Abstract
Sepsis is a major clinical problem associated with significant organ dysfunction and high mortality. The ATP‐sensitive P2X7 receptor activates the NLRP3 inflammasome and is a key component of the innate immune system. We used a fluid‐resuscitated rat model of fecal peritonitis and acute kidney injury (AKI) to investigate the contribution of this purinergic receptor to renal dysfunction in sepsis. Six and 24 h time‐points were chosen to represent early and established sepsis, respectively. A selective P2X7 receptor antagonist (A‐438079) dissolved in dimethyl sulfoxide (DMSO) was infused 2 h following induction of sepsis. Compared with sham‐operated animals, septic animals had significant increases in heart rate (−1(−4 to 8)% vs. 21(12–26)%; P = 0.003), fever (37.4(37.2–37.6)°C vs. 38.6(38.2–39.0)°C; P = 0.0009), and falls in serum albumin (29(27–30)g/L vs. 26(24–28); P = 0.0242). Serum IL‐1β (0(0–10)(pg/mL) vs. 1671(1445–33778)(pg/mL); P < 0.001) and renal IL‐1β (86(50–102)pg/mg protein vs. 200 (147–248)pg/mg protein; P = 0.0031) were significantly elevated in septic compared with sham‐operated animals at 6 h. Serum creatinine was elevated in septic animals compared with sham‐operated animals at 24 h (23(22–25) μmol/L vs. 28 (25–30)μmol/L; P = 0.0321). Renal IL‐1β levels were significantly lower in A‐438079‐treated animals compared with untreated animals at 6 h (70(55–128)pg/mg protein vs. 200(147–248)pg/mg protein; P = 0.021). At 24 h, compared with untreated animals, A‐438079‐treated animals had more rapid resolution of tachycardia (22(13–36)% vs. −1(−6 to 7)%; P = 0.019) and fever (39.0(38.6–39.1)°C vs. 38.2(37.6–38.7)°C; P < 0.024), higher serum albumin (23(21–25)g/L vs. (27(25–28)g/L); P = 0.006), lower arterial lactate (3.2(2.5–4.3)mmol/L vs. 1.4(0.9–1.8)mmol/L; P = 0.037), and lower serum creatinine concentrations (28(25–30)μmol/L vs. 22(17–27)μmol/L; P = 0.019). P2X7A treatment ameliorates the systemic inflammatory response and renal dysfunction in this clinically relevant model of sepsis‐related AKI.
Keywords: Acute kidney injury, inflammasome, sepsis
Introduction
Sepsis is a common and serious global health issue, accounting for 20% of all admissions to intensive care units (ICU), and is the leading cause of death in noncardiac ICUs (Angus et al. 2001). Within the intensive care unit (ICU) up to 5% of critically ill patients require renal replacement therapy (RRT) (Bagshaw et al. 2005; Uchino et al. 2005), and sepsis is implicated in half the cases of AKI (Uchino et al. 2005). The relative risk of mortality associated with AKI requiring RRT is over sixfold compared with patients without AKI (Ricci et al. 2008). Despite this, there has been a stark failure to develop any effective therapeutic agent for sepsis or sepsis‐induced AKI.
The hallmark of severe sepsis includes inflammation, a dysregulated host response, and organ dysfunction. The NLRP3 inflammasome is integral to the innate immune system and is characterized best in immune cells, including monocytes and macrophages. While the NLRP3 inflammasome is crucial for host immunity, it is unclear if excessive activation of the inflammasome in sepsis may have detrimental effects on organ function and survival. NLRP3 inhibition or genetic deletion in sepsis models has been shown to be protective in preclinical models of sepsis (Li et al. 1995) (Mariathasan et al. 2006).
The functional significance of de novo organ‐specific inflammasome expression in systemic inflammatory diseases such as sepsis is unclear. Intrinsic renal cells demonstrate components of the inflammasome in various proinflammatory diseases, while pharmacological inhibition and/or genetic deletion have shown protective effects in many preclinical models of acute kidney injury (AKI) (Turner et al. 2014). The notion that NLRP3 inhibition may be beneficial in sepsis‐induced AKI was demonstrated by caspase‐1 deficiency in a lipopolysaccharide (LPS) model of endotoxaemia (Wang et al. 2005). Similarly, P2X7 knockout (KO) mice subjected to cecal ligation and puncture have an attenuated inflammatory response and reduced lung injury compared with wild‐type mice (Santana et al. 2015).
In a murine model of unilateral ureteric obstruction (UUO), P2X7 was upregulated in cortical tubular epithelial cells, and genetic deletion protected against macrophage infiltration, collagen deposition and apoptosis (Goncalves et al. 2006). This contrasts with the predominant glomerular P2X7 expression seen in a glomerulonephritis model, which was also protected by genetic deletion or by P2X7 receptor antagonism (Taylor et al. 2009). The close association between the site of renal injury and the presence of P2X7, along with the protective effect of a P2X7 antagonist or genetic deletion, strongly supports a role for P2X7 in AKI pathogenesis. A similar relationship may hold for sepsis‐induced AKI.
We hypothesized that upregulation of the ATP‐sensitive P2X7 receptor contributes to renal injury in sepsis through an inflammatory response with local production of cytokines. We aimed to define the expression of this receptor within the kidney, and its relationship with cytokine production, and renal function; we sought to assess effects of P2X7 antagonism in a rat model of sepsis‐induced AKI. The primary objective of this study was to evaluate the effect of a selective P2X7 antagonist (A‐438079) in preventing renal dysfunction (as defined by an elevated serum creatinine) in an experimental model of sepsis.
Methods
Ethics
All animal experiments were performed under a Home Office Project License (PPL 70/7029) and local UCL Ethics Committee approval.
Monocyte isolation and culture
We conducted ex vivo experiments to assess the biological activity of DMSO and the specific P2X7 receptor antagonist (A‐438079) dissolved in DMSO. Details on monocyte isolation from male Wistar rats are detailed in the supplementary data. Monocytes require priming with LPS (signal 1) prior to release of IL‐1β by co‐stimulation with ATP (signal 2). The duration of LPS‐priming is variable, although 4 h has been shown to be adequate (Qu et al. 2007). Stimulation by ATP is only required for 30 min after LPS priming (Mehta et al. 2001).
