TO THE EDITOR
As a major thermoregulatory organ, sweat glands are widely distributed across the human body, secreting up to several liters of sweat per day (Sato et al., 1989). Evaporation of sweat from skin surfaces effectively dissipates heat generated by hot environment or physical activity. Regarding the secretory mechanism, current data indicate that sweating is initiated by acetylcholine release from periglandular sympathetic nerves. Acetylcholine initiates a rapid increase in calcium concentration in the cytosol of secretory cells, which activates calcium-dependent K+ and Cl− channels to effect KCl efflux. The efflux-induced chemical gradient in turn activates NKCC1 cotransporters and Na+/K+ pumps, resulting in massive ion and subsequent water movement across the secretory cells – i.e., robust sweat secretion (Cui and Schlessinger, 2015, Lu and Fuchs, 2014, Sato et al., 1989). Thus, calcium is crucial to drive sweating; but calcium dynamics in sweat glands remains only partially understood.
Calcium rise in other exocrine glands has been shown to be mediated by calcium channels on the endoplasmic reticulum (ER) and the plasma membrane (PM) (Ambudkar, 2014). Extensive studies in salivary glands showed that calcium required for saliva secretion is acquired by two distinct though related routes: early intracellular calcium release and later calcium influx from the extracellular interstitium (Ambudkar, 2014). Intracellular calcium release exploits the ER calcium storehouse in secretory cells. When acetylcholine binds to CHRM receptors, they recruit and activate receptor-coupled G proteins on the PM. The G proteins then activate phospholipase C, which cleaves PIP2, a cell membrane component, into soluble IP3 and membrane bound diacylglycerol. IP3 moves to the ER membrane and binds to IP3Rs (R1–R3), the calcium channels, leading to calcium release from ER to cytosol (Ambudkar, 2014). In sweat glands, it was recently shown that the IP3R2 subtype is the main effector of intracellular calcium release (Klar et al., 2014).
Calcium released from the ER can initiate fluid secretion, but is not sufficient for sustained secretion (Putney and Bird, 2014). To maintain elevated calcium stores in the secretory cells, the second route, calcium influx from interstitium across the PM, is required. In saliva secretory cells, it was shown that calcium influx is mediated by “SOCE” (store-operated calcium entry) (Ambudkar, 2014). SOCE requires two major components, the calcium binding ER transmembrane STIM proteins (STIM1 and STIM2) (Liou et al., 2005) and calcium release-activated calcium channels on the PM -- e.g., ORAI transmembrane proteins (ORAI1–3) (Vig and Kinet, 2007). When calcium is depleted in the ER, STIM proteins dissociate from calcium and move to the PM to open ORAI channels. This gating permits massive calcium influx (Soboloff et al., 2006). Thus, STIM proteins act as a calcium sensor indispensable for saliva secretion.
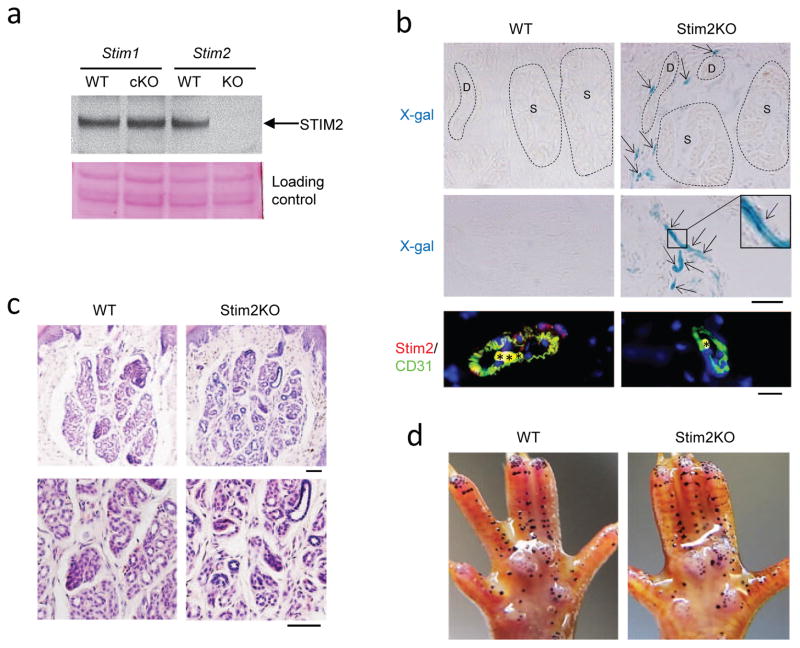
Recently SOCE was shown to operate in sweat glands as well (Concepcion et al., 2016). Stim1/Stim2 double knockout mice showed impaired calcium signaling and severe hypohidrosis. However, the function of individual STIM proteins in sweat glands was not determined. To test whether both or either of the STIM proteins is required for sweat secretion, we analyzed mice ablated separately for STIM1 or STIM2. All animal study protocols were approved by the NIA Institutional Review Board (Animal Care and Use Committee). STIM2 localization and function in sweat glands had not been assessed. We analyzed mice in which the Stim2 gene was knocked out using a LacZ cassette inserted into Exon 1 of the gene (Stim2KO). Thus, STIM2 protein expression was abolished but β-galactosidase expression was enabled in cells that normally express STIM2. Western blotting showed that STIM2 protein was absent in Stim2KO footpad skin, but was normally expressed in wild-type or Stim1cKO mice (Fig. 1a, arrow). To assess the localization of STIM2 we carried out X-gal staining. Positive signals were found in blood vessels surrounding sweat glands, but not in sweat glands in Stim2KO mice (Fig. 1b, upper panels). Stronger signals were seen in blood vessels in dermis (Fig. 1b, middle panels). Immunostaining with an endothelial cell marker, CD31, showed that STIM2 was expressed in blood vessels in wild-type, but not in Stim2KO skin (Fig. 1b, lower panels). Histology showed full formation of sweat glands in the Stim2KO mice (Fig. 1c), and calcium imaging with Fluo4 showed comparable activity in wild-type and Stim2KO sweat glands after acetylcholine stimulation (Fig. 2e, see Supplementary Materials and Methods). Finally, Stim2KO mice sweated comparably to wild-type controls (Fig. 1d). Thus, our data show that STIM2 is not expressed in sweat glands, and corresponding calcium signaling and sweat secretion are not affected in its absence.
