Abstract
Background
Although there has been inconsistency in recommendations regarding the optimal time for introducing complementary foods, most experts agree that introduction should not occur before 4 months. Despite recommendations, studies suggest that 20% to 40% of US infants are introduced to foods at younger than 4 months. Previous studies focused on the introduction of solid foods and are not nationally representative.
Objective
Our aims were to provide a nationally representative estimate of the timing of introduction of complementary foods and to describe predictors of early (<4 months) introduction.
Design
We conducted a cross-sectional analysis of 2009–2014 National Health and Nutrition Examination Survey data.
Participants
The study included 1,482 children aged 6 to 36 months.
Main outcome measures
Timing of first introduction to complementary foods (anything other than breast milk or formula) was analyzed.
Statistical analyses performed
Prevalence estimates of first introduction to complementary foods are presented by month. Logistic regression was used to assess characteristics associated with early (<4 months) introduction.
Results
In this sample, 16.3% of US infants were introduced to complementary foods at <4 months, 38.3% between 4 and <6 months, 32.5% between 6 and <7 months, and 12.9% at ≥7 months of age. In unadjusted analyses, early introduction varied by breastfeeding status; race/Hispanic origin; Special Supplemental Nutrition Program for Women, Infants, and Children participation; and maternal age. In adjusted analyses, only breastfeeding status remained significant; infants who never breastfed or stopped at <4 months were more likely (odds ratio 2.27; 95% CI 1.62 to 3.18) to be introduced to complementary foods early than infants who breastfed ≥4 months.
Conclusions
Despite using a broader definition of complementary foods, this analysis found a lower prevalence of early introduction in this nationally representative sample than previous studies that included only solids. However, many young children were still introduced to complementary foods earlier than recommended. Strategies to support caregivers to adhere to infant feeding guidelines may be needed.
Keywords: National Health and Nutrition Examination Surveys, Complementary feeding, Infant feeding, Infant feeding decisions, Solid food introduction
In recent years, there has been some inconsistency in the recommended timing of the introduction of complementary foods to infants. The term complementary foods refers to anything other than breast milk and infant formula, both solids and liquids, that are needed when milk feeds alone are no longer sufficient to meet the nutritional requirements of infants.1 The Dietary Guidelines for Americans (DGA) serve as federal level diet and nutrition guidance for the population ≥2 years of age; however, there is no federal guidance for children under 2 years.2 The 2020–2025 DGA are expected to begin incorporating or releasing federal dietary guidelines for this age group.2 Some groups that do have guidelines recommend infants be introduced to complementary foods between 4 and 6 months of age3 and others recommend waiting until about 6 months1,4,5; however, most agree that introduction before 4 months is too early and introduction after 8 months is too late. Currently, the American Academy of Pediatrics recommends that infants be introduced to complementary foods at about 6 months of age.5 Early introduction has been found to be a risk factor for obesity, although the evidence for this is inconsistent.6 Both early and late introduction are thought to be risk factors for celiac disease and type 1 diabetes.7 Late introduction has been associated with allergies, micronutrient deficiency, and poor eating patterns at later ages.7,8
Previous studies in the United States have reported that 20% to 40% of infants are introduced to complementary foods before 4 months.9–11 These studies were not nationally representative and focused on introduction to solid foods without consideration for liquids. In addition, two of these studies are more than 10 years old. The timing of when non-milk liquids are introduced is important to consider, as early introduction of non-milk liquids is thought to compromise adequate intake of nutrients that come from breast milk and infant formula, and reduce the duration of breastfeeding among breastfed infants.12 One Canadian study that focused on the timing of introduction of liquids other than breast milk and infant formula found 45% of infants were introduced to other liquids before 6 months.13
The objective of this study was to examine the timing of first introduction of complementary foods (including both solids and liquids other than breast milk and infant formula) among a nationally representative sample of US children. In addition, breastfeeding status and the sociodemographic predictors of early (<4 months) introduction are described. These data may be relevant to the development of the DGA for this age group.2
METHODS
Data Source and Study Sample
This study used data from the 2009–2014 continuous National Health and Nutrition Examination Survey (NHANES). NHANES is conducted in 2-year cycles by the Centers for Disease Control and Prevention’s National Center for Health Statistics. Each 2-year cycle obtains nationally representative data on the health and nutrition status of the non-institutionalized civilian population in the United States and uses a complex, stratified, multistage probability design to select participants. Respondents participate in both a household interview and a physical examination at the Mobile Examination Center; only data from the household interview questionnaires were used for this study. The unweighted response rate for the included NHANES survey years ranged from 82.2% to 88.8% for children aged <1 year and 79.0% to 90.4% for children aged 1 to 5 years.14 The National Center of Health Statistics Research Ethics Review Board approved NHANES protocol and all participants in NHANES provide written informed consent or by proxy for those who are <7 years of age.