Previously published data demonstrates a 3 μmol/L concentration of A‐438079 blocks 75% of IL‐1β release from cultured peritoneal macrophages stimulated with LPS and BzATP (3 μg/mL LPS priming for 2 h followed by 30 min stimulation with 0.3–3.0 μmol/L BzATP) (McGaraughty et al. 2007). A quantity of 10 μmol/L of A‐438079 was selected for this experiment to ascertain any effect of the drug over and above that of DMSO. Experiments were repeated with at least 5 replicates per condition.
P2X7 receptor antagonist pharmacokinetics
The P2X7 antagonist (A‐438079) was kindly donated by Abbvie (Abbott Park, IL). Based on previously published data and personal communication with Abbvie, the pharmacokinetic profile of A‐438079 was determined. A‐438079 blocked BzATP (10 μmol/L)–mediated changes in intracellular Ca++ concentrations at rat P2X7 receptors but not at other P2 receptors (McGaraughty et al. 2007).
Unpublished data from Abbvie Pharmaceuticals demonstrates that the half‐life of intravenous A‐438079 is 0.69 h (41 min). An intravenous dose of 10 μg/kg can achieve a peak serum concentration of 4000 ng/mL. We aimed for a peak concentration of at least 3 μmol/L which would block 75% of IL‐1β release from macrophages in vitro. An intravenous bolus of 10 μg/kg (giving a peak concentration of 4000 ng/mL) would achieve therapeutic levels in serum. We therefore administered a dose of 10 μg/kg. The antagonist was given as a single bolus to achieve a peak (therapeutic) concentration, followed by 4× the dose (i.e.,: 40 μg/kg) for the first half‐life, followed by 2× the dose for the second half life, followed by 10 μg/(kg·h) thereafter.
Dimethylsulfoxide
On dissolving of A‐438079 in water for a final concentration of 20 μmol/L, the pH at room temperature fell to 2.2, and at 38°C was 1.6. Dimethylsulfoxide (DMSO) was therefore selected as a drug solvent. Due to its amphipathic properties, DMSO is an effective solvent for water‐insoluble compounds and is a hydrogen‐bound disrupter. As such it is a commonly used solvent for many drugs in pharmacological studies. This is a commonly used drug solvent, however, itself has biological activity (see Discussion). The strength of DMSO used varied from 3 to 15% depending on the concentration of P2X7 required.
Animal model of sepsis
Male Wistar rats (Charles River, Margate, United Kingdom) weighing 300–375 g were used throughout. All experiments were performed in accordance with relevant guidelines and regulations and are detailed in the supplementary data and our characterization manuscript (Arulkumaran et al. 2017). The 72 h characterization study informed time points for the antagonist study (Arulkumaran et al. 2017). Serum creatinine was significantly elevated at 24 h in septic animals compared with sham animals. Therefore, 24 h was used as a predetermined time point.
In vivo activity was defined as the ability of A‐438079 to reduce renal and systemic IL‐1β release in the rat model of sepsis. Serum IL‐1β was elevated by 3 h and remained elevated for 48 h, whereas renal IL‐1β was elevated at 6 h (Arulkumaran et al. 2017). Six hours was selected as a time point to assess biological activity of A‐438079. As urinary IL‐18 was only elevated after a rise in serum creatinine, this was not used as an endpoint, since the aim was to prevent the onset of AKI in sepsis.
Drug treatment was commenced 2 h after the induction of sepsis. It was necessary to dissolve the P2X7 antagonist in DMSO to achieve sufficient concentration for in vivo injection. Since DMSO itself has anti‐inflammatory effects, we included DMSO injected rats as controls.
Experimental groups (n = 6–12 per group) initially comprised sham‐operated and untreated septic animals at both 6 h and 24 h time points, and DMSO ± A‐438079 treatment at 6 h and 24 h. On review of the results, we undertook further studies where twice the dose of A‐438079 was administered.
Immunohistochemistry
Histological sections from the in vivo experiments were analyzed for P2X7 expression and evidence of acute tubular injury. Details of methods for immunohistological analyses are included in the supplementary data. For each section, ten random fields of view were analyzed and mean fluorescence intensity calculated. One section from each sample was incubated with antibody diluent instead of primary antibody and served as a control.
ELISA
DuoSet ELISA kits (R&D Systems, Minneapolis, MN) were used according to the manufacturer's instructions. The antibody supplied in the IL‐1β kit does not cross‐react with pro–IL‐1β. ELISA was performed to assess the presence of cytokines within serum and renal tissue homogenate. Tissue cytokine levels were expressed relative to the total protein content. Absorbance was read at 450 nm using a spectrophotometric ELISA plate reader (Anthos HTII; Anthos Labtec, Salzburg, Austria).
Western blot
Protein was extracted and estimated from whole kidney tissue. Four animals were randomly selected from each of the sham and sepsis groups, for analysis. Kidney homogenate were assessed for expression of Caspase‐1, and IL‐1β in sham and septic animals at 24 h. Details of methods for Western blot are included in the supplementary data.
Statistics
The number of antagonist‐treated animals and placebo‐treated controls was calculated based on previous laboratory experience. We regarded a 20% change in a tested variable as an important biological effect. The study was designed to have a power of 90% and a significance level of 5%. At least 6 replicate animals per group per time point.
Graphpad Prism (GraphPad Software, Version 5.0d) was used for statistical analyses and graphs. All data were regarded as nonparametric due to small sample sizes (6–12 per group). Continuous variables are presented as median (interquartile range). Data were analyzed in prospectively defined groups. All 6 h experiments were analyzed as a predefined group, as were all 24 h experiments. For comparison of continuous variables between more than two groups, Kruskal–Wallis test with post hoc Dunn's test is used. Mann–Whitney U test was used for comparison of continuous variables between 2 groups. A P < 0.05 was taken as statistically significant.
Results
Cell culture
LPS and adenosine triphosphate (ATP) stimulation of cultured monocytes resulted in significant IL‐1β production (P = 0.029). Addition of 2% DMSO significantly inhibited the release of IL‐1β (P = 0.025). Addition of 10 μmol/L A‐438079 had no added effect on the reduction in IL‐1β release. 5 μmol/L brilliant blue G (BBG) had a significant inhibitory effect on monocyte IL‐1β release (P = 0.029) (Fig. 1).
Figure 1.
