Abstract
Arginine metabolism via the arginine deiminase system (ADS) of oral bacteria generates ammonia, which can increase the pH of oral biofilms and decrease the risk for dental caries. Antagonistic interactions between ADS-positive and cariogenic bacteria in oral biofilms may be an important ecological determinant of caries. This study investigated the antagonistic potential and mechanisms of clinical isolates of arginolytic streptococci on and by Streptococcus mutans UA159, a well-characterized cariogenic human isolate. Low-passage isolates of Streptococcus gordonii, Streptococcus sanguinis, Streptococcus parasanguinis, Streptococcus australis and Streptococcus cristatus inhibited the growth of S. mutans to various degrees when they were inoculated on growth media first or simultaneously with S. mutans. The antagonistic effects of arginolytic strains against S. mutans and the production of H2O2 by these strains were enhanced during growth in a less-rich medium or when galactose was substituted for glucose as the primary carbohydrate source. Pyruvate oxidase was the dominant pathway for H2O2 production by arginolytic strains, but lactate oxidase activity was also detected in some strains of S. gordonii and S. cristatus. UA159 inhibited the growth of all tested arginolytic strains when inoculated first, especially in aerobic conditions. However, the antagonistic effects of S. mutans on certain strains of S. gordonii and S. australis were not observed during anaerobic growth in the presence of arginine. Thus, arginolytic commensal streptococci may have a synergistically positive impact on the ecology of oral biofilms by moderating biofilm pH while antagonizing the growth and virulence of caries pathogens.
Keywords: dental caries, bacteria, biofilm, arginine, antagonism
INTRODUCTION
Dental caries is a classic biofilm-mediated disease that develops when changes in the oral environment favor the growth of cariogenic bacteria, which are highly efficient at converting carbohydrates to organic acids that demineralize tooth enamel [Lemos and Burne, 2008; Huang et al., 2012a]. Decades of research, including microbiological assessment of caries-active sites in humans and studies with experimental animals, have identified Streptococcus mutans as being involved in the caries process [Tanzer et al., 2001; Lemos and Burne, 2008; Takahashi and Nyvad, 2011; Lemos et al., 2013]. S. mutans is either not detectable or is present only in minor proportions on healthy tooth surfaces. In contrast, when conditions are favorable for the development of dental caries, generally as the consumption of dietary carbohydrate by the host increases and acidification of oral biofilms becomes more frequent and sustained, the proportions of S. mutans in oral biofilms significantly increase. S. mutans has multiple virulence attributes that make it an effective caries-associated organism [Kreth et al., 2005], the most important of which appears to be its capacity to produce large quantities of organic acids (acidogenicity) from a wide range of carbohydrates, its ability to tolerate environmental stresses, particularly low pH (aciduricity), and its synthesis of multiple secreted proteins and water-insoluble glucan exopolysaccharides that together promote bacterial adhesion and accumulation on the tooth surface and with other bacteria [Lemos and Burne, 2008; Takahashi and Nyvad, 2011; Lemos et al., 2013].
To compete for limited space and nutritional resources, oral bacteria have developed a range of strategies to survive and persist in the oral cavity. For example, S. mutans can lower the pH to the point where it is inhibitory to the growth of beneficial commensal organisms and synthesizes a variety of bacteriocins, often called mutacins, which can inhibit the growth of multiple species of bacteria commonly found in the human mouth. The production of mutacins is regulated by environmental factors, of which the most influential appear to be cell density, nutrient source and oxygen [Merritt and Qi, 2012]. Notably, growth in the presence of oxygen significantly enhances transcription of bacteriocin-related genes, which may reflect evolutionary pressure on the organism to compete with commensals in relatively immature, oxygen-rich biofilms [Ahn et al., 2007]. Other factors, for example phosphate, can significantly delay or even inhibit mutacin production [Nguyen et al., 2009], whereas growth in diluted medium (e.g. half-strength BHI) yields greater mutacin operon transcription and mutacin production, compared to cells grown in a richer medium [Kreth et al., 2005; Merritt and Qi, 2012]. Other streptococcal species can also produce and secrete antimicrobial substances that suppress the growth of S. mutans, including hydrogen peroxide (H2O2). For example, Streptococcus sanguinis and Streptococcus gordonii produce sufficient amounts of H2O2 to inhibit growth of S. mutans [Kreth et al., 2005], mainly via a pyruvate oxidase enzyme (Pox) encoded by the spxB gene. H2O2 production by S. gordonii [Zheng et al., 2011b], but not S. sanguinis, seems to be inversely correlated with carbohydrate availability [Zhu and Kreth, 2012]. S. oligofermentans can also produce H2O2 from lactic acid through lactate oxidase (Lox) [Tong et al., 2007] and less efficiently using L-amino acid oxidase enzymes (LAAO). Moreover, facultatively anaerobic lactic-acid bacteria, including commensal oral streptococci, were shown to have vigorous oxygen metabolism catalyzed by flavin-containing NADH oxidases, some of which yield H2O2 as an end product [Marquis, 1995].