The dietary behavior and nutrition questionnaire asks participants 0 to 6 years of age questions pertaining to infant feeding. A proxy, typically the child’s parent, responds to these questions. The survey asks how old the infant was when he or she “was first fed anything other than breast milk or formula,” including “juice, cow’s milk, sugar water, baby food, or anything else that [the infant] might have been given, even water.” The sample was limited to children who were reportedly first fed complementary foods before 12 months of age, as it is unlikely a child would receive only breast milk or formula beyond 12 months of life. Breastfeeding status was based on the following questions: “Was [name of the infant/child] ever breastfed or fed breast milk?” and “How old was [name of the infant/child] when [he/she] completely stopped breastfeeding or being fed breast milk? Breastfeeding was categorized as those who received breast milk for 4 or more months or those who did not (ie, never breastfed or stopped breastfeeding before 4 months). For both timing of complementary food introduction and breastfeeding duration, respondents could have answered in days, weeks, or months. NHANES then standardized these variables into days using the conversion factors: 7 days/week, 30.4 days/month, and 365 days/year.
Information on age; sex; race/Hispanic origin; participation in the Special Supplemental Nutrition Program for Women, Infants and Children (WIC); poverty-to-income ratio; maternal age; and maternal smoking were collected during the in-home interview.15 Race and Hispanic origin were based on answers to questions on race and Hispanic origin and were reported in the following categories: non-Hispanic white, non-Hispanic black, and Hispanic. Estimates from the Other race/ethnicity category are not presented as a separate group for race/Hispanic origin specific estimates; however, these data are included in all analyses. WIC status was based on child’s participation in WIC within the past 12 months and poverty-to-income ratio data; a poverty-to-income ratio ≤1.85 was used to determine WIC income eligibility.16 WIC status was reported as not income-eligible, eligible-received, and eligible-not received. Maternal age was categorized as ≥25 years and ≤24 years and maternal smoking during pregnancy was categorized as no or yes.
Two sets of analyses were completed, one including children who were 6 to 24 months of age at the time of the NHANES interview and one including children who were 6 to 36 months of age at the time of the NHANES interview. Results were similar between the narrower and expanded age groups, suggesting recall bias was not greater when including slightly older children. Therefore, in order to increase sample size, the expanded age group (6 to 36 months) was used as the analytic sample. The sample was limited to children at least 6 months of age as participants younger than 6 months of age may not yet be receiving complementary foods. In addition, the sample was limited to children no older than 36 months of age, as reporting of timing of introduction of foods and beverages may be less accurate among older children due to the potential for recall bias.
Data were available for 1,747 children 6 to 36 months of age. Children were excluded from analyses if they were reported to have been introduced to complementary foods after 12 months of age (n=109), had not yet been introduced to complementary foods (n=16), refused to answer the question pertaining to complementary food introduction or did not know when the participant was introduced to complementary foods (n=5), or were missing data on breastfeeding status or key sociodemographic variables (n=135). This resulted in a final analytic sample of 1,482.
Statistical Analysis
The distribution of the percentage of infants with reported first introduction to complementary foods is described by month of age. NHANES reported both age of introduction and duration of breastfeeding in days. For this study, age in days was converted to age in months; 1 month was defined by NHANES as 30.4 days. Because of small sample sizes, infants introduced to complementary foods at 0 to <2 months were collapsed into one category, as were infants introduced at ≥9 months.
