Abstract
Background
Plasma uric acid levels rise in chronic kidney disease (CKD) and may lead to tubular injury, endothelial dysfunction, oxidative stress, and intra-renal inflammation. Whether uric acid levels are associated with kidney failure and death in CKD is unknown.
Study Design
A prospective, observational, cohort study.
Settings & Participants
3885 individuals with CKD stages 2–4 enrolled in the Chronic Renal Insufficiency Cohort (CRIC) between June 2003 and September 2008, and followed up through March 2013.
Predictor
Baseline serum uric acid levels.
Outcomes
Kidney failure (initiation of dialysis or transplantation) and all-cause mortality.
Results
During a median follow-up of 7.9 years, 885 participants progressed to kidney failure, and 789 participants died. After adjustment for demographic, cardiovascular, and kidney-specific covariates, higher levels of uric acid were independently associated with risk of kidney failure in participants with an estimated glomerular filtration rate ≥ 45 mL/min/1.73 m2 (adjusted HR per 1–standard deviation [SD] greater baseline uric acid, 1.40; 95% CI, 1.12–1.75), but not in those with eGFR < 30 mL/min/1.73 m2. There was a nominally higher HR in participants with an eGFR of 30–44 (adjusted HR, 1.13; 95% CI, 0.99–1.29), but this did not reach statistical significance. The relationship between uric acid and all-cause mortality was J-shaped (P = 0.007).
Limitations
Potential residual confounding through unavailable confounders; lack of follow-up measurements to adjust for changes in uric acid levels over time.
Conclusions
Uric acid is an independent risk factor for kidney failure in earlier stages of CKD, and has a ‘J-shaped’ relationship with all-cause mortality in CKD. Adequately powered randomized, placebo-controlled trials in CKD are needed to test whether urate lowering may prove to be an effective approach to prevent complications and progression of CKD.
Keywords: uric acid, hyperuricemia, chronic kidney disease (CKD), end-stage renal disease (ESRD), Death, kidney failure, CKD progression, eGFR decline, Chronic Renal Insufficiency Cohort (CRIC)
Introduction
Uric acid, the end-product of purine metabolism in humans, is excreted largely by the kidneys. In chronic kidney disease (CKD), plasma uric acid levels rise due to reductions in glomerular filtration rate (GFR). Hyperuricemia is a hallmark of gout and is also a suspected risk factor for conditions accompanying the metabolic syndrome such as hypertension 1,2, diabetes mellitus 3, and cardiovascular diseases 4–6. Uric acid can cause acute kidney injury, most notably in tumor lysis syndrome through precipitation and obstruction in tubules 7. Uric acid may also lead to CKD and its progression by causing endothelial dysfunction 8–11, activation of the renin-angiotensin-aldosterone system (RAAS) 8,12, inflammation 13,14, and oxidative stress 15,16.
Several studies have suggested that higher uric acid levels are associated with the development of CKD 17–19. Less is known about the association of uric acid levels with outcomes in CKD 20–22, and whether uric acid is simply a marker of lower estimated GFR (eGFR) or casually associated with adverse outcomes in CKD 23. The distinction is important because uric acid lowering has been proposed as a therapeutic strategy in CKD to prevent CKD progression and cardiovascular events 24–27. We therefore studied whether uric acid levels are associated with adverse events in the Chronic Renal Insufficiency Cohort (CRIC), a prospective cohort study of individuals with established CKD.
Methods
Study Population
The CRIC study is a multicenter, prospective, observational cohort study of individuals with mild to severe CKD that was designed to investigate risk factors for progression of CKD, cardiovascular disease, and mortality 28. The CRIC study enrolled 3939 men and women aged 21 to 74 years between June 2003 and September 2008 across 7 clinical centers in the United States. Individuals were included if they met specific age-defined criteria for eGFR of 20–70 mL/min/1.73 m2. Exclusion criteria included inability to provide consent, institutionalization, enrollment in competing studies, pregnancy, New York Heart Association class III or IV congestive heart failure, human immunodeficiency virus infection, multiple myeloma, polycystic kidney disease, renal cancer, cirrhosis, recent chemotherapy or immunosuppressive therapy, organ transplantation, or prior dialysis treatment for at least 1 month 28–30.
The study protocol was approved by the institutional review boards of the participating centers and is in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all participants enrolled in CRIC. For the purposes of this study, data were obtained from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Data Repository.
Exposure and Outcomes
The primary exposure was baseline serum uric acid, which was measured at baseline in 3885 of the 3939 participants. Serum uric acid was determined by standard laboratory procedures using the uricase/peroxidase enzymatic methods (DAX96; Bayer Diagnostics, Milan, Italy), and measured at the CRIC Central Clinical Laboratory 31. The outcomes were kidney failure, defined as initiation of dialysis or kidney transplantation, and all-cause mortality. Ascertainment of kidney failure was confirmed by cross-linkage of participants with the US Renal Data System 28. Participants were followed up until the occurrence of death, voluntary study withdrawal, loss to follow-up, or March 2013.
Covariates
Data obtained at the baseline visit included demographics, detailed medical history, comprehensive medication lists, standardized blood pressure measurements, and anthropometric measurements. History of cardiovascular disease including coronary artery disease, congestive heart failure, stroke, and peripheral vascular disease were ascertained by self-report with use of questionnaires administered by study staff at study visits. Blood samples were collected for testing of comprehensive metabolic panels and urine samples were collected for assessment of urinary albumin-creatinine ratio (UACR) 30. We used the CKD-EPI (CKD Epidemiology Collaboration) creatinine equation to calculate eGFR 32.
