Abstract
During the course of adolescence, reductions occur in cortical thickness and gray matter (GM) volume, along with a 65% reduction in slow wave (delta) activity during sleep (SWA) but empirical data linking these structural brain and functional sleep differences, is lacking. Here we investigated specifically whether age-related differences in cortical thickness and GM volume and cortical thickness accounted for the typical age-related difference in slow wave (delta) activity (SWA) during sleep. 132 healthy participants (age: 12–21 years) from the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA) study were included in this cross-sectional analysis of baseline polysomnographic, electroencephalographic (EEG) and magnetic resonance imaging (MRI) data. By applying mediation models we identified a large, direct effect of age on SWA in adolescents, which explained 45% of the variance in ultra-SWA (0.3 to 1Hz) and 52% of the variance in delta-SWA (1Hz to <4Hz), where SWA was lower in older adolescents, as has been reported previously. In addition, we provide evidence that the structure of several, predominantly frontal and parietal brain regions, partially mediated this direct age effect, Models including measures of brain structure explained an additional 3–9% of the variance in ultra-SWA and 4–5% of the variance in delta-SWA, with no differences between sexes. Replacing age with pubertal status in models produced similar results. As reductions in GM volume and cortical thickness likely indicate synaptic pruning and myelination, these results suggest that diminished SWA in older, more mature adolescents may largely be driven by such processes within a number of frontal and parietal brain regions.
Adolescence is a period of development associated with a wide range of biological and behavioral changes, characterized by physical growth, hormonal changes, sexual maturation (Pinyerd & Zipf, 2005) and extensive changes to brain structure, function and connectivity (Blakemore, Burnett, & Dahl, 2010; Casey, Jones, & Hare, 2008; Gogtay et al., 2004; Mills et al., 2016; Pfefferbaum et al., 2015; Pohl et al., 2016; Sowell, Thompson, & Toga, 2004; Sullivan et al., 2011). Dramatic changes to sleep architecture also occur during adolescence (see Tarokh, Saletin, and Carskadon (2016) for a recent review), which is most apparent for slow wave sleep (SWS), a stage of sleep dominated by slow delta (0.3 to <4Hz) waves. Typically, advancing adolescence is associated with reductions in sleep-related slow wave activity (SWA) (Baker, Turlington, & Colrain, 2012; Baker et al., 2016; Dube et al., 2015; Feinberg & Campbell, 2010, 2013; Feinberg, Higgins, Khaw, & Campbell, 2006; Jenni & Carskadon, 2004; Tarokh & Carskadon, 2010). SWA (also called delta power) represents power density (µV2.Hz−1) values which are averaged across the frequency band for SWA (0.3 to <4Hz). SWA undergoes a steep decline of approximately 65% during adolescence, typically beginning at ages 11–12 years (Campbell et al., 2011; Campbell & Feinberg, 2009). Furthermore, this trajectory is advanced (i.e., begins earlier) for girls (Feinberg et al., 2006). Similarly, sex differences in puberty timing have been found to account for substantial variance in the timing of SWA decline (Campbell, Grimm, de Bie, & Feinberg, 2012). Recent evidence suggests that there are brain regional effects on these reductions in SWA with age, with reductions being steepest at occipital sites (Baker et al., 2012; Baker et al., 2016; Feinberg, de Bie, Davis, & Campbell, 2011).
These dramatic SWA changes are commonly thought to reflect processes of brain maturation; namely synaptic pruning (Feinberg & Campbell, 2013; Feinberg, Thode, Chugani, & March, 1990; Tarokh & Carskadon, 2010). Through this process, the number of synapses, which are ‘overproduced’ at the beginning of life, are reduced (‘pruned’), likely to facilitate more efficient brain processing (Feinberg et al., 1990). The link between SWA and synapse density was first made when the developmental trajectory of frontal synaptic density as identified by Huttenlocher (1979) was found to follow a very similar trajectory to that identified for SWA (Feinberg, Hibi, & Carlson, 1977) (i.e., lowest at birth, a steep increase in the first years of life to a maximum in childhood, and then a further decline across adolescence). Additionally, as electroencephalography (EEG) signals comprise summed inhibitory and excitatory post-synaptic signals (Lopes da Silva & Rotterdam, 1998), EEG may reflect a functional correlate of synaptic density and thus brain maturation (Colrain & Baker, 2011; Feinberg & Campbell, 2010). Therefore, greater synaptic density (during the first years of life) may facilitate greater synchronous neural activity, resulting in greater EEG power. Conversely, diminished synaptic density during adolescence may facilitate asynchronous neural activity and weaker EEG and thus reduced SWA. Simultaneously, authors have also posited that reduced SWA across adolescence may be driven by a reduced homeostatic need for SWS (Campbell et al., 2011). It has long been established that as the duration of wake increases, progressively greater SWA is exhibited, and that SWA peaks at sleep onset and declines as a function of time spent asleep (See Hanlon, Vyazovskiy, Faraguna, Tononi, and Cirelli (2011)). This phenomenon is thought to reflect a homeostatic process, whereby some substrate produced by waking brain activity is built up and then restored via SWS (Borbely, 1982). More recently scientists have suggested that plastic process, such as learning and memory, result in net increases in synaptic strength throughout the day which are energy demanding and unsustainable long-term. Therefore, according to this synaptic homeostasis hypothesis, sleep is important to renormalize synaptic strength to a baseline level that is sustainable and beneficial for memory and performance (Hanlon et al., 2011). Therefore, reduced synchronous neural activity, caused by fewer interconnected neurons due to synaptic pruning, may be a mechanism by which waking brain activity may decrease during adolescence (Campbell et al., 2011; Feinberg & Campbell, 2010) and thus reduce the homeostatic need for SWS.
Two studies have provided evidence that waking brain activity may feasibly be reduced during adolescence. Kennedy and Sokoloff (1957) identified that children had 25% greater waking brain metabolism rates (CMRO2) compared to young adults (mean age= 24.5 years). This suggests that (older) adolescence is associated with lower waking brain activity levels compared to childhood, as brain metabolic rates are proportional to its functional activity. (Campbell et al., 2011)Similarly, a positron emission tomography (PET) study which identified a 50% reduction in glucose uptake across adolescence (Chugani, Phelps, & Mazziotta, 1987), which is a similar trajectory to the decline in SWA, suggesting that the two processes may be associated. Indeed, when analyzed together, the three datasets: CMRO2, glucose uptake and SWA amplitude, were all fit by a gamma distribution model and all exhibited extremely similar trajectories, i.e. low at birth, markedly increases during childhood and then steeply declines across adolescence. (Feinberg et al., 1990).
Taken together, these results suggest that it reductions in synaptic density may cause either direct (i.e. less neuronal synchrony leads to weaker EEG power) and/or indirect (i.e. less neuronal synchrony leads to less waking activity, which in turn leads to reduced homeostatic drive for SWS) reductions in SWA. However, although the exact mechanism by which reduced synaptic pruning results in less SWA is not entirely understood, the assumption that there is a relationship between synaptic density and SWA is largely undisputed.
Although synaptic density cannot be directly measured in humans, animal studies have determined that cortical thickness increases with greater synaptic density (Schuz & Palm, 1989) and structural brain measures, therefore, can be used to infer differences in synaptic density. Studies have identified developmental trajectories of gray matter (GM) volume and cortical thickness that appear to be analogous to those of synaptic density and SWA (Gogtay et al., 2004; Gogtay & Thompson, 2010; Jernigan, Trauner, Hesselink, & Tallal, 1991; Sowell, Thompson, & Toga, 2004). For example, Lenroot and Giedd (2010) identified U-shaped curves of GM development, with the timing of peak GM volume occurring 1–2 years earlier for females. Similarly, Shaw and colleagues (2008) analyzed longitudinal data from 375 children and adolescents and also identified non-linear (quadratic and cubic) cortical thickness developmental trajectories for the vast majority of cortex, particularly frontal regions. Results from these in-vivo, human studies illustrate that patterns of both GM volume loss and cortical thinning, particularly for the frontal, parietal and temporal lobes, are consistent with the postmortem evidence of synaptic pruning during adolescence and early adulthood in humans (Huttenlocher, 1979) and non-human primates (Bourgeois, Goldman-Rakic, & Rakic, 1994; Goldman-Rakic, 1987). To date, few studies have directly compared MRI derived results with histology. One such study by Kassem et al. (2013) recently identified that, in the rat brain, major changes in GM volume (assessed by MRI) in the anterior cingulate cortex and the hippocampus following stress were accounted for by loss of dendrites and their synapses. Animal research has also provided evidence that cortical thickness increases with increases in synaptic density (Schuz & Palm, 1989), suggesting that the two measures are associated. Furthermore, Whitaker et al. (2016) used a new MRI sequence, developed for myelin mapping in humans, and reported consistent, significant correlations between intra-cortical myelination and cortical thickness, and that myelination partially mediated measures of cortical thickness, thus suggesting that measures of cortical thickness are, at least in part, driven by the degree of myelination within a cortical region.
Few studies have measured both structural measures of brain development (e.g., cortical thickness and GM volume) and SWA during sleep, from EEG, in the same individuals. Buchmann and colleagues (2011) identified that, for a sample of 36 children and adolescents (8–19 years), GM volume of posterior cingulate, bilateral parietal cortex, left dorsolateral prefrontal cortex, sensorimotor, occipital and posterior temporal cortex positively correlated with SWA measured at site C4, which is over the right central cortex. Further, these regions showed the largest age-related decreases in GM volume. The combined parietal, occipital and temporal GM volumes accounted for 45% of SWA variability. The authors also report similar associations for measures of cortical thickness for the same brain regions, with the addition of more pre-frontal regions. However, two studies of young adults have found opposing effects, with one study finding no association between GM volume or cortical thickness and SWA in 20 adults (18–35 years old) (Buchmann, Kurth, et al., 2011) and another study reporting that frontal GM volume was associated with SWA, in general, and slow wave amplitude in particular in 22 adults (19–26 years old) (Saletin, van der Helm, and Walker (2013). Finally, Ringli and others (2013) identified in another sample of 22 boys and girls that girls exhibited both greater SWA and cortical thickness for a temporal brain region, although they did not report the association between the two measures. Taken together, previous studies have provided evidence that an association between brain structure and sleep SWA exists in relatively small samples of participants. However, it remains to be seen exactly how much of the age-related difference in SWA during adolescence is accounted for by both GM volume and cortical thickness and whether sex differences moderate this association. Beyond the period of rapid change across adolescence, a limited number of studies have examined SWA-brain structural relationships in older adults. Dube and colleagues (2015) reported that a lower amount of SWA in older (50–70 years) relative to younger (20–30 years) adults was explained by the indirect effect of age on cortical thickness. Further, the cortical thinning of insula, middle frontal gyrus, superior temporal lobe and inferior parietal lobule, areas previously identified as important for production of SWA, was found to drive SWA in frontal, central and parietal locations (Murphy et al., 2009).