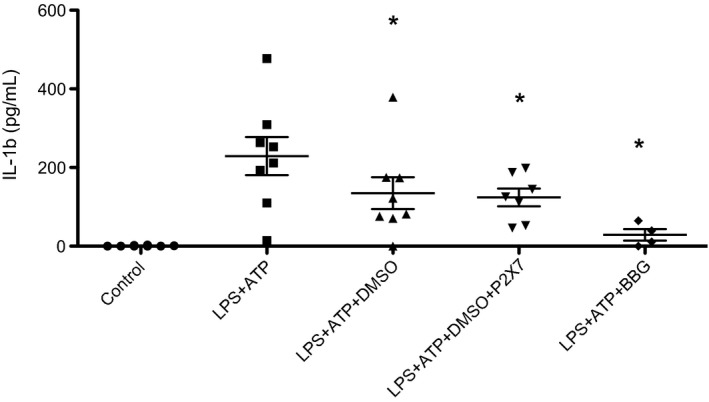
Effect of DMSO and P2X7 antagonist on monocyte IL‐1β release. LPS priming followed by ATP stimulation resulted in significant IL‐1β release from PBMCs. IL‐1β release was inhibited by the addition of 2% DMSO prior to ATP exposure. Addition of 10 μmol/L P2X7 antagonist had no added effect on the reduction in IL‐1β release. 5 μmol/L BBG had a significant inhibitory effect on monocyte IL‐1β release. Experiments were conducted twice. Data plotted represents mean and standard deviation. (P < 0.05 compared with LPS + ATP).
Characterization
Clinical, biochemical, and cytokine changes in the septic model are detailed in our characterization manuscript (Arulkumaran et al. 2017). Changes consistent with systemic inflammation were evident at 6 h following induction of sepsis (Table 1, Fig. 2). Compared with sham‐operated animals, septic animals had significant increases in heart rate from baseline (−1(−4 to 8)% vs. 21(12–26)%; P = 0.003), fever (37.4(37.2–37.6)°C vs. 38.6(38.2–39.0)°C; P = 0.0009), and falls in serum albumin (29(27–30)g/L vs. 26(24–28); P = 0.0242). Serum IL‐1β (0(0–10)(pg/mL) vs. 1671(1445–33778)(pg/mL); P < 0.001) and renal IL‐1β (86(50–102)pg/mg protein vs. 200 (147–248)pg/mg protein; P = 0.0031) were significantly elevated in septic compared with sham‐operated animals at 6 h. Serum creatinine, however, remained unchanged at 6 h (25(23–27)μmol/L vs. 23(21–28) μmol/L; P = 0.9155) in sham‐operated compared with septic animals.
Table 1.
Physiological and biochemical variables of sham‐operated, untreated septic, and treated septic animals at 6 h and 24 h
| Groups | T‐Test | ANOVA | Post analysis Dunn's T3 test | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sham‐operated | Untreated Sepsis | DMSO‐ treated | DMSO/A‐438079‐ treated | Sham vs. Untreated | Untreated vs. Treated | Sepsis vs. DMSO | Sepsis vs. DMSO/A‐438079 | DMSO vs. DMSO/A438079 | |
| 6 h | |||||||||
| Change in heart rate (%) | −5 (−7−1) | 21 (12–26) | 15 (7–20) | 15 (14–17) | 0.0003 | 0.139 | 0.284 | 0.153 | 0.955 |
| Temperature (°C) | 37.4 (37.2–37.6) | 38.6 (38.2–39.0) | 38.4 (38.3–39.1) | 38.7 (38.6–38.8) | 0.0009 | 0.688 | 0.993 | 0.649 | 0.899 |
| Albumin (g/L) | 29 (27–30) | 26 (24–28) | 25 (23–17) | 26 (25–29) | 0.0242 | 0.62 | 0.790 | 0.961 | 0.589 |
| Lactate (mmol/L) | 1.1 (1.0–1.3) | 1.2 (1.0–1.3) | 1.0 (0.9–1.0) | 1.2 (1.1–1.3) | 0.4984 | 0.051 | 0.033 | 0.999 | 0.084 |
| Creatinine (μmol/L) | 25 (23–27) | 23 (21–28) | 19 (15–25) | 25 (24–27) | 0.9155 | 0.547 | 0.401 | 0.997 | 0.034 |
| Renal IL‐1β(pg/mg protein) | 86 (50–102) | 200 (147–248) | 126 (98–220) | 70 (55–128) | 0.0031 | 0.077 | 0.372 | 0.021 | 0.372 |
| Serum IL‐1β(pg/mL) | 0 (0–10) | 1671 (1445–3378) | 1924 (1806–2325) | 1989 (1214–2362) | <0.0001 | 0.749 | 0.807 | 1.00 | 0.840 |
| 24 h | |||||||||
| Change in heart rate (%) | −1 (−4–8) | 22 (13–36) | 5 (−2–16) | −1 (−6–7) | 0.0015 | <0.001 | 0.047 | 0.019 | 0.769 |
| Temperature (°C) | 37.5 (37.4–37.8) | 39.0 (38.6–39.1) | 38.3 (37.8–38.5) | 38.2 (37.6–38.7) | 0.0002 | 0.011 | 0.031 | 0.024 | 0.996 |
| Albumin (g/L) | 30 (29–31) | 23 (21–25) | 24 (22–25) | 27 (25–28) | 0.0003 | 0.002 | 0.785 | 0.006 | 0.016 |
| Lactate (mmol/L) | 0.9 (0.2–1.2) | 3.2 (2.5–4.3) | 2.2 (2.1–2.2) | 1.4 (0.9–1.8) | 0.0004 | <0.001 | 0.037 | 0.001 | 0.001 |
| Creatinine (μmol/L) | 23 (22–25) | 28 (25–30) | 23 (20–26) | 22 (17–27) | 0.0321 | 0.005 | 0.047 | 0.019 | 0.769 |
| Renal IL‐1β(pg/mg protein) | 168 (154–176) | 186 (149–280) | 219 (193–260) | 224 (147–348) | 0.953 | 0.980 | 0.999 | 1.000 | 0.996 |
| Serum IL‐1β(pg/mL) | 0 (0–10) | 1463 (927–2541) | 1809 (964–2678) | 768 (702–1690) | <0.0001 | 0.141 | 1.000 | 0.261 | 0.207 |
Figure 2.