Statistical Analysis
Descriptive statistics were summarized as mean ± standard deviation or median (interquartile range) for continuous variables, and frequency distribution is presented with percentages for categorical variables. For skewed data distributions, we performed natural logarithmic transformation as appropriate. We assessed associations between uric acid and two-group comparisons using t-test and multiple-group comparison using ANOVA. We used Pearson or Spearman correlations between baseline uric acid levels and normally or non-normally distributed laboratory values, respectively. We used chi-square tests to compare uric acid quartiles with categorical variables, and ANOVA or Kruskal-Wallis tests for normally or non-normally distributed continuous variables, respectively. We evaluated the independent predictors of uric acid with multivariable linear regression. We also evaluated the correlation between uric acid and measured GFR (mGFR) in a subset of the cohort assessed by urinary clearance of 125I-iothalamate.29
We performed time-to-event analyses to examine the risk of the outcomes evaluating uric acid as a continuous variable (per 1-SD increase) and as quartiles (lowest quartile as reference group). We used Cox proportional hazards regression to investigate the unadjusted and multivariable adjusted associations between uric acid and outcomes. For each outcome of interest, we fitted a series of hierarchically adjusted models: model 1 (unadjusted); model 2 was stratified by site and includes age, sex, race, systolic blood pressure, diabetes mellitus, prior cardiovascular disease, smoking status, and body mass index (BMI); model 3 included model 2 and further adjusted for medications (angiotensin-converting enzyme [ACE] inhibitor or angiotensin receptor blocker [ARB], β-blocker, statin, anti-platelet agent, urate-lowering medicines, and diuretic) and pertinent laboratory markers (hemoglobin, serum albumin, and natural logarithm-transformed UACR); model 4 included model 3 and further adjusted for baseline eGFR. We examined the possibly non-linear relation between uric acid and each primary outcome with restricted cubic-splines. Tests for non-linearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic-spline terms 33. We tested for statistical interaction between sex, urate lowering medicines, BMI, and eGFR and uric acid in Cox models through multiplicative interaction terms. Fewer than 3.5% of covariate data were missing, and therefore we did not use imputation techniques. The proportional hazard assumption was assessed in all models by the Kolmogorov-type supremum test, and the functional forms of the covariates were assessed by checking the martingale residuals. Follow-up for the primary analysis was censored at death for the outcomes of kidney failure and all-cause mortality. Statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc, Cary, NC, USA). All statistical tests were two-sided and P values < 0.05 were considered significant.
Sensitivity Analyses
Since the primary analysis censored for death with the outcome of kidney failure, and death precludes the ability to reach the outcome of interest, we utilized sub-distribution hazards models in a sensitivity analysis. 34 In additional sensitivity analyses for mortality as an outcome, we censored at the onset of kidney failure because the onset of kidney failure may alter the baseline hazard. We also repeated the primary analyses for both outcomes in the subset of participants with mGFR assessed by urinary clearance of 125I-iothalamate.
Results
Study Participants
Baseline characteristics are presented in Table 1 for the overall cohort and by uric acid quartiles. Mean uric acid levels were higher in males (7.7 versus 7.0 mg/dL), blacks (7.8 versus 7.1 mg/dL in whites), participants with a history of diabetes (7.6 versus 7.2 mg/dL), history of cardiovascular disease (7.7 versus 7.3 mg/dL), diuretic users (7.9 versus 6.7 mg/dL), and users of ACE inhibitors or ARBs (7.6 versus 6.9 mg/dL); uric acid levels were lower in participants on urate-lowering medications (6.9 versus 7.5 mg/dL); for all these comparisons, P < 0.001. Uric acid correlated with age (rp = 0.05; P = 0.004), systolic blood pressure (rs = 0.05; P = 0.004), BMI (rp = 0.21; P <0.001), hemoglobin (rp = −0.07; P <0.001), albumin (rp = 0.03; P = 0.04), UACR (rs = 0.16; P <0.001), and eGFR (rp = −0.36; P <0.001). Mean uric acid differed by CKD stage (CKD stages 2–3a: 6.8 ± 1.8 [SD] mg/dL; CKD stage 3b: 7.8 ± 1.8 mg/dL; CKD stage 4: 8.3 ± 2.0 mg/dL; P <0.001). In the sub-cohort (n = 1405) where GFR was assessed by urinary clearance of 125I-iothalamate, we found a similar correlation with uric acid (rp = −0.33; P <0.001). Table S1 (provided as online supplementary material) demonstrates the results of a multivariable linear regression model with uric acid as the dependent variable.