Although relatively under-investigated as a function of adolescence, it is possible to decompose the SWA EEG band into ‘ultra-slow’, or ‘slow delta’ (typically defined as either <1 or <2Hz) and ‘delta’, or ‘fast delta’ (typically 1 to 4Hz or 2 to 4Hz) (Benoit, Daurat, & Prado, 2000; Dang-Vu et al., 2008; Le Bon & Linkowski, 2013; Le Bon et al., 2012; Moroni et al., 2014; Perrault, Carrier, Desautels, Montplaisir, & Zadra, 2014; Steriade, Nunez, & Amzica, 1993a).The basis for this distinctions is derived from the fact that intracellular recordings have identified that a slow (<1Hz) oscillation, caused by rhythmic cortical neuronal states which alternate between sustained neuronal firing and neuronal silence, is generated by a large proportion of cortical neurons (~88%) and occurs simultaneously with a delta rhythm that is temporally very similar to the slow oscillation (Amzica & Steriade, 1998; Destexhe, Contreras, & Steriade, 1999; Steriade, Nunez, & Amzica, 1993b; Steriade, Timofeev, & Grenier, 2001). Specifically, the surface negativity in the delta signal corresponds to the down state of cortical neurons, characterized by neuronal silence and membrane hyperpolarization, and it has been suggested that this slow oscillation plays a pivotal role in organizing delta waves and sleep spindles in animals (Steriade, Nunez, et al., 1993b). This distinction between slow and delta waves has also been confirmed in human sleep EEG, which has also revealed that EEG power densities within slow and delta frequency bands differ in their dynamics through the night (Achermann & Borbely, 1997). Specifically, the typical homeostatic reduction in EEG throughout the NREM period only occurred within the delta EEG band. Similarly, while delta EEG power increased with greater sleep pressure (i.e. longer time awake), slow EEG power showed the inverse relationship and decreased with greater sleep pressure, furthering the argument for the two frequency bands supporting two distinct processes. Furthermore, it has been identified that slow waves are generated directly within cortical circuits, whereas delta is derived from intrinsic properties of thalamocortical cells and from intracortical network interactions (Steriade, Contreras, Curro Dossi, & Nunez, 1993; Steriade & McCarley, 2005), furthering the argument that the two SWA bands represent distinct processes. However, others have suggested that the two bands are different representations of the same process, where the amplitude and slope of the waves reflect the level of synchronization achieved in cortical neuronal populations (Esser, Hill, & Tononi, 2007). More recently, authors have suggested that assessing slow waves independently may be more informative when investigating sleep disorders or patient populations, compared to when they are combined with delta rhythm (Le Bon et al., 2012). In light of the evidence for the existence of two distinct oscillations within the typically investigated ‘SWA frequency band’ (0.3 to <4Hz), we investigated the relationship between age and brain structure for these two SWA frequency bands (ultra-SWA: 0.3 to 1Hz and delta-SWA:1 to <4Hz), which, to our knowledge, has not been specifically investigated in adolescents.
The purpose of this study was to investigate whether age-related differences in cortical brain structure accounted for age-related reductions in SWA in a large sample of adolescents. We hypothesized that age would have a direct effect on SWA, but that age may also affect SWA indirectly due to its known influence on cortical thinning and GM volume decline. We assessed whether measures of both cortical thickness and GM volume accounted for variance in SWA or whether the association was largely driven by just one of these variables. Although both of these measures are imperfect measures of synaptic density and myelination, both have previously been independently associated with the reduction in SWA during adolescence. Therefore, we included both measures as a way of investigating whether either MRI derived measure of brain maturation was a better predictor of the age-SWA relationship known to exist during adolescence. In addition, we examined whether sex moderated the relationship between age and brain structure and thus whether the indirect effect of age on SWA differed between the sexes.
Method
Participants
Adolescents aged between 12 and 21.9 years, drawn from a large ongoing multi-site longitudinal study (NCANDA), participated in the sleep substudy, at SRI International and the University of Pittsburgh. Sample characteristics for this subset of participants are described elsewhere (2016). A full description of the five-site NCANDA baseline sample characteristics and protocol has also been previously presented (Brown et al., 2015; Pfefferbaum et al., 2015; Pohl et al., 2016; Sullivan et al., 2016). All participants had a phone interview and in-person screening session including the Semi-Structured Assessment for the Genetics of Alcoholism (Bucholz et al., 1994). Participants were healthy; none had severe medical conditions (e.g., heart disease, epilepsy, traumatic brain injury) or current/past major DSM-IV Axis I disorders (e.g., major depression, generalized anxiety disorders), or were using medications known to affect sleep or the central nervous system. None of the participants showed evidence of sleep-disordered breathing, periodic limb movement disorder or narcolepsy, as assessed by a clinical sleep evaluation in the laboratory. Six participants were excluded from analysis due to extremely poor sleep (> 3 SD from the mean for sleep onset latency or wakefulness after sleep onset) or short time in bed (< 6 hours). For this study, a further seven participants were excluded due to a lack of imaging data, resulting in a final sample size of 132 (59 male, 73 female), with an average age of 15.6 years (range: 12–21.9 years). The majority of participants in this sub-sample were right handed (n=107), 24 were left handed and 1 participant was ambidextrous, as assessed by the Edinburgh Inventory (Oldfield, 1971). By design, the majority (~85%) of participants in the NCANDA study at baseline met age-appropriate alcohol and drug use criteria for no-to-low exposure and the remaining participants exceeding these national norms (Brown et al., 2015). In the analysis presented here, 112 participants met criteria for no/low alcohol use and 20 participants exceeded these criteria. Separate analyses (results not presented here) confirmed that neither drinking status nor handedness moderated any of the results presented here, therefore, the participants that exceeded the no-to-low exposure criteria were not excluded from group analysis and left and right handed participants were considered as one group.
The study was approved by the Institutional Review Boards at SRI International and University of Pittsburgh. Adult participants consented to participate and minors provided written assent in addition to consent from a parent/legal guardian. Participants and parents were compensated for participation.
Procedure
All but eight participants had a clinical polysomnographic screening/adaptation night before the sleep architecture recording. Recordings were made at SRI International (n = 105) or the University of Pittsburgh (n = 27) NCANDA sites in sound-attenuated, temperature-controlled bedrooms. All participants went to bed in the laboratory at their self-reported typical bed-times. Each night, negative breath alcohol (S75 Pro, BACtrack Breathalyzers, San Francisco, CA, USA) and urine drug (10 Panel iCup drug test kit, Instant Technologies, Inc.) tests confirmed the absence of recent alcohol or drug use. Girls who were post-menarche were studied irrespective of menstrual cycle phase. Pubertal status was determined by self-assessment with the Pubertal Development Scale (PDS) (Petersen, Crockett, Richards, & Boxer, 1988), a validated measure of pubertal stage that shows modest concordance with a physical exam and that correlates with basal gonadal hormone levels (Shirtcliff, Dahl, & Pollak, 2009). An average pubertal development score was calculated for each participant by summing scores on 5 self-report items for boys and girls, each with scores ranging from 1 to 4. Within one month of the sleep sessions, all participants also completed a separate MRI session.
SWA analysis
Sleep EEG data within non-rapid eye movement (NREM) sleep (N2 and N3 combined) were analyzed on selected electrodes (F3, F4, C3, C4, P3, and P4) using the EEGLAB toolbox (Delorme & Makeig, 2004) for MATLAB (MathWorks, Natick, MA, USA), as previously described (Baker et al., 2016; Willoughby, de Zambotti, Baker, & Colrain, 2015). N1 sleep was excluded due to the transitional nature of the stage, during which frequent arousals can occur. EEG was re-referenced to the average mastoid and filtered at 0.3–36 Hz with half-amplitude cutoffs at 0.15 and 36.15Hz. Fast Fourier Transform analysis was conducted on each 30s epoch using a 4s sliding Hanning window to calculate power density values with 0.125 Hz resolution. Power density (µV2.Hz−1) values were then averaged across the frequency band for ultra-SWA (0.3 to 1Hz) and delta (>1Hz to <4Hz) to investigate how brain structure may differentially mediate the age-SWA relationship for the two SWA bands. Although there is no physiological evidence to set a cut-off at 1Hz, we chose this cut-off as it is typically used by other researchers (Campbell et al., 2011; Perrault et al., 2014). Furthermore, a study by Bersagliere and Achermann (2010) identified peaks in the EEG power density spectrum of SWS at 0.8 and 0.9 Hz, indicating that slow oscillations on average, have a frequency of below 1Hz. As many researchers combine these two bands and use 0.3 to <4Hz for SWA, we also performed analysis on this ‘combined’ SWA band and present the results in the supplementary material, for interest (see Tables S1a and S1b).
Epochs containing arousals, as defined accordingly to the American Academy of Sleep Medicine (AASM guidelines) were removed from analysis. In the time and frequency domains, an automated process was applied to reject outlier epochs from N2 and N3 sleep. In the time domain, if the EEG signal dropped for longer than 5s during the 30s epoch, or if the maximum value exceeded the median by 10 times the median absolute deviation, that epoch was removed. In the frequency domain, if the power of any band exceeded the median by 15 times the median absolute deviation, that epoch was also removed. Fewer than 5% of epochs were rejected with this process.