A selected group of oral bacteria can produce ammonia (NH3) from arginine metabolism to increase intracellular pH and the pH of oral biofilms [Huang et al., 2015]. Arginine metabolism occurs primarily via the arginine deiminase system (ADS) and plays major roles in plaque pH homeostasis and in the inhibition of caries [Burne and Marquis, 2000; Nascimento and Burne, 2014]. Clinical and laboratory evidence supports a positive relationship between ADS activity in oral biofilms and dental health [Stephan, 1944; Turtola and Luoma, 1972; Kleinberg, 1978; Sissons and Cutress, 1988; Margolis et al., 1988; Nascimento et al., 2009; Nascimento et al., 2012]. Known oral arginolytic (ADS-positive) bacteria include S. sanguinis, S. gordonii, Streptococcus ratti, Streptococcus parasanguinis, Streptococcus intermedius, Streptococcus australis, Streptococcus cristatus, certain Lactobacillus species and a few spirochetes [Burne and Marquis, 2000; Huang et al., 2015; Huang et al., 2016]. Studies using laboratory strains of streptococci indicate that the expression of ADS genes is typically inducible by arginine and sensitive to carbohydrate catabolite repression (CCR), but also that low pH and anaerobic conditions enhance ADS gene expression [Curran et al., 1998; Dong et al., 2002; Dong et al., 2004; Liu et al., 2008; Huang et al., 2012b]. A survey of a spectrum of supragingival clinical isolates demonstrated that ADS gene expression can be highly variable within and between species, and that the variability was attributable to both constitutional and environmentally-modulated differences in the expression of ADS activity [Huang et al., 2015].
Antagonism between beneficial commensals and cariogenic bacteria is a major factor that affects the composition and ecology of supragingival biofilms. Oral bacteria that can moderate plaque acidification and interfere with the growth and virulence of caries pathogens play central roles in promoting the formation and stability of health-associated oral biofilm communities. A better understanding of the mechanisms used by certain commensals to compete with cariogenic bacteria will support the development of new strategies for caries risk assessments and interventions. Considering that isolates that have a high potential to catabolize arginine would be particularly beneficial to the host if they could also suppress the growth of caries pathogens, this study aimed to examine the capacity of selected oral arginolytic isolates to antagonize the growth of S. mutans or to be inhibited by S. mutans.
MATERIALS AND METHODS
Bacterial Strains, Media, and Growth Conditions
The reference strains S. mutans UA159 and S. gordonii DL1, along with 56 ADS-positive low-passage clinical isolates were routinely grown in brain heart infusion (BHI) broth (3.7% containing a final concentration of 2g of glucose/L; Difco Laboratories, Detroit, MI, USA) or on BHI agar plates. The arginolytic strains were previously isolated from supragingival dental plaque samples of caries-free (CF) and caries-active (CA) subjects [Huang et al., 2015]. The strains were characterized by 16S rRNA gene sequencing and by ADS expression patterns under normal growth conditions and in response to pH, oxygen, and the availability of arginine and carbohydrate (Table S1) [Huang et al., 2015]. Based on complete 16S rRNA gene sequence, the arginolytic clinical strains used in this study were most similar (>98% rRNA sequence identity) to the following species: S. sanguinis (n=38), S. parasanguinis (n=1), S. intermedius (n=5), S. gordonii (n=5), S. australis (n=2) and S. cristatus (n=5). Several modified agar media were formulated with different carbohydrate sources and nutrient composition: TY (3% tryptone and 0.5% yeast extract) [Liu et al., 2008] supplemented with 25mM galactose (TY-25mM galactose) or 25mM glucose (TY-25mM glucose), a more dilute version of TY (1% tryptone, 0.2% yeast extract) supplemented with 25 mM galactose (TY-D-25mM galactose), half-strength TY-25mM galactose (1.5% tryptone, 0.25% yeast extract and 12.5mM galactose) with or without 20mM arginine, and half-strength BHI (1.85% BHI with a final concentration of 1g of glucose/L).
Preparation of gradient agar plates
Gradient BHI or TY-25mM galactose agar plates were used to detect the influence of nutritional gradients on the antagonistic capacity of the tested strains. Briefly, 15 mL of agar (constituted in dH2O and without nutrients) were poured into the plates and the plates were tilted. After the agar had solidified (approximately 1 hour after pouring the agar), the plates were placed in a horizontal position and another 15 mL of BHI or TY-25mM galactose agar were poured as the top layer.
Preparation of saliva agar
Saliva agar plates were used for bacterial competition assays and prepared as previously described [De Jong et al., 1986], with certain modifications. Whole stimulated saliva was collected from members of our laboratory by asking them to chew on sterile paraffin wax. Informed consent was obtained from all saliva donors under a protocol reviewed and approved by the Institutional Review Board of the University of Florida Health Science Center. The saliva donors were not users of oral hygiene products containing compounds with strong antimicrobial activities (prescription antimicrobials), nor were they being treated with antimicrobial drugs at the time of saliva collection. The saliva samples were pooled and subsequently diluted with sterile demineralized water in a 2:1 ratio. Dithiothreitol (DTT) was added to the saliva mix to a final concentration of 2.5 mmol/L and then the saliva mix was slowly stirred for 10 min. To inactive catalase and peroxidase enzymes, the saliva mix was heated at 60°C for 10 min, centrifuged and filter-sterilized. Glucose, galactose or arginine was added to the treated saliva to a final concentration of 5 mM. Finally, 5 ml of melted 6% agar (approximately at 50°C and constituted in dH2O) was mixed with 15 ml of the saliva media and immediately poured into sterile petri dishes.
Competition assays
TY-25mM galactose, TY-25mM glucose, half-strength BHI and BHI agar plates were used for competition assays between the arginolytic strains and S. mutans UA159. Overnight cultures of each strain grown in BHI broth had their optical density at 600 nm (OD600) adjusted to 0.5. Each strain (6 µl) was then inoculated adjacent to one another on agar plates as follows: (i) arginolytic strains first, followed by UA159 24 h later; (ii) UA159 first, followed by arginolytic strains 24 h later; or (iii) arginolytic strains and UA159 inoculated at the same time. The plates were incubated for an additional 24 h at 37°C, in 5% CO2 and 95% air. AlphaEaseFC software was used to measure the zones of growth inhibition on all tested agar media.