Table 1.
Baseline Characteristics of CRIC Participants by Uric Acid Quartiles
| Characteristics | All Participants^ (N = 3885) | Q1: 1.9–6.0 mg/dL (n = 952) | Q2: 6.1–7.3 mg/dL (n = 1019) | Q3: 7.4–8.6 mg/dL (n = 945) | Q4: 8.7–15.2 mg/dL (n = 969) | P* |
|---|---|---|---|---|---|---|
|
| ||||||
| Demographics and Clinical | ||||||
|
| ||||||
| Age, y | 58.2 (11.0) | 57.4 (11.0) | 58.1 (11.0) | 58.4 (11.1) | 58.9 (10.9) | 0.04 |
|
| ||||||
| Female sex | 1749 (45.0) | 554 (58.2) | 484 (47.5) | 370 (39.2) | 341 (35.2) | <0.001 |
|
| ||||||
| Race | <0.001 | |||||
| White | 1616 (41.6) | 498 (52.3) | 427 (41.9) | 367 (38.8) | 324 (33.4) | |
| Black | 1624 (41.8) | 292 (30.7) | 420 (41.2) | 412 (43.6) | 500 (51.6) | |
|
| ||||||
| BMI, kg/m2 | 32.1 (7.8) | 29.8 (7.1) | 31.7 (7.5) | 32.7 (7.6) | 34.1 (8.5) | <0.001 |
|
| ||||||
| Systolic BP, mm Hg | 128.5 (22.2) | 126.2 (22.6) | 130.1 (23.1) | 129.0 (21.1) | 128.5 (21.7) | 0.002 |
|
| ||||||
| Comorbid Conditions | ||||||
|
| ||||||
| Hypertension | 3342 (86.0) | 708 (74.4) | 870 (85.4) | 863 (91.3) | 901 (93.0) | <0.001 |
|
| ||||||
| Diabetes mellitus | 1885 (48.5) | 407 (42.8) | 485 (47.6) | 465 (49.2) | 528 (54.5) | <0.001 |
|
| ||||||
| MI or prior revascularization | 849 (21.9) | 165 (17.3) | 207 (20.3) | 218 (23.1) | 259 (26.7) | <0.001 |
|
| ||||||
| Congestive Heart Failure | 375 (9.7) | 61 (6.4) | 69 (6.8) | 86 (9.1) | 159 (16.4) | <0.001 |
|
| ||||||
| Stroke | 385 (9.9) | 83 (8.7) | 100 (9.8) | 93 (9.8) | 109 (11.3) | 0.3 |
|
| ||||||
| Peripheral Vascular Disease | 256 (6.6) | 48 (5.0) | 62 (6.1) | 72 (7.6) | 74 (7.6) | 0.06 |
|
| ||||||
| Any Cardiovascular Disease | 1295 (33.3) | 257 (27.0) | 322 (31.6) | 326 (34.5) | 390 (40.3) | <0.001 |
|
| ||||||
| Current Smoker | 504 (13.0) | 123 (12.9) | 137 (13.4) | 126 (13.3) | 18 (12.2) | 0.8 |
|
| ||||||
| Medications | ||||||
|
| ||||||
| ACE inhibitors or ARBs | 2647 (68.6) | 544 (57.4) | 675 (66.7) | 686 (73.5) | 742 (77.0) | <0.001 |
|
| ||||||
| β-Blockers | 1903 (49.3) | 366 (38.6) | 468 (46.3) | 477 (51.1) | 592 (61.4) | <0.001 |
|
| ||||||
| Statins | 2121 (46.0) | 471 (49.7) | 547 (54.1) | 546 (58.5) | 557 (57.8) | <0.001 |
|
| ||||||
| Anti-platelet drugs | 1775 (46.0) | 399 (42.1) | 464 (45.9) | 450 (48.2) | 462 (47.9) | 0.03 |
|
| ||||||
| Diuretics | 2297 (59.5) | 385 (40.6) | 567 (56.0) | 606 (64.9) | 739 (76.7) | <0.001 |
|
| ||||||
| Urate-lowering medications | 383 (9.9) | 131 (13.8) | 100 (9.9) | 95 (10.2) | 57 (5.9) | <0.001 |
|
| ||||||
| Laboratory Data | ||||||
|
| ||||||
| Serum Creatinine, mg/dl | 1.8 (0.6) | 1.5 (0.5) | 1.8 (0.6) | 1.9 (0.6) | 2.2 (0.7) | <0.001 |
|
| ||||||
| eGFR, ml/min/1.73m2 | 44.3 (15.0) | 51.6 (16.3) | 45.8 (14.7) | 42.2 (12.8) | 37.7 (12.4) | <0.001 |
| eGFR category | ||||||
| ≥45 ml/min/1.73m2 | 1755 (45.2) | 622 (16.0) | 506 (13.0) | 372 (9.6) | 255 (6.6) | |
| 30–44 ml/min/1.73m2 | 1415 (36.4) | 242 (6.2) | 360 (9.3) | 403 (10.4) | 410 (10.6) | |
| <30 ml/min/1.73m2 | 715 (18.4) | 88 (2.3) | 153 (3.9) | 170 (4.4) | 304 (7.8) | |
|
| ||||||
| UACR, mg/g | 51.7 [8.6 – 456.4] | 19.0 [5.9 – 167.8] | 54.7 [8.4 – 598.6] | 83.8 [11.4 – 612.0] | 106.9 [15.5 – 496.2] | <0.001 |
|
| ||||||
| Albumin, g/dL | 3.9 (0.5) | 3.9 (0.5) | 3.9 (0.5) | 3.9 (0.5) | 4.0 (0.4) | 0.009 |
|
| ||||||
| Hemoglobin, g/dl | 12.6 (1.8) | 12.7 (1.7) | 12.6 (1.8) | 12.6 (1.9) | 12.4 (1.8) | 0.005 |
Note: Values for categorical variables are given as count (percentage); values for continuous variables, as mean ± standard deviation or median [interquartile range]. Conversion factor for serum creatinine in mg/dL to μmol/L, ×88.4.
Mean uric acid, 7.4 ± 1.9 mg/dL.
P-values represent differences across uric acid quartiles.
Abbreviations: CRIC, Chronic Renal Insufficiency Cohort; BMI, body mass index; BP, blood pressure; MI, myocardial infarction; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; Q, quartile of uric acid; UACR, urinary albumin-creatinine ratio;