MRI data acquisition and analysis
T1-weighted, 3D images were obtained on systems from 2 manufacturers: 3T General Electric (GE) Discovery MR750 (SRI) and 3T Siemens TIM TRIO (University of Pittsburgh). The GE site (SRI) used an Array Spatial Sensitivity Encoding Technique (ASSET) for parallel and accelerated imaging with an 8-channel head coil and acquired an Inversion Recovery-SPoiled Gradient Recalled (IR-SPGR) echo sequence (TR = 5.912 ms, TI = 400 ms, TE = 1.932 ms, flip angle = 11°, NEX = 1, matrix = 256 × 256, FOV = 24 cm, voxel dimensions = 1.2 × 0.9375 × 0.9375 mm, 146 slices). The Siemens site (University of Pittsburgh) used a 12-channel head coil and parallel imaging and temporal acceleration with iPAT and acquired an MPRAGE sequence (TR = 1900 ms, TI = 900 ms, TE = 2.92 ms, flip angle = 9°, NEX = 1, matrix = 256 × 256, FOV = 24 cm, voxel dimensions = 1.2 × 0.9375 × 0.9375 mm, 160 slices).
All T1 -weighted images were pre-processed as described in (Pfefferbaum et al., 2015). Following skull-stripping, FreeSurfer (Dale, Fischl, & Sereno, 1999) was then used to estimate GM volume for all regions from the Desikan-Killiany (2006) cortical atlas, plus the insular cortex (as this region has been implicated in the generation of sleep slow waves (Murphy et al., 2009)). For measures of cortical thickness, the FreeSurfer pipeline consisted of: separating the two hemispheres, tesselation of the gray/white matter boundary (Fischl, Liu, & Dale, 2001), topology correction (Segonne, Pacheco, & Fischl, 2007), and surface deformation, which follows the intensity gradients and thus ensures optimal placement of the white/gray matter and gray matter/cerebrospinal fluid borders (Dale et al., 1999; Fischl et al., 2001). The surfaces were then inflated to a sphere and registered to a spherical atlas (Fischl, Sereno, & Dale, 1999; Fischl, Sereno, Tootell, & Dale, 1999), after which the cortex was parcellated into units based on the gyral and sulcal structure of the Desikan-Killany atlas (Desikan et al., 2006). The cortical thickness value at each vertex on the tessellated surface is the closest distance from the gray/white boundary to the gray/CSF boundary (Fischl & Dale, 2000). An average cortical thickness measure was then derived for each region in the Desikan-Killany (2006) cortical atlas. Utilizing a cortical atlas in this way allowed us to adopt an exploratory approach to evaluate how the cortical thickness and GM volume of regions across the entire cortex may mediate slow wave sleep density in adolescence. Regions of interest consisted of superior temporal cortex, cingulate cortex (caudal anterior, isthmus, rostral), caudal middle frontal gyrus, cuneus, entorhinal cortex, fusiform gyrus, inferior frontal gyrus (pars opercularis, pars orbitalis, pars triangularis), inferior parietal cortex, inferior temporal gyrus, lateral occipital cortex, lateral orbitofrontal cortex, middle temporal gyrus, parahippocampal gyrus, paracentral lobule, pericalcarine cortex, postcentral gyus, posterior cingulate cortex, precentral gyrus, precuneus, rostral middle frontal gyrus, superior frontal gyrus, superior parietal cortex, superior temporal gyrus, supramarginal gyrus, temporal pole and transverse temporal cortex. For each region, measures of cortical thickness (mm) and GM volume (cc) were extracted. These data can be found in the data release ‘NCANDA_DATA_00011_V2’ created by the software platform Scalable Informatics for Biomedical Imaging Studies (https://sibis.sri.com) (Nichols & Pohl, 2015; Pohl et al., 2016).
Statistical analysis
Confounding variables
Site and scanner manufacturer were included as confounding variables in all statistical models. This was because slight differences existed in the sleep protocol between collection sites (at SRI, participants woke up at their typical weekday times but at the University of Pittsburgh, participants were allowed to wake up when they chose) and because Pfefferbaum and colleagues (2015) identified that scanner manufacturer was found to explain a significant proportion of the variance in brain structure, for the larger, complete NCANDA dataset that includes data from three GE sites and two Siemens sites. Further analysis, conducted on the SRI International sample only (n=105), identified that results from the larger group (n=132) were replicated in this smaller group, confirming that any differences in MRI data due to different scanner manufacturers did not affect our results (see Supplementary Material, Table S2). Furthermore, supratentorial volume (svol) was also controlled for in all statistical models after it was identified previously that svol accounted for sex effects, and markedly attenuated ethnicity effects, on brain structure (Pfefferbaum et al 2015).
Assessing mediation effects
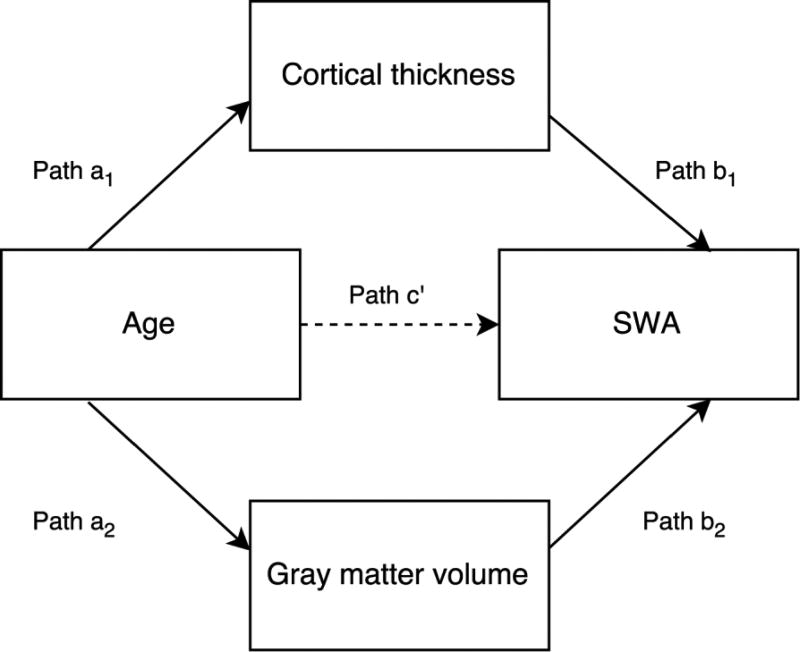
Mediation models were used to assess the mediating effect of cortical thickness and GM volume of cortical brain regions on the relationship between age and SWA. Mediation analyses investigate whether the causal effect of an independent variable (X) on a dependent variable (Y) is fully, or partly, driven by a mediating variable (M). In other words, X exerts its effect on Y because X affects M, which in turn, affects Y (see Figure 1 for the conceptual model diagram). Mediation analyses were first discussed in the 1980’s (Judd & Kenny, 1981)and are commonly used in psychological research (MacKinnon, Fairchild, & Fritz, 2007). The principals of mediation analyses are explained in detail in a number of publications (Hayes, 2013; Judd, Kenny, & McClelland, 2001; Preacher, Rucker, & Hayes, 2007). Recently mediation models have been used to investigate the relationship between cortical thickness and SWA in older adults (Dube et al., 2015). Here, we used parallel mediation models, as described by Hayes (2013), to allow for the inclusion of multiple mediator variables within the same model (i.e., cortical thickness and GM volume). This method established the total effect of age on SWA, which was decomposed into the direct effect (path c’) and the indirect effects (cortical thickness: path a1* path b1 and GM volume: path a2* path b2) for each brain region. In the diagram (Figure 1), a1 & a2 denote the slope coefficient of M1+M2 regressed on X, and b1+b2 and c are the coefficients of Y regressed on M1+M2 and X, respectively, when both are included as simultaneous predictors of Y. The indirect effects are calculated by multiplying paths a1 and b1 and a2 and b2. Mediation occurs when the relationship between X and Y (age and SWA) drops significantly when entering the mediators (cortical thickness and GM volume) as cofactors in the model. Thus, a significant indirect effect indicates that a significant proportion of the total age effect on SWA is driven by cortical thickness and/or GM volume of a brain region. An indirect effect was deemed significant (p<0.05) when the 95% confidence interval of the bootstrapped (10,000 permutations) regression coefficient did not include zero. The PROCESS toolbox (Hayes, 2013) was used to run all mediation models and mediation effect sizes, within IBM SPSS Statistics for Windows (Version 23.0). SWA was log-transformed before being included in the mediation models, following identification of a positively skewed distribution (p>0.05 Shapiro-Wilk after log-transformation). All non-dichotomous variables (i.e., variables other than sex and site) were z-transformed before mediation analysis was applied. For each brain region, the left and right hemispheres were investigated separately. Results derived from the mediation models are presented for frontal electrode sites (SWA averaged across C3 and C4), although the pattern of results was consistent across other electrode sites (F3, F4, P3, P4; results not presented here). For each analysis (e.g. ultra-SWA, delta-SWA) p-values for the indirect effects were adjusted for the number of mediation models computed. Only significant results with a false positive rate (FPR) < 5% are reported.
Figure 1.
A graphical representation of how brain structure may mediate the relationship between age and SWA. We posit that the total effect of age on SWA (path c) is partly driven by the indirect effect of age on cortical thickness and gray matter (GM) volume. These indirect effects of age are calculated by a1*b1 (cortical thickness) and a2*b2 (GM volume). Finally, path c’ depicts the remaining direct effect of age on SWA, that is not mediated by brain structure.
Assessing moderating effects
For the brain regions where a significant mediating effect was identified, we ran secondary analyses, which assessed whether sex moderated the mediating effect of brain structure on the age-SWA relationship. Specifically, this allowed us to assess whether the mechanism of the indirect age effect on SWA differed for the two groups (e.g., whether cortical thickness was more/less of a mediator for one sex), or whether specific brain regions were more/less important in predicating SWA for one sex. As any sex-specific differences in brain volume were controlled for in all models (svol), we can be confident that a significant moderating effect of sex was not simply related to differences in brain volume.
Using the PROCESS plug-in, with the same model specification as above, sex was included as a moderating variable. A moderating effect was deemed significant (p<0.05) when the 95% confidence interval of the bootstrapped (10,000 permutations) regression coefficient for the age*sex interaction did not include zero.
Assessing pubertal status
Finally, we ran all mediation models with age replaced by pubertal status to assess whether brain structure would better predict SWA changes associated with pubertal status, than chronological age. As Baker and others (2016) reported that pubertal development and SWA differences largely overlapped with the age-related differences, we did not expect the total effect of pubertal status on SWA to differ considerably from the effect of age. However, it remained to be seen whether pubertal status would be more or less associated with brain structure and any mediating effects on SWA. Pubertal status data were missing for four participants, resulting in a final sample size of 128 for this additional analysis.