Of the 56 arginolytic strains tested, 10 strains were selected for further assessments of the environmental factors that may influence their antagonistic capacity. The selection criteria were based primarily on differences in species and antagonistic capacities against S. mutans UA159, as compared to the reference strain S. gordonii DL1. The 16S rRNA gene sequences of the selected strains were most similar (>98% identity) to five bacterial species: S. sanguinis, S. parasanguinis, S. gordonii, S. australis, and S. cristatus.
Half-strength TY-25mM galactose plates with or without 20 mM arginine were used to assess the effect of arginine availability on antagonistic potential. Competition assays were also performed in anaerobic (85% N2, 5% CO2, 10% H2) or aerobic (5% CO2, 95% air) conditions to assess the impact of oxygen. To further explore the mechanisms of growth inhibition by S. mutans UA159, 2.5 µg/µL of catalase (2950 units/mg solid, Sigma, St. Louis, MO, USA) or 1 µg/µL proteinase K (7.5 units/mg solid, Sigma, St. Louis, MO, USA) was added to the agar plates. Specifically, 6 µl of overnight cultures of arginolytic bacteria were spotted on the agar plates. Next, 5 µl phosphate-buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4·7H2O, 1.4 mM KH2PO4, pH 7.3), or catalase or proteinase K in PBS, was applied adjacent to the inoculation spot on the agar plate or before spotting 5 µl of an overnight culture of UA159 as follows: (a) arginolytic bacteria first, followed by UA159 24 h later or (b) arginolytic bacteria first, followed by UA159 immediately after.
H2O2 production
To test the capacity of the strains to produce H2O2, overnight cultures of selected arginolytic bacteria were diluted (1:20) into TY-25mM glucose, TY-25mM galactose or TY-D-25mM galactose and allowed to grow to OD600=0.3 in static culture in a 37°C aerobic incubator. The cultures were then shaken at 200 RPM in a 37°C aerobic shaker/incubator for 30 min. The assay for H2O2 production was performed as previously described [Tong et al., 2007] with some modifications. First, 0.6 ml of culture supernate was added to 0.6 ml of a solution containing 2.5 mM of 4-amino-antipyrine (4-amino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one; Sigma) and 0.17 M phenol (Fisher Scientific, Pittsburgh, PA, USA). After 4 min of incubation at room temperature, horseradish peroxidase (Pierce, Thermo Scientific, Grand Island, NY, USA) diluted in 0.2 M potassium phosphate buffer (pH 7.2) was added to the reaction solution at a final concentration of 13 mU/ml. After 20 min of incubation at room temperature, the culture absorbance was measured at OD510. A standard curve with known concentrations of fresh H2O2 (30% w/w, Fisher Scientific) was generated at each time the assays were performed.
Activity of H2O2-generating oxidase enzymes
To measure the activity of certain H2O2-generating enzymes, BHI overnight cultures of the selected arginolytic bacteria were centrifuged at 4,000 × g for 10 min, washed twice with 2 ml of PBS and the pelleted cells were resuspended in 2 ml of PBS. Aliquots of the cell suspension were used for determining lactate oxidase activity, and the remaining cell suspensions were permeabilized for determination of pyruvate oxidase, L-arginine oxidase, and NADH oxidase activities by mixing the cell suspension with 0.02 volumes of toluene-acetone (1:9, v/v) and vortexing the mixture for 2 min. The protein concentration in the cell preparations was determined by using a Pierce BCA protein assay kit (Waltham, MA, USA) with bovine serum albumin as the standard [Liu et al., 2012; Huang et al., 2016].
Pyruvate oxidase activity was determined by measuring the production of acetyl phosphate (AcP) [Fowler et al., 2011; Liu et al., 2012; Huang et al., 2016], with some modifications. The reaction mixture consisted of 0.45 ml of the permeabilized cell suspension and 0.45 ml of a solution containing 50 mM potassium phosphate buffer (KPO4; pH 6.0), 10 µM MgCl2, 0.2 µM thiamine pyrophosphate (Sigma), 50 mM potassium pyruvate, and 12 µM flavin adenine dinucleotide (FAD; Sigma). Controls included reactions without permeabilized cells or without potassium pyruvate. The reaction mixtures were incubated at 37°C for 30 min with shaking at 250 rpm, and the amount of acetyl phosphate generated during the reaction was then measured as follows. Aliquots of cell suspensions (0.3 ml) were pre-incubated at 37°C for 1 min in a 111002 heat block (Boekel Scientific, Feasterville, PA, USA) followed by the addition of 50 µL of 2 M hydroxylamine hydrochloride solution, and further incubation at 60°C for 5 min in the heat block. Next, 100 µl of a development solution containing equal volumes of 0.5 M ferric chloride in 5 M HCl and 30% trichloroacetic acid was added to the reaction solution, and color was allowed to develop for at least 1 min at room temperature. The samples were centrifuged and the absorbance of the supernates was measured at OD540. A standard curve was generated with known concentrations of lithium potassium acetyl phosphate (Sigma).