Future Development of Kidney Failure
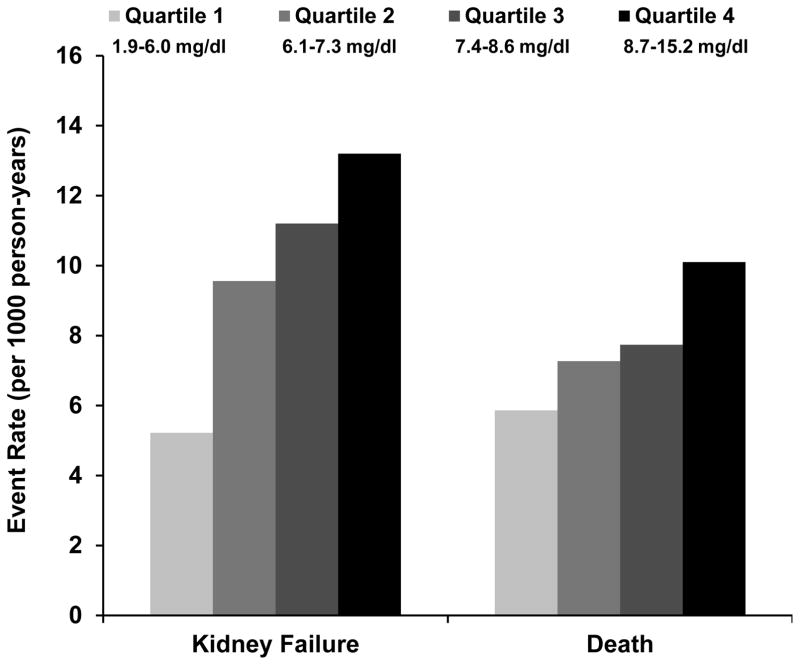
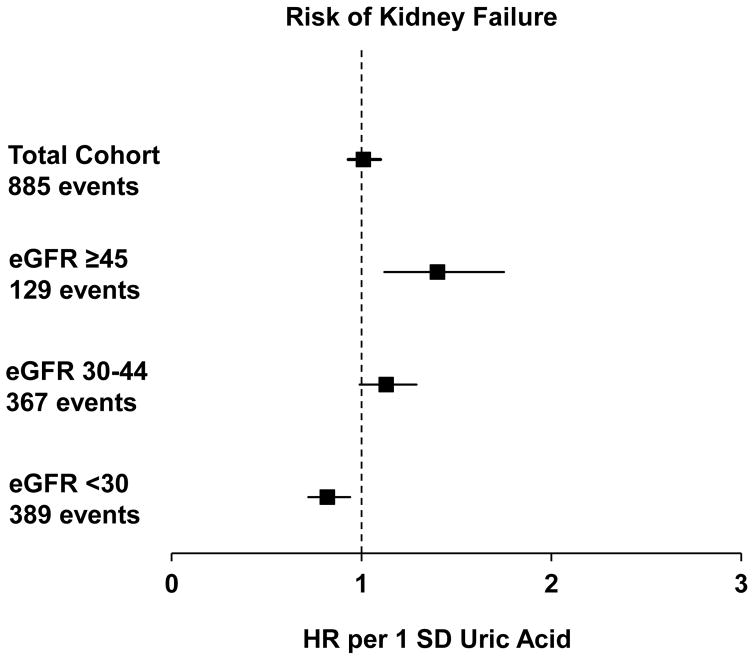
During a median follow-up of 7.9 years, 885 participants reached the outcome of kidney failure (Fig 1). Table 2 shows the unadjusted and multivariable adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) according to baseline uric acid as a continuous variable and by quartiles. Adjustment for demographics, co-morbidities, medications, and laboratory data mildly attenuated the association between baseline uric acid and subsequent kidney failure both as a continuous variable and in quartiles. The associations between uric acid (both as a continuous variable and quartiles) and kidney failure were significantly confounded by eGFR. We also noted effect modification by eGFR (P for interaction = 0.001), but not by sex (P = 0.1), urate-lowering medicine (P = 0.6), or BMI (P = 0.1). Fig 2 presents the multivariable-adjusted associations between uric acid (as a continuous variable, per 1-SD greater amount) and kidney failure in participants with eGFR ≥ 45 (CKD stage 2 or 3a), 30–44 (CKD stage 3b), and < 30 mL/min/1.73m2 (CKD stage 4). Among individuals with CKD stage 2 or 3a, each 1-SD higher level of uric acid was independently associated with a 40% higher risk of subsequent kidney failure (adjusted HR, 1.40; 95% CI, 1.12–1.75). We found a nominally higher, but statistically non-significant, risk for each 1-SD higher level of uric acid among individuals with CKD stage 3b (adjusted, 1.13; 95% CI, 0.99–1.29). In participants with CKD stage 4, uric acid appeared to be protective, with each 1-SD higher level of uric acid independently associated with an 18% lower risk of subsequent kidney failure (adjusted HR, 0.82; 95% CI, 0.72–0.94). When using sub-distribution hazard models in the primary analysis for the outcome of kidney failure, the results did not qualitatively change (Table S2). Using sub-distribution hazard models for the stratified analysis yielded similar results, except that the association in CKD stage 4 was no longer statistically significant (HR, 0.92; 95% CI, 0.81–1.05) (Table S3). Substituting eGFR with mGFR assessed by urinary 125I-iothalamate clearance did not qualitatively change the results of the primary analysis (Table S4).
Fig. 1. Primary Outcome Event Rates by Uric Acid Quartiles.
Event rates (per 1000 person-years) of participants reaching the outcomes by uric acid quartile.
Table 2.
Uric Acid and the Risk of Kidney Failure by Cox Proportional Hazards
| HR (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Uric Acid | No. of Events | Events per 1000 person-y | Model 1 | Model 2 | Model 3 | Model 4 |
| Continuous | 885 | 39.2 | 1.42 (1.33 – 1.51) | 1.31 (1.23 – 1.41) | 1.24 (1.15 – 1.34) | 1.01 (0.93 – 1.10) |
| Categorical | ||||||
| Q1 | 118 | 5.22 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 216 | 9.56 | 1.83 (1.47 – 2.26) | 1.72 (1.38 – 2.14) | 1.36 (1.08 – 1.72) | 1.05 (0.84 – 1.33) |
| Q3 | 253 | 11.2 | 2.28 (1.85 – 2.81) | 2.03 (1.64 – 2.52) | 1.56 (1.24 – 1.97) | 1.03 (0.82 – 1.30) |
| Q4 | 298 | 13.2 | 2.81 (2.29 – 3.45) | 2.35 (1.90 – 2.92) | 1.85 (1.46 – 2.34) | 1.07 (0.84 – 1.37) |
Note: Model 1 is Unadjusted; Model 2 is stratified by center and adjusts for age, sex, race, systolic blood pressure, diabetes, body mass index, any cardiovascular disease; Model 3 is Model 2 plus further adjustment for urate-lowering medicines, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use, diuretics, β-blockers, statins, anti-platelet drugs, hemoglobin, serum albumin, and log(urinary albumin-creatinine ratio); Model 4 is Model 3 plus further adjustment for baseline estimated glomerular filtration rate
HRs are per 1-SD greater uric acid
CI, confidence interval; HR, hazard ratio; Q, quartile; SD. standard deviation
Fig. 2. Uric Acid and Risk of Kidney Failure by Baseline Kidney Function.
Multivariable adjusted hazard ratios of kidney failure per 1SD greater baseline uric acid in all participants and stratified by baseline eGFR. See Model 4 in Table 2 for adjusted covariates. Adjusted HRs are as follows: total cohort, 1.01 (95% CI, 0.93–1.10); eGFR ≥45 ml/min/1.73 m2, 1.40 (95% CI, 1.12–1.75); eGFR of 30–44 ml/min/1.73 m2, 1.13 (95% CI, 0.99–1.29); eGFR < 30 ml/min/1.73 m2, 0.82 (95% CI, 0.72–0.94).