Results
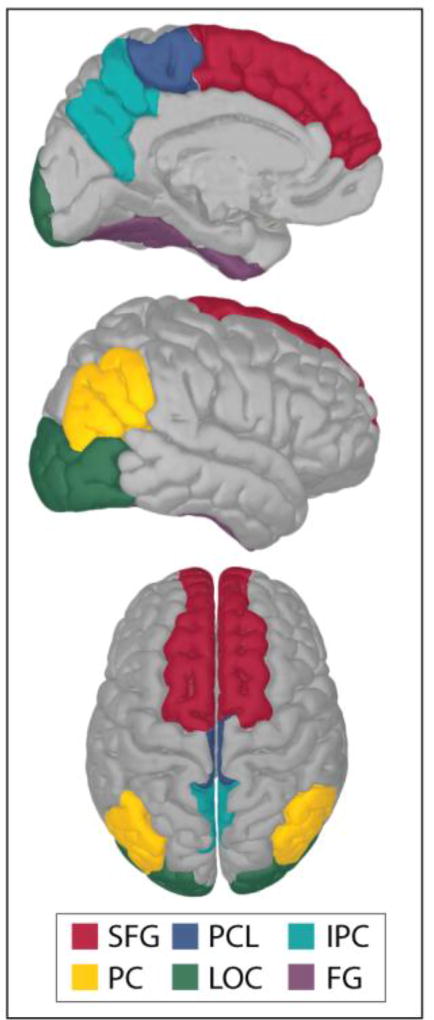
Of the 34 regions included in the analysis, the cortical thickness and/or GM volume of 6 regions (fusiform gyrus (FG), inferior parietal cortex (IPC), lateral occipital cortex (LOC), paracentral lobule (PCL), precuneus (PC) and superior frontal gyrus (SFG)) significantly mediated the relationship between age and SWA. Figure 2 depicts these brain regions, overlaid on an average, inflated brain template. The results from the mediation models for these 7 regions are reported below and specific statistical outcomes for each region are presented in Table 1a and Table 1b.
Figure 2.
Brainstorm (Tadel, Baillet, Mosher, Pantazis, & Leahy, 2011) was used to create the embedded figure, which depicts each of these cortical regions (from the Desikan-Killiany cortical atlas (Desikan et al., 2006)) on an inflated, average, brain template. SFG= Superior frontal gyrus, PCL= paracentral lobule, IPC= inferior parietal cortex, PC= precuneus, LOC= lateral orbital cortex and FG= fusiform gyrus.
Table 1.
Mediation model outcomes for each of the brain regions where cortical thickness (CT) and/or GM volume (GMV) was found to significantly mediate the age-SWA relationship for the two SWA analyses: ultra-SWA (1a) and delta-SWA (1b).
| a: ultra-SWA (0.3 to 1Hz) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Total age effect (path c): b= −0.57, R2= 0.45, p<0.001 | |||||||
|
| |||||||
| ROI | Hem | M | δR2 | Path a1 or a2 | Path b1 or b2 | Path c’ | Indirect effect (CI) |
| FG | L | CT | 0.03 | b= −0.20, p= 0.028 | b= 0.20, p= 0.009 | b= −0.53 p <0.001 | b= −0.04 (−0.111, −0.005) |
| IPC | L | GMV | 0.06 | b= −0.25, p <0.001 | b= 0.29, p= 0.004 | b= −0.46, p <0.001 | b= −0.07 (−0.153, −0.022) |
| R | CT | 0.06 | b= −0.48, p <0.001 | b= 0.24, p= 0.003 | b= −0.42, p <0.001 | b= −0.12 (−0.225, −0.036) | |
| LOC | L | CT | 0.04 | b= −0.35, p <0.001 | b= 0.24, p= 0.004 | b= −0.54 p <0.001 | b= −0.08 (−0.171, −0.022) |
| R | CT | 0.05 | b= −0.32, p <0.001 | b= 0.24, p =0.002 | b= −0.52, p <0.001 | b= −0.08 (−0.156, −0.024) | |
| PCL | R | CT | 0.04 | b= −0.39, p <0.001 | b= 0.23, p= 0.005 | b= −0.49, p <0.001 | b= −0.09 (−0.178, −0.034) |
| PC | R | GMV | 0.09 | b= −0.24, p <0.001 | b= 0.38, p= 0.003 | b= −0.38, p <0.001 | b= −0.09 (−0.181, −0.037) |
| SFG | R | GMV | 0.04 | b= −0.16, p= 0.002 | b= 0.32, p= 0.016 | b= −0.51, p <0.001 | b= −0.05 (−0.129, −0.007) |
| b: delta-SWA (1Hz to <4Hz) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Total age effect t (path c): b = −0.64, R2 = 0.52, p<0.001 | |||||||
|
| |||||||
| ROI | Hem | M | δR2 | Path a1 or a2 | Path b1 or b2 | Path c’ | Indirect effect (CI) |
| IPC | L | GMV | 0.05 | b= −0.25, p <0.001 | b= 0.32, p= 0.001 | b= −0.54, p <0.001 | b= −0.08 (−0.150, −0.032) |
| LOC | R | CT | 0.04 | b= −0.32, p <0.001 | b= 0.21, p =0.004 | b= −0.59, p <0.001 | b= −0.07 (−0.148, −0.016) |
| SFG | L | GMV | 0.04 | b= −0.20, p <0.001 | b= 0.32, p= 0.009 | b= −0.56, p <0.001 | b= −0.07 (−0.146, −0.017) |
| R | GMV | 0.04 | b= −0.16, p= 0.002 | b= 0.35, p= 0.004 | b= −0.58, p <0.001 | b= −0.06 (−0.136, −0.008) | |
Each colored row indicates one brain region of interest (ROI), column ‘Hem’ then indicates whether the significant effect was found to be bilateral or unilateral. M indicates whether the significant mediator for that region was cortical thickness, GM volume or both (both measures were included in each mediation model). δR2 is the increase in R2 caused by adding cortical thickness (a1+b1) or GM volume (a2+b2) as mediators in the model, compared to the total effect model (c’), which included age as the only predictor of SWA. The indirect effect indicates how much of the total effect of age (i.e., b= −0.57 and b= −0.64) was accounted for by the indirect effect of age, via the mediating variable (i.e., cortical thickness and/or GM volume). 95% confidence intervals of the indirect effect are also displayed to indicate significance.
Effects of age on cortical thickness and gray matter volume (paths a1 and a2)
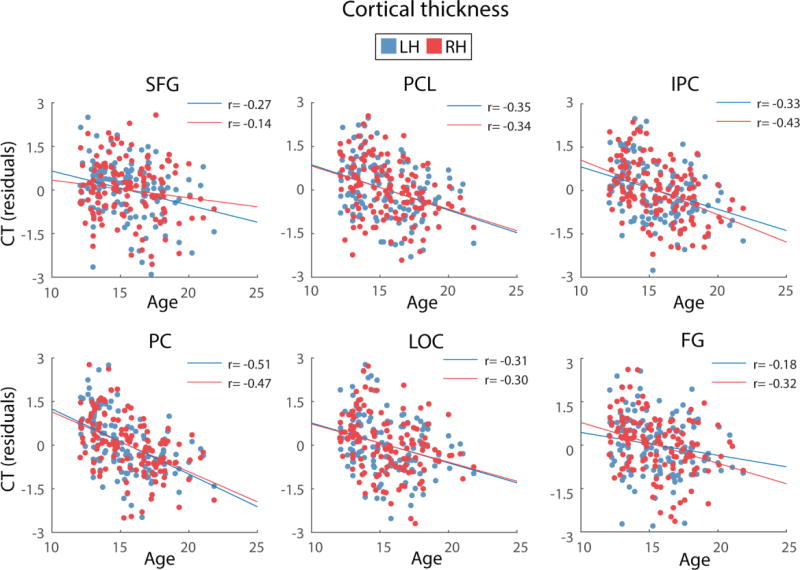
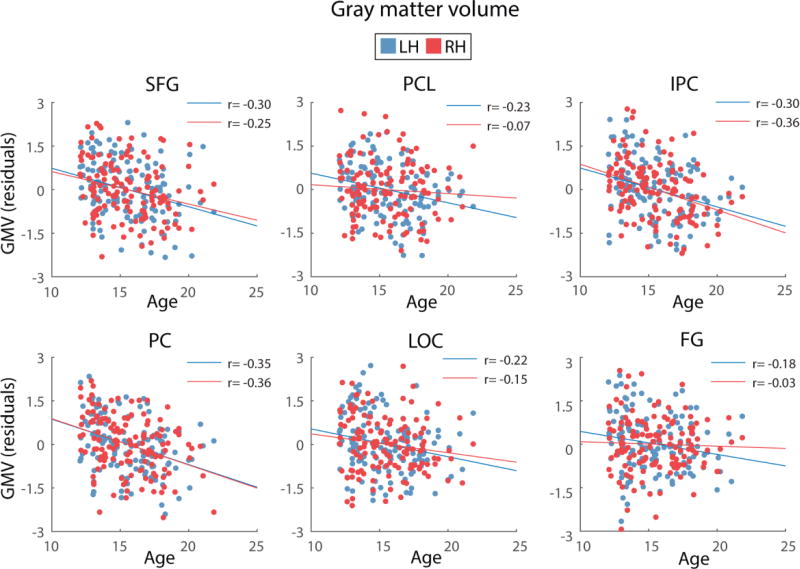
For all regions, age was found to be a significant predictor of cortical thickness and GM volume, after controlling for site and svol. For the left IPC, b was −0.25 for GM volume. For the right hemisphere, b ranged from −0.32 to −0.39 for cortical thickness and b ranged from −0.16 to 0.24 for GM volume. In addition, age, whilst controlling for site and svol, explained a significant proportion of variance in cortical thickness (R2 = 0.15 to 0.24 for the right hemisphere) and GM volume (R2 = 0.56 for the left hemisphere and R2 = 0.22 to 0.71 for the right hemisphere). Across regions, cortical thickness and GM volume were greater for younger than older adolescents, as previously shown in the larger NCANDA cohort (n = 808) (Pfefferbaum et al., 2015). The relationships between cortical thickness and age and GM volume and age are depicted in Figures 3 and 4.
