Abstract
The tolerability of antidepressants is poorly characterized in children and adolescents with depressive and anxiety disorders. Among adverse events that affect the tolerability of antidepressants in youth is activation, a cluster of symptoms that represent a hyperarousal event characterized by impulsivity, restlessness, and/or insomnia. This cluster of symptoms was first identified as a side effect of selective serotonin and selective serotonin norepinephrine inhibitors (SSRIs and SSNRIs) in the early 1990s; however, activation remains poorly characterized in terms of prevalence, risk factors, and pathophysiology. This paper describes the pathophysiology of antidepressant-related activation, predictors of activation and clinical management in youth with depressive and anxiety disorders.
Keywords: Selective Serotonin Reuptake Inhibitor (SSRI), Selective Norepinephrine Reuptake Inhibitor (SNRI), antidepressants, adverse events, activation
Introduction
Despite the efficacy of selective serotonin reuptake inhibitors (SSRIs) and selective serotonin norepinephrine reuptake inhibitors (SSNRIs) in youth with anxiety1,2 and depressive disorders,3–7 antidepressant tolerability remains poorly characterized. The tolerability of these medications is related to their side effect profiles, and class-specific side effects occur frequently in antidepressant-treated pediatric patients.1,8,9 Indeed, adverse events associated with antidepressant treatment received considerable attention in the lay media, following the United States Food and Drug Administration (USFDA) implementing a black box warning in 2004 that highlighted one potential adverse event, treatment-associated suicidality.10 However, other antidepressant-related adverse events have received significantly less attention, despite the fact that the tolerability of these medications represents a significant concern among pediatricians and may subtend qualitative factors that increase pediatricians’ reluctance to utilize these medications.11 Antidepressant-related adverse events affect adherence, increase the likelihood of medication discontinuation and potentiate symptomatic impairment in youth.12 One particular adverse event, activation, is recognized by pediatricians as problematic and limits the use of antidepressant medications in the primary care setting.11 Activation represents a hyperarousal event that is typically characterized by specific symptoms including an increase in activity, impulsivity, disinhibition, restlessness and insomnia.13 This paper focuses on activation-related adverse events as well as risk factors, phenomenology, pathophysiology, temporal course and management of adverse events.
What is Activation?
Despite the recognition of antidepressant-related activation as a risk factor for medication discontinuation and suicidality, the activation syndrome lacks a clear definition.2,14 Currently, several symptoms are considered to be activation-related adverse events: disinhibition, impulsivity, insomnia, restlessness, hyperactivity, and irritability. Moreover, these symptoms frequently co-occur. This overlap among activation cluster symptoms complicates the assessment and measurement of activation. In fact, only recently has a scale been developed to assess the activation syndrome. This scale, the Treatment-Emergent Activation and Suicidality Assessment Profile (TEASAP),15 is a parent-rated scale consisting of five subscales: irritability, akathisia, disinhibition/impulsivity, mania, and self-injury/suicidality15 and includes items from other rating scales that measure these symptoms: suicidality—Columbia Suicide Severity Scale;16 akathisia—Barnes Akathisia Scale) and mania—Young Mania Rating Scale.17 At present, the TEASAP has been utilized to track activation-related adverse events in antidepressant-treated pediatric patients with obsessive compulsive disorder (OCD) and is being used in two prospective, randomized controlled trials of the SSRI escitalopram in the treatment of adolescents with generalized anxiety disorder (NCT02818751) and in adolescents with depressive and anxiety disorders who are at risk for developing bipolar disorder (NCT02553161).
The Temporal Course of Activation in Antidepressant-Treated Children and Adolescents
Antidepressant-related activation emerges early in treatment or following an increase in dose18 and symptoms resolve when the antidepressant dose is decreased or when the antidepressant is discontinued.13,19 This temporal pattern is consistent with one prospective study of the SSRI fluvoxamine in which higher plasma fluvoxamine concentrations were associated with a greater likelihood of activation. The rate of symptom resolution relates to the rate of activation symptoms onset.19 In a retrospective review of SSRI-treated children with major depressive disorder (MDD) or obsessive-compulsive disorder (OCD, N=82), 22% (n=18) of patients developed “treatment emergent psychiatric adverse events,” many of which were activation-related (e.g., irritability (15%, n=12), anxiety (10%, n=8), mania (6%, n=5), aggression (1%, n=1), and insomnia (17%, n=14)). No statistically significant disorder-specific differences in these psychiatric adverse events were observed and all psychiatric adverse events remitted following discontinuation of the SSRI. Further, nearly half (44%, n=8) of patients with a prior antidepressant-related adverse event developed another psychiatric adverse event upon re-exposure to an SSRI.19
How Common is Antidepressant-Related Activation in Youth?
Across studies of SSRIs in youth, adverse events (including activation-related events) are frequently associated with discontinuation.20,21,22,23,24 In a placebo-controlled study of fluoxetine in pediatric anxiety disorders, 5 of the 7 patients experiencing activation-related symptoms (e.g, excitement, giddiness, or disinhibition) discontinued treatment due to these symptoms.25 Even when activation-related symptoms do not result in discontinuation, at least one prospective study of sertraline in pediatric patients with OCD revealed that the presence of activation symptoms decreased treatment response.12
Antidepressant-Induced Activation and Suicidality
While activation in and of itself is problematic and results in a greater likelihood of discontinuing treatment, activation may also be linked to suicidality. The potential for activation symptoms as predictors of suicidal ideation has been proposed, although conflicting evidence exists to support such a link. A 2004 USFDA review of placebo-controlled clinical trials of SSRIs in pediatric patients reported that patients reporting agitation or hostility as adverse events were two to three times more likely to experience suicidal ideation or behavior.26 However, results from Treatment for Adolescents With Depression Study (TADS), a 36 week randomized clinical trial to evaluate the effectiveness of fluoxetine, cognitive-behavior therapy or a combination of treatments in adolescents aged 12–17 years, do NOT support an association between activation symptoms (specifically insomnia, irritability, or mania) and suicidal behavior. In the study, only one patient experienced symptoms of behavioral activation (insomnia, mood lability and irritability) in the two weeks prior to a suicidal event.27 At present, the relationship between antidepressant-induced activation and suicidality remains unclear. The limited and inconsistent evidence for determining a clinically important relationship between activation and suicidal behavior warrants further evaluation.