All-Cause Mortality
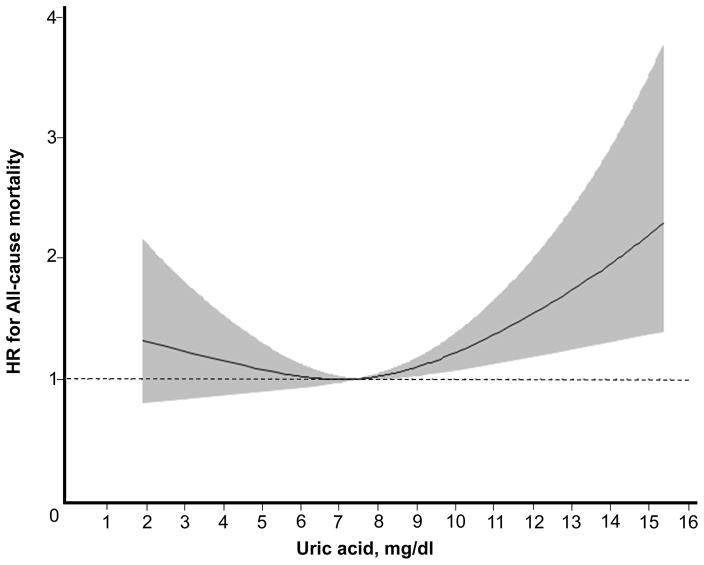
over a median follow-up time of 7.9 years, 789 participants died (Fig 1). We observed a non-linear relationship in a ‘J-shape’ between uric acid and all-cause mortality (P = 0.007) after adjustment for demographic information, co-morbidities, medications, and pertinent laboratory data including eGFR (Fig 3). There was no evidence of statistical interaction between uric acid by sex (P for interaction = 0.1), urate-lowering medicines (P = 0.2), BMI (P = 0.4), or eGFR (P = 0.7) for the outcome of all-cause mortality. In the sensitivity analysis, substituting eGFR with mGFR did not qualitatively change the results of the primary analysis (Table S4). Similarly, repeating the analysis censoring at the onset of kidney failure did not qualitatively change the non-linear relationship between uric acid and all-cause mortality (Fig S1).
Fig. 3. Association between Uric Acid and All-Cause Mortality.
Restricted cubic spline model reflecting fully adjusted model for covariates described in Model 4 of Table 2 (P for non-linear association = 0.007). Mean uric acid (7.4 mg/dl) is the reference.
Discussion
The two major findings in this prospective study of serum uric acid levels in nearly 4000 individuals with CKD were: 1) higher levels of uric acid were independently associated with a higher risk of subsequent kidney failure in individuals with CKD stage 3a or earlier; and 2) uric acid demonstrated a ‘J-shaped’ relationship with all-cause mortality. As expected for a small filtered metabolite, uric acid was inversely correlated with eGFR, which strongly confounded the relationship of uric acid with subsequent kidney failure and mortality. Stratified analyses revealed evidence for a potentially protective effect at CKD stage 4 or greater between higher levels of uric acid and subsequent kidney failure.
The biological plausibility of uric acid as a kidney or cardiovascular toxin is supported by a number of in vitro and in vivo studies on the capability of uric acid to cause inflammation 13,14, oxidative stress 15,16, endothelial dysfunction 8–11, and activation of RAAS 8,12. However, uric acid is also a potent anti-oxidant 35,36, and treatment with inosine to increase plasma levels of uric acid is being tested in clinical trials in Parkinson’s disease 37. Lowering uric acid levels with uricosuric agents or xanthine oxidase inhibitors for the primary or secondary prevention of cardiovascular diseases or kidney disease has been the subject of a number of completed and ongoing clinical trials 38,39. Our study adds to the literature by demonstrating the relationship between uric acid and adverse clinical outcomes in CKD, a setting in which uric acid levels rise due to impaired clearance by the kidneys.
Previous studies on the association between uric acid and incident CKD or its progression have yielded inconsistent results. In population-based cohort studies such as the Cardiovascular Health Study (CHS) and the Atherosclerosis Risk in Communities (ARIC) Study, higher levels of uric acid were associated with incident CKD or CKD progression 17,20. Only a few epidemiology studies have examined uric acid specifically as a risk factor for progression in established CKD. In 227 individuals with mild to moderate kidney disease, uric acid was found not to be associated with doubling of serum creatinine or need of renal replacement therapy after adjustment for eGFR and proteinuria 40. In the MDRD (Modification of Diet in Renal Disease) Study, a randomized controlled trial designed to test low versus usual protein intake on CKD progression in participants with eGFR of 13–55 mL/min/1.73m2, no association was observed between uric acid and CKD progression, defined as the requirement of dialysis or transplantation, but no stratified analyses were performed to assess for effect modification by levels of kidney function 21.
Our finding of effect modification—that uric acid was associated with the future risk of kidney failure only in those with higher baseline levels of kidney function—suggests that higher uric acid levels at preserved eGFR have more relevance for kidney failure than at a lower eGFR. In settings of preserved GFR, the deleterious effects of uric acid may be more pathogenic and easier to discern than at lower levels of kidney function, when other factors that govern the rise of uric acid levels and also contribute to morbidity may be more important. Among those with CKD stage 4, we found uric acid levels to be seemingly protective against kidney failure, possibly due to residual confounding by malnutrition. Our paradoxical findings in advanced CKD are reminiscent of the findings in the Dialysis Outcomes and Practice Patterns Study (DOPPS): Latif and colleagues reported in 5827 long-term hemodialysis patients a lower risk of all-cause and cardiovascular disease mortality with higher uric acid levels 41. Another potential explanation may be related to volume status, with lower uric acid levels reflective of inadequate diuretic use and volume control, for which our multivariable adjustment may not have been adequate.