Figure 3.
Depiction of the relationship between age and cortical thickness (CT) for the brain regions where structure was found to significantly mediate the age-SWA relationship. Cortical thickness values presented in the figures are residuals, after regressing the effects of site and svol. Blue and red circles represent the cortical thickness of left and right hemispheres, respectively, for each region. SFG= Superior frontal gyrus, PCL= paracentral lobule, IPC= inferior parietal cortex, PC= precuneus, LOC= lateral orbital cortex and FG= fusiform gyrus.
Figure 4.
Depiction of the relationship between age and GM volume (GMV) for the brain regions where structure was found to significantly mediate the age-SWA relationship. GM volume values presented in the figures are residuals, after regressing the effects of site and svol. Blue and red circles represent the GM volume of left and right hemispheres, respectively, for each region. SFG= Superior frontal gyrus, PCL= paracentral lobule, IPC= inferior parietal cortex, PC= precuneus, LOC= lateral orbital cortex and FG= fusiform gyrus.
Effects of cortical thickness and gray matter volume on SWA (paths b1 and b2)
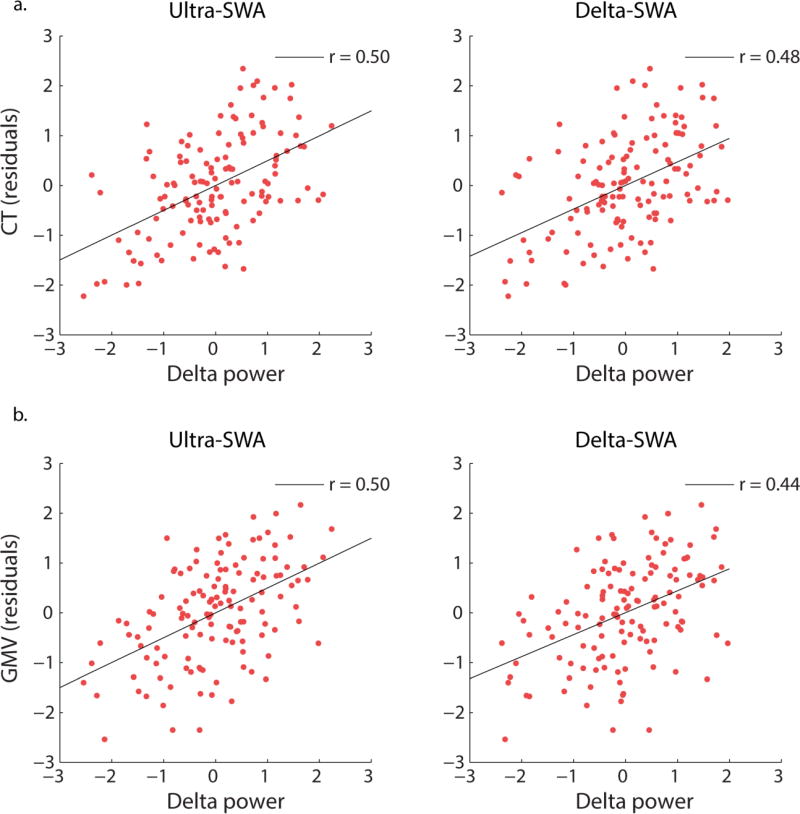
For all regions, cortical thickness or GM volume was a significant predictor of SWA. Across regions, greater cortical thickness or GM volume was associated with greater SWA. For ultra-SWA, b ranged from 0.20 to 0.24 for left-hemisphere cortical thickness and was 0.29 for left IPC GM volume. For the right hemisphere, b ranged from 0.23 to 0.24 for cortical thickness and −0.32 to 0.38 for GM volume. For delta-SWA, b was 0.32 for left IPC and SFG GM volume, 0.21 for right LOC cortical thickness and 0.35 for right SFG GM volume. Furthermore, cortical thickness or GM volume accounted for a significant proportion of variance in SWA, combined with age (R2 ranged from 0.48 to 0.54 for ultra-SWA and 0.56 to 0.57 for delta-SWA). Figures 5a and 5b depict these typical relations. See Table 1a and Table 1b, column ‘Path b’ for significant statistical outcomes for each brain region.
Figure 5.
Figures depicting the significant relationships between log transformed SWA averaged across electrodes C3 and C4 (residuals after regressing site) and a) IPC cortical thickness (residuals after regressing site and svol) and b) PC gray matter (GM) volume (residuals after regressing site and svol). These figures depict the typical associations between cortical thickness/GM and SWA for the regions where a significant mediating effect was identified.
Total effect of age on SWA (path c)
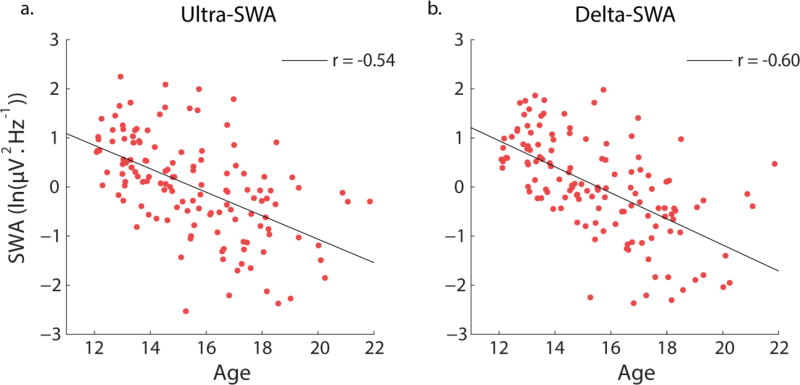
The total effect of age on SWA was assessed by a regression model that predicted SWA by age only (i.e., no measures of cortical thickness or GM volume included), whilst still controlling for site and svol. Age alone significantly predicted ultra-SWA (b= −0.57, t(128)= −8.11, p<0.001) accounting for 45% of the variance (R2= 0.45, F(3, 128)=34.52, p<0.001) and delta-SWA, (b= −0.64, t(128)= −9.64, p<0.001 accounting for 52% of the variance (R2= 0.52, F(3, 128)=46.05, p<0.001). Older age was associated with less ultra-SWA and delta-SWA. Figure 6 illustrates the correlation between SWA and age.
Figure 6.
The significant association between age and log transformed SWA for: a) ultra-SWA (0.3 to 1Hz) and b) delta-SWA (1Hz to <4Hz). Values are residuals after controlling for site.
Indirect effects of age on SWA via cortical thickness (path a1*b1) and GM volume (path a2*b2)
For ultra-SWA, adding cortical thickness and GM volume to the model, significantly increased the explained variance in SWA to 48–54%, compared to the total effect model (45%), depending on the region. For delta-SWA, adding cortical thickness and GM volume to the model, significantly increased the explained variance in SWA to 56–57%, compared to the total effect model (52%), depending on the region.
Left hemisphere
For ultra-SWA, the GM volume of left IPC significantly mediated the age-SWA relationship. with a 6% increase in explained variance of SWA, relative to age alone. The indirect effect of the total effect of age (b= −0.57) was b= −0.08 for left IPC GM volume. For this region, this mediation effect was specific to GM volume. Although cortical thickness was also included in each model, cortical thickness did not significantly mediate the age-delta relationship in the same manner as GM volume. The CT of left LOC and left FG also significantly mediated the age-ultra-SWA relationship with a 4% increase in explained with LOC and a 3% increase with FG, relative to age alone. The indirect effect of the total effect of age (b= −0.57) was b= −0.08 for left LOC and b= −0.04 for left FG cortical thickness. For these regions, this mediation effect was specific to cortical thickness. Although GM volume was also included in each model, it did not significantly mediate the age-delta relationship in the same manner as cortical thickness. For delta-SWA, the GM volume of left IPC and left SFG significantly mediated the age-SWA relationship with a 5% increase in explained variance with IPC and a 5% increase with SFG, relative to age alone. The indirect effect of the total effect of age (b= −0.64) was b= −0.08 for left IPC and b= −0.07 for left SFG GM volume. For these regions, this mediation effect was specific to GM volume. Although cortical thickness was also included in each model, cortical thickness did not significantly mediate the age-delta relationship in the same manner as GM volume.
Right hemisphere
For ultra-SWA, right IPC, LOC PCL, PC and SFG cortical thickness significantly mediated the age-SWA relationship. Including IPC cortical thickness in the mediation model resulted in a 6% increase, LOC cortical thickness a 5% increase and PCL cortical thickness a 4% increase in explained variance in SWA, compared to age alone. The indirect effect of the total effect of age (b= −0.57) was b= −0.12 for right IPC, b= −0.08 for right LOC and b= −0.09 for right PCL cortical thickness. Despite GM volume being included in this mediation model, the significant effects were specific to cortical thickness.
Including PC GM volume in the mediation model resulted in a 9% increase in explained variance and including SFG GM volume a 4% increase in explained SWA variance, compared to age alone. The indirect effect of the total effect of age (b= −0.57) was b= −0.09 for right PC GM volume and b= −0.05 for right SFG GM volume. Despite cortical thickness also being included in these mediation models, the significant effects were specific to GM volume.
For delta-SWA, right LOC and SFG significantly mediated the age-SWA relationship. For both right, LOC and SFG, including cortical thickness and GM volume in the mediation model resulted in a 4% increase in explained variance in SWA, compared to age alone. The indirect effect of the total effect of age (b= −0.57) was b= −0.07 for right LOC cortical thickness and b= −0.06 for right SFG GM volume. Despite both cortical thickness and GM volume being included in these mediation models, the significant effect was specific to CT for right LOC and GMV for right SFG.
The direct effect of age on SWA (path c’)
For all regions, a significant direct effect (i.e., an effect of age not mediated by cortical thickness/GM volume) of age on SWA remained, even when controlling for cortical thickness and GM volume. In all cases, older age was associated with lower SWA, as expected. For the brain regions identified as significant mediators of the age-SWA relationship, the direct effect of age ranged from b= −0.38 to −0.54 of the total effect of age (b= −0.57) for ultra-SWA and b= −0.54 to −0.59 of the total effect of age (b= −0.64) for delta-SWA. These results indicate the mediating effects identified are partial, rather than full. In other words, age-related differences in cortical thickness and/or GM volume did not entirely account for age-related differences in SWA.