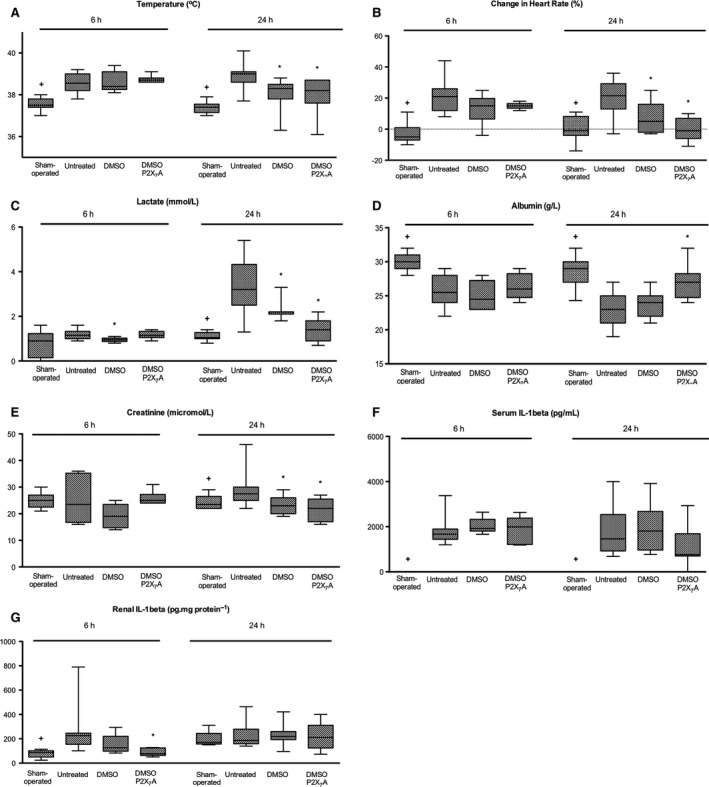
Systemic variables following induction of experimental sepsis in Wistar rats (that have received either no drug, DMSO, or A‐438079 (P2X7 antagonist) in DMSO) at 6 h and 24 h. Box plots represent median and interquartile range and whiskers represent minimum and maximum, respectively. Experiments include at least 6 animals per group for 6 h time point and 10–12 animals per group for 24 h time point. Septic animals have a significantly elevated body temperature and tachycardia, at 6 h and 24 h compared with sham‐operated animals. Serum albumin falls by 24 h and remains low at 24 h, whereas lactate is significantly elevated at 24 h in septic animals compared with sham‐operated animals. A‐438079 treatment reduced fever at 6 h, and improved resolution of tachycardia, serum albumin, and lactate at 24 h. Septic animals have a significantly elevated serum IL‐1β compared with sham animals at 6 h and 24 h. Renal IL‐1β Is significantly elevated by 6 h, followed by a rise in serum creatinine at 24 h. Treatment with A‐438079 after the onset of sepsis abrogated the rise in serum creatinine at 24 h with reduced renal IL‐1β expression at 6 h. (+P < 0.05 for sham vs. untreated septic animals. *P < 0.05 for treated vs. untreated septic animals).
At 24 h, septic animals had persistent changes of systemic inflammation (Table 1, Fig. 2). Compared with sham‐operated animals, septic animals had significant increases in heart rate from baseline (−5(−7 to 1)% vs. 22(13–36)%; P = 0.0015), fever (37.5(37.4–37.8)°C vs. 39.0(38.6–39.1)°C; P = 0.0002), and fall in serum albumin (30(29–31)g/L vs. 23(21–25)g/L; P = 0.0003). Serum IL‐1β (0(0–10)(pg/mL) vs. 1463(927–2541)(pg/mL); P < 0.001) remains significantly elevated in septic compared with sham‐operated animals at 24 h although renal IL‐1 β levels were similar (168(154–176)pg/mg protein vs. 186(149–280)pg/mg protein; P = 0.053). Serum creatinine was significantly elevated in septic animals compared with sham‐operated animals at 24 h (23(22–25)μmol/L vs. 28 (25–30)μmol/L; P = 0.0321).
There was histological evidence of focal tubular injury as evidenced by flattening of tubular epithelia and tubular cell vacuolation in septic animals at 24 h (Fig. 3). Changes were minimal and sporadic. Naïve and sham‐operated animals showed minimal staining for P2X7 on immunohistochemistry: there was minimal constitutive P2X7 expression limited to distal tubular epithelial cells (Fig. 3). Increased renal P2X7 expression was detected in both proximal tubules and distal tubules, and was localized to the cytoplasm in septic compared with sham‐operated animals. The increased renal P2X7 expression reached statistical significance by 24 h (0.015 ± 0.002 vs. 0.007 ± 0.003; P = 0.003) (Fig. 3).
Figure 3.
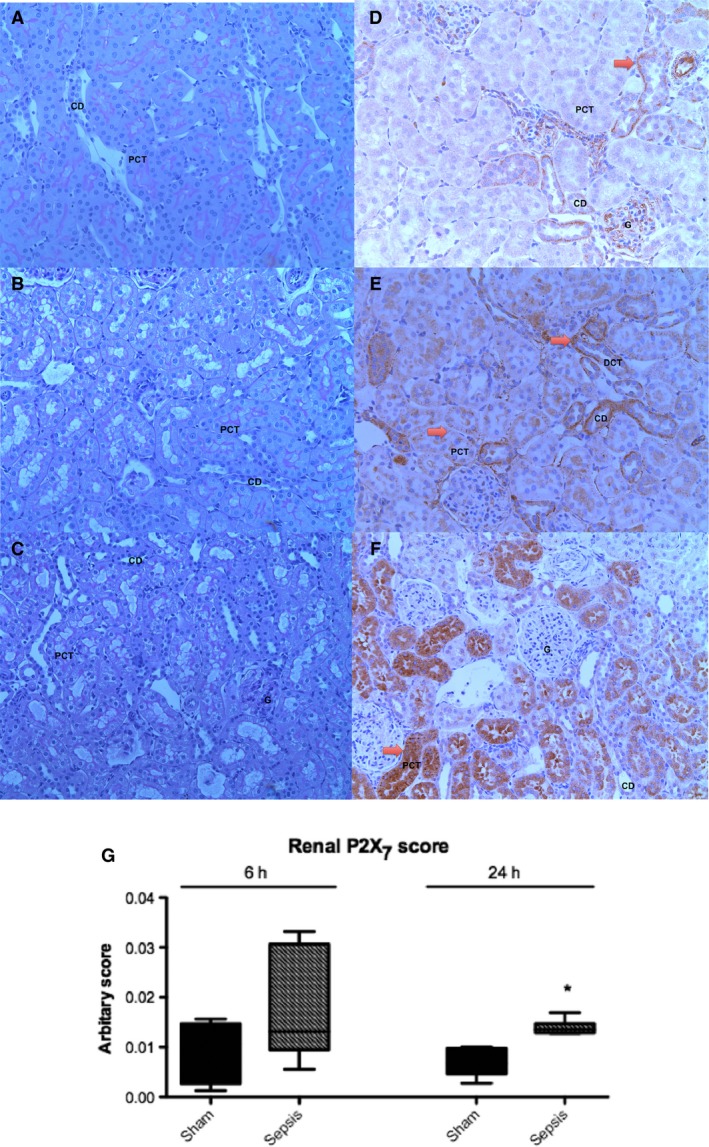
Histological analysis of kidney sections from naïve, 6 h septic, and 24 h septic animals. All images taken at x20 magnification. (A) Naive kidney with no tubular injury (B) Kidney demonstrating minimal tubular injury 6 h following induction of sepsis (C) Kidney demonstrating minimal tubular injury 24 h following induction of sepsis (D) Naive kidney with minimal tubular P2X7, (E) Kidney from a 6 h septic animal with minimal P2X7, mainly within the basolateral regions of tubules (F) Kidney from a 24 h septic animal with a similar pattern as the 6 h septic animals but greater intensity and wider distribution (G) Immunohistochemistry image analysis units for P2X7 expression in sham and septic animals (n = 6–8 per group). There is significantly increased renal tubular P2X7 expression at 24 h in septic animals compared with sham animals. Box plots represent median and interquartile range and whiskers represent minimum and maximum. (*P < 0.05 sham vs. septic animals). G, glomerulus; PCT, proximal convoluted tubule; DCT, distal convoluted tubule; CD, collecting duct; Arrow, P2X7 staining.