Causality is not possible to determine in observational studies, but whether uric acid is causally associated with CKD progression has been tested in Mendelian randomization studies. In a study of 755 individuals with CKD stages 2–5 and a median 3 years of follow-up, Testa et al found that polymorphims in the gene encoding the GLUT9 urate transporter that were strongly associated with higher uric acid levels were associated with a 2.35-fold higher risk of CKD progression, defined as > 30% decrease in GFR or need for renal replacement therapy. The association remained statistically significant after adjustment for baseline eGFR, proteinuria, and other risk factors 42. In another study of 3895 individuals with type 1 diabetes, Ahola and colleagues measured uric acid levels at baseline and also calculated a genetic risk score for uric acid levels based on 23 single nucleotide polymorphisms (SNPs) that were shown in other studies to predict uric acid levels. They found higher uric acid levels were associated with CKD progression (defined as deterioration to more advanced CKD stages) in adjusted models with an average of 7 years’ follow-up. They found no cross-sectional association of the 23-SNP score with albuminuria or eGFR-based nephropathy status. However, analyses of the 23-SNP score with subsequent CKD progression were not reported 43.
We also tested uric acid as a risk factor for all-cause mortality and did not find evidence of effect modification by baseline eGFR as we did for the outcome of kidney failure. In a study including 15,336 ARIC participants, Naveenathan et al. found evidence for an association between uric acid and those with eGFR ≥ 60 mL/min/1.73 m2, with no association in the 461 individuals with eGFR < 60 mL/min/1.73 m2, but power was limited 44. The finding of a ‘J-shaped’ relationship between baseline uric acid and all-cause mortality is similar to previous reports in CKD stage 545, incident hemodialysis 46, and long-term hemodialysis patients 47.
Whether uric acid lowering is effective in improving outcomes in CKD requires adequately powered, placebo-controlled randomized controlled trials. Our findings suggest that for CKD progression, individuals with advanced CKD (stages 3b–4) may not benefit from uric acid lowering. There have been only a handful of trials of urate lowering in CKD. Previous small trials suggest that xanthine oxidase inhibitor (allopurinol or febuxostat) therapy may slow CKD progression, but statistical power was limited due to small sample sizes 25,26. The largest clinical trial evaluating CKD progression (n = 113) also suggested a slowing of progression (defined as an eGFR decline ≥ 0.2 mL/min/1.73 m2 per month) and lower risk of cardiovascular events in participants with CKD (eGFR <60 mL/min/1.73 m2) randomized to allopurinol 100 mg daily for 24 months 24. Uric acid–lowering trials involving surrogate measures of endothelial dysfunction have also yielded inconsistent results 48,49. In a 9 month, randomized, placebo-controlled trial of allopurinol in CKD stage 3, Kao et al reported that allopurinol treatment led to reduced endothelial dysfunction assessed by flow-mediated brachial dilation and reduced left ventricular hypertrophy on cardiac magnetic resonance imaging 50. However, the most recent trial evaluating endothelial dysfunction in CKD stage 3 (n = 80) demonstrated no improvement in endothelial dysfunction by lowering uric acid with allopurinol 51. The Preventing Early Renal Loss in Diabetes (PERL) trial is an ongoing multicenter, double-blind, placebo-controlled, randomized trial of allopurinol in type 1 diabetics with eGFR ≥ 45 ml/min/1.73 m2 and albuminuria (planned enrollment n = 400), and is powered to detect a difference in eGFR decline between treatment arms of at least 1 mL/min/1.73 m2 per year 52. The results of this trial are eagerly awaited and should answer questions regarding the benefit of uric acid lowering in CKD progression.
The most important limitations of our study relate to the observational design, which makes it impossible to infer causality between the observed associations between uric acid and CKD progression and mortality. Residual confounding is always a concern even after multivariable adjustment. We analyzed uric acid levels only at baseline and did not have access to follow-up measurements to adjust for changes in uric acid levels over time. Nevertheless, we believe our study is the largest to date to report on the prospective association of uric acid levels with adverse events in individuals with CKD.
In conclusion, hyperuricemia in CKD is associated with mortality in a ‘J-shaped’ relationship and, among those with eGFR ≥ 45 ml/min/1.73 m2, with higher risk of subsequent kidney failure. Adequately powered randomized, placebo-controlled trials in CKD are needed to test whether urate lowering may prove to be an effective approach to prevent complications and progression of CKD.
Supplementary Material
Figure S1: Association between uric acid and all-cause mortality (censored for kidney failure).
Table S1: Independent predictors of uric acid in CRIC.
Table S2: Association between uric acid and risk of kidney failure (death as competing risk).
Table S3: Association of uric acid with kidney failure stratified by CKD stage (death as competing risk).
Table S4: Association of uric acid with kidney failure and all-cause mortality using mGFR.
Acknowledgments
Support: This work was supported by NIDDK grants F32DK111066 (Dr Srivastava) and U01DK085660 (Dr Waikar). The authors are supported by NHLBI grant R01HL105440 (Dr McMullan), and NIDDK grants R01DK110087 (Dr Isakova) and R01DK093574, R01DK103784, and U01DK104308 (Dr Waikar). CRIC was conducted by the CRIC Investigators and supported by the NIDDK. The funders of this study had no role in the analysis, interpretation of data, writing of the manuscript, or the decision to submit the report for publication.
The data from the CRIC study reported herein were supplied by the NIDDK Central Repositories.
Footnotes
Authors’ Contributions: Study design: AS, SSW; data acquisition/analysis: AS, ADK, SSW; data interpretation: all authors. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Disclaimer: This manuscript was not prepared in collaboration with Investigators of the CRIC study and does not necessarily reflect the opinions or views of the CRIC study, the NIDDK Central Repositories, or the NIDDK.
Peer Review: Received ______. Evaluated by three external peer reviewers, with editorial input from a Statistics/Methods Editor and an Acting Editor-in-Chief (International Editor Magdalena Madero, MD). Accepted in revised form ________. The involvement of an Acting Editor-in-Chief to handle the peer-review and decision-making processes was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Supplementary Material Descriptive Text for Online Delivery
Supplementary Figure S1 (PDF). Association between uric acid and all-cause mortality (censored for kidney failure).
Supplementary Table S1 (PDF). Independent predictors of uric acid in CRIC.
Supplementary Table S2 (PDF). Association between uric acid and risk of kidney failure (death as competing risk).
Supplementary Table S3 (PDF). Association of uric acid with kidney failure stratified by CKD stage (death as competing risk).