Activation in Children and Adolescents at Risk for Developing Bipolar Disorder
Some,28,29 but not all,30 reports suggest that SSRIs decrease the age of onset of manic spectrum symptoms in susceptible individuals.31 Given this potential association, some have conceptualized activation and other hyperarousal events as related to the pathophysiology of mood dysregulation and bipolar disorder. Understanding the prevalence of activation in children and adolescents at risk for developing bipolar disorder represents a particularly important area of research. In a longitudinal study of children and adolescents who were at risk for developing bipolar disorder (N=118), high rates of antidepressant related adverse events were common.32 Many of these adverse events were consistent with activation. In general, younger patients were more likely to experience antidepressant-induced adverse events (including activation and manic symptoms) compared to older patients and the likelihood of antidepressant discontinuation secondary to an adverse event was inversely associated with age.32 Martin and colleagues (2004)28 observed that in patients 5 to 29 years of age (N=87,920), younger age was associated with a higher risk for antidepressant associated manic symptoms. Regarding the presence of baseline and treatment-emergent manic symptoms in pediatric patients, SSRI-resistant MDD was recently identified as a specific risk factor for poor outcome.33 However, in our study of youth at risk for developing bipolar disorder, only trends toward higher irritability and motor hyperactivity were observed in patients who subsequently developed adverse events with antidepressant treatment consistent with the notion that sub-syndromal manic symptoms (or behavioral activation) predict poor treatment response across diagnoses.34 Finally, in youth who are risk for developing bipolar disorder, comorbidity contributes to the likelihood of antidepressant adverse events leading to discontinuation. In this regard, the presence of ADHD was associated with an odds ratio of 2 for an antidepressant-related adverse event, but secondary to the small sample size this unadjusted odds ratio should be interpreted with caution.32
Activation in Children and Adolescents with ADHD
Given the symptomatic overlap between ADHD and activation symptoms, there has been speculation that the risk of activation may be increased in pediatric patients with ADHD. Indeed, some evidence suggests that stimulant treatment may precipitate or exacerbate externalizing symptoms in susceptible individuals (e.g., oppositional behavior) and that amphetamine-based stimulants may be uniquely associated with treatment emergent irritability.35 In this regard, a recent meta-analysis of irritability in stimulant treated youth found amphetamine derivatives to be associated iwht a significant increase in irritability (risk ratio = 2.9, CI: 1.26–6.71, p<0.01) whereas methylphenidate-based medications decreased irritability relative to placebo (risk ratio = 0.89, CI: 0.82–0.96, p<0.004).35 Further, our group reported that bipolar adolescents with a history of stimulant exposure had an earlier age at onset of bipolar disorder than those without prior stimulant exposure, independent of co-occurring ADHD.36 Bipolar adolescents treated with at least two stimulant medications had a younger age at onset compared with those who were treated with one stimulant. A behavioral sensitization model may account for this observation. A retrospective case review found that 58% of adolescents with bipolar disorder who were treated with antidepressants or psychostimulants met criteria for treatment-emergent mania within an average of 14 days, and treatment-emergent mania was more frequently observed following treatment with antidepressants than stimulants (44% vs. 18%).37
Direct evidence regarding this putative association is limited and conflicted. A 6-week open-label study of fluoxetine in pediatric patients with ADHD (n=19; age range: 7–15 years; mean age: 10.9 years), only one patient reported an activation-related adverse event (akathisia).38 In an 8-week double blind study of methylphenidate with fluvoxamine or placebo in pediatric patients age 6 to 17 co-occurring ADHD and generalized, separation and/or social anxiety disorder, activation-related adverse events did not occur at a significantly higher rate in the stimulant with fluvoxamine arm compared to the stimulant with placebo arm (restlessness: 33% vs. 20%, p=0.39; behavioral activation 33% vs. 20%, p=0.39; mood liability: 20 vs. 40%, p=0.29).39
Risk Factors for Activation
Diagnosis
Meta-analytic evaluations of antidepressants in youth have yielded inconsistent results with regard to the risk of activation cluster symptoms associated with antidepressants. A recent meta-analysis failed to find significant differences in the relative risk of “excessive mood elevation” observed in studies (κ=42) of patients with anxiety (κ=17) or depressive disorders (κ=25).40 In this meta-analysis, the “mood elevation” symptoms included insomnia, restlessness, agitation, irritability, sustained anger and mania—a broad definition of activation. The relative risk of “excessive mood elevation” did not differ among trials involving depressed or anxious youth (p=0.546) suggests that the risk of the activation syndrome does not differ between the two diagnostic groups (i.e., depressive disorders compared to anxiety disorders),40 although other co-morbidity patterns (e.g., pre-treatment manic symptoms, ADHD) may contribute to an increased risk of activation.
Age
When compared to adolescents, children experience more total adverse events, more activation-related adverse events, and have higher numbers of discontinuation due to activation-related adverse events when treated with antidepressants.41,42 In the Child/Adolescent Anxiety Multimodal Study,24 the total number of psychiatric adverse events was significantly higher in children than adolescents (31.7% vs. 23.1%, p<0.05), including a number of activation-related adverse events (disinhibition: 5.8% vs. 0%; increased motor activity 4.1% vs. 0.8%; restless/fidgety: 2.8% vs. 1.6%; significance thresholds for individual events not reported). Similar rates of insomnia were observed between children and adolescents while anxiety and agitation were numerically more common in adolescents compared to children.41 A sample of youth with OCD (n=17), aged 7–17 years of age, who were treated with fluvoxamine, activation occurred in 3/17 patients and all of these patients were <12 years of age.43 Similarly, in a retrospective study of young children (N=39) treated with SSRIs who were <7 years of age, approximately 20% (n=8) experienced behavioral activation (e.g., irritability), the most common adverse event, and the reason for discontinuation in 6 out of 7 early termination patients.44 Finally, Safer and Zito26 observed that SSRI-related restlessness was less common in older children compared with younger children.
Drug Concentration
Higher plasma concentrations of SSRIs as well as rapid increases in SSRI plasma concentrations may represent a risk factor for activation-related adverse events. In a randomized controlled trial of fluvoxamine for pediatric anxiety disorders in which 10 of 22 patients (45%) experienced symptoms of activation compared to only 1 of 23 (4%) who received placebo, differences in plasma fluvoxamine concentrations were noted between those patients with activation-related adverse events and those who did not experience activation. Plasma fluvoxamine concentrations (at endpoint, 8 weeks), in this randomized controlled trial of flexibly-dosed fluvoxamine, were higher in patients who experienced an activation-related adverse event (381.7±232.0 ng/mL) compared to those who did not (127.5±68.5 ng/mL; p=0.04) (Figure 3). However, it is of interest that the medication dose between patients who experienced activation and those who did not was not significantly different (activation: 134±22 mg/day; no activation 150±22 mg/day, p=0.63). This difference between dose and plasma drug concentration underscores the importance of “unknown” factors affecting drug concentration and also the relationship between drug concentration and activation (Figure 1). Several SSRIs are used to treat adolescents with MDD and anxiety disorders are metabolized by cytochromes. There is significant ethnic and inter-individual variability, polymorphisms in these cytochromes that have been associated with altered clearance.45 Indeed, in a pharmacokinetic study of paroxetine in youth, the patient with the lowest clearance of paroxetine, who was also a poor-metabolizer with regard to P4502D6. The patient developed severe activation resulting in discontinuation of paroxetine.45
FIGURE 3.
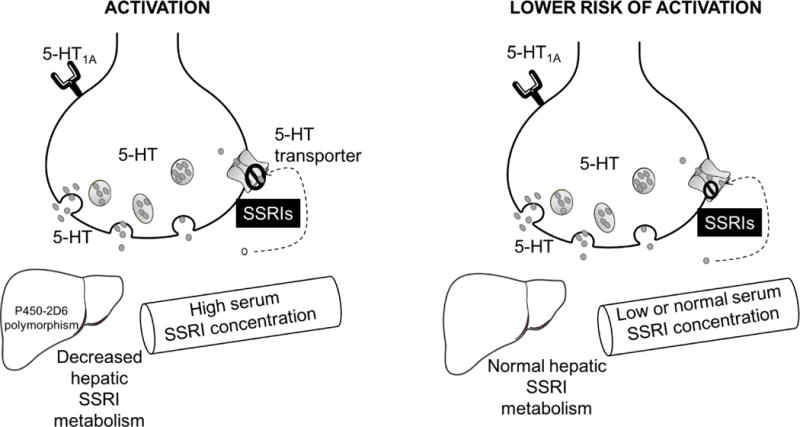
Patient-specific pharmacologic factors that have been implicated in the development of activation-related adverse events. While many SSRIs and SNRIs are metabolized through cytochrome P450 systems, P4502D6 is primarily responsible for metabolism of fluoxetine, paroxetine and venlafaxine. Importantly, 5–10% of the Caucasian populations are poor metabolizers which may result in increased concentration of these SSRIs and increases the blockade of the serotonin (5-HT) transporter.