The activity of lactate, L-arginine and NADH oxidases was assessed by measuring H2O2 production [Liu et al., 2012; Huang et al., 2016]. For lactate oxidase assays, 0.45 ml of intact cells was added to 0.45 ml of 0.2 M sodium phosphate buffer (pH 7.0) containing 20 mM sodium L-lactate. The mixtures were incubated at 37°C with shaking at 250 rpm for 30 min. For L-arginine oxidase assays, 0.45 ml of permeabilized cell suspensions was added to 0.45 ml of 0.2 M sodium phosphate buffer (pH 7.0) containing 20 mM L-arginine. The mixtures were incubated at 37°C with shaking at 250 rpm for 2 h. For NADH oxidase assays, 0.45 ml of permeabilized cell suspensions was added to 0.45 ml of 0.2 M sodium phosphate buffer (pH 7.0) containing 13 mM NADH. The mixtures were incubated at 37°C with shaking at 250 rpm for 3 h, and the concentration of H2O2 in the supernates was determined. Controls included reaction solutions without sodium salts of L-lactate, L-arginine or NADH, respectively.
Statistical analysis
For descriptive analysis, the distribution of percentages and means were calculated when appropriate. Student’s t test was used to analyze the influence of growth conditions (TY-25mM galactose versus TY-25mM glucose, ½ strength BHI versus BHI), source of the isolate (CF versus CA subjects), and the zones of inhibition of S. mutans grown with or without arginine. Two-way ANOVA was used to analyze the influence of the type of strain and growth conditions, and Dunnet test in one-way ANOVA was used to compare the strains growth in a given condition. The level of significance was determined at p < 0.05.
RESULTS
Arginolytic strains have a highly variable capacity to inhibit the growth of S. mutans UA159
Table 1 shows the mean average of the growth inhibition zones of the closest relative species representing the 56 arginolytic strains tested for antagonistic capacity on and by S. mutans UA159. The growth of all arginolytic strains was inhibited when S. mutans was inoculated first on the different media tested. However, clinical strains of S. sanguinis, S. parasanguinis, S. gordonii, S. australis and S. cristatus could inhibit the growth of UA159 to various degrees when inoculated first on the different media tested. Strains of S. intermedius were the only group that was not able to inhibit the growth of S. mutans when inoculated first or simultaneously on the various agar media. When inoculated first on TY-25mM galactose, strains of S. gordonii, S. australis and S. cristatus showed greater inhibition of S. mutans, compared to the reference strain S. gordonii DL1. When inoculated first on TY-25mM glucose plates, strains of S. australis and S. cristatus also presented greater inhibition of S. mutans, compared to the reference strain. Overall, S. australis displayed the strongest inhibitory effects on S. mutans when inoculated first onto TY agar, whereas DL1 displayed the strongest inhibitory effects when inoculated first onto BHI agar.
Table 1.
Mean average of growth inhibition zones of the arginolytic bacterial strains on or by S. mutans UA159.
| TY-25mM galactose | TY-25mM glucose | ½ strength BHI | BHI | |
|---|---|---|---|---|
| On S. mutans UA159 | ||||
| S. gordonii DL1 | 0.7±0.0a | 0.6±0.1 | 1.4±0.3 | 1.3±0.2 |
| S. parasanguinis | 0.5±0.0a | 0.3±0.1 | 1.0±0.3 | 0.9±0.2 |
| S. intermedius | 0 | 0 | 0 | 0 |
| S. gordonii | 1.0±0.7a | 0.4±0.2 | 0.6±0.4 | 0.5±0.4 |
| S. australis | 2.2±0.3a | 0.9±0.1 | 1.3±0.3 | 1.0±0.5 |
| S. sanguinis | 0.7±0.6a | 0.3±0.4 | 0.8±0.6b | 0.5±0.4 |
| S. cristatus | 1.0±0.7 | 0.8±0.4 | 0.4±0.4 | 0.5±0.3 |
| By S. mutans UA159 | ||||
| S. gordonii DL1 | −6.5±0.2a | −2.4±0.9 | −1.7±0.3 | −1.6±0.4 |
| S. parasanguinis | −5.6±0.4a | −1.2±0.2 | −1.9±0.7 | −1.7±0.6 |
| S. intermedius | −8.0±0 | −7.7±0.6 | −7.6±1.0 | −7.2±1.4 |
| S. gordonii | −5.6±2.1a | −1.5±1.5 | −4.8±1.3 | −4.3±1.4 |
| S. australis | −6.3±0.5a | −2.7±1.0 | −3.3±0.3 | −4.1±0.4 |
| S. sanguinis | −6.4±2.2a | −4.5±2.2 | −4.4±2.3 | −5.3±2.2 |
| S. cristatus | −8.0±0a | −4.8±1.9 | −6.5±0.5b | −7.6±0.6 |
| Simultaneously | ||||
| S. gordonii DL1 | 0.7±0.1a | 0.4±0.0 | 0.4±0.1 | 0.4±0.0 |
| S. parasanguinis | 0.3±0.1a | 0.1±0.1 | 0 | 0 |
| S. intermedius | −4.0±1.2 | −3.7±0.8 | −2.1±0.6 | −2.1±0.5 |
| S. gordonii | 0.6±0.4 | −0.2±−0.2 | 0.1±0.3b | −0.2±0.4 |
| S. australis | 0.8±0.1 | 1.0±0.3 | 0.3±0.2 | 0.3±0.2 |
| S. sanguinis | 0.3±0.7a | −0.1±0.6 | −0.3±0.7b | −0.6±0.7 |
| S. cristatus | −0.6±1.1 | −0.4±1.2 | −1.5±1.5b | −2.5±1.1 |
Mean average of inhibition zones ± standard deviation (millimeter). The 56 arginolytic strains tested are grouped and listed by their closest relative species. Competition assays occurred as follows: (1) arginolytic strain first followed by S. mutans UA159 24 h later; (2) S. mutans UA159 first followed by arginolytic strain 24 h later, and (3) arginolytic strain and S. mutans UA159 simultaneously. Positive values show that arginolytic strains inhibit the growth of S. mutans UA159 while negative values show that the growth of arginolytic strains was inhibited by S. mutans UA159.
indicates significant differences between TY-25mM galactose and TY-25mM glucose inhibition zones in a given species.
indicates significant differences between ½ strength BHI and BHI inhibition zones in a given species (p<0.05).
