Abstract
Melatonin, an evolutionarily ancient derivative of serotonin with hormonal properties, is the main neuroendocrine secretory product of the pineal gland. Although melatonin is best known to regulate circadian rhythmicity and lower vertebrate skin pigmentation, the full spectrum of functional activities of this free radical-scavenging molecule, which also induces/promotes complex antioxidative and DNA repair systems, includes immunomodulatory, thermoregulatory, and antitumor properties. Because this plethora of functional melatonin properties still awaits to be fully appreciated by dermatologists, the current review synthesizes the main features that render melatonin a promising candidate for the management of several dermatoses associated with substantial oxidative damage. We also review why promises to be useful in skin cancer prevention, skin photo- and radioprotection, and as an inducer of repair mechanisms that facilitate the recovery of human skin from environmental damage. The fact that human skin and hair follicles not only express functional melatonin receptors but also engage in substantial, extrapineal melatonin synthesis further encourages one to systematically explore how the skin’s melatonin system can be therapeutically targeted in future clinical dermatology and enrolled for preventive medicine strategies.
MELATONIN: A JOURNEY THROUGH TIME
The methoxyindole, melatonin (N-acetyl-5-methoxytryptamine), is produced by at least three clades of bacteria, all clades of eucarya including dinoflagellates, unicellular and multicellular fungi, at least 120 plant species, and numerous animal species including simple and complex vertebrates and invertebrates (Back et al., 2016; Hardeland, 2016; Hardeland et al., 1995; Pöggeler et al., 1991; Tan et al., 2013, 2014).
The presence of melatonin in Alphaproteobacteria and Cyanobacteria indicates an early appearance of this multifunctional serotonin derivative in the evolution of life (Tan et al., 2012, 2013). The discovery of antioxidative properties of melatonin (Hardeland, 2005, 2017; Hardeland et al., 2011; Reiter, 1998; Tan et al., 2002) is consistent with an ancient role of this molecule in contributing to survival under high oxygen levels or exposure to UVR. Because of melatonin production in mitochondria (Reiter et al., 2017b; Suofu et al., 2017; Tan et al., 2013), scavenging of free oxygen radicals has likely been a primary role of melatonin in evolution (Hardeland et al., 1995) originating around 2.5–3 billion years ago (Reiter et al., 2017; Tan et al., 2015).
In all organisms, melatonin is formed from tryptophan via serotonin, with taxon-specific variations in the sequence of steps and intermediates (Back et al., 2016; Hardeland, 2015, 2016; Tan et al., 2012, 2014, 2015, 2016) (Supplementary Text S1 online).
In vertebrates, melatonin is mainly perceived as the hormone of the pineal gland (Lerner et al., 1958 Reiter, 1991). The mammalian pineal gland represents the major source of melatonin in the blood, and in the cerebrospinal fluid of the third ventricle of the brain, where it contributes to the regulation of the circadian system (Reiter et al., 2014). Melatonin is also synthesized in numerous extrapineal sites such as brain, Harderian gland, retina, lens, cochlea, immune system, lungs, gastrointestinal tract, liver, kidney, thyroid, pancreas, thymus, spleen, carotid body, reproductive tract, endothelial cells, and skin (Acuña-Castroviejo et al., 2014; Hardeland et al., 2011; Slominski et al., 2008 Vanegas et al., 2012). Melatonin levels are regulated by its rapid metabolism in the liver or peripheral organs including skin (Slominski et al., 2017b).
Since its origin in early unicells, melatonin, while protecting against oxidative stress, has acquired numerous functions that are taxon, species, and tissue specific. In vertebrates, these functions are manifold and complex, with melatonin acting as a pleiotropic regulator of numerous parameters that orchestrate complex cell and tissue responses, both directly and indirectly (via the circadian system) (Hardeland et al., 2011; Tan et al., 2015). A full record of these functions is summarized elsewhere (Hardeland et al., 2011; Pandi-Perumal et al., 2006; Tan et al., 2015).
MELATONIN IN THE SKIN
Production and metabolism
Normal and pathological skin not only expresses enzymatic elements of the pathway but can also produce serotonin, N-acetylserotonin, and melatonin with its metabolites (Kim et al., 2013, 2015a, 2015b; Kobayashi et al., 2005; Nordlind et al., 2008; Schallreuter et al., 2012; Semak et al., 2004; Slominski et al., 1996, 2002a, 2002b, 2002c, 2003a, 2005c, 2008) (Supplementary Figure S1 online). Skin can also synthesize/recycle the (6R)-l-erythro-5,6,7,8-tetrahydrobiopterin, a cofactor for tryptophan hydroxylase (TPH; Grando et al., 2006; Schallreuter et al., 1997, 1998). Hydroxytryptophan can also be generated in the skin nonenzymatically through H2O2 and UVA-induced free-radical-mediated oxidation of l-tryptophan (Schallreuter et al., 2008). In addition to widespread TPH1 gene expression in skin cells (Slominski et al., 2002b, 2003a, 2005c), we also detected TPH2 in melanocytes, dermal fibroblasts (Slominski et al., 2014b), and retinal pigment epithelium (Zmijewski et al., 2009). Skin can also express alternatively spliced forms of the melatonin-synthesizing pathway: TPH, arylalkylamine N-acetyltransferase, and N-acetylserotonin-O-methyltransferase (Slominski et al., 2002b). Cutaneous N-acetylserotonin is produced by both arylalkylamine N-acetyltransferase and arylamine N-acetyltransferase (Semak et al., 2004; Slominski et al., 2002a, 2003b).
Melatonin in skin cells is rapidly metabolized through indolic, kynuric, and P450-dependent pathways or via nonenzymatic processes induced by UVR or free radicals (Fischer et al., 2006a; Kim et al., 2013; Slominski et al., 1996, 2017b) (Supplementary Figure S1).
Although immunocytochemistry identified N-acetylserotonin and melatonin antigens in epidermal and follicular keratinocytes and melanocytes, adnexal structures, fibroblasts, endothelial cells, and mast cells (Kobayashi et al., 2005; Slominski et al., 2005c, 2008), only recently mass spectrometry quantified melatonin and its metabolites in human epidermis (Kim et al., 2015a, 2015b). Epidermal melatonin production depends on race, gender, and age with the highest melatonin levels in African Americans. Among metabolites, 6-hydroxymelatonin showed the highest levels followed by 5-methoxytryptamine, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), and N1-acetyl-5-methoxykynuramine (Kim et al., 2015a, 2015b). Levels of AFMK and N1-acetyl-5-methoxykynuramine were the highest in African Americans, and no racial difference was seen for 6-hydroxymelatonin and 5-methoxytryptamine. However, skin pathology-related changes in the epidermal content of melatonin and its metabolites remain to be systematically explored to dissect the relationship between their endogenous production and metabolic consumption and defined skin disorders.
Mechanism of action
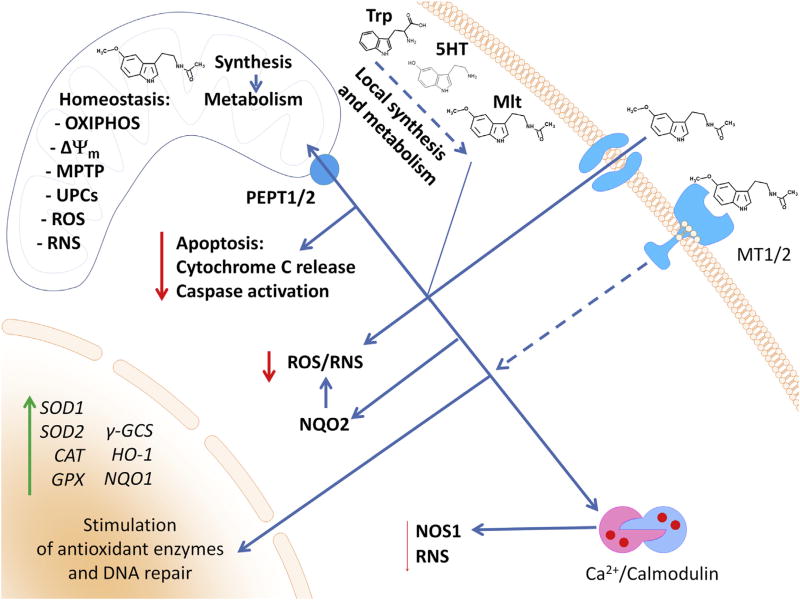
The potential intracellular targets for melatonin action are shown in Figure 1. The receptor-dependent regulatory functions of melatonin are mediated through interactions with G protein-coupled melatonin type 1 and 2 receptors (MT1 and MT2) (Cecon et al., 2017; Slominski et al., 2012). The latter predominantly work by inhibiting production of second messengers (cAMP, cGMP), thereby modifying signaling pathways downstream of protein kinases A and C, and cAMP response element-binding protein (Dubocovich et al., 2010; Slominski et al., 2012) and by activating MAP kinases (discussed in Hardeland, 2009). MT2 shows a 60% homology in structure to MT1 (Reppert et al., 1996). MT1 and MT2 homodimerize as well as heterodimerize (Jockers et al., 2008), which can affect the pharmacological properties of the receptors (Jockers et al., 2008; Legros et al., 2014). In addition, their activity is modulated by C-terminal phosphorylation, and, in the case of MT1, by stabilization via the scaffolding protein MUPP1 and inhibition by heterodimerization with GPR50 (Hardeland, 2009).