FIGURE 1. Factors that may contribute to activation-related adverse events in pediatric patients.
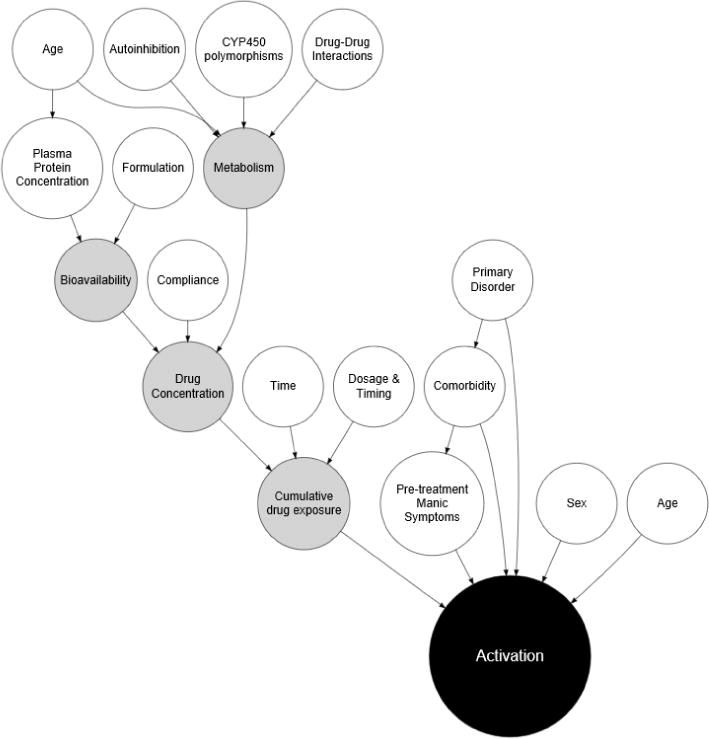
In this functional causal diagram, white circles represent clinical and demographic risk factors as well as attributes of the medication or its interactions. Gray circles reflect intrinsic metabolism and pharmacologic factors.
Dose Titration
To date, one double blind, randomized controlled study of 56 children and adolescents (mean age: 11.7±3.3 years) with OCD evaluated SSRI (sertraline) dose on activation and examined the impact of activation and treatment outcome. In this placebo-controlled study, children and adolescents were randomized to receive either (1) slow or (2) regular sertraline titration over 9 weeks. For patients randomized to slow titration sertraline was initiated at 25 mg/day and was continued at this dose for the first two weeks, then sertraline was titrated to 50 mg/day for the next two weeks followed by, 75 mg/day for the 5th and 6th week of treatment, 100 mg/day for week 7, then 150 mg/day for week 8, and 200 mg/day for week 9. Regular titration consisted of initiating sertraline at 25 mg/day and increasing, as tolerated to 200 mg/day over 9 weeks. In both treatment groups, if patients experienced adverse effects (including activation), the sertraline dose could be decreased. Interestingly, activation occurred at similar rates in both groups and activation appeared to moderate treatment outcome. In this regard, 74% of those with low severity of activation syndrome, as assessed by Treatment-Emergent Activation and Suicidality Assessment Profile (TEASAP), 37% of those with average activation syndrome, and 5% of those with high activation syndrome experienced a 50% reduction in obsessive-compulsive symptoms. Of the 5 activation cluster symptoms (i.e., irritability, akathisia, disinhibition, mania and self-harm), only an increase in irritability from one session to the next predicted an increase in obsessive-compulsive symptoms.12
Pharmacogenomic Factors
The serotonin transporter promotor (SLC6A4 or 5-HT) exists as a long or short variant (the result of a 44 base pair insertion or deletion), which results in an individual’s genotype being: homozygous short (ss), heterozygous (sl), or homozygous long (ll). The short allele may be associated with an increased risk of depressive and anxiety disorders and may—in some samples—be associated with decreased response to SSRIs (see Wehry et al, 2017–this issue).46,47 However, given that these polymorphisms relate to the ‘site of action’ for antidepressants, there has been some investigation of their role in SSRI/SNRI related adverse effects. Recently, an 8-week open-label trial of citalopram in pediatric patients aged 7 to 18 with MDD and/or anxiety disorders, patients who had an ss genotype had a smaller decrease in CDRS-R scores than the sl/ll genotype (p=0.04) but also reported less treatment-related agitation relative to patients with the sl/ll genotype (6.3 vs. 32.8%, p=0.05).47 In a study of 64 offspring of bipolar parents and 51 healthy controls, within the subset of un-medicated bipolar offspring, those with the ss and ls genotype had higher anxiety than those with the ll genotype.48 In an open label study of 87 pediatric outpatients (7–18 years of age) with MDD and/or anxiety disorders treated citalopram for 8 weeks, three polymorphisms were analyzed: 5-HTR21 T102C, 5-HTR1Dβ G861C, and 5-HTR2C G86C. Only the 5-HTR1Dβ G861C was associated with and increased risk of activation, with the CC genotype experiencing significantly more agitation (71.4%) compared to the CG (33%) and GG (18.1%) genotypes (p=0.02).49
Medication Specific Factors
The mechanism of SSRIs and their characteristic delayed onset of therapeutic benefit may relate to activation. Specifically, during the 2–4 week period that often precedes clinical benefit, increased synaptic 5-HT may activate presynaptic 5HT1A receptors to inhibit release of 5-HT.50 Such paradoxical decreases in serotonergic tone has been associated with impulsive and violent behavior similar to that of activation symptoms in some samples.51,52 Therefore, the early onset of activation-related adverse events may be linked to 5HT1A-associated depression of 5-HT release. In support of this, in a mouse model of antidepressant-induced activation, administration of a 5-HT1A antagonist, decreased SSRI-related “anxiogenic behavior.”53 This finding is of interest in light of the fact that modulation of the 5-HT1A receptor may underlie akathisia associated with some second generation antipsychotics (e.g., compared to first generation antipsychotics)54 and given observations that 5-HT1A binding is decreased in patients with anxiety disorders.55,56 This study also demonstrated an inverse relationship between time to reach maximum drug concentration and “anxiogenic behavior” in the rodent model of antidepressant-induced activation (Figure 3).