Strains of S. parasanguinis, S. sanguinis, S. gordonii, and S. australis could inhibit the growth of S. mutans to different degrees when inoculated simultaneously on TY-25mM galactose agar. Strains of S. gordonii and S. australis could also inhibit the growth of S. mutans when inoculated simultaneously onto half-strength BHI agar. In fact, strains of S. australis were the only group of clinical isolates capable of inhibiting S. mutans when inoculated simultaneously onto the media tested. Of note, S. australis showed greater inhibitory effects on UA159 than did S. gordonii DL1 when the clinical strains were inoculated simultaneously with UA159 on TY-25mM galactose or TY-25mM glucose plates.
Two strains of S. sanguinis isolated from CF subjects showed differences in their anatagonistic capacity against S. mutans. Specifically, one strain of S. sanguinis (A17), for which the best BLAST match on 16S rRNA was S. sanguinis SK36, showed significant inhibition of S. mutans (2.1±0.2 mm zone of inhibition when colonizing first, 1.3±0.1mm when colonizing simultaneously) on TY-25mM galactose plates, while another strain of S. sanguinis (A51), with a 16S rRNA sequence most similar to S. sanguinis ChDC B357, showed no capacity to inhibit the growth of S. mutans under any condition. Table 2 shows the mean of the zones of growth inhibition on S. mutans by strains with similar 16S rRNA gene sequence matches, but that were isolated from either from CF or CA subjects. Strains isolated from CF subjects showed a significantly stronger inhibitory effect on S. mutans than those from CA subjects in the media tested, except for strains most similar to S. sanguinis JCM 5708 grown on TY-25mM glucose and BHI plates.
Table 2.
Mean average of growth inhibition zones of arginolytic strains on S. mutans UA159 by type of isolation source.
| Closest relative species | Caries Status | TY-25mM galactose | TY-25mM glucose | ½ strength BHI | BHI |
|---|---|---|---|---|---|
| S. gordonii str. Challis substr. CH1 strain | CF | 2.3±0.2* | 0.7±0.1* | 1.3±0.3* | 1.2±0.1* |
| CA | 0.6±0.1 | 0.3±0.1 | 0.4±0.1 | 0.3±0.0 | |
|
| |||||
| S. gordonii strain ATCC 10558 | CF | 1.3±0.2* | 0.6±0.1* | 0.8±0.1* | 0.5±0.1* |
| CA | 0.5±0.1 | 0.3±0.1 | 0.4±0.1 | 0.3±0.1 | |
|
| |||||
| S. sanguinis JCM 5708 | CF | 0.6±0.5* | 0.2±0.3 | 0.6±0.4* | 0.4±0.4 |
| CA | 0.3±0.3 | 0.4±0.4 | 0.3±0.2 | 0.3±0.2 | |
Mean average of inhibition zones ± standard deviation (millimeter). Isolation source refers to the caries status of the subjects from which the strains were isolated: CF (caries-free) and CA (caries-active).
indicates statistically significant difference between the inhibition zones of strains isolated from CF versus CA within the same closest relative species and growth media (p<0.05).
Table 3 shows the mean average of the zones of growth inhibition on and by S. mutans of 10 arginolytic strains that were chosen to investigate in more detail some environmental factors that may affect their antagonistic capacities. All strains inhibited the growth of S. mutans to different degrees when inoculated first, except for A49 (best 16S rRNA gene sequence match: S. sanguinis JCM 5708). Growth in TY-25mM galactose, half-strength BHI or TY-D-25mM galactose under aerobic conditions appeared to favor the antagonistic effects of most arginolytic strains against S. mutans when they were inoculated first. S. mutans inhibited the growth of all arginolytic strains when it was inoculated first, especially in aerobic conditions. All strains inhibited the growth of S. mutans to differing degrees when inoculated simultaneously with UA159 on TY-25mM galactose, expect for A52 (best 16S rRNA gene sequence match: S. cristatus ATCC 51100). However, all strains, including A52, inhibited the growth of S. mutans when inoculated simultaneously with UA159 on TY-D-25mM galactose under aerobic conditions.
Table 3.
Mean average of growth inhibition zones of arginolytic strains on or by S. mutans UA159.