Moderating effects
Sex was not a significant moderator of the relationship between age and SWA, for any region, for either ultra-SWA or delta-SWA. This result indicates that neither the particular regions involved in mediating age-SWA nor the variable underlying the mediation (i.e., cortical thickness or GM volume) for each region, differed between the two sexes.
Pubertal status
As above, the total effect of pubertal status on SWA was assessed by a regression model that predicted SWA by pubertal status only (i.e., no measures of cortical thickness or GM volume included), whilst still controlling for site and svol. Pubertal status alone significantly predicted ultra-SWA (b= −0.50, t(124)= −6.71, p<0.001) and accounted for 39% of the its variance (R2= 0.39, F(3, 124)=26.26, p<0.001). Pubertal status alone significantly predicted delta-SWA (b= −0.49, t(124)= −6.82, p<0.001) and accounted for 42% of its variance (R2= 0.42, F(3, 124)=29.38, p<0.001). For both SWA bands, pubertal status accounted for less variance than that predicted by chronological age, with advanced pubertal stage nonetheless being associated with less SWA.
Results from mediation models, which used pubertal status to predict SWA, were almost identical to those using chronological age. Similarly, the effect of pubertal status on cortical thickness and GM volume (i.e., paths a1 and a2) matched those of chronological age.
Discussion
This study investigated how differences in brain structure across adolescence may contribute to differences in SWA during sleep between older and younger adolescents. The results indicated that the brain structure of several, mainly frontal and parietal, regions partially mediated the relationship between age and SWA and that the effects were similar in boys and girls. Interestingly, we found a larger number of brain regions producing mediating effects for ultra-SWA, compared to delta-SWA. While age alone explained more variance in delta-SWA compared to ultra-SWA (52% vs. 45%), the increase in explained variance caused by adding brain structure to the model was slightly greater for ultra-SWA (up to a 9% increase, compared to 4–5% increase for delta-SWA). Taken together, these results suggest that a stronger mediating effect of brain structure changes on the typical age-SWA association during adolescence for ultra-SWA than for delta-SWA. In addition, structural estimates for a larger network of brain regions mediating the age-related differences in ultra-SWA, with a smaller but overlapping set of regions mediating the age-related differences in delta-SWA. Our ongoing research will investigate the longitudinal trajectories of these two SWA bands and how they are related to longitudinal changes in brain structure. However, these results should be considered in light of the fact that while a number of studies have investigated these two sub-bands of SWA, based on the hypothesis that they reflect distinct processes derived from different brain circuits (Steriade & McCarley, 2005), others have questioned whether the difference between ultra-slow waves and delta waves is qualitative or quantitative (Dang-Vu et al., 2008; Knyazev, 2012). Nonetheless, a number of studies have employed this distinction between SWA bands and have purported results to suggest their distinction exists (Benoit et al., 2000; Le Bon & Linkowski, 2013; Le Bon et al., 2012; Moroni et al., 2014). Future research should attempt to further characterize the distinction and to investigate the differences in terms of adolescent development, which, to our knowledge, this paper is the first to explore.
The cortical regions for which brain structure significantly mediated the relationship between age and SWA corresponded with those reported by Buchmann and colleagues (2011), who identified that the reduction in SWA across adolescence was significantly correlated with cortical thickness and GM volume loss largely for frontal and parietal regions. Specifically, the cortical regions consistently identified across the two studies were fusiform gyrus (FG), superior frontal gyrus (SFG), precuneus (PC), inferior parietal cortex (IPC), and paracentral lobule (PCL).
Although we identified a number of instances where cortical thickness or GM volume mediated the effect of age on SWA, a significant direct effect of age remained in each mediation model. This suggests that brain structural differences across adolescence do not fully account for the age-related decline of SWA during adolescence. Interestingly, Dube and colleagues (2015) identified full mediation of the age-slow wave density relationship via cortical thickness of a number of brain regions for a sample of young and older adults. However, despite identifying that differences in cortical thickness were responsible for the age-related differences in slow wave density, overall, age explained less variance and was less of a predictor (R2=0.32 and b = −34) than observed in the current study (R2=0.50 and b= −0.62). The major difference between these two studies is the population studied. The magnitude of differences in cortical thickness and/or GM volume as well as SWA may differ in an adolescent group, across a single decade (12–21 years), compared to differences in adults, across multiple decades (20–30 vs. 50–70 years), affecting variance in mediation models. Also, the processes underlying differences in brain structure and SWA across adolescence versus with aging in adults may differ.
By including standardized measures of both cortical thickness and GM volume within the mediation models we were able to ascertain whether one measure explained more variance than the other, which to our knowledge, has not been explored before in this direct manner. We did not find conclusive evidence to identify cortical thickness or GM volume as the more influential mediator; of the 17 significant mediating effects identified, 8 were driven by cortical thickness and 9 were driven by GM volume. For IPC both GM volume and cortical thickness were found to be significant mediators of the effect of age on ultra-SWA. For all other regions, only one mediator (i.e., either cortical thickness or GM volume) was found to mediate the relationship.
GM volume and cortical thickness may be indirect measures of synaptic density, as their developmental trajectories also feature pre-pubescent increases and post-pubescent decreases, particularly for frontal regions (Lenroot & Giedd, 2010; Shaw et al., 2008), which could feasibly reflect synaptic pruning (Gogtay & Thompson, 2010). Cortical thinning during adolescence may also be due to an increased proportion of myelinated axons, as opposed to a reduction in synaptic density or loss of neuronal matter (Sowell, Thompson, Leonard, et al., 2004), for which non-human studies have provided some supporting evidence (Hammelrath et al., 2016; Mengler et al., 2014). A recent study by Whitaker and others (2016) used a new MRI sequence, based on magnetization transfer (MT), which has been developed for myelin mapping in humans and validated with post-mortem imaging (Glasser, Goyal, Preuss, Raichle, & Van Essen, 2014; Schmierer et al., 2007). Their study assessed myelination and cortical thickness at differing layers of the cortex and identified that the greatest age-related difference in myelination was seen in lamina layers V and VI, which are deep cytoarchitectonic layers that consist of pyramidal or projection neurons. Furthermore, they reported consistent, significant correlations between intra-cortical myelination and cortical thickness, and that myelination partially mediated measures of cortical thickness. This suggests that measures of cortical thickness are, at least in part, driven by the degree of myelination within a cortical region. However, determining causality is a fundamental issue within the in-vivo brain imaging field. MRI is an indirect measure of a complex architecture of glia, dendrites, vasculature, and neurons and current MRI resolution cannot ascertain the exact cellular processes that underlie cortical thinning or GM volume loss. The development of new MRI protocols, such as that listed above to assess myelination and recent advances that allow the identification of layer-specific cortical changes (Wagstyl et al., 2016), will go some way to advancing our understanding of how the brain changes during adolescence. However, integrated, multifaceted research studies are perhaps the only way to ascertain how findings from different fields of brain imaging research (i.e. cellular and MRI) are related to each other and such scientific collaboration is being advocated by researchers in the field (Jernigan, Brown, Bartsch, Dale, 2016).
However, assuming that cortical thickness and GM volume are, at least in part, indirect measures of synaptic density and myelination (cortical thickness only), our finding of significant mediating effects of cortical thickness and GM volume on SWA, suggests that synaptic density and/or myelination are responsible for SWA. Consequently, we speculate that the observed changes in SWA across adolescence, mediated by cortical thickness and GM volume, are driven by reductions in synaptic density and increased myelination, processes marking adolescent development (Keshavan, Anderson, & Pettergrew, 1994).
Replacing age with pubertal status in mediation models produced similar results. Since age and pubertal status are tightly coupled, it is challenging to differentiate whether differences in sleep or brain structure across adolescence are related to differences in pubertal maturation processes versus other age-specific differences. Changes in pubertal hormones may feasibly interact with sleep quality and architecture directly (Campbell et al., 2012; Pinyerd & Zipf, 2005) or through changes to brain structure (Bava et al., 2011; Bramen et al., 2012; Bramen et al., 2011; Herting, Gautam, Spielberg, Dahl, & Sowell, 2015; Herting et al., 2014; Herting, Maxwell, Irvine, & Nagel, 2012) and connectivity (Sisk & Foster, 2004). A number of studies have provided evidence that hormonal changes may drive changes to the circadian system (Hagenauer & Lee, 2012) and may also influence sleep patterns and architecture (de Zambotti, Colrain, & Baker, 2015; Hagenauer & Lee, 2013).
The results obtained here need to be interpreted in light of the study limitations. This study was cross-sectional in design, as we present results from the first year of the NCANDA study, which may have led to an over-simplification of the association between brain structure and SWA during adolescence. Although the linear decline in SWA we observed are consistent with other cross-sectional studies (Baker et al., 2012; Baker et al., 2016; Dube et al., 2015; Feinberg & Campbell, 2010, 2013; Feinberg et al., 2006; Jenni & Carskadon, 2004; Tarokh & Carskadon, 2010), longitudinal studies have identified non-linear declines in SWA, which suggested potentially biologically relevant inflection points for brain maturational processes (Campbell & Feinberg, 2009; Campbell, Grimm, de Bie, Feinberg, 2012). As we continue to assess both SWA and brain structure across the course of the NCANDA study, we will be able to compare our cross-sectional results to those we obtain longitudinally, to allow us to better characterize the trajectory of both SWA decline and cortical thinning throughout adolescence. In addition, our age group ranged from 12–21 years, thus missing earlier development in brain structure and sleep. Consequently, our sample may have lower variability compared to the true adolescent population that could result in Type II errors. Furthermore, the lack of younger adolescents in our sample may have limited our ability to identify moderating sex differences because of sex differences in pubertal development, with girls developing earlier than boys. The assessment of pubertal development was also limited by the use of the PDS (Petersen, Crockett, Richards, & Boxer, 1988), rather than a physical exam or measurement of gonadal hormone levels.