Mounting evidence suggests that dietary deficits in omega-3 (n-3) polyunsaturated fatty acids, including eicosapenaenoic acid (EPA) and docosahexaenoic acid (DHA), are associated with mood dysregulation.57 Similar to adults with mood disorders, children and adolescents with major depressive disorder58,59 exhibit blood n-3 polyunsaturated fatty acids deficits compared with healthy youth, and increasing blood n-3 polyunsaturated fatty acids levels has been found to promote SSRI response.60,61 Preclinical studies further indicate that n-3 fatty acid deficiency during development produces long-standing alterations in central serotonin neurotransmission62 that may modify the central actions of SSRI medications. For example, abnormal increases in behavioral activation were observed in n-3 fatty acid deficient rats following chronic fluoxetine treatment associated with alterations in 5-HT1A and alpha2A adrenergic receptor expression.63 The latter findings suggest that n-3 polyunsaturated fatty acids deficiency may promote behavioral activation in response to SSRI treatment by augmenting noradrenergic tone. N-3 polyunsaturated fatty acids have anti-inflammatory properties, and n-3 polyunsaturated fatty acids deficiency is associated with inflammation-induced irritability in human subjects with the TNF-α A-308G polymorphism.64 The latter study also found that SSRI treatment was associated with inflammation-induced irritability. Additional studies are warranted to characterize the role of n-3 polyunsaturated fatty acids status and inflammation in SSRI-induced activation.
Relative Risk of SSRI-Related Activation in Pediatric Mood and Anxiety Disorders
Across six published studies of antidepressants in non-OCD anxiety disorders, summarized by Strawn and colleagues,24,25,65–68 activation-related adverse events ranged from 3 to 55%. Given concerns that antidepressant related activation may depend on the disorder being treated, activation cluster (e.g., activation, restlessness, irritability, nervousness, or hypersensitivity) were compared between studies of SSRIs in youth with MDD and those with anxiety disorders. For trials of SSRIs in pediatric patients with anxiety disorders, the average relative risk of activation was 1.8 (range 0.7 [duloxetine] to 5.2 [paroxetine]) and the average number needed to harm (NNH) was 17.6 (range −71 [duloxetine] to 24 [sertraline]). By contrast in studies of these medications in adolescents with MDD,8 the average activation rates in depressed adolescents treated with SSRIs was 0.65–9.5% with the average relative risk of activation being 2 (range: 0.6 [fluoxetine] to 4.5 [fluoxetine]) (Figure 4). Regarding insomnia, a specific symptom within the activation cluster, similar relative risk was observed for the two disorders. In studies of pediatric patients with anxiety disorders who were treated with SSRIs, insomnia was reported in 8.3–19% of patients with the average relative risk of insomnia being 1.59 (range: 0.92 [fluvoxamine] to 2.31 [paroxetine]) and average NNH being 23 (range: −63 [fluvoxamine] to 26 [duloxetine]). By contrast, in studies of pediatric patients with MDD, 2.8–23.8% of patients reported insomnia, with the average relative risk of insomnia being 2 (range: 1.2 [citalopram] to 4.8 [fluoxetine]) and average NNH being 19 (range: 9 [paroxetine] to 53 [fluoxetine]). However, while there are numerical differences between the magnitude of relative risk across studies of pediatric patients with MDD and non-OCD anxiety disorders, factors other than the primary disorder under study may contribute to these differences. For example, patients with co-occurring MDD are excluded from most randomized controlled trials of antidepressants in pediatric patients with anxiety disorders. Anxiety disorders are often co-morbid in youth with MDD and this comorbidity does not generally result in the exclusion of patients with MDD from clinical trials of antidepressants in youth. Antidepressant treatment studies may under-represent the presence of activation in youth with anxiety disorders.
FIGURE 4.
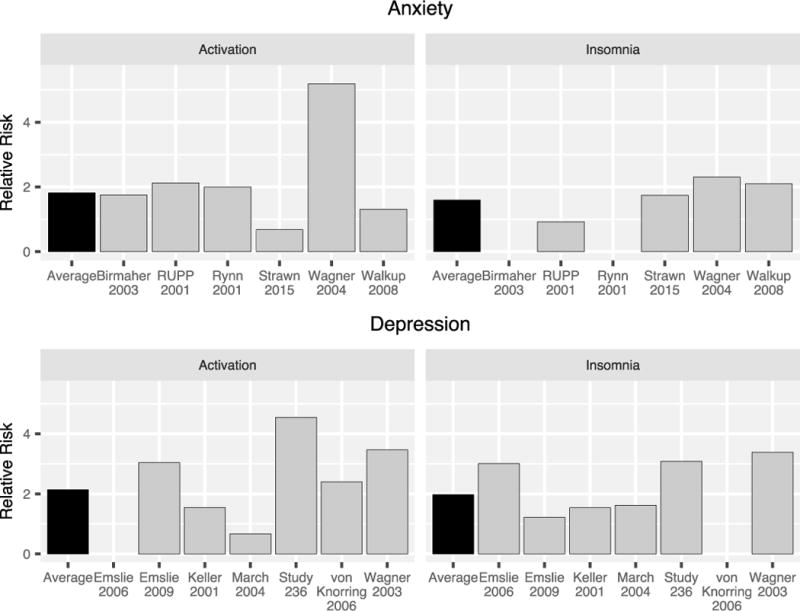
Relative risk of activation and insomnia across randomized controlled trials of SSRIs in pediatric patients with generalized, separation and or social anxiety disorders (top) or major depressive disorder (bottom). The average relative risk of an activation-related adverse event was 1.8 in studies of anxiety disorders and 2.1 in pediatric patients with MDD. The average relative risk of insomnia (black bars) was 1.6 in studies of anxiety disorders and 2 in studies of MDD.