| Closest relative species | Caries status |
TY-25mM galactose | TY-25mM glucose | ½ strength BHI | BHI | TY-D-25mM galactose aerobic |
TY-D-25mM galactose anaerobic | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||||||
| Strains | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | ||
| DL1 | S. gordonii DL1 | 0.7±0.0 | −6.5±0.2 | 0.7±0.1 | 0.6±0.1 | −2.4±0.9 | 0.4±0.0 | 1.4±0.3 | −1.7±0.3 | 0.4±0.1 | 1.3±0.2 | −1.6±0.4 | 0.4±0.0 | 1.7±0.2 | −3.2±0.2 | 0.9±0.1 | 0.0±0.0 | −1.0±0.1 | 0.0±0.0 | |
| A1 | S. parasanguinis ChDC B356 | CF | 0.5±0.0 | −5.6±0.4 | 0.0±0.0 | 0.3±0.1 | −1.2±0.2 | 0.0±0.0 | 1.0±0.3 | −1.9±0.7 | 0.0±0.0 | 0.9±0.2 | −1.7±0.6 | 0.0±0.0 | 2.2±0.7* | −4.6±0.9 | 2.0±0.5* | 1.0±0.1* | −1.6±0.1 | 0.0±0.0 |
| A10 | S. gordonii ATCC 10558 | CA | 0.5±0.1 | −7.2±1.0 | 0.4±0.1 | 0.4±0.1 | −0.5±0.1 | 0.2±0.0 | 0.5±0.1 | −4.6±0.3 | 0.2±0.1 | 0.2±0.1 | −3.0±0.0 | 0.1±0.0 | 0.4±0.1 | −5.0±2.0 | 0.6±0.3 | 0.0±0.0 | −0.5±0.0 | 0.0±0.0 |
| A11 | S. gordonii ATCC 10558 | CF | 1.3±0.2* | −4.0±0.0 | 0.3±0.1 | 0.6±0.1 | −4.0±0.0 | 0.3±0.1 | 0.8±0.1 | −4.1±0.4 | 0.2±0.1 | 0.5±0.1 | −4.0±0.4 | −0.9±0.1 | 2.0±0.1* | −4.7±0.7 | 1.0±0.2* | 0.5±0.5* | −0.8±0.3 | 0.0±0.0 |
| A12 | S. australis AI-1 | CF | 2.1±0.1* | −6.5±0.6 | 0.9±0.1* | 0.9±0.1* | −3.5±0.4 | 1.2±0.1* | 1.6±0.1* | −3.3±0.2 | 0.5±0.1* | 1.5±0.2* | −4.1±0.3 | 0.5±0.1* | 2.6±0.7* | −4.4±1.5 | 1.6±0.2* | 0.4±0.1* | −2.0±0.0 | 0.0±0.0 |
| A13 | S. australis AI-1 | CF | 2.3±0.4* | −6.0±0.2 | 0.7±0.1 | 0.8±0.0* | −1.6±0.3 | 0.7±0.1* | 1.1±0.2 | −3.3±0.3 | 0.2±0.1 | 0.5±0.1 | −4.1±0.4 | 0.1±0.1 | 2.4±0.7* | −4.3±1.5 | 1.4±0.2* | 0.2±0.1* | −1.8±0.0 | 0.0±0.0 |
| A17 | S. sanguinis SK36 | CF | 2.1±0.2* | −1.0±0.3 | 1.3±0.1* | 1.5±0.2* | −1.5±0.3 | 0.4±0.0 | 2.0±0.3* | −1.7±0.3 | 0.5±0.1* | 1.0±0.2 | −3.1±0.1 | 0.1±0.0 | 1.4±0.3 | −4.6±1.1 | 1.3±0.3* | 0.4±0.1* | −2.0±0.1 | 0.3±0.1* |
| A42 | S. sanguinis JCM 5708 | CF | 1.2±0.1* | −6.8±0.3 | 0.6±0.6 | 0.6±0.1 | −8.0±0.0 | −2.1±0.2 | 1.3±0.1 | −8.0±0.0 | −2.8±0.2 | 0.9±0.2 | −8.0±0.0 | −0.3±0.1 | 1.1±0.3 | −8.0±0.0 | 0.7±0.5 | 0.2±0.3* | −6.±0.33 | −1.2±0.3 |
| A49 | S. sanguinis JCM 5708 | CA | 0.0±0.0 | −6.9±0.2 | 0.0±0.0 | 0.0±0.0 | −3.4±0.5 | −0.5±0.1 | 0.0±0.0 | −5.2±0.2 | −0.5±0.1 | 0.0±0.0 | −4.7±0.4 | −0.6±0.1 | 0.0±0.0 | −5.6±0.5 | 0.5±0.2 | 0.0±0.0 | −3.3±0.2 | 0.0±0.0 |
| A52 | S. cristatus ATCC 51100 | CA | 1.6±0.1* | −8.0±0.0 | −1.9±0.5 | 1.7±0.3* | −4.0±0.0 | −0.5±0.1 | 0.9±0.1 | −4.9±0.2 | −2.1±0.4 | 1.2±0.2 | −7.4±1.2 | −2.8±0.4 | 3.1±0.3* | −7.1±0.4 | 1.8±0.6* | 0.3±0.0* | −5.6±0.5 | −0.8±0.9 |
| A55 | S. cristatus F0329 | CA | 1.7±0.1* | −8.0±0.0 | 0.4±0.1 | 0.9±0.1* | −4.0±0.0 | 0.6±0.1* | 0.9±0.1 | −6.5±0.4 | −0.7±0.2 | 0.7±0.2 | −8.0±0.0 | −2.3±0.3 | 1.3±0.1 | −8.0±0.0 | 2.0±0.6* | 0.5±0.1* | −5.7±0.5 | −1.2±0.7 |
Mean average of inhibition zones ± standard deviation (millimeter). Competition assays occurred as follows: (1) arginolytic strain first followed by S. mutans UA159 24 h later; (2) S. mutans UA159 first followed by arginolytic strain 24 h later, and (3) arginolytic strain and S. mutans UA159 simultaneously. Positive values show that arginolytic strains inhibit the growth of S. mutans UA159 while negative values show that the growth of arginolytic strains was inhibited by S. mutans UA159.
indicates that the inhibition zone of the arginolytic clinical strain on S. mutans UA159 is greater than that of S. gordonii DL1 on S. mutans UA159 in a given growth media (p<0.05).