Western blot of whole kidney homogenate at the 24 h time point revealed increased renal Caspase‐1 (relative expression 1.77(1.67–2.27) vs. 1.35(1.25–1.37); P = 0.029) protein expression in septic animals compared with sham‐operated animals (Fig. 4).
Figure 4.
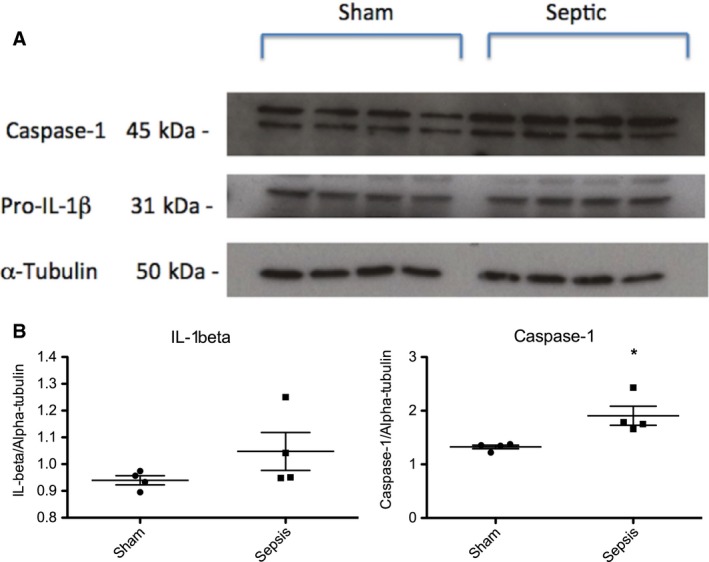
Western blot of whole kidney homogenate from sham‐operated or septic rats at 24 h. (A) Representative western blot showing the expression of renal Caspase‐1, and IL‐1β protein at 24 h in sham‐operated and septic animals. (B) Quantification band intensity from WB by densitometry. Septic animals have increased renal Caspase‐1 expression compared with sham operated animals (*P < 0.05 compared with sham).
Effect of A‐438079 (in DMSO vehicle)
Initial in vivo experiments targeting a peak concentration of 3 μmol/L did not result in any clinical or biochemical differences between untreated and A‐438079‐treated septic animals (data not shown). We therefore undertook further studies where twice the dose of A‐438079 was administered.
At 6 h there was no difference among the groups in core temperature, change in heart rate, serum creatinine, or serum IL‐1β and between treated and untreated animals (Table 1, Fig. 2). Renal IL‐1β levels were significantly lower in A‐438079‐treated animals compared with untreated animals at 6 h (70(55–128)pg/mg protein vs. 200(147–248)pg/mg protein; P = 0.021). Arterial lactate concentration was different between DMSO‐treated and untreated animals (1.0 (0.9–1.0)mmol/L vs. 1.2(1.0–1.3)mmol/L; P = 0.033), although remained within the normal clinical range in all groups of animals.
At 24 h, there were a number of significant differences between A‐438079 treated animals and untreated septic animals (Table 1, Fig. 2). Compared with untreated animals, A‐438079‐treated animals had more rapid resolution of tachycardia (22(13–36)% vs. −1(−6 to 7)%; P = 0.019) and fever (39.0(38.6–39.1)°C vs. 38.2(37.6–38.7)°C; P < 0.024), higher serum albumin (23(21–25)g/L vs. (27(25–28)g/L); P = 0.006), lower arterial lactate (3.2(2.5–4.3)mmol/L vs. 1.4(0.9–1.8)mmol/L; P = 0.037), and lower serum creatinine concentrations (28(25–30)μmol/L vs. 22(17–27)μmol/L; P = 0.019).
At 24 h, compared with DMSO‐treated animals, A‐438079‐treated animals had higher serum albumin (24(22–25)g/L vs. 27(25–28)g/L; P = 0.016), and lower arterial lactate (2.2 (2.1–2.2)mmol/L vs. 1.4(0.9–1.8)mmol/L; P = 0.001). Serum IL‐1β and renal IL‐1β were similar in A‐438079‐treated, DMSO‐treated, and untreated animals at 24 h.
Discussion
Our fluid‐resuscitated rat model of sepsis recapitulates many of the hemodynamic, biochemical, and inflammatory features seen in clinical sepsis. Various components of the NLRP3 inflammasome, including P2X7 and IL‐1β, are expressed de novo within the renal tubular epithelial cells in this septic model. This is consistent with published data showing that LPS‐primed Madin–Darby canine kidney (MDCK) renal tubular epithelial cells express Toll‐like receptor 4, NLRP3, caspase‐1, and IL‐1β mRNA (Jalilian et al. 2012). Tubular injury in sepsis is subtle and focal, despite significant renal dysfunction (Langenberg et al. 2008; Lerolle et al. 2010). Changes in renal function in sepsis are likely to be functional, rather than due to structural injury or hemodynamic insufficiency, with reductions in renal tubular epithelial ion channel or transporter expression in response to inflammatory stimuli, including IL‐1β (Schmidt et al. 2007).