Figure 1. Targets for melatonin action in skin cells.
Melatonin, depending on the concentration, binds to membrane-bound receptor MT1 or MT2. Subsequent activation of signal transduction cascades stimulates the expression of antioxidative enzymes and DNA repair. Melatonin might also be transported to cytoplasm; however, the detailed mechanism is not fully understood. In a cell, melatonin at concentrations higher than 1 nM interacts with the calcium/calmodulin complex, which inhibits NOS1-mediated generation of RNS. On the other hand, melatonin can also interact with NQO2 (previously described as MT3) with potential inhibition of ROS/RNS levels. Recently, peptide transporter PEPT1/2 was found to be responsible for melatonin transport to mitochondria (Huo et al., 2017). Melatonin improves mitochondrial membrane potential (Δψm) by inhibition of the mitochondrial permeability transition pore (MPTP) and stimulation of uncoupling proteins (UCPs). This results in an elevated production of ATP by oxidative phosphorylation (OXIPHOS). These effects will also depend on its local synthesis (tryptophan (Trp) → serotonin (5TH) → melatonin (Mlt)) and metabolism. NQO2, quinone reductase 2; ROS, reactive oxygen species, RNS, reactive nitrogen species.
MT1 and MT2 receptors have been detected in mammalian skin (Fischer et al., 2008a; Kobayashi et al., 2005; Singh and Jadhav, 2014; Slominski et al., 1994, 2005c, 2008). Human skin predominantly transcribes the MT1 gene, with MT2 showing restricted or conditional expression (Slominski et al., 2003c, 2005a). Gene expression and production of alternatively spliced or aberrant forms of MT receptors is modulated by UVB and skin pathology (Slominski et al., 2003c, 2005a). In contrast, murine skin showed exclusive expression of the MT2 gene (Kobayashi et al., 2005; Slominski et al., 2004a). By immunocytochemistry, MT1 was detected in the differentiating layers of the epidermis, outer and inner root sheaths of the hair follicle (HF), eccrine glands, and blood vessels, whereas MT2 was detected in inner root sheath (IRS), eccrine glands, and blood vessels of human skin (Fischer et al., 2008a; Slominski et al., 2005a, 2005c).
It remains to be clarified whether there is also a nuclear receptor for melatonin, because the originally proposed nuclear melatonin receptor candidate, RORα, which is expressed in skin and HFs (Brozyna et al., 2016; Kobayashi et al., 2005; Slominski et al., 2005a, 2014a), has turned out to be a receptor for sterols and secosteroids, but not for melatonin (Slominski et al., 2014a, 2016c, 2017a).
Melatonin and its metabolites act as free radical scavengers and protectors against oxidative stress (Fernández et al., 2015; Fischer et al., 2006c; Galano et al., 2013; Hardeland, 2017; Hardeland et al., 2011). Melatonin and its precursor N-acetylserotonin bind to several regulatory proteins including quinone reductase 2 (Jockers et al., 2008; Nosjean et al., 2000). Quinone reductase 2 protects cells against oxidative stress (Boutin, 2016; Hardeland, 2009), against dimethylbenz(a)anthracene-induced skin cancer (Shen et al., 2010), and is required for tumor necrosis factor-α-induced apoptosis in keratinocytes (Ahn et al., 2007). The capability of melatonin binding to this enzyme (see Kleszczynski et al., 2016) requires additional studies.
Calmodulin is another melatonin-binding protein, which may gain physiologically relevant affinity after Ca2+ binding and interaction with calmodulin-controlled enzymes, such as calmodulin kinase II and calcineurin, which regulate intracellular calcium homeostasis (Fernández et al., 2015; Fukunaga et al., 2002; Hardeland et al., 2009; León et al., 2000; Lu et al., 2015; Romero et al., 1998). These observations may partially explain the roles of melatonin in the endoplasmic reticulum stress response, regulation of apoptosis and autophagy, and mitochondrial homeostasis (Fernández et al., 2015). Melatonin also regulates mitochondrial functions that affect cellular homeostasis (Acuña Castroviejo et al., 2011; Semak et al., 2005).
FUNCTIONS OF MELATONIN IN THE SKIN
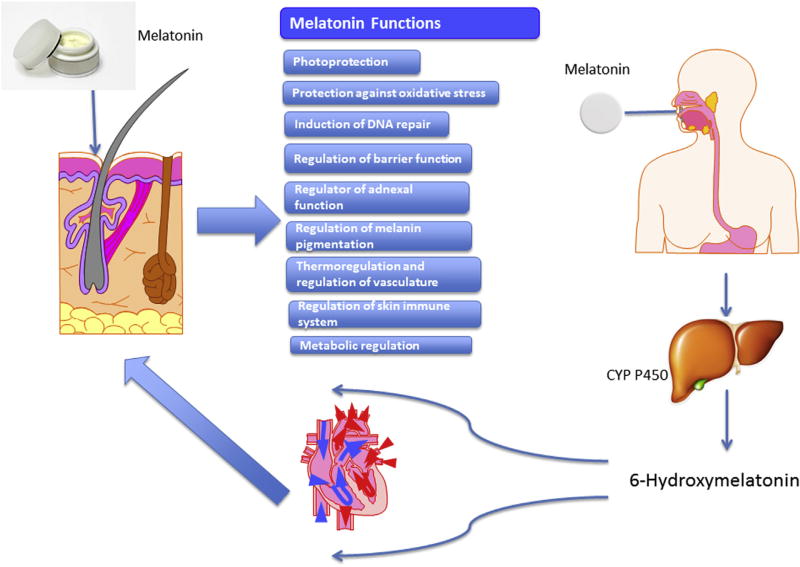
Figure 2 summarizes melatonin effects on the skin, which depend on the route of delivery.
Figure 2. Topically or orally administered melatonin affects different skin functions.
To compensate inadequate intracutaneous levels of melatonin secondary to environmentally induced degradation or suboptimal local production or transport from the pineal gland, melatonin can be delivered to the skin via different routes. Orally ingested melatonin is rapidly metabolized in the liver by CYP450 into 6-hydroxymelatonin while sublingual (transmucosal) melatonin administration can bypass liver metabolism. Transdermal application of melatonin appears to be optimal for local application due to slow absorption, deposition in the skin, lack of identifiable site effects, and availability of different formulations (Flo et al., 2016, 2017; Milan et al., 2017; Romic et al., 2016; Scheuer et al., 2016b; Zetner et al., 2016). Because there is a cutaneous melatonin metabolism with metabolites sharing similar activities as melatonin (Slominski et al., 2017b), the effects summarized in the central column may also be secondary to the action of its metabolites. Of note, 6-hydroxymelatonin, 4-hydroxymelatonin, 2-hydroxymelatonin, AFMK, or AMK being produced by different species, in addition to the human body, may fulfill the definition of natural products for topical applications to improve healthy skin status. Importantly, the functional effects depicted here may also be exerted by endogenous melatonin synthesized in the skin and hair follicles (see main text). AFMK, N1-acetyl-N2-formyl-5-methoxykynuramine; AMK, N1-acetyl-5-methoxykynuramine; CYP, cytochrome P450.
Photoprotection
The ancient functions of melatonin and its metabolites as antioxidants or inducers of responses against oxidative stress or as protectors of genomic integrity are efficiently utilized in the human skin, an organ exposed to different stressors including solar radiation (Fischer et al., 2008b; Slominski et al., 2012, 2014b). Because protective effects of melatonin against UVR were extensively discussed in Fischer et al. (2008b), Kleszczynski and Fischer (2012), Ndiaye et al. (2014), and Slominski et al. (2014b, 2014c), we will focus on the most important and novel aspects of melatonin radioprotection.
Melatonin and AFMK protect human epidermal keratinocytes both in cell and organ culture against UVB (Fischer et al., 2006a, 2006b, 2008b, 2008c, 2013; Kleszczynski et al., 2011, 2013, 2015, 2016). In addition to melatonin, N-acetylserotonin, 5-methoxytryptamine, 6-hydroxymelatonin, AFMK, and N1-acetyl-5-methoxykynuramine protect against or reverse UVB-induced damage in keratinocytes and melanocytes (Janjetovic et al., 2014, 2017). Similarly, melatonin protects dermal fibroblasts against UVA and UVB (Izykowska et al., 2009; Lee et al., 2003; Rezzani et al., 2014; Ryoo et al., 2001).
Although the mechanisms underlying these radioprotective and antioxidative activities are not fully understood yet (Fischer et al., 2006a; Slominski et al., 2005c, 2014b), recent studies implicate the involvement of nuclear erythroid 2-related factor 2 (Janjetovic et al., 2017; Kleszczynski et al., 2016) and sirtuin 1 (Sirt1) (Lee et al., 2016; Ranieri et al., 2015), major regulators of human skin and HF oxidative stress responses, and skin aging (Haslam et al., 2017; Jadkauskaite et al., 2017; Vidali et al., 2016). Clinically, topically applied melatonin can indeed attenuate skin erythema in healthy human subjects exposed to either artificial UVR (Bangha et al., 1996, 1997; Fischer et al., 1999) or natural sunlight (Scheuer, 2017; Scheuer et al., 2016a), thus demonstrating melatonin’s clinical potential as a protector against photodamage.