Fig. 1.
STIM2 is not expressed in mouse sweat glands. a, Western blot showing that STIM2 is ablated in Stim2KO footpad skin. STIM2 is normally expressed in wild-type or Stim1cKO footpad skin. b, X-gal staining showed positive signals in the blood vessels surrounding sweat glands, but not in sweat glands in Stim2KO mice, neither in the secretory portion nor in the sweat duct (upper right panel). Strong signals were seen in dermal blood vessels in Stim2KO mice (middle right panel). No signals were found at all in wild-type sweat glands (upper left panel) or dermis (middle left panel). Immunostaining with an endothelial cell marker, CD31, showed that STIM2 was localized in blood vessels in wild-type, but not in Stim2KO (lower panels). Asterisks in lower panels indicate non-specific staining of erythrocytes. D, sweat duct; S, secretory portion. Scale bars, 200μm. c, Histology showed normal formation of sweat glands in Stim2KO mice. Scale bars, 200μm. d, Sweat tests showed comparable sweating capacity between Stim2KO mice and wild-type controls (KO, n=5; WT, n=5).
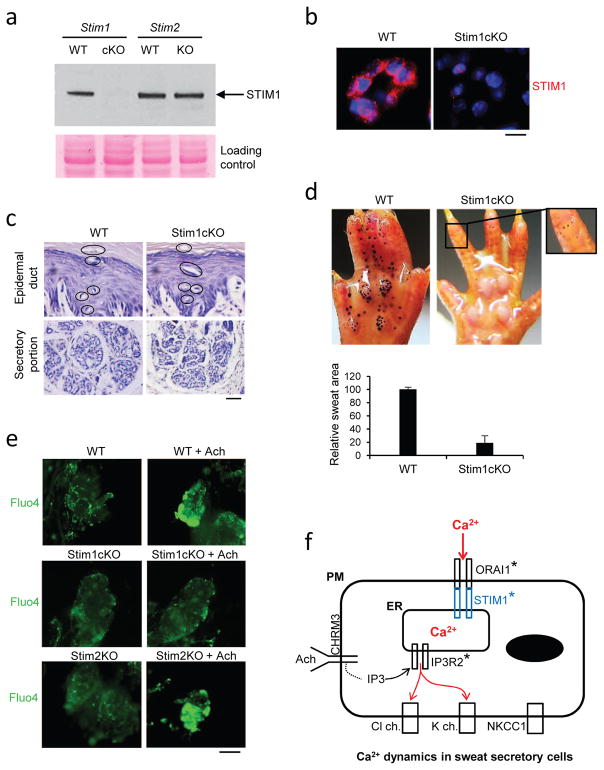
Fig. 2.