Pathophysiology of Activation
The complex pathophysiology of antidepressant-induced activation likely involves genetic and neurochemical factors as well as circuit-level processes that interact with psychological and pharmacologic aspects of the patient and treatment (Figure 3).49 However, given the difficulty assessing and defining activation, it is not surprising that it has been difficult to elucidate the pathophysiology of activation with clarity. Some hypothesize that activation—like affective instability—represents a variant of the manic phase of bipolar disorder. Others propose that activation results of a developmental sensitivity to the increase in serotonergic tone associated with acute serotonin reuptake inhibition within arousal-regulating circuits. Based on a nascent literature implicating prefrontal-amygdala circuits in dysfunctional arousal, and studies that suggest that SSRIs are associated with functional changes within structures contained in these circuits, antidepressants may precipitate activation through facilitating effects within these circuits in vulnerable youth. It is noteworthy that studies of these same structures suggest that SSRIs may normalize activation within these regions.69,70 Thus, with regard to activation, it is likely that the relationship between activity within regions or circuits and treatment response as well as treatment-related adverse effects is complex and characterized by temporal and developmental nuance. It is possible that these antidepressant-related activation effects might only emerge at specific medication threshold concentrations (i.e., exposure); consistent with the observation that activation may be related to SSRI plasma levels in some studies.14 Specifically, antidepressants have been shown to initially increase and then decrease amygdala activity and increases prefrontal inhibition of the amygdala.71,72 Thus, the degree to which a particular antidepressant, in a particular patient, might potentiate vs dampen activity within this circuitry is not well understood. Some individuals (e.g., patients with a family history of bipolar disorder) may be primed to react to antidepressants within these arousal-regulating circuits.42 While the neurocircuitry of activation is unknown, findings from neuroimaging studies of antidepressant dysfunctional hyperarousal—primarily in youth with a family history of bipolar disorder—have been extrapolated to propose a similar neurocircuitry that may underlie activation. Decreased amygdala volumes73 and increased amygdala activation during emotional processing tasks74 have been implicated in the early development of chronic hyperarousal. 5-HT receptor blockade by SSRI administration increases amygdala reactivity to salient stimuli in a dose dependent manner in adolescents.75 While antidepressant administration to pediatric patients generally increases functional activity in prefrontal structures that inhibit the amygdala,76 there is a possibility that antidepressants may act differently in a limbic system that is primed, as may be the case for some patients who are at higher risk of developing antidepressant-related hyperarousal77,78
Management of Antidepressant-Related Activation
Only one study evaluated slow and standard titration strategies for sertraline dosing in youth with OCD.12 Case reports79,80 as well as a few controlled trials suggest that activation is more likely to occur with higher doses of SSRIs as well as rapid titration of antidepressants. Common clinical strategies for prevention of activation syndrome, as well as other side effects, include starting SSRIs at low doses with slow, planned titrations. Clinical practice guidelines (e.g., GLAD toolkit, www.glad-pc.org/)81 are available, that outline typical starting doses as well as suggestions for titration of medications in children. If a patient develops activation once he or she begins a higher dose of the medication, there is some evidence82 that decreasing total daily dose or stopping the medication can be effective in reducing this side effect. Because activation may be related to high plasma concentrations of SSRIs, switching to an extended-release form of medication (when available) may also be helpful in that the peak plasma drug concentration (i.e., Cmax) for the medication will generally be reduced with extended release. Another treatment strategy could be to switch to a different antidepressant, keeping in mind that patients who have experienced activation with one agent may be more likely to experience this side effect again. Pharmacotherapy to target specific symptoms within activation syndrome such as treating insomnia with melatonin is another option for managing symptoms. Compliance with medication should also be assessed; withdrawal symptoms may mimic the symptoms seen in activation syndrome in patients who do not consistently take their medications. Specifically, venlafaxine, which has a half-life of about 6 hours, has a significant discontinuation syndrome that includes insomnia, irritability and worsening anxiety. Paroxetine with its lack of active metabolites is more likely to cause significant discontinuation syndromes compared to other SSRIs.83
Discussion
Activation cluster symptoms represent a particularly important adverse effect that decreases the efficacy of antidepressant treatment in youth and increases the likelihood of antidepressant discontinuation. Moreover, our prior mixed-methods (i.e., quantitative/qualitative) evaluation of antidepressant prescribing in pediatricians in youth with anxiety and depression suggests that activation is widely recognized by pediatricians, but that pediatricians often feel uncomfortable managing this side effect.84 Current data suggest that, because activation is likely related to age and plasma level as well as to factors that may accentuate the action of an antidepressant (e.g., alterations in serotonin transporter expression), the likelihood of activation may be reduced by initiating antidepressant treatment at low doses, particularly in younger (e.g., prepubertal) patients and in those with a family history of bipolar disorder. Should activation emerge, pediatricians may wish to consider decreasing dose or hold the antidepressant dose; this strategy—in one prospective study and in clinical practice—resolves activation-related adverse events in most SSRI-treated pediatric patients.14
Risk factors for antidepressant-related activation are critically important for child health clinicians, particularly when knowledge of these risk factors is accompanied by an understanding of the impact of dosing and the temporal course of antidepressant-related activation. For example, in treating a pre-pubertal girl with generalized anxiety disorder and comorbid ADHD who might be at higher risk of developing antidepressant-related activation, a clinician might choose to initiate antidepressant treatment at a particularly low dose and might choose to titrate the antidepressant more slowly. A clinician treating this patient might consider more frequent monitoring of the patient and might aggressively utilize non-pharmacologic strategies to decrease specific anxiety and activation-related symptoms. For example, the clinician treating such a patient might consider melatonin (3–6 mg at bedtime or 0.5 mg after dinner and 2 mg at bedtime) and the use of a sleep diary for the management of her insomnia as well as psychotherapy to decrease family conflict and associated irritability in addition to ADHD-specific interventions to manage ADHD-related impulsivity and hyperactivity.
In sum, antidepressant-induced activation likely involves genetic and neurochemical factors as well as circuit-level processes that interact with psychological and pharmacologic aspects of the patient and treatment. This constellation of adverse events frequently emerges early in treatment or following an increase in dose,18 but can be effectively managed. Activation frequently resolves when the antidepressant dose is decreased or when the antidepressant is discontinued13,19 and should be monitored, particularly in patients who may be at increased risk for developing activation. Finally, several practices including starting SSRIs at low doses with slow, planned titrations may decrease the likelihood of treatment-related activation
FIGURE 2.
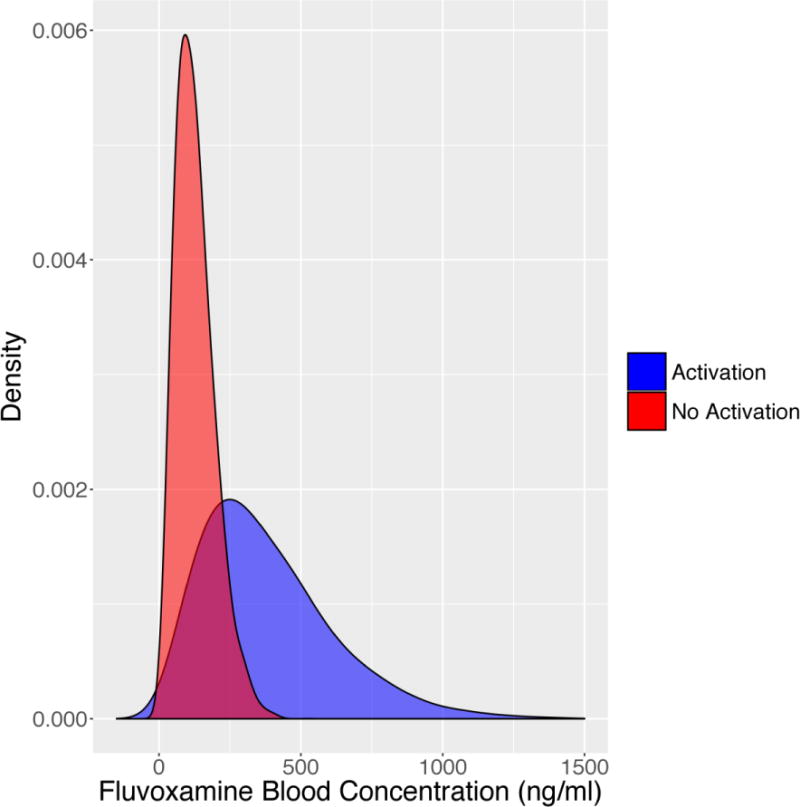
Modeled density (probability) of activation of fluvoxamine in patients who developed activation-related adverse effects compared to those who did not. Mean and standard deviation were extracted from Reibblatt et al, 2009 and a t-distribution was modeled from bootstrapped fluvoxamine plasma concentrations for patients with (blue) and without (red) activation adverse events. Mean fluvoxamine blood levels were higher in subjects that experienced an activation-related adverse event (p=0.04).
Acknowledgments
Drs. Strawn, DelBello and McNamara received support from the NIMH (JRS: MH106037-01; RKM and MPD: MH105464, MH107378 and MH097818).