Supplementary Table S4 (PDF). Association of uric acid with kidney failure and all-cause mortality using mGFR.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003;42(4):474–480. doi: 10.1161/01.HYP.0000091371.53502.D3. [DOI] [PubMed] [Google Scholar]
- 2.Cirillo P, Sato W, Reungjui S, Heinig M, Gersch M, Sautin Y, et al. Uric acid, the metabolic syndrome, and renal disease. J Am Soc Nephrol. 2006;17(12 Suppl 3):S165–168. doi: 10.1681/ASN.2006080909. [DOI] [PubMed] [Google Scholar]
- 3.Nakanishi N, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, Tatara K. Serum uric acid and risk for development of hypertension and impaired fasting glucose or Type II diabetes in Japanese male office workers. Eur J Epidemiol. 2003;18(6):523–530. doi: 10.1023/a:1024600905574. [DOI] [PubMed] [Google Scholar]
- 4.Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37(6):1503–1507. doi: 10.1161/01.STR.0000221716.55088.d4. [DOI] [PubMed] [Google Scholar]
- 5.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silbernagel G, Hoffmann MM, Grammer TB, Boehm BO, Marz W. Uric acid is predictive of cardiovascular mortality and sudden cardiac death in subjects referred for coronary angiography. Nutr Metab Cardiovasc Dis. 2013;23(1):46–52. doi: 10.1016/j.numecd.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Howard S, Jones D, Ching-Hon P. The Tumor Lysis Syndrome. N Engl J Med. 2011;364:1844–1854. doi: 10.1056/NEJMra0904569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazzali M, Hughes J, Kim Y, Jefferson J, Kang D, Gordan K, et al. Elevated Uric Acid Increases Blood Pressure in the Rat by a Novel Crystal-Independent Mechanism. Hypertension. 2001;38(5):1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Lozada LG, Lanaspa MA, Cristobal-Garcia M, Garcia-Arroyo F, Soto V, Cruz-Robles D, et al. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp Nephrol. 2012;121(3–4):e71–78. doi: 10.1159/000345509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabadi MM, Kuo MC, Ghaly T, Rabadi SM, Weber M, Goligorsky MS, et al. Interaction between uric acid and HMGB1 translocation and release from endothelial cells. Am J Physiol Renal Physiol. 2012;302(6):F730–741. doi: 10.1152/ajprenal.00520.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoccali C, Maio R, Mallamaci F, Sesti G, Perticone F. Uric acid and endothelial dysfunction in essential hypertension. J Am Soc Nephrol. 2006;17(5):1466–1471. doi: 10.1681/ASN.2005090949. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Lozada L, Tapia E, Santamaria J, Avila-Casado C, Soto V, Nepomuceno T, et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67(1):237–247. doi: 10.1111/j.1523-1755.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y, Fang L, Jiang L, Wen P, Cao H, He W, et al. Uric acid induces renal inflammation via activating tubular NF-kappaB signaling pathway. PLoS One. 2012;7(6):e39738. doi: 10.1371/journal.pone.0039738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurts C. A crystal-clear mechanism of chronic kidney disease. Kidney Int. 2013;84(5):859–861. doi: 10.1038/ki.2013.251. [DOI] [PubMed] [Google Scholar]
- 15.Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens. 2008;26(2):269–275. doi: 10.1097/HJH.0b013e3282f240bf. [DOI] [PubMed] [Google Scholar]
- 16.Yu MA, Sanchez-Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. 2010;28(6):1234–1242. [PubMed] [Google Scholar]
- 17.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008;19(6):1204–1211. doi: 10.1681/ASN.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu CY, Iribarren C, CM, JD, AG Risk Factors for End-Stage Renal Disease: 25 year Follow-up. Arch Intern Med. 2009;169(4):342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnan E, Akhras KS, Sharma H, Marynchenko M, Wu E, Tawk RH, et al. Serum urate and incidence of kidney disease among veterans with gout. J Rheumatol. 2013;40(7):1166–1172. doi: 10.3899/jrheum.121061. [DOI] [PubMed] [Google Scholar]
- 20.Chonchol M, Shlipak MG, Katz R, Sarnak MJ, Newman AB, Siscovick DS, et al. Relationship of uric acid with progression of kidney disease. Am J Kidney Dis. 2007;50(2):239–247. doi: 10.1053/j.ajkd.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Madero M, Sarnak MJ, Wang X, Greene T, Beck GJ, Kusek JW, et al. Uric acid and long-term outcomes in CKD. Am J Kidney Dis. 2009;53(5):796–803. doi: 10.1053/j.ajkd.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ficociello LH, Rosolowsky ET, Niewczas MA, Maselli NJ, Weinberg JM, Aschengrau A, et al. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes Care. 2010;33(6):1337–1343. doi: 10.2337/dc10-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishani A, Grandits GA, Grimm RH, Svendsen KH, Collins AJ, Prineas RJ, et al. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol. 2006;17(5):1444–1452. doi: 10.1681/ASN.2005091012. [DOI] [PubMed] [Google Scholar]
- 24.Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincon A, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5(8):1388–1393. doi: 10.2215/CJN.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47(1):51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Sircar D, Chatterjee S, Waikhom R, Golay V, Raychaudhury A, Chatterjee S, et al. Efficacy of Febuxostat for Slowing the GFR Decline in Patients With CKD and Asymptomatic Hyperuricemia: A 6-Month, Double-Blind, Randomized, Placebo-Controlled Trial. Am J Kidney Dis. 2015;66(6):945–950. doi: 10.1053/j.ajkd.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Levy G, Rashid N, Fang N, Cheetham C. Effect of Urate-lowering Therapies on Renal Disease Progression in Patients with Hyperuricemia. J Rheumatol. 2014;41(5):955–962. doi: 10.3899/jrheum.131159. [DOI] [PubMed] [Google Scholar]