Anticancer activity
The potent anticancer activities of melatonin through a direct action or regulation of circadian rhythms have been discussed in depth elsewhere (Ma et al., 2016; Reiter et al., 2017; Rondanelli et al., 2013; Su et al., 2017). In vitro and in vivo antimelanoma effects of melatonin are established in rodent models (Kadekaro et al., 2004; Narita and Kudo, 1985; Otalora et al., 2008; Slominski and Pruski, 1993; Stanberry et al., 1983). Melatonin and its metabolites also inhibit the growth of cultured human melanomas (Cabrera et al., 2010; Fischer et al., 2006c; Roberts et al., 2000; Souza et al., 2003; Yi et al., 2014; Ying et al., 1993). This raises the question whether topically applied melatonin and its metabolites may be used as adjuvants in difficult-to-resect lentigo maligna or mucosal melanomas.
Clinical trials with high doses of melatonin in late stage of metastatic melanoma suggested its beneficial effect through either reduction of side effects of chemotherapy/chemoimmunotherapy or by enhancing their efficacy (Gonzalez et al., 1991; Lissoni et al., 1997, 2002). Also, an improvement of disease-free survival was observed in patients treated with melatonin after lymph node dissection (Lissoni et al., 1996). Therefore, given its low cost, very favorable toxicological profile, and expected low or absent adverse effects, systematic clinical testing of melatonin and/or its metabolites as adjuvants in melanoma therapy is both warranted and long overdue.
Melatonin also attenuated benzo[a]pyrene-induced cutaneous sarcomas (Vesnushkin et al., 2006), squamous cell carcinomas (Deriabina et al., 2010), and papillomas (Kumar and Das, 2000) in mice. Patients with basal cell and squamous cell carcinomas had lower urinary indicators of systemic melatonin production in comparison to controls (Ghaderi et al., 2014). Together with its radioprotective function, these additional observations identify melatonin as a promising natural product for clinical use in skin cancer prevention and oncological therapy, which awaits systematic clinical testing.
Epidermal barrier function and wound healing
Exogenously applied melatonin may enhance the skin barrier (Kim et al., 2013). Specifically, melatonin and AFMK in human skin organ culture stimulated expression of involucrin and keratin-10 and keratin-14. It also increased proliferative activity of keratinocytes in ex vivo skin explants (Kim et al., 2013), consistent with earlier observations on murine skin (Slominski et al., 1994). Melatonin can also promote skin wound healing (Lee et al., 2014; Ozler et al., 2010; Pugazhenthi et al., 2008; Romic et al., 2016; Song et al., 2016; Soybir et al., 2003), and can improve the antimicrobial action of wound dressing (Romic et al., 2016). Of note, a novel melatonin-mitochondria axis has been proposed recently as an important regulator of epidermal homeostasis where melatonin and its metabolites coordinate the mitochondrial regulation of epidermal cell destiny by impacting on the decision between cell survival, entry into terminal differentiation as a part of epidermal barrier formation, or to death by apoptosis to evade malignant transformation (Slominski et al., 2017c).
In view of melatonin’s reactive oxygen species-scavenging functions on the one hand, and the recently appreciated physiological key role of a tightly controlled burst of reactive oxygen species production in tissue repair and regeneration on the other (Kimmel et al., 2016; Love et al., 2013), one of the functions of the cutaneous melatonin system (Slominski et al., 2008, 2017c) may also be to control and fine-tune reactive oxygen species availability during wound healing. Thus, preclinical studies on the beneficial role of melatonin and its metabolites on epidermal barrier formation and wound healing are warranted.
Pigmentation
Lightening effects of melatonin on the skin of lower vertebrates and inhibition of pigmentation in some furry animals are well appreciated (reviewed in Slominski et al., 2004b, 2005c). Seasonal changes in hair pigmentation have been attributed in part to melatonin (reviewed in Fischer et al., 2008a; Slominski et al., 2005b). Attenuation of melanogenesis was demonstrated in cultured rodent melanomas (Slominski and Pruski, 1993; Valverde et al., 1995) and in organ-cultured murine skin (Slominski et al., 1994).
In humans, melatonin and some metabolites showed moderate inhibition of tyrosinase and of proliferation of cultured epidermal melanocytes (Kim et al., 2015b). Hardman et al. (2015) have shown that the cutaneous circadian clock elements regulate melanogenesis and melanocyte activities in human epidermis and HFs. Thus, although there are conflicting results on melatonin functions in human hair and skin pigmentation (McElhinney et al., 1994; Slominski et al., 2004b, 2005c), locally produced melatonin may play a role in the regulation of melanocytic activities via its impact on the peripheral clock. Thus, testing of topically applied melatonin during defined circadian windows as an external modulator of intracutaneous clock activity is warranted (Slominski et al., 2015).
Finally, in light of the autodestruction and oxidative stress theories of vitiligo pathogenesis (Lerner, 1971; Schallreuter et al., 2008), melatonin and serotonin might play a protective role in vitiligo pathogenesis (see viewpoint 4 in Schallreuter et al., 2008). This is further supported by low expression of TPH1 in vitiligo (Schallreuter et al., 2012). Thus, topical supplementation of melatonin and its precursors may be beneficial by ameliorating the oxidative environment of vitiligo skin.
Hair follicle
Melatonin can regulate hair growth directly or indirectly (e.g., via modulating the prolactin serum level) in several nonhuman species (Fischer et al., 2008a; Slominski et al., 2008), and the MT2 expression levels of murine HFs change during HF cycling (Kobayashi et al. 2005). Human scalp HFs also synthesize melatonin, and this intrafollicular melatonin synthesis can be stimulated by noradrenaline, just as in the pineal gland (Kobayashi et al. 2005). Moreover, melatonin may modulate human HF responses to estrogens, because it downregulates intrafollicular estrogen receptor expression (Kobayashi et al. 2005). Studies on human volunteers have raised the possibility that topically applied melatonin may inhibit androgenetic alopecia in women (Fischer et al., 2004, 2012).
Given that melatonin stimulates nuclear erythroid 2-related factor 2 (Janjetovic et al., 2017; Kleszczynski et al., 2016) whereas nuclear erythroid 2-related factor 2 activation protects human HFs against oxidative stress-induced hair growth inhibition (Haslam et al., 2017), it deserves further exploration whether melatonin can protect human hair growth via nuclear erythroid 2-related factor 2 induction. That melatonin renders human HFs ex vivo less susceptible to chemotherapy-induced damage (Kobayashi et al., 2005) may be related in part to this putative protective mechanism. Furthermore, involvement of circadian clock proteins in the control of human HF cycling (Al-Nuaimi et al., 2014) and of both epidermal and HF pigmentation (Hardman et al., 2015) raises the question whether melatonin can impact on the control of human hair growth and pigmentation via modulating peripheral clock activity in the skin, just as it does centrally.
Inflammatory dermatoses
The role of melatonin in immunodermatology remains insufficiently defined because both immunostimulatory and anti-inflammatory actions of melatonin have been reported (Carrillo-Vico et al., 2013; Jahanban-Esfahlan et al., 2017). Yet, key innate skin immunocytes such as mast cells express melatonin receptors (Theoharides, 2017) and melatonin plays an important role in T-cell fate determination, T-cell-based immune pathologies (Ren et al., 2017), and macrophage function (Kadena et al., 2017; Yi and Kim, 2017).
Despite a shortage of information on the role of melatonin in inflammatory skin disorders, beneficial effects of melatonin have been suggested in atopic dermatitis (Calvo and Maldonado, 2016; Marseglia et al., 2014, 2015; Park et al., 2017) and seborrheic dermatitis (Fischer et al., 2012), and disturbances in serum melatonin levels were reported in patients with psoriasis (Kartha et al., 2014; Mozzanica et al., 1988). Thus, studies that define the role of melatonin in chronic inflammatory skin diseases are warranted, and may lead to a discovery of beneficial effects similar to those recently described for multiple sclerosis (Farez et al., 2015).
Thermoregulation
Melatonin directly or via its circadian effects influences core body and skin temperature (Atkinson et al., 2005; Cuesta et al., 2017; Filadelfi and Castrucci, 1996; Kräuchi et al., 1997; van den Heuvel et al., 1999). It can modify the cutaneous vasodilator response to heat (Aoki et al., 2006) and is involved in the fine-tuning of vascular tone and modifies cutaneous vasoconstrictor response to whole body skin cooling (Aoki et al., 2008; Kräuchi et al., 2006). However, it remains unknown whether melatonin also plays a role in sweat gland physiology.
CONCLUSIONS AND TRANSLATIONAL PERSPECTIVES
That mammalian skin and HFs not only are prominent melatonin targets but also produce and rapidly metabolize this multifunctional methoxyindole to biologically active metabolites is of considerable dermatological interest and potentially of great clinical importance for future dermatological therapy. Here, we have delineated why sufficient levels of locally produced melatonin and its metabolites are required for both optimal skin homeostasis, thus preventing skin pathology, and optimal responses to environmental stressors including UVR. Therefore, physiological concentration of melatonin will depend on the biological context. In fact, the serum melatonin level may be largely irrelevant, because skin produces a much larger amount of melatonin for its own use than can be detected in serum (Kim et al., 2015b; Slominski et al., 2008), similar to the gastrointestinal system that contains several hundred times more melatonin than the pineal gland itself (Bubenik, 2002).