In conclusion, we provide evidence that the significant association between age and SWA in a large group of adolescents was partially mediated by age-related differences in brain structure (cortical thickness and GM volume) of several, predominantly frontal and parietal, brain regions. As reductions in cortical thickness and GM volume likely are indicative of synaptic pruning and myelination, combined with other brain microscopic maturational processes, these results suggest that diminished SWA in adolescence may largely be driven by synaptic pruning and myelination within a number of cortical brain regions. Furthermore, we provided evidence that brain structure may differentially mediate ultra-SWA (0.3 to 1Hz) compared to delta-SWA (1Hz to <4Hz). The longitudinal nature of the NCANDA study, together with its large sample size and multimodal imaging techniques, will allow us to continue to investigate how biological and external factors interact to drive changes in sleep architecture throughout adolescence.
Supplementary Material
Table 2.
For each significant mediation effect, the full model statistics are reported for the regression models used to calculate paths b1, b2 and c’, which included age, CT and GM volume as dependent variables and site and svol as confounding variables.
| Ultra-SWA | ||
|---|---|---|
| ROI | Hem | Full model statistics |
| FG | L | R2= 0.48, F(5,126)=23.56, p<0.001 |
| IPC | L | R2= 0.51, F(5,126)=26.51, p<0.001 |
| R | R2= 0.51, F(5,126)=26.54, p<0.001 | |
| LOC | L | R2= 0.49, F(5,126)=23.78, p<0.001 |
| R | R2= 0.50, F(5,126)=24.96, p<0.001 | |
| PCL | R | R2= 0.49, F(5,126)=23.79, p<0.001 |
| PC | R | R2= 0.54, F(5,126)=29.83, p<0.001 |
| SFG | R | R2= 0.49, F(5,126)=23.84, p<0.001 |
|
| ||
| Delta-SWA | ||
| ROI | Hem | Full model statistics |
|
| ||
| IPC | L | R2= 0.57, F(5,126)=33.70, p<0.001 |
| LOC | R | R2= 0.56, F(5,126)=31.48, p<0.001 |
| SFG | L | R2= 0.56, F(5,126)=32.10, p<0.001 |
| R | R2= 0.56, F(5,126)=31.50, p<0.001 | |
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank our lab manager, Stephanie Claudatos, and research assistants, David Dresser, David Sugarbaker, Justin Greco, Sarah Inkelis, Lena Kardos, Devika Nair, and Leonardo Rosas, for their effort in collecting data for this project, and all research participants.
Financial statement: This study was supported by the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA); grants: AA021690 (DBC), AA021697 (AP+KMP), AA021697-04S1 (KMP) and AA021696 (IMC+FCB).
Footnotes
Conflict of interest: The authors declare no conflicts of interest
References
- Achermann P, Borbely AA. Low-frequency (< 1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;81(1):213–222. doi: 10.1016/s0306-4522(97)00186-3. [DOI] [PubMed] [Google Scholar]
- Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroencephalogr Clin Neurophysiol. 1998;107(2):69–83. doi: 10.1016/s0013-4694(98)00051-0. [DOI] [PubMed] [Google Scholar]
- Baker FC, Turlington SR, Colrain I. Developmental changes in the sleep electroencephalogram of adolescent boys and girls. J Sleep Res. 2012;21(1):59–67. doi: 10.1111/j.1365-2869.2011.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker FC, Willoughby AR, de Zambotti M, Franzen PL, Prouty D, Javitz H, Hasler B, Clark DB, Colrain IM. Age-Related Differences in Sleep Architecture and Electroencephalogram in Adolescents in the National Consortium on Alcohol and Neurodevelopment in Adolescence Sample. Sleep. 2016;39(7):1429–1439. doi: 10.5665/sleep.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Boucquey V, Goldenberg D, Thayer RE, Ward M, Jacobus J, Tapert SF. Sex differences in adolescent white matter architecture. Brain Res. 2011;1375:41–48. doi: 10.1016/j.brainres.2010.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit O, Daurat A, Prado J. Slow (0.7–2 Hz) and fast (2–4 Hz) delta components are differently correlated to theta, alpha and beta frequency bands during NREM sleep. Clin Neurophysiol. 2000;111(12):2103–2106. doi: 10.1016/s1388-2457(00)00470-3. [DOI] [PubMed] [Google Scholar]
- Bersagliere A, Achermann P. Slow oscillations in human non-rapid eye movement sleep electroencephalogram: effects of increased sleep pressure. J Sleep Res. 2010;19(1 Pt 2):228–237. doi: 10.1111/j.1365-2869.2009.00775.x. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum Brain Mapp. 2010;31(6):926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4(1):78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Chen J, Rosso C, Forbes EE, Dinov ID, Worthman CM, Sowell ER. Sex matters during adolescence: testosterone-related cortical thickness maturation differs between boys and girls. PLoS One. 2012;7(3):e33850. doi: 10.1371/journal.pone.0033850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, Dinov ID, Worthman CM, Sowell ER. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex. 2011;21(3):636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Brumback T, Tomlinson K, Cummins K, Thompson WK, Nagel BJ, De Bellis MD, Hooper SR, Clark DB, Chung T, Hasler BP, Colrain IM, Baker FC, Prouty D, Pfefferbaum A, Sullivan EV, Pohl KM, Rohlfing T, Nichols BN, Chu W, Tapert SF. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): A Multisite Study of Adolescent Development and Substance Use. J Stud Alcohol Drugs. 2015;76(6):895–908. doi: 10.15288/jsad.2015.76.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann A, Kurth S, Ringli M, Geiger A, Jenni OG, Huber R. Anatomical markers of sleep slow wave activity derived from structural magnetic resonance images. J Sleep Res. 2011;20(4):506–513. doi: 10.1111/j.1365-2869.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- Buchmann A, Ringli M, Kurth S, Schaerer M, Geiger A, Jenni OG, Huber R. EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb Cortex. 2011;21(3):607–615. doi: 10.1093/cercor/bhq129. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55(2):149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Darchia N, Higgins LM, Dykan IV, Davis NM, de Bie E, Feinberg I. Adolescent Changes in Homeostatic Regulation of EEG Activity in the Delta and Theta Frequency Bands during NREM Sleep. Sleep. 2011;34(1):83–91. doi: 10.1093/sleep/34.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci U S A. 2009;106(13):5177–5180. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IG, Grimm KJ, de Bie E, Feinberg I. Sex, puberty, and the timing of sleep EEG measured adolescent brain maturation. Proc Natl Acad Sci U S A. 2012;109(15):5740–5743. doi: 10.1073/pnas.1120860109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The Adolescent Brain. Annals of the New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Annals of neurology. 1987;22(4):487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Baker FC. Sleep EEG, the clearest window through which to view adolescent brain development. Sleep. 2011;34(10):1287–1288. doi: 10.5665/sleep.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, Schabus M, Desseilles M, Albouy G, Boly M, Darsaud A, Gais S, Rauchs G, Sterpenich V, Vandewalle G, Carrier J, Moonen G, Balteau E, Degueldre C, Luxen A, Phillips C, Maquet P. Spontaneous neural activity during human slow wave sleep. Proc Natl Acad Sci U S A. 2008;105(39):15160–15165. doi: 10.1073/pnas.0801819105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zambotti M, Colrain IM, Baker FC. Interaction between reproductive hormones and physiological sleep in women. J Clin Endocrinol Metab. 2015;100(4):1426–1433. doi: 10.1210/jc.2014-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J Neurosci. 1999;19(11):4595–4608. doi: 10.1523/JNEUROSCI.19-11-04595.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube J, Lafortune M, Bedetti C, Bouchard M, Gagnon JF, Doyon J, Evans AC, Lina JM, Carrier J. Cortical thinning explains changes in sleep slow waves during adulthood. J Neurosci. 2015;35(20):7795–7807. doi: 10.1523/jneurosci.3956-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser SK, Hill SL, Tononi G. Sleep homeostasis and cortical synchronization: I. Modeling the effects of synaptic strength on sleep slow waves. Sleep. 2007;30(12):1617–1630. doi: 10.1093/sleep/30.12.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I, Campbell IG. Sleep EEG changes during adolescence: an index of a fundamental brain reorganization. Brain Cogn. 2010;72(1):56–65. doi: 10.1016/j.bandc.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Campbell IG. Longitudinal sleep EEG trajectories indicate complex patterns of adolescent brain maturation. Am J Physiol Regul Integr Comp Physiol. 2013;304(4):R296–303. doi: 10.1152/ajpregu.00422.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I, de Bie E, Davis NM, Campbell IG. Topographic differences in the adolescent maturation of the slow wave EEG during NREM sleep. Sleep. 2011;34(3):325–333. doi: 10.1093/sleep/34.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I, Hibi S, Carlson VR. Changes in EEG Amplitude During Sleep with Age. In: Nandy K, Sherwin I, editors. The Aging Brain and Senile Dementia. Boston, MA: Springer US; 1977. pp. 85–98. [Google Scholar]
- Feinberg I, Higgins LM, Khaw WY, Campbell IG. The adolescent decline of NREM delta, an indicator of brain maturation, is linked to age and sex but not to pubertal stage. Am J Physiol Regul Integr Comp Physiol. 2006;291(6):R1724–1729. doi: 10.1152/ajpregu.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I, Thode HC, Jr, Chugani HT, March JD. Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. J Theor Biol. 1990;142(2):149–161. doi: 10.1016/s0022-5193(05)80218-8. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Goyal MS, Preuss TM, Raichle ME, Van Essen DC. Trends and properties of human cerebral cortex: correlations with cortical myelin content. Neuroimage. 2014;(93 Pt 2):165–175. doi: 10.1016/j.neuroimage.2013.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Thompson PM. Mapping Gray Matter Development: Implications for typical development and vulnerability to psychopathology. Brain Cogn. 2010;72(1):6. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child Dev. 1987:601–622. [PubMed] [Google Scholar]