- 28.Feldman H, Appel L, Chertow G, Cifelli D, BC, JD, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. Journal of the American Society of Nephrology. 2003;14(90002):148S–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 29.Denker M, Boyle S, Anderson AH, Appel LJ, Chen J, Fink JC, et al. Chronic Renal Insufficiency Cohort Study (CRIC): Overview and Summary of Selected Findings. Clin J Am Soc Nephrol. 2015;10(11):2073–2083. doi: 10.2215/CJN.04260415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhatnagar V, Richard EL, Wu W, Nievergelt CM, Lipkowitz MS, Jeff J, et al. Analysis of ABCG2 and other urate transporters in uric acid homeostasis in chronic kidney disease: potential role of remote sensing and signaling. Clin Kidney J. 2016;9(3):444–453. doi: 10.1093/ckj/sfw010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levey A, Stevens L, Schmid C, Zhang Y, CA, Feldman H, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Govindarajulu U, Malloy E, Ganguli B, Spiegelman D, Eisen E. The comparison of alternative smoothing methods for fitting non-linear exposure-response relationships with Cox models in a simulation study. Int J Biostat. 2009;5(1):1557–4679. doi: 10.2202/1557-4679.1104. Article 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fine J, Gray R. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94(466):496–509. [Google Scholar]
- 35.Ames BCR, Schwiers E, Hocstein P. Uric Acid provides an antioxidant defense in humans against oxidant- and radical-cause aging and cancer: A hypothesis. Proc Natl Acad Sci U S A. 1981 Nov;11:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies K, Sevanian A, Muakkassah-Kelly S, Hochstein P. Uric acid-iron ion complexes: A new aspect of the antioxidant functions of uric acid. Biochem J. 1986;235:747–754. doi: 10.1042/bj2350747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parkinson Study Group S-PDI. Schwarzschild MA, Ascherio A, Beal MF, Cudkowicz ME, Curhan GC, et al. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol. 2014;71(2):141–150. doi: 10.1001/jamaneurol.2013.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson RJ, Nakagawa T, Jalal D, Sanchez-Lozada LG, Kang DH, Ritz E. Uric acid and chronic kidney disease: which is chasing which? Nephrol Dial Transplant. 2013;28(9):2221–2228. doi: 10.1093/ndt/gft029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumagai T, Ota T, Tamura Y, Chang WX, Shibata S, Uchida S. Time to target uric acid to retard CKD progression. Clin Exp Nephrol. 2016;21(2):182–192. doi: 10.1007/s10157-016-1288-2. [DOI] [PubMed] [Google Scholar]
- 40.Sturm G, Kollerits B, Neyer U, Ritz E, Kronenberg F, Group MS. Uric acid as a risk factor for progression of non-diabetic chronic kidney disease? The Mild to Moderate Kidney Disease (MMKD) Study. Exp Gerontol. 2008;43(4):347–352. doi: 10.1016/j.exger.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Latif W, Karaboyas A, Tong L, Winchester JF, Arrington CJ, Pisoni RL, et al. Uric acid levels and all-cause and cardiovascular mortality in the hemodialysis population. Clin J Am Soc Nephrol. 2011;6(10):2470–2477. doi: 10.2215/CJN.00670111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Testa A, Mallamaci F, Spoto B, Pisano A, Sanguedolce MC, Tripepi G, et al. Association of a polymorphism in a gene encoding a urate transporter with CKD progression. Clin J Am Soc Nephrol. 2014;9(6):1059–1065. doi: 10.2215/CJN.11041013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahola AJ, Sandholm N, Forsblom C, Harjutsalo V, Dahlstrom E, Groop PH, et al. The serum uric acid concentration is not causally linked to diabetic nephropathy in type 1 diabetes. Kidney Int. 2017;91(5):1178–1185. doi: 10.1016/j.kint.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 44.Navaneethan SD, Beddhu S. Associations of serum uric acid with cardiovascular events and mortality in moderate chronic kidney disease. Nephrol Dial Transplant. 2009;24(4):1260–1266. doi: 10.1093/ndt/gfn621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suliman ME, Johnson RJ, Garcia-Lopez E, Qureshi AR, Molinaei H, Carrero JJ, et al. J-shaped mortality relationship for uric acid in CKD. Am J Kidney Dis. 2006;48(5):761–771. doi: 10.1053/j.ajkd.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 46.Lee SM, Lee AL, Winters TJ, Tam E, Jaleel M, Stenvinkel P, et al. Low serum uric acid level is a risk factor for death in incident hemodialysis patients. Am J Nephrol. 2009;29(2):79–85. doi: 10.1159/000151292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsukuma Y, Masutani K, Tanaka S, Tsuchimoto A, Fujisaki K, Torisu K, et al. A J-shaped association between serum uric acid levels and poor renal survival in female patients with IgA nephropathy. Hypertens Res. 2017;40(3):291–297. doi: 10.1038/hr.2016.134. [DOI] [PubMed] [Google Scholar]
- 48.Yelken B, YC, Gorgulu N, Altun I, Yilmaz A, Yazici H, et al. Reduction of uric acid levels with allopurinol treatment improves endothelial function in patients with chronic kidney disease. Clin Nephrol. 2012;77(4):275–282. doi: 10.5414/cn107352. [DOI] [PubMed] [Google Scholar]
- 49.Kanbay M, Huddam B, Azak A, Solak Y, Kadioglu GK, Kirbas I, et al. A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6(8):1887–1894. doi: 10.2215/CJN.11451210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kao MP, Ang DS, Gandy SJ, Nadir MA, Houston JG, Lang CC, et al. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol. 2011;22(7):1382–1389. doi: 10.1681/ASN.2010111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jalal D, Decker E, Perrenoud L, Nowak K, Bispham N, Mehta T, et al. Vascular Function and Uric Acid-Lowering in Stage 3 CKD. J Am Soc Nephrol. 2016;28(3):943–952. doi: 10.1681/ASN.2016050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maahs DM, Caramori L, Cherney DZ, Galecki AT, Gao C, Jalal D, et al. Uric acid lowering to prevent kidney function loss in diabetes: the preventing early renal function loss (PERL) allopurinol study. Curr Diab Rep. 2013;13(4):550–559. doi: 10.1007/s11892-013-0381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Association between uric acid and all-cause mortality (censored for kidney failure).
Table S1: Independent predictors of uric acid in CRIC.
Table S2: Association between uric acid and risk of kidney failure (death as competing risk).
Table S3: Association of uric acid with kidney failure stratified by CKD stage (death as competing risk).
Table S4: Association of uric acid with kidney failure and all-cause mortality using mGFR.