Although we have reviewed here how melatonin and its metabolites can impact on multiple aspects of human skin physiology, challenging open questions include how exactly these agents affect human skin pigmentation, hair growth, and the development of melanoma and nonmelanoma skin cancers mechanistically and under clinically relevant circumstances. This still needs to be clarified definitively in appropriately designed clinical studies. The translational perspectives that emanate from melatonin’s unique properties in multiple oxidative damage control and DNA repair systems and its immunomodulatory properties render melatonin an especially attractive candidate agent in the future management of dermatoses associated with substantial oxidative, photo-, and radiation-induced damage. Melatonin also promises to be useful in skin cancer prevention. In addition, in view of melatonin's capability to induce multiple damage reparative mechanisms in skin, the potential anti-skin aging properties of melatonin deserve systemic study.
Given that melatonin is essentially nontoxic, readily available over the counter in different formulations, and that many of its metabolites meet the definition of natural products, their topical and transepidermal delivery is a promising area for full exploration in future dermatotherapy and preventive skin medicine.
Supplementary Material
Acknowledgments
Writing of this review was supported in part by NIH grants 1R01AR056666-01A2 and 1R01AR071189-01A1 to ATS and by support from the NIHR Manchester Biomedical Research Centre to RP.
Abbreviations
- AFMK
N1-acetyl-N2-formyl-5-methoxykynuramine
- HF
hair follicle
- MT1 and 2
melatonin type 1 and 2 receptors
- TPH1 and 2
tryptophan hydroxylase 1 and 2
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at https://doi.org/10.1016/j.jid.2017.10.025.
References
- Acuña-Castroviejo D, Escames G, Venegas C, Diaz-Casado ME, Lima-Cabello E, Lopez LC, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71:2997–3025. doi: 10.1007/s00018-014-1579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuña-Castroviejo D, Lopez LC, Escames G, Lopez A, Garcia JA, Reiter RJ. Melatonin-mitochondria interplay in health and disease. Curr Top Med Chem. 2011;11:221–40. doi: 10.2174/156802611794863517. [DOI] [PubMed] [Google Scholar]
- Ahn KS, Gong X, Sethi G, Chaturvedi MM, Jaiswal AK, Aggarwal BB. Deficiency of NRH:quinone oxidoreductase 2 differentially regulates TNF signaling in keratinocytes: up-regulation of apoptosis correlates with downregulation of cell survival kinases. Cancer Res. 2007;67:10004–11. doi: 10.1158/0008-5472.CAN-07-2213. [DOI] [PubMed] [Google Scholar]
- Al-Nuaimi Y, Hardman JA, Biro T, Haslam IS, Philpott MP, Tóth B, et al. A meeting of two chronobiological systems: circadian proteins Period1 and BMAL1 modulate the human hair cycle clock. J Invest Dermatol. 2014;134:610–9. doi: 10.1038/jid.2013.366. [DOI] [PubMed] [Google Scholar]
- Aoki K, Stephens DP, Zhao K, Kosiba WA, Johnson JM. Modification of cutaneous vasodilator response to heat stress by daytime exogenous melatonin administration. Am J Physiol Regul Integr Comp Physiol. 2006;291:R619–24. doi: 10.1152/ajpregu.00117.2006. [DOI] [PubMed] [Google Scholar]
- Aoki K, Zhao K, Yamazaki F, Sone R, Alvarez GE, Kosiba WA, et al. Exogenous melatonin administration modifies cutaneous vasoconstrictor response to whole body skin cooling in humans. J Pineal Res. 2008;44:141–8. doi: 10.1111/j.1600-079X.2007.00501.x. [DOI] [PubMed] [Google Scholar]
- Atkinson G, Holder A, Robertson C, Gant N, Drust B, Reilly T, et al. Effects of melatonin on the thermoregulatory responses to intermittent exercise. J Pineal Res. 2005;39:353–9. doi: 10.1111/j.1600-079X.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- Back K, Tan DX, Reiter RJ. Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J Pineal Res. 2016;61:426–37. doi: 10.1111/jpi.12364. [DOI] [PubMed] [Google Scholar]
- Bangha E, Elsner P, Kistler GS. Suppression of UV-induced erythema by topical treatment with melatonin (N-acetyl-5-methoxytryptamine). A dose response study. Arch Dermatol Res. 1996;288:522–6. doi: 10.1007/BF02505248. [DOI] [PubMed] [Google Scholar]
- Bangha E, Elsner P, Kistler GS. Suppression of UV-induced erythema by topical treatment with melatonin (N-acetyl-5-methoxytryptamine). Influence of the application time point. Dermatology. 1997;195:248–52. doi: 10.1159/000245953. [DOI] [PubMed] [Google Scholar]
- Boutin JA. Quinone reductase 2 as a promising target of melatonin therapeutic actions. Expert Opin Ther Targets. 2016;20:303–17. doi: 10.1517/14728222.2016.1091882. [DOI] [PubMed] [Google Scholar]
- Brozyna AA, Jozwicki W, Roszkowski K, Filipiak J, Slominski AT. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget. 2016;7:17844–53. doi: 10.18632/oncotarget.7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci. 2002;47:2336–48. doi: 10.1023/a:1020107915919. [DOI] [PubMed] [Google Scholar]
- Cabrera J, Negrin G, Estevez F, Loro J, Reiter RJ, Quintana J. Melatonin decreases cell proliferation and induces melanogenesis in human melanoma SK-MEL-1 cells. J Pineal Res. 2010;49:45–54. doi: 10.1111/j.1600-079X.2010.00765.x. [DOI] [PubMed] [Google Scholar]
- Calvo JR, Maldonado MD. The role of melatonin in autoimmune and atopic diseases. AIMS Mol Sci. 2016;3:158–86. [Google Scholar]
- Carrillo-Vico A, Lardone PJ, Alvarez-Sanchez N, Rodriguez-Rodriguez A, Guerrero JM. Melatonin: buffering the immune system. Int J Mol Sci. 2013;14:8638–83. doi: 10.3390/ijms14048638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecon E, Oishi A, Jockers R. Melatonin receptors: molecular pharmacology in the context of system bias. Br J Pharmacol. 2017 doi: 10.1111/bph.13950. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta M, Boudreau P, Cermakian N, Boivin DB. Skin temperature rhythms in humans respond to changes in the timing of sleep and light. J Biol Rhythms. 2017;32:257–73. doi: 10.1177/0748730417702974. [DOI] [PubMed] [Google Scholar]
- Deriabina ON, Plotnikova NA, Anisimov VN. Melatonin and metformin inhibit skin carcinogenesis induced by benz(a)pyrene in mice. Vopr Onkol. 2010;56:583–7. [PubMed] [Google Scholar]
- Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62:343–80. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farez MF, Mascanfroni ID, Mendez-Huergo SP, Yeste A, Murugaiyan G, Garo LP, et al. Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell. 2015;162:1338–52. doi: 10.1016/j.cell.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández A, Ordóñez R, Reiter RJ, González-Gallego J, Mauriz JL. Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis. J Pineal Res. 2015;59:292–307. doi: 10.1111/jpi.12264. [DOI] [PubMed] [Google Scholar]
- Filadelfi AM, Castrucci AM. Comparative aspects of the pineal/melatonin system of poikilothermic vertebrates. J Pineal Res. 1996;20:175–86. doi: 10.1111/j.1600-079x.1996.tb00256.x. [DOI] [PubMed] [Google Scholar]
- Fischer T, Bangha E, Elsner P, Kistler GS. Suppression of UV-induced erythema by topical treatment with melatonin. Influence of the application time point. Biol Signals Recept. 1999;8:132–5. doi: 10.1159/000014581. [DOI] [PubMed] [Google Scholar]
- Fischer TW, Burmeister G, Schmidt HW, Elsner P. Melatonin increases anagen hair rate in women with androgenetic alopecia or diffuse alopecia: results of a pilot randomized controlled trial. Br J Dermatol. 2004;150:341–5. doi: 10.1111/j.1365-2133.2004.05685.x. [DOI] [PubMed] [Google Scholar]
- Fischer TW, Kleszczynski K, Hardkop LH, Kruse N, Zillikens D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2′-deoxyguanosine) in ex vivo human skin. J Pineal Res. 2013;54:303–12. doi: 10.1111/jpi.12018. [DOI] [PubMed] [Google Scholar]
- Fischer TW, Slominski A, Tobin DJ, Paus R. Melatonin and the hair follicle. J Pineal Res. 2008a;44:1–15. doi: 10.1111/j.1600-079X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- Fischer TW, Slominski A, Zmijewski MA, Reiter RJ, Paus R. Melatonin as a major skin protectant: from free radical scavenging to DNA damage repair. Exp Dermatol. 2008b;17:713–30. doi: 10.1111/j.1600-0625.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- Fischer TW, Sweatman TW, Semak I, Sayre RM, Wortsman J, Slominski A. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J. 2006a;20:1564–6. doi: 10.1096/fj.05-5227fje. [DOI] [PubMed] [Google Scholar]