Factors affecting the antagonistic capacity of arginolytic bacteria
Carbohydrate source
To explore how the source of carbohydrate on which the organisms were grown affected the antagonistic capacity of the arginolytic strains on or by S. mutans, the zones of inhibition on TY- 25mM galactose were compared to those on TY-25mM glucose agar plates (Tables 1 and 3). Clearly, a stronger antagonistic capacity against S. mutans was observed when the strains were grown on galactose.
Nutrient availability
Interbacterial antagonism was evaluated in diluted and modified versions of the full-strength media tested. The concentration of nutrients affected antagonistic interactions, with greater antagonism of S. mutans being evident on TY-D-25mM galactose agar, compared to that seen on full-strength TY-25mM galactose (Table 3); TY-D contains 1/3 of the tryptone and yeast extract found in TY medium. For example, S. parasanguinis A1 could not inhibit S. mutans when inoculated simultaneously onto TY-25mM galactose, but displayed strong inhibitory effects on UA159 onto TY-D-25mM galactose agar. An increased antagonistic capacity against UA159 was also observed when S. gordonii A11, S. australis A13, and S. cristatus A52 were inoculated simultaneously with S. mutans onto TY-D-25mM galactose agar. Gradient TY-25mM galactose (25 agar plates were also used, and larger inhibiton zones were evident in the regions of the plates that contained lower nutrient concentrations (Figure S2). Specifically, growth of S. mutans was completely inhibited by S. sanguinis A17 and A42 (Figure S2) in the area of the plates containing lower concentrations of nutrients.
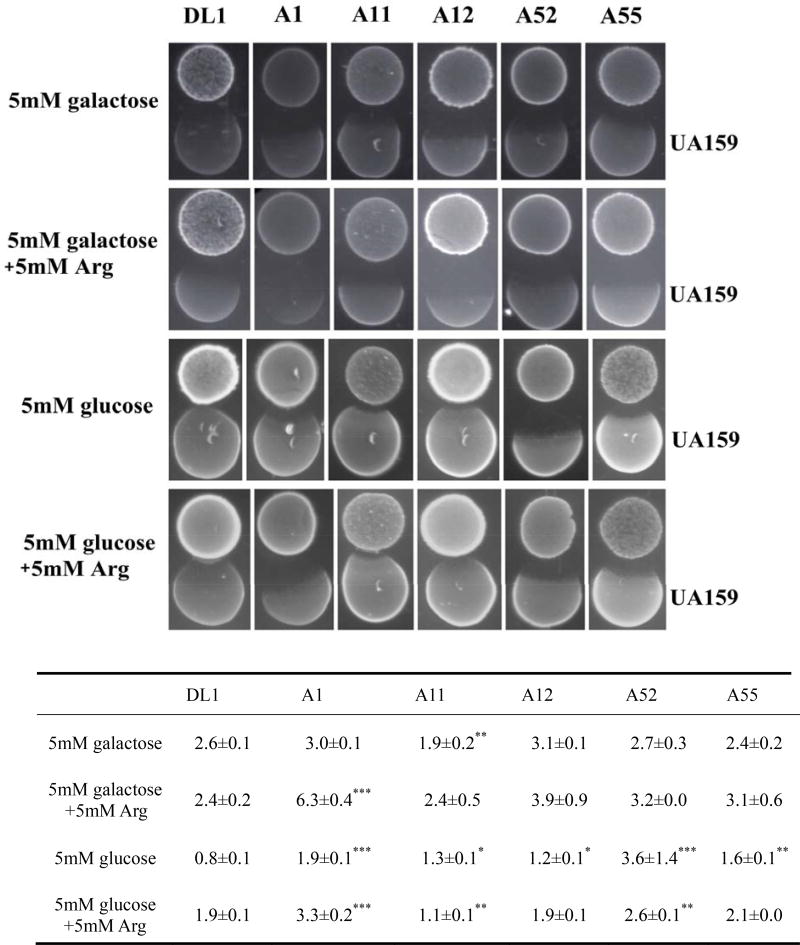
Saliva agar plates supplemented with galactose or glucose, and with or without arginine, were used to mimic nutritional conditions in the oral cavity. All strains displayed substantial antagonistic capacity against S. mutans on saliva agar (Fig. 1), but antagonism was more pronounced when galactose was the primary carbohydrate source, as compared with glucose. The addition of arginine to the saliva plates clearly enhanced the antagonistic capacity of the strains, especially when the medium was supplemented with galactose instead of glucose.
Figure 1.
Growth inhibition zones of arginolytic strains on S. mutans UA159 on saliva agar plates under different growth conditions. Arginolytic strains were inoculated first and S. mutans UA159 was inoculated 24 h later. The table below the figure shows the mean average of inhibition zones ± standard deviation in millimeters. (*) indicates that the inhibition zone of the arginolytic strain on S. mutans UA159 is significantly different compared to S. gordonii DL1 in a given growth condition.
Oxygen
While anaerobes do persist, and in some cases thrive in the oral cavity, the microorganisms in all habitats of the human mouth are exposed continuously to relatively high concentrations of oxygen [Marquis, 1995]. To explore how the presence of oxygen influenced the antagonistic capacity of the arginolytic strains on and by S. mutans, growth on TY-D-25mM galactose was compared under aerobic and anaerobic conditions (Table 3). In all cases, growth in aerobic conditions favors the antagonistic capacity of the arginolytic strains against UA159.