- Hagenauer MH, Lee TM. The neuroendocrine control of the circadian system: adolescent chronotype. Front Neuroendocrinol. 2012;33(3):211–229. doi: 10.1016/j.yfrne.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH, Lee TM. Adolescent sleep patterns in humans and laboratory animals. Horm Behav. 2013;64(2):270–279. doi: 10.1016/j.yhbeh.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammelrath L, Skokic S, Khmelinskii A, Hess A, van der Knaap N, Staring M, Lelieveldt BP, Wiedermann D, Hoehn M. Morphological maturation of the mouse brain: An in vivo MRI and histology investigation. Neuroimage. 2016;125:144–152. doi: 10.1016/j.neuroimage.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Hanlon EC, Vyazovskiy VV, Faraguna U, Tononi G, Cirelli C. Synaptic potentiation and sleep need: clues from molecular and electrophysiological studies. Curr Top Med Chem. 2011;11(19):2472–2482. doi: 10.2174/156802611797470312. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press; 2013. [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Dahl RE, Sowell ER. A longitudinal study: changes in cortical thickness and surface area during pubertal maturation. PLoS One. 2015;10(3):e0119774. doi: 10.1371/journal.pone.0119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Kan E, Dahl RE, Sowell ER. The role of testosterone and estradiol in brain volume changes across adolescence: a longitudinal structural MRI study. Hum Brain Mapp. 2014;35(11):5633–5645. doi: 10.1002/hbm.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Maxwell EC, Irvine C, Nagel BJ. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb Cortex. 2012;22(9):1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27(4):774–783. [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114(Pt 5):2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Judd CM, Kenny DA. Process analysis: Estimating mediation in treatment evaluations. Evaluation review. 1981;5(5):602–619. [Google Scholar]
- Judd CM, Kenny DA, McClelland GH. Estimating and testing mediation and moderation in within-subject designs. Psychol Methods. 2001;6(2):115–134. doi: 10.1037/1082-989x.6.2.115. [DOI] [PubMed] [Google Scholar]
- Kassem MS, Lagopoulos J, Stait-Gardner T, Price WS, Chohan TW, Arnold JC, Hatton SN, Bennett MR. Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Mol Neurobiol. 2013;47(2):645–661. doi: 10.1007/s12035-012-8365-7. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Sokoloff L. An adaptation of the nitrous oxide method to the study of the cerebral circulation in children; normal values for cerebral blood flow and cerebral metabolic rate in childhood. J Clin Invest. 1957;36(7):1130–1137. doi: 10.1172/jci103509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Anderson S, Pettergrew JW. Is Schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. Journal of Psychiatric Research. 1994;28(3):239–265. doi: 10.1016/0022-3956(94)90009-4. doi: http://dx.doi.org/10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Knyazev GG. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci Biobehav Rev. 2012;36(1):677–695. doi: 10.1016/j.neubiorev.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Le Bon O, Linkowski P. Absence of systematic relationships between REMS duration episodes and spectral power Delta and Ultra-Slow bands in contiguous NREMS episodes in healthy humans. J Neurophysiol. 2013;110(1):162–169. doi: 10.1152/jn.00020.2013. [DOI] [PubMed] [Google Scholar]
- Le Bon O, Neu D, Berquin Y, Lanquart JP, Hoffmann R, Mairesse O, Armitage R. Ultra-slow delta power in chronic fatigue syndrome. Psychiatry Res. 2012;200(2–3):742–747. doi: 10.1016/j.psychres.2012.06.027. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain Cogn. 2010;72(1):46. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes da Silva FH, Rotterdam Av. Biophysical aspects of EEG and magnetoencephalogram generation 1998 [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengler L, Khmelinskii A, Diedenhofen M, Po C, Staring M, Lelieveldt BP, Hoehn M. Brain maturation of the adolescent rat cortex and striatum: changes in volume and myelination. Neuroimage. 2014;84:35–44. doi: 10.1016/j.neuroimage.2013.08.034. [DOI] [PubMed] [Google Scholar]
- Mills KL, Goddings A-L, Herting MM, Meuwese R, Blakemore S-J, Crone EA, Dahl RE, Güroğlu B, Raznahan A, Sowell ER, Tamnes CK. Structural brain development between childhood and adulthood: Convergence across four longitudinal samples. Neuroimage. 2016;141:273–281. doi: 10.1016/j.neuroimage.2016.07.044. doi: http://dx.doi.org/10.1016/j.neuroimage.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni F, Nobili L, Iaria G, Sartori I, Marzano C, Tempesta D, Proserpio P, Lo Russo G, Gozzo F, Cipolli C, De Gennaro L, Ferrara M. Hippocampal slow EEG frequencies during NREM sleep are involved in spatial memory consolidation in humans. Hippocampus. 2014;24(10):1157–1168. doi: 10.1002/hipo.22299. [DOI] [PubMed] [Google Scholar]
- Murphy M, Riedner BA, Huber R, Massimini M, Ferrarelli F, Tononi G. Source modeling sleep slow waves. Proc Natl Acad Sci U S A. 2009;106(5):1608–1613. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols BN, Pohl KM. Neuroinformatics Software Applications Supporting Electronic Data Capture, Management, and Sharing for the Neuroimaging Community. Neuropsychol Rev. 2015;25(3):356–368. doi: 10.1007/s11065-015-9293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Perrault R, Carrier J, Desautels A, Montplaisir J, Zadra A. Electroencephalographic slow waves prior to sleepwalking episodes. Sleep Med. 2014;15(12):1468–1472. doi: 10.1016/j.sleep.2014.07.020. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc. 1988;17(2):117–133. doi: 10.1007/bf01537962. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Pohl KM, Lane B, Chu W, Kwon D, Nolan Nichols B, Brown SA, Tapert SF, Cummins K, Thompson WK, Brumback T, Meloy MJ, Jernigan TL, Dale A, Colrain IM, Baker FC, Prouty D, De Bellis MD, Voyvodic JT, Clark DB, Luna B, Chung T, Nagel BJ, Sullivan EV. Adolescent Development of Cortical and White Matter Structure in the NCANDA Sample: Role of Sex, Ethnicity, Puberty, and Alcohol Drinking. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinyerd B, Zipf WB. Puberty-timing is everything! J Pediatr Nurs. 2005;20(2):75–82. doi: 10.1016/j.pedn.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Pohl KM, Sullivan EV, Rohlfing T, Chu W, Kwon D, Nichols BN, Zhang Y, Brown SA, Tapert SF, Cummins K, Thompson WK, Brumback T, Colrain IM, Baker FC, Prouty D, De Bellis MD, Voyvodic JT, Clark DB, Schirda C, Nagel BJ, Pfefferbaum A. Harmonizing DTI measurements across scanners to examine the development of white matter microstructure in 803 adolescents of the NCANDA study. Neuroimage. 2016;130:194–213. doi: 10.1016/j.neuroimage.2016.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, Hayes AF. Addressing moderated mediation hypotheses: Theory, methods, and prescriptions. Multivariate behavioral research. 2007;42(1):185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- Ringli M, Kurth S, Huber R, Jenni OG. The sleep EEG topography in children and adolescents shows sex differences in language areas. Int J Psychophysiol. 2013;89(2):241–245. doi: 10.1016/j.ijpsycho.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Saletin JM, van der Helm E, Walker MP. Structural brain correlates of human sleep oscillations. Neuroimage. 2013;83:658–668. doi: 10.1016/j.neuroimage.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer K, Tozer DJ, Scaravilli F, Altmann DR, Barker GJ, Tofts PS, Miller DH. Quantitative magnetization transfer imaging in postmortem multiple sclerosis brain. J Magn Reson Imaging. 2007;26(1):41–51. doi: 10.1002/jmri.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuz A, Palm G. Density of neurons and synapses in the cerebral cortex of the mouse. J Comp Neurol. 1989;286(4):442–455. doi: 10.1002/cne.902860404. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26(4):518–529. doi: 10.1109/tmi.2006.887364. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28(14):3586–3594. doi: 10.1523/jneurosci.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: correspondence between hormonal and physical development. Child Dev. 2009;80(2):327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7(10):1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–8231. doi: 10.1523/jneurosci.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10(4):372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Curro Dossi R, Nunez A. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993;13(8):3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCarley R. Brain control of wakefulness and sleeping. Springer; New York: 2005. [Google Scholar]
- Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993a;13(8):3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993b;13(8):3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85(5):1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Brumback T, Tapert SF, Fama R, Prouty D, Brown SA, Cummins K, Thompson WK, Colrain IM, Baker FC, De Bellis MD, Hooper SR, Clark DB, Chung T, Nagel BJ, Nichols BN, Rohlfing T, Chu W, Pohl KM, Pfefferbaum A. Cognitive, emotion control, and motor performance of adolescents in the NCANDA study: Contributions from alcohol consumption, age, sex, ethnicity, and family history of addiction. Neuropsychology. 2016;30(4):449–473. doi: 10.1037/neu0000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, Rohlfing T, Baker FC, Padilla ML, Colrain IM. Developmental change in regional brain structure over 7 months in early adolescence: comparison of approaches for longitudinal atlas-based parcellation. Neuroimage. 2011;57(1):214–224. doi: 10.1016/j.neuroimage.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: A User-Friendly Application for MEG/EEG Analysis. Computational Intelligence and Neuroscience. 2011;2011:13. doi: 10.1155/2011/879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L, Carskadon MA. Developmental changes in the human sleep EEG during early adolescence. Sleep. 2010;33(6):801–809. doi: 10.1093/sleep/33.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L, Saletin JM, Carskadon MA. Sleep in adolescence: Physiology, cognition and mental health. Neurosci Biobehav Rev. 2016 doi: 10.1016/j.neubiorev.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstyl K, Ronan L, Whitaker KJ, Goodyer IM, Roberts N, Crow TJ, Fletcher PC. Multiple markers of cortical morphology reveal evidence of supragranular thinning in schizophrenia. Transl Psychiatry. 2016;6:e780. doi: 10.1038/tp.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker KJ, Vertes PE, Romero-Garcia R, Vasa F, Moutoussis M, Prabhu G, Weiskopf N, Callaghan MF, Wagstyl K, Rittman T, Tait R, Ooi C, Suckling J, Inkster B, Fonagy P, Dolan RJ, Jones PB, Goodyer IM, Bullmore ET. Adolescence is associated with genomically patterned consolidation of the hubs of the human brain connectome. Proc Natl Acad Sci U S A. 2016;113(32):9105–9110. doi: 10.1073/pnas.1601745113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby AR, de Zambotti M, Baker FC, Colrain IM. Partial K-Complex Recovery Following Short-Term Abstinence in Individuals with Alcohol Use Disorder. Alcohol Clin Exp Res. 2015;39(8):1417–1424. doi: 10.1111/acer.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.