Most preliminary studies using knockout mice have demonstrated a potential benefit of P2X7 inhibition/genetic deletion in sepsis (Santana et al. 2015; Savio et al. 2016, 2017). However, others have demonstrated increased mortality with E. coli urosepsis in P2X7‐deficient mice (Greve et al. 2017). However, the role of a selective P2X7 receptor inhibitor has not been evaluated in a clinically relevant model of sepsis in which treatment is administered following the onset of sepsis. We demonstrate that P2X7 inhibition with A‐438079 after the onset of sepsis reduced renal IL‐1β early expression and abrogated the rise in serum creatinine concentration at 24 h. In addition, A‐438079 treatment reduced fever and improved resolution of tachycardia, increased serum albumin and reduced lactate levels at 24 h.
Serum IL‐1β with A‐438079 treatment
Other investigators have found that P2X7‐deficient mice produce less IL‐1β in response to cecal ligation and puncture (Santana et al. 2015; Savio et al. 2016, 2017). Despite treatment with A‐438079, we did not demonstrate any reduction in serum IL‐1β levels at either 6 h or 24 h. A significant amount of IL‐1β is likely to have been released prior to the initiation of treatment with A‐438079. Moreover, IL‐1β release following a P2X7 receptor antagonist given after the induction of sepsis cannot directly be compared with similar experiments in animals with genetic deletion. Furthermore, it is unlikely that IL1‐β is released by only one mechanism in polymicrobial sepsis. Monocytes are capable of IL‐1β release independent of P2X7 receptor activation (e.g., via TLR 7/8 ligands), though P2X7 activation accelerates mature IL‐1β release from monocytes (Ward et al. 2010). Splenic dendritic cells are able to release mature IL‐1β in the absence of P2X7 activation, but require the NLRP3 inflammasome. Intraperitoneal administration of LPS‐induced IL‐1β production in serum, which was abrogated in Nlrp3‐null mice, but unaffected in P2X7‐deficient mice (He et al. 2013). IL‐1β may be released independently of NLRP3 activity. NLRP3‐deficient mice had detectable (but significantly reduced) levels of IL‐1β (Mariathasan et al. 2006). In fact, P2X7 deficient mice inoculated with α‐hemolysin (HlyA)‐producing Escherichia coli had markedly lower survival, higher cytokine levels (including IL‐1β), and activated intravascular coagulation compared with wildtype mice (Greve et al. 2017). These paradoxical findings were explained by caspase‐8‐dependent IL‐1β production during sepsis with uropathogenic E. coli.
Inflammasome‐independent actions of P2X7
Despite similar serum IL‐1β levels at 6 h and 24 h in A‐438079‐treated and untreated animals, there were clinical features suggestive of an attenuated inflammatory response in A‐438079‐treated animals. The improvement in systemic inflammation and organ function must also be explained by mechanisms independent of the inflammasome and IL‐1β release. Arterial lactate at 24 h was lower with DMSO/A‐438079 treatment compared with untreated animals, suggesting improved mitochondrial function. Prevention of excessive P2X7 activation may have promoted mitochondrial health associated with improved oxidative phosphorylation and lower lactate. Basal expression of P2X7 promotes cell growth in P2X7‐transfected HEK293 (Adinolfi et al. 2005). However, an ATP challenge resulted in mitochondrial fragmentation mediated by increases in ER Ca2+ concentration and subsequent cell death (Adinolfi et al. 2009).
The reduction in serum albumin levels in sepsis is due in part to increased microvascular permeability/capillary leak. Extracellular ATP and ADP activate NF‐κB and induce endothelial cell apoptosis mediated via the P2X7R (von Albertini et al. 1998). Animals treated with A‐438079 had a significantly higher serum albumin compared with untreated animals, which may have been mediated by improved endothelial barrier function.
Systemic versus local IL‐1β production
There were systemic effects of A‐438079 treatment in sepsis over and above renal‐specific effects. At 24 h, animals treated with A‐438079 had a lower temperature and more rapid resolution of tachycardia compared with untreated animals. The resolution of tachycardia at 24 h associated with A‐438079 might be explained by a reduction in cardiac inflammatory mediator levels.
It is difficult to ascertain the relative contribution of systemic versus renal inflammasome activation in sepsis‐induced kidney dysfunction, with variable evidence in the literature. Mice with functional TLR4 deficiency (C3H/HeJ mice) transplanted with wild‐type kidneys were protected from LPS‐induced AKI, whereas wild‐type mice transplanted with C3H/HeJ kidneys developed severe LPS‐induced AKI. This suggests that TLR4 expression in circulating cells propagates injury in septic AKI, rather than intra‐renal TLR4 (Cunningham et al. 2004). In contrast, chimeria mice deficient of renal TLR4 but with wild‐type bone marrow were protected against LPS‐induced renal proximal tubular oxidative stress. Chimera mice generated through bone marrow transfer from TLR4 KO into WT recipient (KO/WT), however, exhibited oxidative stress similar to WT mice, suggesting a pathogenic role for renal TLR4 over systemic TLR4 (Kalakeche et al. 2011). In a murine model of UUO, use of bone marrow chimeras revealed that NLRP3 mediates injurious/inflammatory processes in both hematopoietic and nonhematopoietic cellular compartments (Vilaysane et al. 2010).
Limitations
Various drug solvents such as ethanol and MF59 can affect the inflammasome directly (Nurmi et al. 2013) or indirectly (Seubert et al. 2011). DMSO was the only suitable vehicle for A‐438079, but it also possesses some biological activity. DMSO exerts anti‐inflammatory effects as a hydroxyl radical scavenger (Man et al. 2014). DMSO also decreases IL‐1β mRNA transcription and reduces inflammation (Ahn et al. 2014a). Furthermore, mitochondrial reactive oxygen species can induce IL‐1β and NLRP3 activity, and this is inhibited by DMSO (Ahn et al. 2014b). However, there are also isolated reports of the proinflammatory action of DMSO in human peripheral blood mononuclear cells (PBMCs) (Xing and Remick 2005) and low‐dose toxicity in a retinal neuronal cell line (Galvao et al. 2014). Differences may be related to the dose of DMSO, cell/species type, and duration of stimulation. DMSO‐treated septic animals were compared with A‐438079 in DMSO. A number of clinical and biochemical parameters were improved at 24 h with A‐438079 with DMSO compared with untreated animals. However, there are trends in improvement in temperature, resolution of tachycardia, and serum creatinine with DMSO alone, suggesting some synergy.