Logistic regression was used to assess whether breast-feeding status and any demographic characteristics predicted early (<4 months) complementary food introduction. Odds ratios were adjusted for all included variables (breastfeeding status, sex, race/Hispanic origin, WIC status, maternal age, and maternal smoking during pregnancy). All analyses were conducted using SAS software17 survey procedures to account for NHANES complex sampling design. A 6-year interview sample weight was created to account for the complex survey design, survey nonresponse and post-stratification as recommended by the National Center for Health Statistics; weighted estimates are presented.18
RESULTS
Table 1 illustrates the breastfeeding status and selected sociodemographic characteristics of the 1,482 children included in the study. The mean age of the children was 16.4 months (standard deviation 7.8 months). Less than half of the children were still receiving breast milk at 4 months of age (43.3%) and just over half of the children were male (51.7%). More than half of the children (52.4%) were non-Hispanic white, 12.5% were non-Hispanic black, and 26.3% were Hispanic. Among children in the study, 50.4% were not eligible for WIC, 42.1% were eligible for and received WIC in the past 12 months, and 7.5% were eligible but not receiving WIC. More than half of the children were born to mothers ≥25 years of age (69.6%) and most were born to mothers who did not report smoking (90.5%).
Table 1.
Breastfeeding status and selected demographic characteristics of US children 6 to 36 months of age,a National Health and Nutrition Examination Surveys, 2009–2014 (n=1,482)
| Characteristic | n | Mean–SDb |
|---|---|---|
| Age, mo | 1,482 | 16.4±7.8 |
| %c (95% CI) | ||
|
| ||
| Breastfeeding status | ||
| ≥4 mo | 558 | 43.3 (39.0–47.5) |
| Never or <4 mo | 924 | 56.7 (52.5–61.0) |
| Sex | ||
| Male | 755 | 51.7 (47.9–55.5) |
| Female | 727 | 48.3 (44.5–52.1) |
| Race/Hispanic origind | ||
| Non-Hispanic white | 479 | 52.4 (46.4–58.5) |
| Non-Hispanic black | 287 | 12.5 (10.2–14.9) |
| Hispanic | 562 | 26.3 (20.3–32.2) |
| WICe status | ||
| Not income-eligible | 546 | 50.4 (46.6–54.2) |
| Eligible-received | 828 | 42.1 (38.0–46.3) |
| Eligible-not received | 108 | 7.5 (5.6–9.3) |
| Maternal age | ||
| ≥25 y | 946 | 69.6 (66.4–72.8) |
| ≤24 y | 536 | 30.4 (27.2–33.6) |
| Maternal smoking during pregnancy | ||
| No | 1,335 | 90.5 (88.0–93.0) |
| Yes | 147 | 9.5 (7.0–12.0) |
At time of interview.
SD=standard deviation.
Data are weighted to account for survey sampling design.
Race/Hispanic origin subanalyses are limited to participants who report being non-Hispanic white, non-Hispanic black, or Hispanic.
WIC=Special Supplemental Nutrition Program for Women, Infants, and Children.
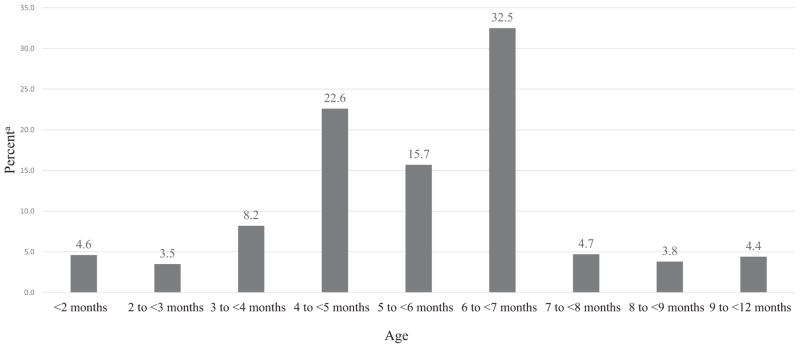
The distribution of the percentage of children aged 6 to 36 months and their reported age of first introduction to complementary foods is presented in the Figure. Among US children 6 to 36 months of age, 16.3% were introduced to complementary foods early (<4 months) and 38.3% were introduced to complementary foods between 4 and <6 months. Overall, 54.6% of infants were introduced to complementary foods before 6 months of age and 32.5% were introduced between 6 and <7 months. Introduction (≥7 months) occurred in 12.9% of infants.