Skin-specific Stim1cKO mice show severe hypohidrosis. a, Western blot showing the lack of STIM1 protein in Stim1cKO sweat glands. STIM1 was normally expressed in wild-type or Stim2KO sweat glands. b, Immunostaining showed that STIM1 was expressed in sweat secretory cells in a dot-like pattern in WT, but not in Stim1cKO sweat glands. Scale bar, 100μm. c, Histology showed that sweat glands were fully formed in Stim1cKO mice. Upper panels show epidermal sweat ducts (circles), and lower panels show secretory potions. Scale bar, 200μm. d, Sweat test showed severe hypohidrosis in Stim1cKO mice (right panel) (n=8), compared to wild-type controls (left panel) (n=4). Tiny sweat drops appear in Stim1cKO mice within the first minute of the sweat test, but they fail to grow bigger (a higher magnification in upper right corner). The sum of the sweat area in Stim1cKO mice calculated by an ImageJ program was less than 20% of the wild-type littermates (Lower panel. KO, n=4; WT, n=2). e, Fluo4 calcium imaging for isolated sweat glands. Significant increase of calcium concentration (green) was seen in wild-type and Stim2KO sweat glands treated with acetylcholine (+Ach). Stim1cKO sweat glands did not respond significantly to acetylcholine input. Photos were taken 1 minute following the acetylcholine application. Scale bar, 1000μm. f, A schematic representation of calcium signaling in sweat glands. Acetylcholine activates CHRM3 receptor to produce IP3 in the cytosol of sweat secretory cells, which in turn activates IP3R2 calcium channel on the ER membrane to induce intracellular calcium release. The intracellular calcium release allows initiation of sweat secretion. Mutations in IP3R2 gene result in severe hypohidrosis (asterisk). Calcium depletion in ER activates STIM1 to gate to ORAI1 on the PM to induce calcium influx from the interstitium. When STIM1 or ORAI1 is ablated, sustained sweating is unattainable due to blocking of calcium influx (asterisks).
As for STIM1, skin-specific Stim1 knockout mice (Stim1cKO) were generated by crossing Stim1-LoxP mice with K14Cre mice. In these mice STIM1 is ablated in skin epidermis and epidermal appendages, including sweat glands. Western blotting confirmed that STIM1 was absent in Stim1cKO footpad skin, but was normally expressed in wild-type or Stim2KO mice (Fig. 2a, arrow). Immunostaining showed STIM1 expressed with a dot-like pattern in the cytoplasm of secretory cells in wild-type mice, but was absent, as expected, in Stim1cKO mice (Fig. 2b). Histology showed fully formed sweat glands in Stim1cKO mice (Fig. 2c). However, severe hypohidrosis was observed in sweat tests (Fig. 2d). Notably, tiny sweat drops appeared within the first minute of the test in the Stim1cKO mice, but unlike the course of sweating in wild-type controls, the drops arrested at small size (Fig. 2d, right panel). By measuring the volume of sweat, we estimate that 80% of sweat production was ablated in Stim1cKO mice (Fig. 2d, lower panel). To further confirm STIM1 function in calcium dynamics, we carried out calcium imaging assays with Fluo4 as a calcium indicator. In contrast to wild-type controls or Stim2KO mice, isolated sweat glands from Stim1cKO mice showed no response to acetylcholine (Fig. 2e). Collectively, the data indicate that sweat secretion was initiated, most likely by intracellular calcium release, but was then arrested, presumably by the failure of subsequent calcium influx, resulting in severe hypohidrosis.
In the current context of calcium dynamics in sweat glands, acetylcholine activation of the CHRM3 receptor activates IP3R2 to induce intracellular calcium release and initial sweat secretion (Fig. 2f). Our data demonstrate that STIM1 is the calcium sensor for prolonged sweat secretion. Calcium depletion in ER activates STIM1 to open ORAI1 gating for calcium influx from the interstitium, but in the absence of STIM1, calcium influx is blocked and sustained sweating precluded.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging, NIH. The authors thank Dr. Dongmei Yang for technical assistance.
Footnotes
Conflict of interests
The authors declare no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambudkar IS. Ca2+ signaling and regulation of fluid secretion in salivary gland acinar cells. Cell Calcium. 2014;55(6):297–305. doi: 10.1016/j.ceca.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concepcion AR, Vaeth M, Wagner LE, Eckstein M, Hecht L, Yang J, et al. Store-operated Ca2+ entry regulates Ca2+-activated chloride channels and eccrine sweat gland function. J Clin Invest. 2016;126(11):4303–18. doi: 10.1172/JCI89056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui CY, Schlessinger D. Eccrine sweat gland development and sweat secretion. Exp Dermatol. 2015;24(9):644–50. doi: 10.1111/exd.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar J, Hisatsune C, Baig SM, Tariq M, Johansson AC, Rasool M, et al. Abolished InsP3R2 function inhibits sweat secretion in both humans and mice. J Clin Invest. 2014;124(11):4773–80. doi: 10.1172/JCI70720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15(13):1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Fuchs E. Sweat gland progenitors in development, homeostasis, and wound repair. Cold Spring Harb Perspect Med. 2014;4(2) doi: 10.1101/cshperspect.a015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW, Bird GS. Calcium signaling in lacrimal glands. Cell Calcium. 2014;55(6):290–6. doi: 10.1016/j.ceca.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Kang WH, Saga K, Sato KT. Biology of sweat glands and their disorders. I. Normal sweat gland function. J Am Acad Dermatol. 1989;20(4):537–63. doi: 10.1016/s0190-9622(89)70063-3. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281(30):20661–5. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- Vig M, Kinet JP. The long and arduous road to CRAC. Cell Calcium. 2007;42(2):157–62. doi: 10.1016/j.ceca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.