The changes found in animals treated with the P2X7 antagonist may have been due to reduced systemic inflammatory response (evidenced by less tachycardia and fever), with better perfusion to organs including the kidney, rather than specific actions over the renal‐inflammasome activation.
ELISAs were performed on whole kidney homogenate for IL‐β measurement, which does not allow localization to specific cell types. Immunohistochemistry would enable localization of these cytokines. Although insights into renal expression of the inflammasome and P2X7 in sepsis have been demonstrated, the expression of the P2X7 in other organs and on immune cells also needs to be evaluated.
Summary
We demonstrate a number of strengths of our experimental model relevant to potential therapeutics in sepsis. Sepsis is associated with an increase in renal IL‐1β and tubular cell P2X7 expression by 6 h and 24 h, respectively. Treatment with A‐438079 after the onset of sepsis abrogated the rise in serum creatinine at 24 h with reduced renal IL‐1β expression early. In addition, A‐438079 treatment reduced fever, improved resolution of tachycardia, increased serum albumin concentration and reduced lactate at 24 h. Given these encouraging results in an in vivo model of sepsis that is relevant to human disease, further studies targeting P2X7 in sepsis are warranted.
Ethics approval and consent to participate
All animal experiments were performed under a Home Office Project License (PPL 70/7029) and local University College London Ethics Committee approval.
Conflict of Interest
Materials: Abbvie pharmaceuticals (Chicago, USA) have provided the selective P2X7 antagonist (A‐438079). FWKT has received research project grants from AstraZeneca Limited, Baxter Biosciences, Boehringer Ingelheim, and MedImmune. He has consultancy agreements with Rigel Pharmaceuticals, Novartis and Baxter Biosciences.
Data Accessibility
Supporting information
Data S1. Supplementary data.
Acknowledgments
The in vivo and some of the ex vivo studies were undertaken at University College London, UK. The ex vivo work including multiplex analysis and histopathology were undertaken at Hammersmith Hospital, Imperial College, UK.
Arulkumaran N., Sixma M. L., Pollen S., Ceravola E., Jentho E., Prendecki M., Bass P. S., Tam F. W. K., Unwin R. J., Singer M.. P2X7 receptor antagonism ameliorates renal dysfunction in a rat model of sepsis. Physiol Rep, 6 (5), 2018, e13622, https://doi.org/10.14814/phy2.13622
Funding Information
Dr. Arulkumaran and his Institution (UCL) received grant support from the Wellcome Trust 093969/10/Z Clinical Research Training Fellowship (grant in support of this project in part, including Dr. Arulkumaran's salary). FWKT is supported by the Diamond Fund from Imperial College Healthcare Charity and Ken and Mary Minton Chair of Renal Medicine.
References
- Adinolfi, E. , Callegari M. G., Ferrari D., Bolognesi C., Minelli M., Wieckowski M. R., et al. 2005. Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serum‐independent growth. Mol. Biol. Cell 16:3260–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinolfi, E. , Callegari M. G., Cirillo M., Pinton P., Giorgi C., Cavagna D., et al. 2009. Expression of the P2X7 receptor increases the Ca2 + content of the endoplasmic reticulum, activates NFATc1, and protects from apoptosis. J. Biol. Chem. 284:10120–10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, H. , Kim J., Jeung E. B., and Lee G. S.. 2014a. Dimethyl sulfoxide inhibits NLRP3 inflammasome activation. Immunobiology 219:315–322. [DOI] [PubMed] [Google Scholar]
- Ahn, H. , Kim J., Lee M. J., Kim Y. J., Cho Y. W., and Lee G. S.. 2014b. Methylsulfonylmethane inhibits NLRP3 inflammasome activation. Cytokine 71:223–231. [DOI] [PubMed] [Google Scholar]
- von Albertini, M. , Palmetshofer A., Kaczmarek E., Koziak K., Stroka D., Grey S. T., et al. 1998. Extracellular ATP and ADP activate transcription factor NF‐kappa B and induce endothelial cell apoptosis. Biochem. Biophys. Res. Commun. 248:822–829. [DOI] [PubMed] [Google Scholar]
- Angus, D. C. , Linde‐Zwirble W. T., Lidicker J., Clermont G., Carcillo J., and Pinsky M. R.. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303–1310. [DOI] [PubMed] [Google Scholar]
- Arulkumaran, N. , Sixma M. L., Jentho E., Ceravola E., Bass P. S., Kellum J. A., et al. 2017. Sequential analysis of a panel of biomarkers and pathologic findings in a resuscitated rat model of sepsis and recovery. Crit. Care Med. 0000:000; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw, S. M. , Laupland K. B., Doig C. J., Mortis G., Fick G. H., Mucenski M., et al. 2005. Prognosis for long‐term survival and renal recovery in critically ill patients with severe acute renal failure: a population‐based study. Crit. Care 9:R700–R709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, P. N. , Wang Y., Guo R., He G., and Quigg R. J.. 2004. Role of Toll‐like receptor 4 in endotoxin‐induced acute renal failure. J. Immunol. 172:2629–2635. [DOI] [PubMed] [Google Scholar]
- Galvao, J. , Davis B., Tilley M., Normando E., Duchen M. R., and Cordeiro M. F.. 2014. Unexpected low‐dose toxicity of the universal solvent DMSO. FASEB J. 28:1317–1330. [DOI] [PubMed] [Google Scholar]
- Goncalves, R. G. , Gabrich L., Rosario A. Jr, Takiya C. M., Ferreira M. L., Chiarini L. B., et al. 2006. The role of purinergic P2X7 receptors in the inflammation and fibrosis of unilateral ureteral obstruction in mice. Kidney Int. 70:1599–1606. [DOI] [PubMed] [Google Scholar]