Figure.
The distribution of the percentage of children 6 to 36 months and their reported age of introduction to complementary foods, National Health and Nutrition Examination Surveys, 2009–2014 (n=1,482). aData are weighted to account for survey sampling design.
In bivariate analyses, the odds of being introduced to complementary foods early (<4 months) were higher among infants never breastfed or breastfed <4 months (odds ratio [OR] 2.46, 95% CI 1.84 to 3.30) compared to infants breastfed for 4 months or longer (Table 2). In addition, the odds of being introduced to complementary foods early were higher among non-Hispanic black infants (OR 1.67, 95% CI 1.08 to 2.57) compared to non-Hispanic white infants, among infants receiving WIC (OR 1.38, 95% CI 1.01 to 1.89) compared to infants not eligible for WIC, and among infants born to mothers ≤24 years of age (OR 1.61, 95% CI 1.16 to 2.22) compared to infants born to mothers 25 years of age or older. After adjusting for covariates, only breastfeeding status remained significant (OR 2.27; 95% CI 1.62 to 3.18).
Table 2.
Prevalence and odds ratios of introduction to complementary foods before 4 months of age by breastfeeding status and demographic characteristics among children 6 to 36 months of age,a National Health and Nutrition Examination Surveys, 2009–2014
| Variable | %b | Unadjusted | Adjustedc |
|---|---|---|---|
| ←–– odds ratio (95% CI) ––→ | |||
| Total | 17.3 | ||
| Breastfeeding status | |||
| ≥4 mo | 9.9 | Reference | Reference |
| Never or <4 mo | 21.2 | 2.46 (1.84–3.30) | 2.27 (1.62–3.18) |
| Sex | |||
| Male | 17.9 | Reference | Reference |
| Female | 14.5 | 0.77 (0.54–1.11) | 0.75 (0.54–1.06) |
| Race/Hispanic origind | |||
| Non-Hispanic white | 15.7 | Reference | Reference |
| Non-Hispanic black | 23.7 | 1.67 (1.08–2.57) | 1.40 (0.87–2.24) |
| Hispanic | 15.8 | 1.01 (0.71–1.44) | 0.94 (0.63–1.40) |
| WICe status | |||
| Not income-eligible | 13.9 | Reference | Reference |
| Eligible-received | 18.1 | 1.38 (1.01–1.89) | 0.97 (0.67–1.41) |
| Eligible-not received | 22.1 | 1.77 (0.83–3.77) | 1.60 (0.76–3.37) |
| Maternal age | |||
| ≥25 y | 14.2 | Reference | Reference |
| ≤24 y | 21.0 | 1.61 (1.16–2.22) | 1.22 (0.85–1.74) |
| Maternal smoking during pregnancy | |||
| No | 15.5 | Reference | Reference |
| Yes | 24.1 | 1.74 (0.88–3.42) | 1.38 (0.64–2.97) |
At the time of interview.
Data are weighted to account for survey sampling design.
Each adjusted odds ratio was adjusted for all other variables presented in the table.
Race/Hispanic origin subanalyses are limited to participants who report being non-Hispanic white, non-Hispanic black, or Hispanic.
WIC=Special Supplemental Nutrition Program for Women, Infants, and Children.