Disclosures: Dr. Strawn has received research support from the National Institutes of Health, Edgemont, Forest Research Laboratories/Allergan, Lundbeck, Neuronetics, and Shire. He has received material support from Assurex/Genesight—a company which performs pharmacogenomic testing. Finally, Dr. Strawn receives royalties, from Springer Publishing, for the publication of two textbooks as well as an UpToDate chapter. Dr. McNamara has received research support from NARSAD, Martek Biosciences Corporation, Ortho-McNeil Janssen, AstraZeneca, Eli Lilly, Kyowa Hakko Bio Co., LTD, Royal DSM Nutritional Products, LLC, and the Inflammation Research Foundation (IRF), was a member of the IRF scientific advisory board, and served as a paid consultant for VAYA Pharma Inc., and Vifor Pharma Inc. DelBello has received research support from the National Institute of Mental Health, Pfizer, Otsuka, Martek, Novartis, Lundbeck, Supernus, Sunovion, Johnson and Johnson, Janssen, and Shire. She has received Consulting/Advisory Board/Honoraria/Travel support from Pfizer, Lundbeck, Sunovian, Akili, Neuronetics, Johnson and Johnson Supernus and Otsuka.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The remaining authors reported no conflicts of interests.
Contributor Information
Marissa J. Luft, University of Cincinnati, College of Medicine Cincinnati, Ohio 45267.
Martine Lamy, Cincinnati Children’s Hospital Medical Center Cincinnati, Ohio 45219.
Melissa P. DelBello, University of Cincinnati, College of Medicine Cincinnati, Ohio 45267.
Robert K. McNamara, University of Cincinnati, College of Medicine Cincinnati, Ohio 45267.
Jeffrey R. Strawn, University of Cincinnati, College of Medicine Cincinnati, Ohio 45267.
References
- 1.Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683–1696. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]
- 2.Strawn JR, Welge JA, Wehry AM, Keeshin B, Rynn MA. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32(3):149–157. doi: 10.1002/da.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walkup JT. Antidepressant efficacy for depression in children and cdolescents: Industry- and NIMH-funded studies. Am J Psychiatry. 2017:1–8. doi: 10.1176/appi.ajp.2017.16091059. [DOI] [PubMed] [Google Scholar]
- 4.Cheung AH, Emslie GJ, Mayes TL. The use of antidepressants to treat depression in children and adolescents. CMAJ. 2006;174:193–200. doi: 10.1503/cmaj.050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.March J, Silva S, Curry J, et al. The treatment for adolescents with depression Study (TADS): outcomes over 1 year of naturalistic follow-up. Am J Psychiatry. 2009;166(10):1141–1149. doi: 10.1176/appi.ajp.2009.08111620. [DOI] [PubMed] [Google Scholar]
- 6.Kennard B, Silva S, Vitiello B, et al. Remission and Residual Symptoms After Short-Term Treatment in the Treatment of Adolescents with Depression Study (TADS) J Am Acad Child Adolesc Psychiatry. 2006;45(12):1404–1411. doi: 10.1097/01.chi.0000242228.75516.21. [DOI] [PubMed] [Google Scholar]
- 7.March J, Silva S, Petrycki S, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA. 2004;292(7):807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- 8.Cipriani A, Zhou X, Giovane C Del, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016:1263–1271. doi: 10.1016/S0140-6736(16)30385-3. [DOI] [PubMed] [Google Scholar]
- 9.Strawn JR, Welge Ja, Wehry AM, Keeshin B, Rynn MA. Efficacy and Tolerability of Antidepressants in Pediatric Anxiety Disorders: a Systematic Review and Meta-Analysis. Depress Anxiety. 2015;32(3):149–157. doi: 10.1002/da.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):332–339. doi: 10.1001/archpsyc.63.3.332. [DOI] [PubMed] [Google Scholar]
- 11.Tulisiak AK, Klein JA, Harris E, et al. Antidepressant Prescribing by Pediatricians: A Mixed-Methods Analysis. Curr Probl Pediatr Adolesc Health Care. 2017;47(1):15–24. doi: 10.1016/j.cppeds.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid AM, McNamara JPH, Murphy TK, et al. Side-effects of SSRIs disrupt multimodal treatment for pediatric OCD in a randomized-controlled trial. J Psychiatr Res. 2015;71:140–147. doi: 10.1016/j.jpsychires.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riddle MA, King RA, Hardin MT, et al. Behavioral Side Effects of Fluoxetine in Children and Adolescents. J Child Adolesc Psychopharmacol. 1990;1(3):193–198. [Google Scholar]
- 14.Reinblatt SP, DosReis S, Walkup JT, Riddle MA. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119–126. doi: 10.1089/cap.2008.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bussing R, Murphy TK, Storch EA, et al. Psychometric properties of the Treatment-Emergent Activation and Suicidality Assessment Profile (TEASAP) in youth with OCD. Psychiatry Res. 2013;205(3):253–261. doi: 10.1016/j.psychres.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133(11):429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 18.Reinblatt SP, DosReis S, Walkup JT, Riddle MA. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119–126. doi: 10.1089/cap.2008.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilens TE, Biederman J, Kwon A, et al. A systematic chart review of the nature of psychiatric adverse events in children and adolescents treated with selective serotonin reuptake inhibitors. J Child Adolesc Psychopharmacol. 2003;13(2):143–152. doi: 10.1089/104454603322163862. [DOI] [PubMed] [Google Scholar]
- 20.Emslie GJ, Wagner KD, Kutcher S, et al. Paroxetine treatment in children and adolescents with major depressive disorder: a randomized, multicenter, double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2006;45(6):709–719. doi: 10.1097/01.chi.0000214189.73240.63. [DOI] [PubMed] [Google Scholar]
- 21.Emslie GJ, Heiligenstein JH, Wagner KD, et al. Fluoxetine for acute treatment of depression in children and adolescents: a placebo-controlled, randomized clinical trial. J Am Acad Child Adolesc Psychiatry. 2002;41:1205–1215. doi: 10.1097/00004583-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Emslie GJ, Rush J, Weinberg WA, et al. A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Arch Gen Psychiatry. 1997;54(11):1031–1037. doi: 10.1001/archpsyc.1997.01830230069010. [DOI] [PubMed] [Google Scholar]
- 23.Wagner KD, Robb AS, Findling RL, Jin J, Gutierrez MM, Heydorn WE. A randomized, placebo-controlled trial of citalopram for the treatment of major depression in children and adolescents. Am J Psychiatry. 2004;161(6):1079–1083. doi: 10.1176/appi.ajp.161.6.1079. [DOI] [PubMed] [Google Scholar]
- 24.Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359:2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birmaher B, Axelson Da, Monk K, et al. Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2003;42(4):415–423. doi: 10.1097/01.CHI.0000037049.04952.9F. [DOI] [PubMed] [Google Scholar]
- 26.Safer DJ, Zito JM. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: children versus adolescents. J Child Adolesc Psychopharmacol. 2006;16(1–2):159–169. doi: 10.1089/cap.2006.16.159. [DOI] [PubMed] [Google Scholar]