- Greve, A. S. , Skals M., Fagerberg S. K., Tonnus W., Ellermann‐Eriksen S., Evans R. J., et al. 2017. P2X1, P2X4, and P2X7 receptor knock out mice expose differential outcome of sepsis induced by alpha‐haemolysin producing Escherichia coli . Front Cell. Infect. Microbiol. 7:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y. , Franchi L., and Nunez G.. 2013. TLR agonists stimulate Nlrp3‐dependent IL‐1beta production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J. Immunol. 190:334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalilian, I. , Spildrejorde M., Seavers A., Curtis B. L., McArthur J. D., and Sluyter R.. 2012. Functional expression of the damage‐associated molecular pattern receptor P2X7 on canine kidney epithelial cells. Vet. Immunol. Immunopathol. 150:228–233. [DOI] [PubMed] [Google Scholar]
- Kalakeche, R. , Hato T., Rhodes G., Dunn K. W., El‐Achkar T. M., Plotkin Z., et al. 2011. Endotoxin uptake by S1 proximal tubular segment causes oxidative stress in the downstream S2 segment. J. Am. Soc. Nephrol. 22:1505–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenberg, C. , Bagshaw S. M., May C. N., and Bellomo R.. 2008. The histopathology of septic acute kidney injury: a systematic review. Crit. Care 12:R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerolle, N. , Nochy D., Guerot E., Bruneval P., Fagon J. Y., Diehl J. L., et al. 2010. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intensive Care Med. 36:471–478. [DOI] [PubMed] [Google Scholar]
- Li, P. , Allen H., Banerjee S., Franklin S., Herzog L., Johnston C., et al. 1995. Mice deficient in IL‐1 beta‐converting enzyme are defective in production of mature IL‐1 beta and resistant to endotoxic shock. Cell 80:401–411. [DOI] [PubMed] [Google Scholar]
- Man, W. , Ming D., Fang D., Chao L., and Jing C.. 2014. Dimethyl sulfoxide attenuates hydrogen peroxide‐induced injury in cardiomyocytes via heme oxygenase‐1. J. Cell. Biochem. 115:1159–1165. [DOI] [PubMed] [Google Scholar]
- Mariathasan, S. , Weiss D. S., Newton K., McBride J., O'Rourke K., Roose‐Girma M., et al. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228–232. [DOI] [PubMed] [Google Scholar]
- McGaraughty, S. , Chu K. L., Namovic M. T., Donnelly‐Roberts D. L., Harris R. R., Zhang X. F., et al. 2007. P2X7‐related modulation of pathological nociception in rats. Neuroscience 146:1817–1828. [DOI] [PubMed] [Google Scholar]
- Mehta, V. B. , Hart J., and Wewers M. D.. 2001. ATP‐stimulated release of interleukin (IL)‐1beta and IL‐18 requires priming by lipopolysaccharide and is independent of caspase‐1 cleavage. J. Biol. Chem. 276:3820–3826. [DOI] [PubMed] [Google Scholar]
- Nurmi, K. , Virkanen J., Rajamaki K., Niemi K., Kovanen P. T., and Eklund K. K.. 2013. Ethanol inhibits activation of NLRP3 and AIM2 inflammasomes in human macrophages–a novel anti‐inflammatory action of alcohol. PLoS ONE 8:e78537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, Y. , Franchi L., Nunez G., and Dubyak G. R.. 2007. Nonclassical IL‐1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J. Immunol. 179:1913–1925. [DOI] [PubMed] [Google Scholar]
- Ricci, Z. , Cruz D., and Ronco C.. 2008. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 73:538–546. [DOI] [PubMed] [Google Scholar]
- Santana, P. T. , Benjamim C. F., Martinez C. G., Kurtenbach E., Takiya C. M., and Coutinho‐Silva R.. 2015. The P2X7 receptor contributes to the development of the exacerbated inflammatory response associated with sepsis. J. Innate. Immun. 7:417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savio, L. E. , Andrade M. G., de Andrade Mello P., Santana P. T., Moreira‐Souza A. C., Kolling J., et al. 2016. P2X7 receptor signaling contributes to sepsis‐associated brain dysfunction. Mol. Neurobiol. 8:6459–6470. [DOI] [PubMed] [Google Scholar]
- Savio, L. E. B. , de Andrade Mello P., Figliuolo V. R., de Avelar Almeida T. F., Santana P. T., Oliveira S. D. S., et al. 2017. CD39 limits P2X7 receptor inflammatory signaling and attenuates sepsis‐induced liver injury. J. Hepatol. 4:716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, C. , Hocherl K., Schweda F., Kurtz A., and Bucher M.. 2007. Regulation of renal sodium transporters during severe inflammation. J. Am. Soc. Nephrol. 18:1072–1083. [DOI] [PubMed] [Google Scholar]
- Seubert, A. , Calabro S., Santini L., Galli B., Genovese A., Valentini S., et al. 2011. Adjuvanticity of the oil‐in‐water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc. Natl Acad. Sci. USA 108:11169–11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, S. R. , Turner C. M., Elliott J. I., McDaid J., Hewitt R., Smith J., et al. 2009. P2X7 deficiency attenuates renal injury in experimental glomerulonephritis. J. Am. Soc. Nephrol. 20:1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, C. M. , Arulkumaran N., Singer M., Unwin R. J., and Tam F. W.. 2014. Is the inflammasome a potential therapeutic target in renal disease? BMC Nephrol. 15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino, S. , Kellum J. A., Bellomo R., Doig G. S., Morimatsu H., Morgera S., et al. 2005. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294:813–818. [DOI] [PubMed] [Google Scholar]
- Vilaysane, A. , Chun J., Seamone M. E., Wang W., Chin R., Hirota S., et al. 2010. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J. Am. Soc. Nephrol. 21:1732–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Faubel S., Ljubanovic D., Mitra A., Falk S. A., Kim J., et al. 2005. Endotoxemic acute renal failure is attenuated in caspase‐1‐deficient mice. Am. J. Physiol. Renal Physiol. 288:F997–F1004. [DOI] [PubMed] [Google Scholar]
- Ward, J. R. , West P. W., Ariaans M. P., Parker L. C., Francis S. E., Crossman D. C., et al. 2010. Temporal interleukin‐1beta secretion from primary human peripheral blood monocytes by P2X7‐independent and P2X7‐dependent mechanisms. J. Biol. Chem. 285:23147–23158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, L. , and Remick D. G.. 2005. Mechanisms of dimethyl sulfoxide augmentation of IL‐1 beta production. J. Immunol. 174:6195–6202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplementary data.