DISCUSSION
This is the first nationally representative report describing the timing of the introduction of complementary foods to US infants. Contrary to previous studies that found 20%9 to 40%10 of infants are being introduced to solids before 4 months of age, this study found that 16.3% are introduced to complementary foods before 4 months and 54.6% of infants are being introduced to complementary foods before the recommended 6 months. Consistent with existing literature, earlier introduction to complementary foods was associated with infants who had not received breast milk or had received it for <4 months.10,19,20 In one analysis that explored reasons for early introduction of complementary foods by milk feeding type, mothers of infants who were formula fed at the time of complementary food introduction were more likely to report that a doctor or other health professional had said that their baby was ready to begin eating solid foods.10 Infants with a longer duration of breastfeeding (ie, 4 months or longer) may be delaying food introduction as a result of the American Academy of Pediatrics recommendation to breast-feed exclusively through 6 months.21
Guidelines for complementary food introduction have changed dramatically over the last 60 years. In 1958, guidelines were released that recommended infants be introduced to solids in the third month,22 in the 1970s, guidelines began recommending solid introduction be delayed until after 4 months of age, and it was not until the 1990s that guidelines began specifying that solids not be introduced until 6 months of age.23 Most recently, in 2017, guidelines were released that recommend infants at high risk for developing allergies be introduced to peanut-containing foods as early as 4 to 6 months to reduce the risk of developing peanut allergy.24 Given these variations, it is not surprising that studies that have focused on the timing of the introduction of solid foods have found a general lack of adherence to current professional guidelines.
The 2002 cross-sectional Feeding Infants and Toddlers Study (FITS) found that among toddlers 12 months and older, 29% were introduced to infant cereal and pureed foods before 4 months of age.11 Another example is the 2005–2007 Infant Feeding and Practices Study II (IFPS II), a longitudinal study of children through 12 months of age, which reported 40% of infants were introduced to solid foods before the age of 4 months.10 Lastly, the US Department of Agriculture’s WIC ITFPS-2 (Special Supplemental Nutrition Program for Women, Infants, and Children’s Infant and Toddler Feeding and Practices Study), which began in 2013, found that 20% of low-income infants enrolled in WIC were introduced to infant cereals, fruits, vegetables, or meats before 4 months.9
There are several potential reasons why the timing of food introduction varied across studies. Studies were conducted at different time points (ie, 2002, 2005–2007, and 2013) and the method for assessing the timing of food introduction has varied across studies. For example, FITS was a cross-sectional survey, and parents of children 12 to 24 months of age were asked about the introduction of specific foods (infant cereal and pureed foods) and reported the timing of introduction in weeks or months.11 However, IFPS II was a longitudinal study with approximately monthly data collection. The age of introduction to solid foods was calculated at the midpoint between the child’s age when the mother reported no solid food consumption and when she first reported that her child had consumed solid foods in the previous 7 days.10 Therefore, if a child was receiving no complementary foods at 4 months, but reported receiving complementary foods in the previous 7 days at 5 months, the child would be considered to have received solid foods at 4.5 months. WIC IFPS-2 is also a longitudinal study and assessed the timing of when a child was first fed various foods at each data collection point, including months 1, 3, 5, 7, 9, 11, and 13 months.25 It is not known how these different methodologies impact the reported percentages of children’s introduction to complementary foods. An assessment between studies on how parents report food introduction (either specific foods or any foods or beverages) and the timing of food introduction (eg, days, months, mid points) may provide more details on the magnitude of these differences, but was beyond the scope of this study. Lastly, the study populations were not always similar. For example, women in FITS were identified from a child-level commercial vendor and were more likely to be white and of higher socioeconomic status.26 Similarly, women in IFPS II were identified from a consumer panel and were more likely to be white, of higher socioeconomic status, able to read English, and have a stable mailing address.27 However, women in WIC ITFPS-2 were drawn from national WIC sites and are representative of children participating in WIC.25
This study had several limitations. Measurement error could have occurred from the diet behavior and nutrition interview data, as they rely on accurate memory of when foods or liquids were first introduced. There is also potential for bias related to the desire to be perceived as following best practices or recommendations. A further limitation is that NHANES does not provide data on what foods are first introduced, the amount, or frequency of introduction. In addition, NHANES has limited data on maternal characteristics.
Strengths of this study include that NHANES is a nationally representative data source and therefore findings are generalizable across the United States and interviews were conducted in person by a trained interviewer.
The timing of when complementary foods are first introduced to infants has implications for public health, as early (before 4 months) and late (after 8 months) introduction has been associated with both short- and long-term adverse health outcomes.28 To develop strategies to improve adherence to complementary feeding guidelines, it is important to understand not just who is introducing early, but why. Clayton and colleagues10 found mothers most commonly reported reasons for introducing early were that they wanted to feed their baby something in addition to breast milk or formula, a doctor or health care provider recommended they begin feeding solids, the baby seemed hungry a lot of the time, and to help the baby sleep longer at night.