- Fischer TW, Trueb RM, Hanggi G, Innocenti M, Elsner P. Topical melatonin for treatment of androgenetic alopecia. Int J Trichology. 2012;4:236–45. doi: 10.4103/0974-7753.111199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer TW, Zbytek B, Sayre RM, Apostolov EO, Basnakian AG, Sweatman TW, et al. Melatonin increases survival of HaCaT keratinocytes by suppressing UV-induced apoptosis. J Pineal Res. 2006b;40:18–26. doi: 10.1111/j.1600-079X.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- Fischer TW, Zmijewski MA, Wortsman J, Slominski A. Melatonin maintains mitochondrial membrane potential and attenuates activation of initiator (casp-9) and effector caspases (casp-3/casp-7) and PARP in UVR-exposed HaCaT keratinocytes. J Pineal Res. 2008c;44:397–407. doi: 10.1111/j.1600-079X.2007.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer TW, Zmijewski MA, Zbytek B, Sweatman TW, Slominski RM, Wortsman J, et al. Oncostatic effects of the indole melatonin and expression of its cytosolic and nuclear receptors in cultured human melanoma cell lines. Int J Oncol. 2006c;29:665–72. doi: 10.3892/ijo.29.3.665. [DOI] [PubMed] [Google Scholar]
- Flo A, Calpena AC, Halbaut L, Araya EI, Fernández F, Clares B. Melatonin delivery: transdermal and transbuccal evaluation in different vehicles. Pharm Res. 2016;33:1615–27. doi: 10.1007/s11095-016-1901-9. [DOI] [PubMed] [Google Scholar]
- Flo A, Cambras T, Diez-Noguera A, Calpena A. Melatonin pharmacokinetics after transdermal administration changes according to the time of the day. Eur J Pharm Sci. 2017;96:164–70. doi: 10.1016/j.ejps.2016.09.020. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Horikawa K, Shibata S, Takeuchi Y, Miyamoto E. Ca2+/calmodulin-dependent protein kinase II-dependent long-term potentiation in the rat suprachiasmatic nucleus and its inhibition by melatonin. J Neurosci Res. 2002;70:799–807. doi: 10.1002/jnr.10400. [DOI] [PubMed] [Google Scholar]
- Galano A, Tan DX, Reiter RJ. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J Pineal Res. 2013;54:245–57. doi: 10.1111/jpi.12010. [DOI] [PubMed] [Google Scholar]
- Ghaderi R, Sehatbakhsh S, Bakhshaee M, Sharifzadeh GR. Urinary melatonin levels and skin malignancy. Iran J Med Sci. 2014;39:64–7. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Sanchez A, Ferguson JA, Balmer C, Daniel C, Cohn A, et al. Melatonin therapy of advanced human malignant melanoma. Melanoma Res. 1991;1:237–43. doi: 10.1097/00008390-199111000-00003. [DOI] [PubMed] [Google Scholar]
- Grando SA, Pittelkow MR, Schallreuter KU. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J Invest Dermatol. 2006;126:1948–65. doi: 10.1038/sj.jid.5700151. [DOI] [PubMed] [Google Scholar]
- Hardeland R. Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine. 2005;27:119–30. doi: 10.1385/endo:27:2:119. [DOI] [PubMed] [Google Scholar]
- Hardeland R. Melatonin: signaling mechanisms of a pleiotropic agent. Biofactors. 2009;35:183–92. doi: 10.1002/biof.23. [DOI] [PubMed] [Google Scholar]
- Hardeland R. Melatonin in plants and other phototrophs: advances and gaps concerning the diversity of functions. J Exp Bot. 2015;66:627–46. doi: 10.1093/jxb/eru386. [DOI] [PubMed] [Google Scholar]
- Hardeland R. Melatonin in plants—diversity of levels and multiplicity of functions. Front Plant Sci. 2016;7:198. doi: 10.3389/fpls.2016.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeland R. Melatonin and the electron transport chain [e-pub ahead of print] Cell Mol Life Sci. 2017;74:3883–3896. doi: 10.1007/s00018-017-2615-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeland R, Balzer I, Poeggeler B, Fuhrberg B, Uria H, Behrmann G, et al. On the primary functions of melatonin in evolution: mediation of photoperiodic signals in a unicell, photooxidation, and scavenging of free radicals. J Pineal Res. 1995;18:104–11. doi: 10.1111/j.1600-079x.1995.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin—a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93:350–84. doi: 10.1016/j.pneurobio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Tan DX, Reiter RJ. Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J Pineal Res. 2009;47:109–26. doi: 10.1111/j.1600-079X.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- Hardman JA, Tobin DJ, Haslam IS, Farjo N, Farjo B, Al-Nuaimi Y, et al. The peripheral clock regulates human pigmentation. J Invest Dermatol. 2015;135:1053–64. doi: 10.1038/jid.2014.442. [DOI] [PubMed] [Google Scholar]
- Haslam IS, Jadkauskaite L, Szabo IL, Staege S, Hesebeck-Brinckmann J, Jenkins G, et al. Oxidative damage control in a human (mini-) organ: Nrf2 activation protects against oxidative stress-induced hair growth inhibition. J Invest Dermatol. 2017;137:295–304. doi: 10.1016/j.jid.2016.08.035. [DOI] [PubMed] [Google Scholar]
- Izykowska I, Cegielski M, Gebarowska E, Podhorska-Okolow M, Piotrowska A, Zabel M, et al. Effect of melatonin on human keratinocytes and fibroblasts subjected to UVA and UVB radiation in vitro. In Vivo. 2009;23:739–45. [PubMed] [Google Scholar]
- Jadkauskaite L, Coulombe PA, Schäfer M, Dinkova-Kostova AT, Paus R, Haslam IS. Oxidative stress management in the hair follicle: Could targeting NRF2 counter age-related hair disorders and beyond? [e-pub ahead of print] Bioessays. 2017;39 doi: 10.1002/bies.201700029. https://doi.org/10.1002/bies.201700029. [DOI] [PubMed] [Google Scholar]
- Jahanban-Esfahlan R, Mehrzadi S, Reiter RJ, Seidi K, Majidinia M, Baghi HB, et al. Melatonin in regulation of inflammatory pathways in rheumatoid arthritis and osteoarthritis: involvement of circadian clock genes. Br J Pharmacol. 2017 doi: 10.1111/bph.13898. https://doi.org/10.1111/bph.13898. [DOI] [PMC free article] [PubMed]
- Janjetovic Z, Jarrett SG, Lee EF, Duprey C, Reiter RJ, Slominski AT. Melatonin and its metabolites protect human melanocytes against UVB-induced damage: involvement of NRF2-mediated pathways. Sci Rep. 2017;7:1274. doi: 10.1038/s41598-017-01305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjetovic Z, Nahmias ZP, Hanna S, Jarrett SG, Kim TK, Reiter RJ, et al. Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. J Pineal Res. 2014;57:90–102. doi: 10.1111/jpi.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockers R, Maurice P, Boutin JA, Delagrange P. Melatonin receptors, heterodimerization, signal transduction and binding sites: what’s new? Br J Pharmacol. 2008;154:1182–95. doi: 10.1038/bjp.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadekaro AL, Andrade LN, Floeter-Winter LM, Rollag MD, Virador V, Vieira W, et al. MT-1 melatonin receptor expression increases the anti-proliferative effect of melatonin on S-91 murine melanoma cells. J Pineal Res. 2004;36:204–11. doi: 10.1111/j.1600-079x.2004.00119.x. [DOI] [PubMed] [Google Scholar]
- Kadena M, Kumagai Y, Vandenbon A, Matsushima H, Fukamachi H, Maruta N, et al. Microarray and gene co-expression analysis reveals that melatonin attenuates immune responses and modulates actin rearrangement in macrophages. Biochem Biophys Res Commun. 2017;485:414–20. doi: 10.1016/j.bbrc.2017.02.063. [DOI] [PubMed] [Google Scholar]
- Kartha LB, Chandrashekar L, Rajappa M, Menon V, Thappa DM, Ananthanarayanan PH. Serum melatonin levels in psoriasis and associated depressive symptoms. Clin Chem Lab Med. 2014;52:e123–5. doi: 10.1515/cclm-2013-0957. [DOI] [PubMed] [Google Scholar]
- Kim TK, Kleszczynski K, Janjetovic Z, Sweatman T, Lin Z, Li W, et al. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 2013;27:2742–55. doi: 10.1096/fj.12-224691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Lin Z, Li W, Reiter RJ, Slominski AT. N1-Acetyl-5-Methoxykynuramine (AMK) is produced in the human epidermis and shows antiproliferative effects. Endocrinology. 2015a;156:1630–6. doi: 10.1210/en.2014-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Lin Z, Tidwell WJ, Li W, Slominski AT. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol Cell Endocrinol. 2015b;404:1–8. doi: 10.1016/j.mce.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HM, Grant A, Ditata J. The presence of oxygen in wound healing. Wounds. 2016;28:264–70. [PubMed] [Google Scholar]
