciences through the Clinical and Translational Science Awards Program (CTSA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health.
Footnotes
Conflict of Interest: Dr. Hebert received a research grant from the Eunice Kennedy Shriver National Institute of Child Health (grant number U10HD047892) and the study was conducted in the University of Washington Clinical Research Unit which is supported by a grant from the National Institutes of Health, National Center for Advancing Translational Sciences through the Clinical and Translational Science Awards Program (CTSA) (grant number UL1TR000423).
Compliance with Ethical Standards
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Pentheroudakis G, Pavlidis N. Cancer and pregnancy: Poena magna, not anymore. European Journal of Cancer. 2006;42(2):126–140. doi: 10.1016/j.ejca.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Müller A, Ihorst G, Mertelsmann R, Engelhardt M. Epidemiology of non-Hodgkin’s Lymphoma (NHL): Trends, geographic distribution and etiology. Annals of Hematology. 2005;84(1):1–12. doi: 10.1007/s00277-004-0939-7. [DOI] [PubMed] [Google Scholar]
- 3.Pereg D, Koren G, Lishner M. The treatment of Hodgkin’s and non-Hodgkin’s lymphoma in pregnancy. Haematologica. 2007;92(9):1230–1237. doi: 10.3324/haematol.11097. [DOI] [PubMed] [Google Scholar]
- 4.Stewart HL, Monto RW. Hodgkin's disease and pregnancy. American Journal of Obstetrics and Gynecology. 1952;63(3):570–578. doi: 10.1016/0002-9378(52)90071-9. [DOI] [PubMed] [Google Scholar]
- 5.Woo SY, Fuller LM, Cundiff JH, Bondy ML, Hagemeister FB, Mclaughlin P, Velasquez WS, Swan F, Alma Rodriguez M, Cabanillas F, Allen PK, Carpenter RJ. Radiotherapy during pregnancy for clinical stages IA-IIA Hodgkin’s disease. International Journal of Radiation Oncology, Biology, Physics. 1992;23(2):407–412. doi: 10.1016/0360-3016(92)90761-6. [DOI] [PubMed] [Google Scholar]
- 6.Reid JM, Kuffel MJ, Miller JK, Rios R, Ames MM. Metabolic activation of dacarbazine by human cytochromes P450: the role of CYP1A1, CYP1A2, and CYP2E1. Clinical Cancer Research. 1999;5(8):2192–2197. [PubMed] [Google Scholar]
- 7.Breithaupt H, Dammann A, Aigner K. Pharmacokinetics of dacarbazine (DTIC) and its metabolite 5-aminoimidazole-4-carboxamide (AIC) following different dose schedules. Cancer Chemother Pharmacol. 1982;9(2):103–109. doi: 10.1007/BF00265388. [DOI] [PubMed] [Google Scholar]
- 8.Loo TL, Housholder GE, Gerulath AH, Saunders PH, Farquhar D. Mechanism of action and pharmacology studies with DTIC (NSC-45388) Cancer Treatment Reports. 1976;60(2):149–152. [PubMed] [Google Scholar]
- 9.Farina P, Benfenati E, Reginato R, Torti L, D'Incalci M, Threadgill MD, Gescher A. Metabolism of the anticancer agent 1-(4-acetylphenyl)-3,3-dimethyltriazene. Biomedical Mass Spectrometry. 1983;10(8):485–488. doi: 10.1002/bms.1200100808. [DOI] [PubMed] [Google Scholar]
- 10.Fiore D, Jackson AJ, Didolkar MS, Dandu VR. Simultaneous determination of dacarbazine, its photolytic degradation product, 2-azahypoxanthine, and the metabolite 5-aminoimidazole-4-carboxamide in plasma and urine by high-pressure liquid chromatography. Antimicrobial Agents and Chemotherapy. 1985;27(6):977–979. doi: 10.1128/aac.27.6.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loo TL, Luce JK, Jardine JH, Frei E. Pharmacologic studies of the antitumor agent 5-(dimethyltriazeno)imidazole-4-carboxamide. Cancer Research. 1968;28(12):2448–2453. [PubMed] [Google Scholar]
- 12.Safgren SL, Reid JM, Rios R, Ames MM. Validated high-performance liquid chromatographic assay for simultaneous determination of dacarbazine and the plasma metabolites 5-(3-hydroxymethyl-3-methyl-1-triazeno)imidazole-4-carboxamide and 5-(3-methyl-1-triazeno)imidazole-4-carboxamide. Journal of Chromatography B: Biomedical Sciences and Applications. 2001;754(1):91–96. doi: 10.1016/s0378-4347(00)00586-7. [DOI] [PubMed] [Google Scholar]