The recommended timing of first introduction to complementary foods has been controversial and varied and historically span the 4- to 6-month time period.3–5,24 The current guidelines from American Academy of Pediatrics state that infants should first be introduced to complementary foods at about 6 months of age.5 Efforts to support caregivers, families, and health care providers may be needed to ensure that US children are achieving recommendations on the timing of food introduction. Inclusion of children <2 years in the 2020–2025 DGA may promote consistent messaging of when children should be introduced to complementary foods.
CONCLUSIONS
These findings provide a national estimate of the timing of first introduction of complementary foods to young children. These data indicate that 16% of US children are introduced to complementary foods too early (before 4 months) and one in two children are introduced to complementary foods before 6 months of age.1,5 Future research could explore which foods and beverages are introduced first and the reasons for variation in early introduction by milk feeding type.
RESEARCH SNAPSHOT.
Research Question
When are infants in the United States first being introduced to complementary foods?
Key Findings
Among US children 6 to 36 months of age in 2009-2014, 16.3% were introduced to complementary foods too early (<4 months). An additional 38.3% were introduced between 4 and <6 months and 32.5% were introduced at the recommended 6 to <7 months of age. The remaining 12.9% were introduced to complementary foods at ≥7 months.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Author contributions: C. M. Barrera contributed to the analytic study design, conducted the analysis, and drafted and revised the final manuscript. C. G. Perrine contributed to the analytic study design and provided critical review of the manuscript. H. C. Hamner contributed to the analytic study design and provided critical review of the manuscript. K. S. Scanlon contributed to the analytic study design and provided critical review of the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Footnotes
STATEMENT OF POTENTIAL CONFLICT OF INTEREST
No potential conflict of interest was reported by the authors.
FUNDING/SUPPORT
There is no funding to disclose.
References
- 1.World Health Organization. Complementary Feeding: Report of the Global Consultation and Summary of Guiding Principles. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- 2.Raiten DJ, Raghavan R, Porter A, Obbagy JE, Spahn JM. “Executive summary: Evaluating the evidence base to support the inclusion of infants and children from birth to 24 mo of age in the Dietary Guidelines for Americans—”the B-24 Project. Am J Clin Nutr. 2014;99:663S–691S. doi: 10.3945/ajcn.113.072140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fewtrell M, Bronsky J, Campoy C, et al. Complementary feeding: A position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2017;64(1):119–132. doi: 10.1097/MPG.0000000000001454. [DOI] [PubMed] [Google Scholar]
- 4.Section on Breasfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 5.Kleinman RE, Greer FR, editors. Pediatric Nutrition Handbook. 7. Elk Grove Village, IL: American Academy of Pediatrics; 2013. [Google Scholar]
- 6.Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. Risk factors for childhood obesity in the first 1,000 days: A systematic review. Am J Prev Med. 2016;50(6):761–779. doi: 10.1016/j.amepre.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Alvisi P, Brusa S, Alboresi S, et al. Recommendations on complementary feeding for healthy, full-term infants. Ital J Pediatr. 2015;41:36. doi: 10.1186/s13052-015-0143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grummer-Strawn LM, Li R, Perrine CG, Scanlon KS, Fein SB. Infant feeding and long-term outcomes: Results from the year 6 follow-up of children in the Infant Feeding Practices Study II. Pediatrics. 2014;134(suppl 1):S1–S3. doi: 10.1542/peds.2014-0646B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May L, Borger C, Weinfield N, et al. WIC Infant and Toddler Feeding Practices Study—2: Infant Year Report. Rockville, MD: Westat; 2017. [Accessed November 28, 2017]. https://fns-prod.azureedge.net/sites/default/files/ops/WIC-ITFPS2-Infant.pdf. [Google Scholar]