- 27.Vitiello B, Silva SG, Rohde P, et al. Suicidal events in the Treatment for Adolescents With Depression Study (TADS) J Clin Psychiatry. 2009;70(5):741–747. doi: 10.4088/jcp.08m04607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin A, Young C, Leckman JF, Mukonoweshuro C, Rosenheck R, Leslie D. Age effects on antidepressant-induced manic conversion. Arch Pediatr Adolesc Med. 2004;158(8):773–780. doi: 10.1001/archpedi.158.8.773. [DOI] [PubMed] [Google Scholar]
- 29.Cicero D, El-Mallakh RS, Holman J, Robertson J. Antidepressant exposure in bipolar children. Psychiatry. 2003;66(4):317–322. doi: 10.1521/psyc.66.4.317.25437. [DOI] [PubMed] [Google Scholar]
- 30.Chang KD, Saxena K, Howe M, Simeonova D. Psychotropic medication exposure and age at onset of bipolar disorder in offspring of parents with bipolar disorder. J Child Adolesc Psychopharmacol. 2010;20:25–32. doi: 10.1089/cap.2009.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldsmith M, Singh M, Chang K. Antidepressants and psychostimulants in pediatric populations: Is there an association with mania? Pediatr Drugs. 2011;13:225–243. doi: 10.2165/11591660-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strawn JR, Adler CM, McNamara RK, et al. Antidepressant tolerability in anxious and depressed youth at high risk for bipolar disorder: a prospective naturalistic treatment study. Bipolar Disord. 2013;16(5):523–530. doi: 10.1111/bdi.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maalouf FT, Porta G, Vitiello B, et al. Do sub-syndromal manic symptoms influence outcome in treatment resistant depression in adolescents? A latent class analysis from the TORDIA study. J Affect Disord. 2012;138(1–2):86–95. doi: 10.1016/j.jad.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strawn JR, Welge JA, Wehry AM, Keeshin B, Rynn MA. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2014;9:1–9. doi: 10.1002/da.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cox EQ, Sowa NA, Meltzer-Brody SE, Gaynes G. Risk of irritability with psychostimulant treatment in children with ADHD: a meta-analysis. J Clin Psychiatry. 2016 Feb;77:1189–1200. [Google Scholar]
- 36.DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord. 2001;3(2):53–57. doi: 10.1034/j.1399-5618.2001.030201.x. [DOI] [PubMed] [Google Scholar]
- 37.Faedda GL, Baldessarini RJ, Glovinsky IP, Austin NB. Treatment-emergent mania in pediatric bipolar disorder: a retrospective case review. J Affect Disord. 2004;82(1):149–158. doi: 10.1016/j.jad.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Barrickman L, Noyes R, Kuperman S, Schumacher E, Verda M. Treatment of ADHD with fluoxetine: a preliminary trial. J Am Acad Child AdolescPsychiatry. 1991;30:762–767. [PubMed] [Google Scholar]
- 39.Abikoff H, McGough J, Vitiello B, et al. Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(5):418–427. doi: 10.1097/01.chi.0000155320.52322.37. [DOI] [PubMed] [Google Scholar]
- 40.Offidani E, Fava GA, Tomba E, Baldessarini RJ. Excessive mood elevation and behavioral activation with antidepressant treatment of juvenile depressive and anxiety disorders: a systematic review. Psychother Psychosom. 2013;82:132–141. doi: 10.1159/000345316. [DOI] [PubMed] [Google Scholar]
- 41.Rynn MA, Walkup JT, Compton SN, et al. Child/Adolescent Anxiety Multimodal Study: evaluating safety. J Am Acad Child Adolesc Psychiatry. 2015;54(3):180–190. doi: 10.1016/j.jaac.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strawn JR, Adler CM, McNamara RK, et al. Antidepressant tolerability in anxious and depressed youth at high risk for bipolar disorder: a prospective naturalistic treatment study. Bipolar Disord. 2014;16(5):523–530. doi: 10.1111/bdi.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris E, Eng HY, Kowatch R, Delgado SV, Saldaña SN. Disinhibition as a side Effect of treatment with fluvoxamine in pediatric patients with obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. 2010;20(4):347–353. doi: 10.1089/cap.2009.0126. [DOI] [PubMed] [Google Scholar]
- 44.Zuckerman ML, Vaughan BL, Whitney J, et al. Tolerability of selective serotonin reuptake inhibitors in thirty-nine children under age seven: a retrospective chart review. J Child Adolesc Psychopharmacol. 2007;17(2):165–174. doi: 10.1089/cap.2007.0086. [DOI] [PubMed] [Google Scholar]
- 45.Findling RL, Nucci G, Piergies AA, et al. Multiple dose pharmacokinetics of paroxetine in children and adolescents with major depressive disorder or obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31(6):1274–1285. doi: 10.1038/sj.npp.1300960. [DOI] [PubMed] [Google Scholar]
- 46.Luddington NS, Mandadapu A, Husk M, El-Mallakh RS. Clinical implications of Genetic variation in the serotonin transporter promoter region. Prim Care Companion J Clin Psychiatry. 2009;11(3):93–102. doi: 10.4088/pcc.08r00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kronenberg S, Apter A, Brent D, et al. Serotonin transporter polymorphism (5-HTTLPR) and citalopram effectiveness and side effects in children with depression and/or anxiety disorders. J Child Adolesc Psychopharmacol. 2007;17(6):741–750. doi: 10.1089/cap.2006.0144. [DOI] [PubMed] [Google Scholar]
- 48.Park M-H, Sanders E, Howe M, et al. Association of anxiety symptoms in offspring of bipolar parents with serotonin transporter-linked polymorphic region (5-HTTLPR) genotype. J Child Adolesc Psychopharmacol. 2015;25(6):458–466. doi: 10.1089/cap.2014.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amitai M, Kronenberg S, Carmel M, et al. Pharmacogenetics of citalopram-related side effects in children with depression and/or anxiety disorders. J Neural Transm. 2016;123(11):1347–1354. doi: 10.1007/s00702-016-1585-7. [DOI] [PubMed] [Google Scholar]
- 50.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38(8):1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 51.Kruesi MJ, Rapoport JL, Hamburger S, et al. Cerebrospinal fluid monoamine metabolites, aggression, and impulsivity in disruptive behavior disorders of children and adolescents. Arch Gen Psychiatry. 1990;47(5):419–426. doi: 10.1001/archpsyc.1990.01810170019003. [DOI] [PubMed] [Google Scholar]
- 52.Placidi GPA, Oquendo MA, Malone KM, Huang YY, Ellis SP, Mann JJ. Aggressivity, suicide attempts, and depression: relationship to cerebrospinal fluid monoamine metabolite levels. Biol Psychiatry. 2001;50(10):783–791. doi: 10.1016/s0006-3223(01)01170-2. [DOI] [PubMed] [Google Scholar]
- 53.Rahn KA, Cao Y-J, Hendrix CW, Kaplin a I. The role of 5-HT1A receptors in mediating acute negative effects of antidepressants: implications in pediatric depression. Transl Psychiatry. 2015;5(5):e563. doi: 10.1038/tp.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newman-Tancredi A, Kleven MS. Comparative pharmacology of antipsychotics possessing combined dopamine D 2 and serotonin 5-HT 1A receptor properties. Psychopharmacology (Berl) 2011;216(4):451–473. doi: 10.1007/s00213-011-2247-y. [DOI] [PubMed] [Google Scholar]