- Kleszczynski K, Fischer TW. Melatonin and human skin aging. Dermatoendocrinol. 2012;4:245–52. doi: 10.4161/derm.22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleszczynski K, Hardkop LH, Fischer TW. Differential effects of melatonin as a broad range UV-damage preventive dermato-endocrine regulator. Dermatoendocrinol. 2011;3:27–31. doi: 10.4161/derm.3.1.14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleszczynski K, Tukaj S, Kruse N, Zillikens D, Fischer TW. Melatonin prevents ultraviolet radiation-induced alterations in plasma membrane potential and intracellular pH in human keratinocytes. J Pineal Res. 2013;54:89–99. doi: 10.1111/j.1600-079X.2012.01028.x. [DOI] [PubMed] [Google Scholar]
- Kleszczynski K, Zillikens D, Fischer TW. Melatonin enhances mitochondrial ATP synthesis, reduces reactive oxygen species formation, and mediates translocation of the nuclear erythroid 2-related factor 2 resulting in activation of phase-2 antioxidant enzymes (gamma-GCS, HO-1, NQO1) in ultraviolet radiation-treated normal human epidermal keratinocytes (NHEK) J Pineal Res. 2016;61:187–97. doi: 10.1111/jpi.12338. [DOI] [PubMed] [Google Scholar]
- Kleszczynski K, Zwicker S, Tukaj S, Kasperkiewicz M, Zillikens D, Wolf R, et al. Melatonin compensates silencing of heat shock protein 70 and suppresses ultraviolet radiation-induced inflammation in human skin ex vivo and cultured keratinocytes. J Pineal Res. 2015;58:117–26. doi: 10.1111/jpi.12197. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kromminga A, Dunlop TW, Tychsen B, Conrad F, Suzuki N, et al. A role of melatonin in neuroectodermal-mesodermal interactions: the hair follicle synthesizes melatonin and expresses functional melatonin receptors. FASEB J. 2005;19:1710–2. doi: 10.1096/fj.04-2293fje. [DOI] [PubMed] [Google Scholar]
- Kräuchi K, Cajochen C, Mori D, Graw P, Wirz-Justice A. Early evening melatonin and S-20098 advance circadian phase and nocturnal regulation of core body temperature. Am J Physiol. 1997;272(Pt 2):R1178–88. doi: 10.1152/ajpregu.1997.272.4.R1178. [DOI] [PubMed] [Google Scholar]
- Kräuchi K, Cajochen C, Pache M, Flammer J, Wirz-Justice A. Thermoregulatory effects of melatonin in relation to sleepiness. Chronobiol Int. 2006;23:475–84. doi: 10.1080/07420520500545854. [DOI] [PubMed] [Google Scholar]
- Kumar CA, Das UN. Effect of melatonin on two stage skin carcinogenesis in Swiss mice. Med Sci Monit. 2000;6:471–5. [PubMed] [Google Scholar]
- Lee JH, Moon JH, Nazim UM, Lee YJ, Seol JW, Eo SK, et al. Melatonin protects skin keratinocyte from hydrogen peroxide-mediated cell death via the SIRT1 pathway. Oncotarget. 2016;7:12075–88. doi: 10.18632/oncotarget.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Lee WS, Suh SI, Kim SP, Lee SR, Ryoo YW, et al. Melatonin reduces ultraviolet-B induced cell damages and polyamine levels in human skin fibroblasts in culture. Exp Mol Med. 2003;35:263–8. doi: 10.1038/emm.2003.35. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Jung YH, Oh SY, Yun SP, Han HJ. Melatonin enhances the human mesenchymal stem cells motility via melatonin receptor 2 coupling with Galphaq in skin wound healing. J Pineal Res. 2014;57:393–407. doi: 10.1111/jpi.12179. [DOI] [PubMed] [Google Scholar]
- Legros C, Devavry S, Caignard S, Tessier C, Delagrange P, Ouvry C, et al. Melatonin MT(1) and MT(2) receptors display different molecular pharmacologies only in the G-protein coupled state. Br J Pharmacol. 2014;171:186–201. doi: 10.1111/bph.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León J, Macías M, Escames G, Camacho E, Khaldy H, Martín M, et al. Structure-related inhibition of calmodulin-dependent neuronal nitric-oxide synthase activity by melatonin and synthetic kynurenines. Mol Pharmacol. 2000;58:967–75. doi: 10.1124/mol.58.5.967. [DOI] [PubMed] [Google Scholar]
- Lerner AB. On the etiology of vitiligo and gray hair. Am J Med. 1971;51:141–7. doi: 10.1016/0002-9343(71)90232-4. [DOI] [PubMed] [Google Scholar]
- Lerner AB, Case JD, Takahashi Y. Isolation of melatonin, a pineal factor that lightens melanocytes. J Am Chem Soc. 1958;80:2587. [Google Scholar]
- Lissoni P, Brivio O, Brivio F, Barni S, Tancini G, Crippa D, et al. Adjuvant therapy with the pineal hormone melatonin in patients with lymph node relapse due to malignant melanoma. J Pineal Res. 1996;21:239–42. doi: 10.1111/j.1600-079x.1996.tb00292.x. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Fumagalli L, Paolorossi F, Rovelli F, Roselli MG, Maestroni GJ. Anticancer neuroimmunomodulation by pineal hormones other than melatonin: preliminary phase II study of the pineal indole 5-methoxytryptophol in association with low-dose IL-2 and melatonin. J Biol Regul Homeost Agents. 1997;11:119–22. [PubMed] [Google Scholar]
- Lissoni P, Vaghi M, Ardizzoia A, Malugani F, Fumagalli E, Bordin V, et al. A phase II study of chemoneuroimmunotherapy with platinum, subcutaneous low-dose interleukin-2 and the pineal neurohormone melatonin (P.I.M.) as a second-line therapy in metastatic melanoma patients progressing on dacarbazine plus interferon-alpha. In Vivo. 2002;16:93–6. [PubMed] [Google Scholar]
- Love NR, Chen Y, Ishibashi S, Kritsiligkou P, Lea R, Koh Y, et al. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nat Cell Biol. 2013;15:222–8. doi: 10.1038/ncb2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Yi X, Cheng X, Sun X, Yang X. Melatonin protects against myocardial hypertrophy induced by lipopolysaccharide. In Vitro Cell Dev Biol Anim. 2015;51:353–60. doi: 10.1007/s11626-014-9844-0. [DOI] [PubMed] [Google Scholar]
- Ma Z, Yang Y, Fan C, Han J, Wang D, Di S, et al. Melatonin as a potential anticarcinogen for non-small-cell lung cancer. Oncotarget. 2016;7:46768–84. doi: 10.18632/oncotarget.8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marseglia L, Cuppari C, Manti S, D’Angelo G, Salpietro C, Reiter RJ, et al. Atopic dermatitis: melatonin as potential treatment. J Biol Regul Homeost Agents. 2015;29(Suppl 1):142–9. [PubMed] [Google Scholar]
- Marseglia L, D’Angelo G, Manti S, Salpietro C, Arrigo T, Barberi I, et al. Melatonin and atopy: role in atopic dermatitis and asthma. Int J Mol Sci. 2014;15:13482–93. doi: 10.3390/ijms150813482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhinney DB, Hoffman SJ, Robinson WA, Ferguson J. Effect of melatonin on human skin color. J Invest Dermatol. 1994;102:258–9. doi: 10.1111/1523-1747.ep12371773. [DOI] [PubMed] [Google Scholar]
- Milan AB, Campmany AC, Naveros BC. Antioxidant nanoplatforms for dermal delivery: melatonin. Curr Drug Metab. 2017;18:437–453. doi: 10.2174/1389200218666170222145908. [DOI] [PubMed] [Google Scholar]
- Mozzanica N, Tadini G, Radaelli A, Negri M, Pigatto P, Morelli M, et al. Plasma melatonin levels in psoriasis. Acta Derm Venereol. 1988;68:312–6. [PubMed] [Google Scholar]
- Narita T, Kudo H. Effect of melatonin on B16 melanoma growth in athymic mice. Cancer Res. 1985;45:4175–7. [PubMed] [Google Scholar]
- Ndiaye MA, Nihal M, Wood GS, Ahmad N. Skin, reactive oxygen species, and circadian clocks. Antioxid Redox Signal. 2014;20:2982–96. doi: 10.1089/ars.2013.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlind K, Azmitia EC, Slominski A. The skin as a mirror of the soul: exploring the possible roles of serotonin. Exp Dermatol. 2008;17:301–11. doi: 10.1111/j.1600-0625.2007.00670.x. [DOI] [PubMed] [Google Scholar]
- Nosjean O, Ferro M, Coge F, Beauverger P, Henlin JM, Lefoulon F, et al. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem. 2000;275:31311–7. doi: 10.1074/jbc.M005141200. [DOI] [PubMed] [Google Scholar]
- Otalora BB, Madrid JA, Alvarez N, Vicente V, Rol MA. Effects of exogenous melatonin and circadian synchronization on tumor progression in melanoma-bearing C57BL6 mice. J Pineal Res. 2008;44:307–15. doi: 10.1111/j.1600-079X.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- Ozler M, Simsek K, Ozkan C, Akgul EO, Topal T, Oter S, et al. Comparison of the effect of topical and systemic melatonin administration on delayed wound healing in rats that underwent pinealectomy. Scand J Clin Lab Invest. 2010;70:447–52. doi: 10.3109/00365513.2010.506926. [DOI] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R. Melatonin: nature’s most versatile biological signal? FEBS J. 2006;273:2813–38. doi: 10.1111/j.1742-4658.2006.05322.x. [DOI] [PubMed] [Google Scholar]
- Park G, Lee SH, Oh DS, Kim YU. Melatonin inhibits neuronal dysfunction-associated with neuroinflammation by atopic psychological stress in NC/Nga atopic-like mouse models. J Pineal Res. 2017;63:e12420. doi: 10.1111/jpi.12420. [DOI] [PubMed] [Google Scholar]