- 13.Hebert MF, Roberts JP, Prueksaritanont T, Benet LZ. Bioavailability of cyclosporine with concomitant rifampin administration is markedly less than predicted by hepatic enzyme induction. Clinical Pharmacology Therapy. 1992;52(5):453–457. doi: 10.1038/clpt.1992.171. [DOI] [PubMed] [Google Scholar]
- 14.Hebert MF, Carr DB, Anderson GD, Blough D, Green GE, Brateng DA, Kantor E, Benedetti TJ, Easterling TR. Pharmacokinetics and Pharmacodynamics of Atenolol During Pregnancy and Postpartum. Journal of Clinical Pharmacology. 2005;45(1):25–33. doi: 10.1177/0091270004269704. [DOI] [PubMed] [Google Scholar]
- 15.Hoppe RT, Advani RH, Ai WZ, Ambinder RF, Aoun P, Bello CM, Bierman PJ, Blum KA, Chen R, Dabaja B, Duron Y, Forero A, Gordon LI, Hernandez-Ilizaliturri FJ, Hochberg EP, Maloney DG, Mansur D, Mauch PM, Metzger M, Moore JO, Morgan D, Moskowitz CH, Poppe M, Pro B, Winter JN, Yahalom J, Sundar H. Hodgkin lymphoma, version 2.2012 featured updates to the NCCN guidelines. Journal of the National Comprehensive Cancer Network. 2012;10(5):589–597. doi: 10.6004/jnccn.2012.0061. [DOI] [PubMed] [Google Scholar]
- 16.Herbst C, Rehan FA, Skoetz N, Bohlius J, Brillant C, Schulz H, Monsef I, Specht L, Engert A. Chemotherapy alone versus chemotherapy plus radiotherapy for early stage Hodgkin lymphoma. The Cochrane Database of Systematic Reviews. 2011;2:CD007110. doi: 10.1002/14651858.CD007110.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Bachanova V, Connors JM. Hodgkin Lymphoma in Pregnancy. Current Hematologic Malignancy Reports. 2013;8(3):211–217. doi: 10.1007/s11899-013-0163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sagan D, Semczuk A, Lampka E. Combination chemotherapy for Hodgkin’s lymphoma during pregnancy: Favorable outcome for mother and child. Journal of Obstetrics and Gynaecology Research. 2010;36(4):882–886. doi: 10.1111/j.1447-0756.2010.01249.x. [DOI] [PubMed] [Google Scholar]
- 19.Ryu RJ, Eyal S, Kaplan HG, Akbarzadeh A, Hays K, Puhl K, Easterling TR, Berg SL, Scorsone KA, Feldman EM, Umans JG, Miodovnik M, Hebert MF. Pharmacokinetics of doxorubicin in pregnant women. Cancer Chemotherapy and Pharmacology. 2014;73(4):789–797. doi: 10.1007/s00280-014-2406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tracy TS, Venkataramanan R, Glover DD, Caritis SN. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. American Journal of Obstetrics and Gynecology. 2005;192(2):633–639. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 21.Kulo A, Peeters MY, Allegaert K, Smits A, Hoon J, Verbesselt R, Lewi L, Velde M, Knibbe CAJ. Pharmacokinetics of paracetamol and its metabolites in women at delivery and post-partum. British Journal of Clinical Pharmacology. 2013;75(3):850–860. doi: 10.1111/j.1365-2125.2012.04402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davison JM, Dunlop W, Ezimokhai M. 24-hour creatinine clearance during the third trimester of normal pregnancy. British Journal of Obstetrics and Gynaecology. 1980;87(2):106–109. doi: 10.1111/j.1471-0528.1980.tb04501.x. [DOI] [PubMed] [Google Scholar]
- 23.Davison JM, Noble MC. Serial changes in 24 hour creatinine clearance during normal menstrual cycles and the first trimester of pregnancy. British Journal of Obstetrics and Gynaecology. 1981;88(1):10–17. doi: 10.1111/j.1471-0528.1981.tb00930.x. [DOI] [PubMed] [Google Scholar]
- 24.Iwamoto T, Hiraku Y, Okuda M, Kawanishi S. Mechanism of UVA-dependent DNA Damage Induced by An Antitumor Drug Dacarbazine in Relation to its Photogenotoxicity. Pharmaceutical Research. 2007;25(3):598–604. doi: 10.1007/s11095-007-9413-2. [DOI] [PubMed] [Google Scholar]
- 25.Beal DD, Skibba JL, Whitnable KK, Bryan GT. Effects of 5-(3,3-dimethyl-1-triazeno)imidazole-4-carboxamide and its metabolites on Novikoff hepatoma cells. Cancer Research. 1976;36(8):2827–2831. [PubMed] [Google Scholar]