- 55.Neumeister A. Reduced Serotonin Type 1A Receptor Binding in Panic Disorder. J Neurosci. 2004;24(3):589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nash JR, Sargent PA, Rabiner EA, et al. Serotonin 5-HT1A receptor binding in people with panic disorder: positron emission tomography study. Br J Psychiatry. 2008;193(3):229–234. doi: 10.1192/bjp.bp.107.041186. [DOI] [PubMed] [Google Scholar]
- 57.Messamore E, McNamara RK. Detection and treatment of omega-3 fatty acid deficiency in psychiatric practice: rationale and implementation. Lipids Health Dis. 2016;15(1):25. doi: 10.1186/s12944-016-0196-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pottala JV, Talley JA, Churchill SW, Lynch DA, von Schacky C, Harris WS. Red blood cell fatty acids are associated with depression in a case-control study of adolescents. Essent Fat Acids. 2012;86(4–5):161–165. doi: 10.1016/j.plefa.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 59.McNamara RK, Jandacek R, Rider T, et al. Effects of fish oil supplementation on prefrontal metabolite concentrations in adolescents with major depressive disorder: A preliminary (1)H MRS study. Nutr Neurosci. 2016;19(4):145–155. doi: 10.1179/1476830514Y.0000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59(10):913. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- 61.Jazayeri S, Tehrani-Doost M, Keshavarz SA, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42(3):192–198. doi: 10.1080/00048670701827275. [DOI] [PubMed] [Google Scholar]
- 62.Kodas E, Galineau L, Bodard S, et al. Serotoninergic neurotransmission is affected by n-3 polyunsaturated fatty acids in the rat. J Neurochem. 2004;89(3):695–702. doi: 10.1111/j.1471-4159.2004.02401.x. [DOI] [PubMed] [Google Scholar]
- 63.Able JA, Liu Y, Jandacek R, Rider T, Tso P, McNamara RK. Omega-3 fatty acid deficient male rats exhibit abnormal behavioral activation in the forced swim test following chronic fluoxetine treatment: Association with altered 5-HT1A and alpha2A adrenergic receptor expression. J Psychiatr Res. 2014;50(1):42–50. doi: 10.1016/j.jpsychires.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lotrich FE, Sears B, McNamara RK. Anger induced by interferon-alpha is moderated by ratio of arachidonic acid to omega-3 fatty acids. J Psychosom Res. 2013;75(5):475–483. doi: 10.1016/j.jpsychores.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(4):283–293. doi: 10.1016/j.jaac.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 66.Pine DS, Walkup JT, Labellarte MJ, et al. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med. 2001;344:1279–1285. doi: 10.1056/NEJM200104263441703. [DOI] [PubMed] [Google Scholar]
- 67.Rynn MA, Riddle MA, Yeung PP, Kunz NR. Efficacy and safety of extended-release venlafaxine in the treatment of generalized anxiety disorder in children and Adolescents: two placebo-controlled trials. AmJ Psychiatry. 2007;164:290–300. doi: 10.1176/ajp.2007.164.2.290. [DOI] [PubMed] [Google Scholar]
- 68.March JS, Entusah AR, Rynn M, Albano AM, Tourian KA. A Randomized controlled trial of venlafaxine ER versus placebo in pediatric social anxiety disorder. Biol Psychiatry. 2007;62(10):1149–1154. doi: 10.1016/j.biopsych.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 69.Maslowsky J, Mogg K, Bradley BP, et al. A preliminary investigation of neural correlates of treatment in adolescents with generalized anxiety disorder. J Child Adolesc Psychopharmacol. 2010;20(2):105–111. doi: 10.1089/cap.2009.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Godlewska B, Norbury R, Selveraj S, Cowen P. Decreased neural response to fear after a short-term SSRI treatment: A 3T functional magnetic resonance imaging study. Eur Neuropsychopharmacol 2011 ECNP Work Neuropsychopharmacol. 2011;21:S73. [Google Scholar]
- 71.Robertson B, Wang L, Diaz MT, et al. Effect of bupropion extended release on negative emotion processing in major depressive disorder: a pilot functional magnetic resonance imaging study. J Clin Psychiatry. 2007;68(2):261–267. doi: 10.4088/jcp.v68n0212. [DOI] [PubMed] [Google Scholar]
- 72.Iordan A, Dolcos S, Dolcos F. Neural signatures of the response to emotional distraction: a review of evidence from brain imaging investigations. Front Hum Neurosci. 2013 Jun;7:1–21. doi: 10.3389/fnhum.2013.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Windischberger C, Lanzenberger R, Holik A, et al. Area-specific modulation of neural activation comparing escitalopram and citalopram revealed by pharmaco-fMRI: A randomized cross-over study. Neuroimage. 2010;49(2):1161–1170. doi: 10.1016/j.neuroimage.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 74.Godlewska BR, Norbury R, Selvaraj S, Cowen PJ, Harmer CJ. Short-term SSRI treatment normalises amygdala hyperactivity in depressed patients. Psychol Med. 2012;42(12):2609–2617. doi: 10.1017/S0033291712000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tao RR, Calley CSCS, Hart JJ, et al. Brain activity in adolescent major depressive disorder before and after fluoxetine treatment. Am J Psychiatry. 2012;169(4):381–388. doi: 10.1176/appi.ajp.2011.11040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maslowsky J, Mogg K, Bradley BP, et al. A preliminary investigation of neural correlates of treatment in adolescents with generalized anxiety disorder. J Child Adolesc Psychopharmacol. 2010;20(2):105–111. doi: 10.1089/cap.2009.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deveney CM, Connolly ME, Jenkins SE, et al. Striatal dysfunction during failed motor inhibition in children at risk for bipolar disorder. Prog Neuro-Psychopharmacology Biol Psychiatry. 2012;38(2):127–133. doi: 10.1016/j.pnpbp.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61(8):781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 79.Guile JM. Sertraline-induced behavioral activation during the treatment of an adolescent with major depression. J Child Adolesc Psychopharmacol. 1996;6(4):281–285. doi: 10.1089/cap.1996.6.281. [DOI] [PubMed] [Google Scholar]
- 80.King RA, Riddle MA, Chappell PB, et al. Emergence of self-destructive phenomena in children and adolescents during fluoxetine treatment. J Am Acad Child Adolesc Psychiatry. 1991;30(2):179–186. doi: 10.1097/00004583-199103000-00003. [DOI] [PubMed] [Google Scholar]
- 81.Cheung AH, Zuckerbrot RA, Jensen PS, Ghalib K, Laraque D, Stein REK. Guidelines for adolescent depression in Primary Care (GLAD-PC): II. Treatment and ongoing management. Pediatrics. 2007;120(5):e1313–e1326. doi: 10.1542/peds.2006-1395. [DOI] [PubMed] [Google Scholar]
- 82.Guile JM. Sertraline-induced behavioral activation during the treatment of an adolescent with major depression. J Child Adolesc Psychopharmacol. 1996;6(4):281–285. doi: 10.1089/cap.1996.6.281. [DOI] [PubMed] [Google Scholar]
- 83.Haddad PM. Antidepressant discontinuation syndromes. Drug Saf. 2001;24(3):183–197. doi: 10.2165/00002018-200124030-00003. [DOI] [PubMed] [Google Scholar]