- Pöggeler B, Balzer I, Hardeland R, Lerchl A. Pineal hormone melatonin oscillates also in the dinoflagellate Gonyaulax polyedra. Naturwissenschaften. 1991;78:268–9. [Google Scholar]
- Pugazhenthi K, Kapoor M, Clarkson AN, Hall I, Appleton I. Melatonin accelerates the process of wound repair in full-thickness incisional wounds. J Pineal Res. 2008;44:387–96. doi: 10.1111/j.1600-079X.2007.00541.x. [DOI] [PubMed] [Google Scholar]
- Ranieri D, Avitabile D, Shiota M, Yokomizo A, Naito S, Bizzarri M, et al. Nuclear redox imbalance affects circadian oscillation in HaCaT keratinocytes. Int J Biochem Cell Biol. 2015;65:113–24. doi: 10.1016/j.biocel.2015.05.018. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991;12:151–80. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. Oxidative damage in the central nervous system: protection by melatonin. Prog Neurobiol. 1998;56:359–84. doi: 10.1016/s0301-0082(98)00052-5. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Rosales-Corral SA, Tan DX, Acuña-Castroviejo D, Qin L, Yang SF, et al. Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int J Mol Sci. 2017a;18:843. doi: 10.3390/ijms18040843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu B. Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas [Epub ahead of print] Cell Mol Life Sci. 2017b;74:3863–3881. doi: 10.1007/s00018-017-2609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Kim SJ, Cruz MH. Delivery of pineal melatonin to the brain and SCN: role of canaliculi, cerebrospinal fluid, tanycytes and Virchow- Robin perivascular spaces. Brain Struct Funct. 2014;219:1878–87. doi: 10.1007/s00429-014-0719-7. [DOI] [PubMed] [Google Scholar]
- Ren W, Liu G, Chen S, Yin J, Wang J, Tan B, et al. Melatonin signaling in T cells: functions and applications. J Pineal Res. 2017;62:e12394. doi: 10.1111/jpi.12394. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Ebisawa T, Mahle CD, Kolakowski LF., Jr Cloning of a melatonin-related receptor from human pituitary. FEBS Lett. 1996;386:219–24. doi: 10.1016/0014-5793(96)00437-1. [DOI] [PubMed] [Google Scholar]
- Rezzani R, Rodella LF, Favero G, Damiani G, Paganelli C, Reiter RJ. Attenuation of ultraviolet A-induced alterations in NIH3T3 dermal fibroblasts by melatonin. Br J Dermatol. 2014;170:382–91. doi: 10.1111/bjd.12622. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Wiechmann AF, Hu DN. Melatonin receptors in human uveal melanocytes and melanoma cells. J Pineal Res. 2000;28:165–71. doi: 10.1034/j.1600-079x.2001.280306.x. [DOI] [PubMed] [Google Scholar]
- Romero MP, García-Pergañeda A, Guerrero JM, Osuna C. Membrane-bound calmodulin in Xenopus laevis oocytes as a novel binding site for melatonin. FASEB J. 1998;12:1401–8. doi: 10.1096/fasebj.12.13.1401. [DOI] [PubMed] [Google Scholar]
- Romic MD, Klaric MS, Lovric J, Pepic I, Cetina-Cizmek B, Filipovic-Grcic J, et al. Melatonin-loaded chitosan/Pluronic(R) F127 microspheres as in situ forming hydrogel: an innovative antimicrobial wound dressing. Eur J Pharm Biopharm. 2016;107:67–79. doi: 10.1016/j.ejpb.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Rondanelli M, Faliva MA, Perna S, Antoniello N. Update on the role of melatonin in the prevention of cancer tumorigenesis and in the management of cancer correlates, such as sleep-wake and mood disturbances: review and remarks. Aging Clin Exp Res. 2013;25:499–510. doi: 10.1007/s40520-013-0118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo YW, Suh SI, Mun KC, Kim BC, Lee KS. The effects of the melatonin on ultraviolet-B irradiated cultured dermal fibroblasts. J Dermatol Sci. 2001;27:162–9. doi: 10.1016/s0923-1811(01)00133-5. [DOI] [PubMed] [Google Scholar]
- Schallreuter KU, Bahadoran P, Picardo M, Slominski A, Elassiuty YE, Kemp EH, et al. Vitiligo pathogenesis: autoimmune disease, genetic defect, excessive reactive oxygen species, calcium imbalance, or what else? Exp Dermatol. 2008;17:139–40. doi: 10.1111/j.1600-0625.2007.00666_1.x. discussion 41–60. [DOI] [PubMed] [Google Scholar]
- Schallreuter KU, Beazley WD, Hibberts NA, Tobin DJ, Paus R, Wood JM. Pterins in human hair follicle cells and in the synchronized murine hair cycle. J Invest Dermatol. 1998;111:545–50. doi: 10.1046/j.1523-1747.1998.00335.x. [DOI] [PubMed] [Google Scholar]
- Schallreuter KU, Salem MA, Gibbons NC, Martinez A, Slominski R, Ludemann J, et al. Blunted epidermal L-tryptophan metabolism in vitiligo affects immune response and ROS scavenging by Fenton chemistry, part 1: epidermal H2O2/ONOO(–)-mediated stress abrogates tryptophan hydroxylase and dopa decarboxylase activities, leading to low serotonin and melatonin levels. FASEB J. 2012;26:2457–70. doi: 10.1096/fj.11-197137. [DOI] [PubMed] [Google Scholar]
- Schallreuter KU, Schulz-Douglas V, Bunz A, Beazley W, Korner C. Pteridines in the control of pigmentation. J Invest Dermatol. 1997;109:31–5. doi: 10.1111/1523-1747.ep12276418. [DOI] [PubMed] [Google Scholar]
- Scheuer C. Melatonin for prevention of erythema and oxidative stress in response to ultraviolet radiation. Dan Med J. 2017;64:B5358. [PubMed] [Google Scholar]
- Scheuer C, Pommergaard HC, Rosenberg J, Gogenur I. Dose dependent sun protective effect of topical melatonin: a randomized, placebo-controlled, double-blind study. J Dermatol Sci. 2016a;84:178–85. doi: 10.1016/j.jdermsci.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Scheuer C, Pommergaard HC, Rosenberg J, Gögenur I. Effect of topical application of melatonin cream 12.5% on cognitive parameters: a randomized, placebo-controlled, double-blind crossover study in healthy volunteers. J Dermatolog Treat. 2016b;27:488–94. doi: 10.3109/09546634.2016.1161154. [DOI] [PubMed] [Google Scholar]
- Semak I, Korik E, Naumova M, Wortsman J, Slominski A. Serotonin metabolism in rat skin: characterization by liquid chromatography-mass spectrometry. Arch Biochem Biophys. 2004;421:61–6. doi: 10.1016/j.abb.2003.08.036. [DOI] [PubMed] [Google Scholar]
- Semak I, Naumova M, Korik E, Terekhovich V, Wortsman J, Slominski A. A novel metabolic pathway of melatonin: oxidation by cytochrome C. Biochemistry. 2005;44:9300–7. doi: 10.1021/bi050202d. [DOI] [PubMed] [Google Scholar]
- Shen J, Barrios RJ, Jaiswal AK. Inactivation of the quinone oxidoreductases NQO1 and NQO2 strongly elevates the incidence and multiplicity of chemically induced skin tumors. Cancer Res. 2010;70:1006–14. doi: 10.1158/0008-5472.CAN-09-2938. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Singh M, Jadhav HR. Melatonin: functions and ligands. Drug Discov Today. 2014;19:1410–8. doi: 10.1016/j.drudis.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Slominski A, Baker J, Rosano TG, Guisti LW, Ermak G, Grande M, et al. Metabolism of serotonin to N-acetylserotonin, melatonin, and 5-methoxytryptamine in hamster skin culture. J Biol Chem. 1996;271:12281–6. doi: 10.1074/jbc.271.21.12281. [DOI] [PubMed] [Google Scholar]
- Slominski A, Chassalevris N, Mazurkiewicz J, Maurer M, Paus R. Murine skin as a target for melatonin bioregulation. Exp Dermatol. 1994;3:45–50. doi: 10.1111/j.1600-0625.1994.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Fischer TW, Zmijewski MA, Wortsman J, Semak I, Zbytek B, et al. On the role of melatonin in skin physiology and pathology. Endocrine. 2005a;27:137–48. doi: 10.1385/ENDO:27:2:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Johansson O, Jing C, Semak I, Slugocki G, et al. Tryptophan hydroxylase expression in human skin cells. Biochim Biophys Acta. 2003a;1639:80–6. doi: 10.1016/s0925-4439(03)00124-8. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Semak I, Sweatman T, Szczesniewski A, Wortsman J. Serotoninergic system in hamster skin. J Invest Dermatol. 2002a;119:934–42. doi: 10.1046/j.1523-1747.2002.00156.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J. Characterization of the serotoninergic system in the C57BL/6 mouse skin. Eur J Biochem. 2003b;270:3335–44. doi: 10.1046/j.1432-1033.2003.03708.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J, Szczesniewski A, et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002b;16:896–8. doi: 10.1096/fj.01-0952fje. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Wortsman J. Expression of genes coding melatonin and serotonin receptors in rodent skin. Biochim Biophys Acta. 2004a;1680:67–70. doi: 10.1016/j.bbaexp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Zbytek B, Tobin DJ, Kauser S, Wortsman J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J Cell Physiol. 2003c;196:144–53. doi: 10.1002/jcp.10287. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pruski D. Melatonin inhibits proliferation and melanogenesis in rodent melanoma cells. Exp Cell Res. 1993;206:189–294. doi: 10.1006/excr.1993.1137. [DOI] [PubMed] [Google Scholar]