- 10.Clayton HB, Li R, Perrine CG, Scanlon KS. Prevalence and reasons for introducing infants early to solid foods: Variations by milk feeding type. Pediatrics. 2013;131(4):e1108–e1114. doi: 10.1542/peds.2012-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briefel RR, Reidy K, Karwe V, Devaney B. Feeding infants and toddlers study: Improvements needed in meeting infant feeding recommendations. J Am Diet Assoc. 2004;104(1 suppl 1):s31–s37. doi: 10.1016/j.jada.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Andreasyan K, Ponsonby AL, Dwyer T, Dear K, Cochrane J. Infant feeding and childhood atopy: Does early introduction of non-milk fluids matter? Pediatr Allergy Immunol. 2007;18(3):250–257. doi: 10.1111/j.1399-3038.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- 13.Fegan S, Bassett E, Peng Y, Steel O’Connor K. Adherence to complementary feeding recommendations for infants and implications for public health. Public Health Nutr. 2016;19(4):638–649. doi: 10.1017/S1368980015001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention, National Center for Health Statistics. 2009–2014 NHANES Response Rates and Population Totals. Atlanta, GA: Centers for Disease Control and Prevention; 2015. [Accessed November 28, 2017]. https://www.cdc.gov/nchs/nhanes/response_rates_cps.htm. [Google Scholar]
- 15.US Department of Agriculture. Infant Nutrition and Feeding: A Guide for Use in the WIC and CSF Programs. Washington, DC: US Department of Agriculture; 2009. Special Supplemental Nutrition Program for Women Infants and Children. [Google Scholar]
- 16.Food and Nutrition Service. WIC Income Eligibility Requirements. Washington, DC: US Department of Agriculture; 2016. [Google Scholar]
- 17.SAS [computer program]. Version 9.3. Cary, NC: SAS Institute; 2011. [Google Scholar]
- 18.Johnson CL, Paulose-Ram R, Ogden CL, et al. National Health and Nutrition Examination Survey: Analytic Guidelines, 1999–2010. Vital Health Stat. 2013 Sep;2(161):1–24. [PubMed] [Google Scholar]
- 19.Kuo AA, Inkelas M, Slusser WM, Maidenberg M, Halfon N. Introduction of solid food to young infants. Matern Child Health J. 2011;15(8):1185–1194. doi: 10.1007/s10995-010-0669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doub AE, Moding KJ, Stifter CA. Infant and maternal predictors of early life feeding decisions. The timing of solid food introduction. Appetite. 2015;92:261–268. doi: 10.1016/j.appet.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics. 2012;129:E827–E841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 22.Comittee on Nutrition. On the feeding of solid foods to infants. Pediatrics. 1958:21. [PubMed] [Google Scholar]
- 23.Koplin JJ, Allen KJ. Optimal timing for solids introduction—Why are the guidelines always changing? Clin Exp Allergy. 2013;43(8):826–834. doi: 10.1111/cea.12090. [DOI] [PubMed] [Google Scholar]
- 24.Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases-Sponsored Expert Panel. Pediatr Dermatol. 2017;34:e1–e21. doi: 10.1111/pde.13093. [DOI] [PubMed] [Google Scholar]
- 25.Harrison GG, Hirschman JD, Owens TA, McNutt SW, Sallack LE. WIC Infant and Toddler Feeding Practices Study: Protocol design and implementation. Am J Clin Nutr. 2014;99(3):742s–746s. doi: 10.3945/ajcn.113.073585. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler P, Briefel R, Clusen N, Devaney B. Feeding Infants and Toddlers Study (FITS): Development of the FITS survey in comparison to other dietary survey methods. J Am Diet Assoc. 2006;106(1 suppl 1):S12–S27. doi: 10.1016/j.jada.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 27.Fein SB, Labiner-Wolfe J, Shealy KR, Li R, Chen J, Grummer-Strawn LM. Infant Feeding Practices Study II: Study methods. Pediatrics. 2008;122(suppl 2):S28–S35. doi: 10.1542/peds.2008-1315c. [DOI] [PubMed] [Google Scholar]
- 28.Robinson SM. Infant nutrition and lifelong health: Current perspectives and future challenges. J Dev Orig Health Dis. 2015;6:384–389. doi: 10.1017/S2040174415001257. [DOI] [PMC free article] [PubMed] [Google Scholar]