- Slominski A, Semak I, Pisarchik A, Sweatman T, Szczesniewski A, Wortsman J. Conversion of L-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Lett. 2002c;511:102–6. doi: 10.1016/s0014-5793(01)03319-1. [DOI] [PubMed] [Google Scholar]
- Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004b;84:1155–228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, Paus R. Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol Metab. 2008;19:17–24. doi: 10.1016/j.tem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. J Invest Dermatol. 2005b;124:13–21. doi: 10.1111/j.0022-202X.2004.23528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005c;19:176–94. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- Slominski AT, Hardeland R, Reiter RJ. When the circadian clock meets the melanin pigmentary system. J Invest Dermatol. 2015;135:943–5. doi: 10.1038/jid.2014.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Kim TK, Hobrath JV, Oak AS, Tang EK, Tieu EW, et al. Endogenously produced nonclassical vitamin D hydroxy-metabolites act as “biased” agonists on VDR and inverse agonists on RORalpha and ROR-gamma. J Steroid Biochem Mol Biol. 2017a;173:42–56. doi: 10.1016/j.jsbmb.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Kim TK, Takeda Y, Janjetovic Z, Brozyna AA, Skobowiat C, et al. RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20, 23-dihydroxyvitamin D. FASEB J. 2014a;28:2775–89. doi: 10.1096/fj.13-242040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Kleszczynski K, Semak I, Janjetovic Z, Zmijewski MA, Kim TK, et al. Local melatoninergic system as the protector of skin integrity. Int J Mol Sci. 2014b;15:17705–32. doi: 10.3390/ijms151017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Semak I, Fischer TW, Kim TK, Kleszczynski K, Hardeland R, et al. Metabolism of melatonin in the skin: why is it important? Exp Dermatol. 2017b;26:563–568. doi: 10.1111/exd.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Jetten AM. RORalpha is not a receptor for melatonin (response to doi 10.1002/bies.201600018) Bioessays. 2016c;38:1193–4. doi: 10.1002/bies.201600204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Semak I, Kim T-K, Janjentovic Z, Slominski RM, Zmijewski J. Melatonin, mitochondria and the skin [e-pub ahead of print] Cell Mol Life Sci. 2017c;74:3913–3925. doi: 10.1007/s00018-017-2617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Semak I, Zbytek B, Pisarchik A, Li W, et al. Cytochromes p450 and skin cancer: role of local endocrine pathways. Anticancer Agents Med Chem. 2014c;14:77–96. doi: 10.2174/18715206113139990308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v–vii. 1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Ren L, Ma H, Hu R, Gao H, Wang L, et al. Melatonin promotes diabetic wound healing in vitro by regulating keratinocyte activity. Am J Transl Res. 2016;8:4682–93. [PMC free article] [PubMed] [Google Scholar]
- Suofu Y, Li W, Jean-Alphonse FG, Jia J, Khattar NK, Li J, et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc Natl Acad Sci USA. 2017;114:E7997–8006. doi: 10.1073/pnas.1705768114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza AV, Visconti MA, Castrucci AM. Melatonin biological activity and binding sites in human melanoma cells. J Pineal Res. 2003;34:242–8. doi: 10.1034/j.1600-079x.2003.02928.x. [DOI] [PubMed] [Google Scholar]
- Soybir G, Topuzlu C, Odabas O, Dolay K, Bilir A, Koksoy F. The effects of melatonin on angiogenesis and wound healing. Surg Today. 2003;33:896–901. doi: 10.1007/s00595-003-2621-3. [DOI] [PubMed] [Google Scholar]
- Stanberry LR, Das Gupta TK, Beattie CW. Photoperiodic control of melanoma growth in hamsters: influence of pinealectomy and melatonin. Endocrinology. 1983;113:469–75. doi: 10.1210/endo-113-2-469. [DOI] [PubMed] [Google Scholar]
- Su SC, Hsieh MJ, Yang WE, Chung WH, Reiter RJ, Yang SF. Cancer metastasis: mechanisms of inhibition by melatonin. J Pineal Res. 2017;62:e12370. doi: 10.1111/jpi.12370. [DOI] [PubMed] [Google Scholar]
- Tan DX, Hardeland R, Back K, Manchester LC, Alatorre-Jimenez MA, Reiter RJ. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: comparisons across species. J Pineal Res. 2016;61:27–40. doi: 10.1111/jpi.12336. [DOI] [PubMed] [Google Scholar]
- Tan DX, Hardeland R, Manchester LC, Korkmaz A, Ma S, Rosales-Corral S, et al. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J Exp Bot. 2012;63:577–97. doi: 10.1093/jxb/err256. [DOI] [PubMed] [Google Scholar]
- Tan DX, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ. Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules. 2015;20:18886–906. doi: 10.3390/molecules201018886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DX, Manchester LC, Liu X, Rosales-Corral SA, Acuña-Castroviejo D, Reiter RJ. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J Pineal Res. 2013;54:127–38. doi: 10.1111/jpi.12026. [DOI] [PubMed] [Google Scholar]
- Tan DX, Reiter RJ, Manchester LCMTY, El-Sawi M, Sainz RM, et al. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002;2:181–97. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- Tan DX, Zheng X, Kong J, Manchester LC, Hardeland R, Kim SJ, et al. Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: relation to their biological functions. Int J Mol Sci. 2014;15:15858–90. doi: 10.3390/ijms150915858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC. Neuroendocrinology of mast cells: challenges and controversies. Exp Dermatol. 2017;26:751–759. doi: 10.1111/exd.13288. [DOI] [PubMed] [Google Scholar]
- Valverde P, Benedito E, Solano F, Oaknin S, Lozano JA, Garcia-Borron JC. Melatonin antagonizes alpha-melanocyte-stimulating hormone enhancement of melanogenesis in mouse melanoma cells by blocking the hormone-induced accumulation of the c locus tyrosinase. Eur J Biochem. 1995;232:257–63. doi: 10.1111/j.1432-1033.1995.tb20807.x. [DOI] [PubMed] [Google Scholar]
- van den Heuvel CJ, Kennaway DJ, Dawson D. Thermoregulatory and soporific effects of very low dose melatonin injection. Am J Physiol. 1999;276(Pt 1):E249–54. doi: 10.1152/ajpendo.1999.276.2.E249. [DOI] [PubMed] [Google Scholar]
- Venegas C, Garcia JA, Escames G, Ortiz F, Lopez A, Doerrier C, et al. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res. 2012;52:217–27. doi: 10.1111/j.1600-079X.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- Vesnushkin GM, Plotnikova NA, Semenchenko AI, Anisimov VN. Dose-dependent inhibitory effect of melatonin on carcinogenesis induced by benzo[a]pyrene in mice. J Exp Clin Cancer Res. 2006;25:507–13. [PubMed] [Google Scholar]
- Vidali S, Chéret J, Giesen M, Haeger S, Alam M, Watson REB, et al. Thyroid hormones enhance mitochondrial function in human epidermis. J Invest Dermatol. 2016;136:2003–12. doi: 10.1016/j.jid.2016.05.118. [DOI] [PubMed] [Google Scholar]
- Yi WJ, Kim TS. Melatonin protects mice against stress-induced inflammation through enhancement of M2 macrophage polarization. Int Immunopharmacol. 2017;48:146–58. doi: 10.1016/j.intimp.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Yi C, Zhang Y, Yu Z, Xiao Y, Wang J, Qiu H, et al. Melatonin enhances the anti-tumor effect of fisetin by inhibiting COX-2/iNOS and NF-kappaB/p300 signaling pathways. PLoS One. 2014;9:e99943. doi: 10.1371/journal.pone.0099943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying SW, Niles LP, Crocker C. Human malignant melanoma cells express high-affinity receptors for melatonin: antiproliferative effects of melatonin and 6-chloromelatonin. Eur J Pharmacol. 1993;246:89–96. doi: 10.1016/0922-4106(93)90084-m. [DOI] [PubMed] [Google Scholar]
- Zetner D, Andersen LP, Rosenberg J. Pharmacokinetics of alternative administration routes of melatonin: a systematic review. Drug Res (Stuttg) 2016;66:169–73. doi: 10.1055/s-0035-1565083. [DOI] [PubMed] [Google Scholar]
- Zmijewski MA, Sweatman TW, Slominski AT. The melatonin-producing system is fully functional in retinal pigment epithelium (ARPE-19) Mol Cell Endocrinol. 2009;307:211–6. doi: 10.1016/j.mce.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.