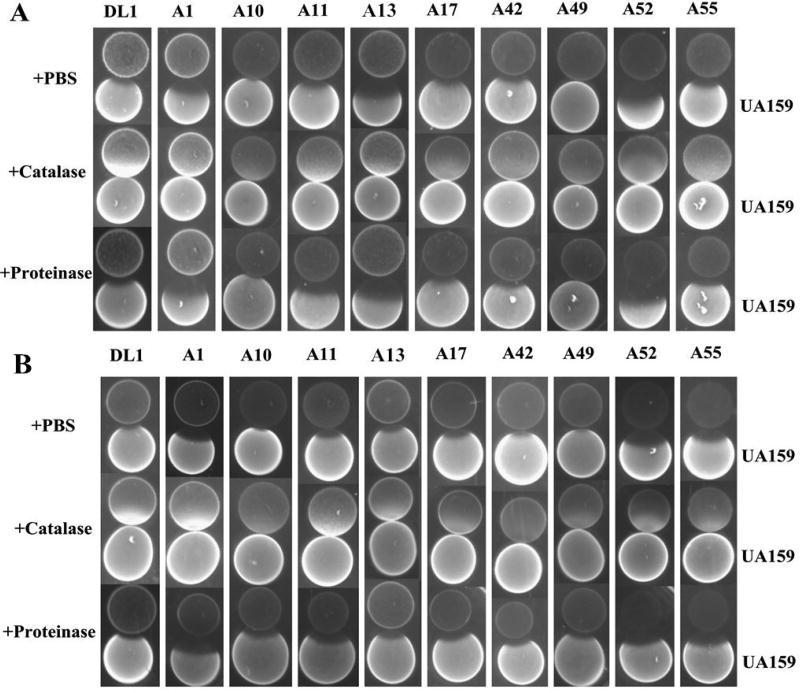
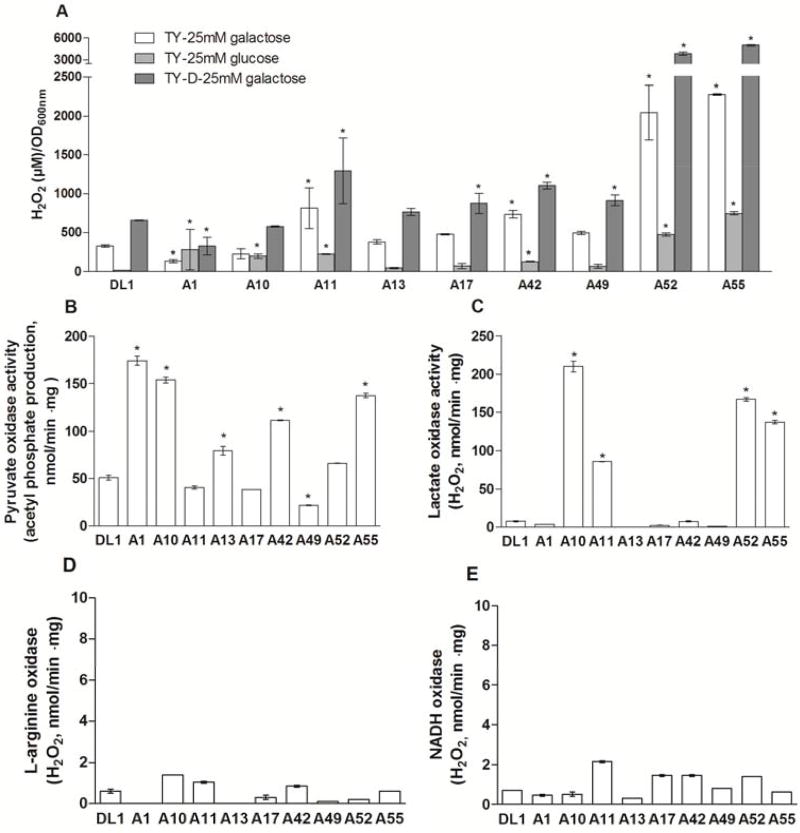
H2O2 is a primary antagonistic compound produced by the arginolytic isolates
Figure 2 illustrates the zones of growth inhibition of the arginolytic strains on and by S. mutans UA159 on agar plats containing catalase or proteinase K, or an equivalent amount of the carrier (PBS) used for these enzymes as control. Irrespective of the inoculation order of commensals and S. mutans, no zones of inhibition were observed in media containing catalase. Figure 3 shows that there was substantial variation in the capacity of the different strains to produce H2O2 under the conditions tested (see methods for details). Two-way ANOVA supported that the type of strain and the growth conditions influenced the production of H2O2 (p<0.001; Figure 3A). Generally speaking, greater levels of H2O2 were measured after growth in TY-D-25mM galactose broth, as compared to TY-25mM galactose broth (p=0.002) and TY-25mM glucose broth (p<0.001). The presence of glucose inhibited H2O2 production by all strains compared to galactose (p=0.022). Of note, the strains A52 (best 16S rRNA gene sequence match: S. cristatus ATCC 51100) and A55 (best 16S rRNA gene sequence match: S. cristatus F0329) produced significantly more H2O2 compared to the other strains in all growth conditions tested (p<0.001).
Figure 2.
Characterization of growth inhibitory substance produced by arginolytic strains on a dilute version of TY (TY-D; 1% tryptone and 0.2% yeast extract) agar plates supplemented with 25 mM galactose. (A) arginolytic strain were inoculated first followed by inoculation of S. mutans UA159 24 h later, and (B) arginolytic strain and UA159 were inoculated simultaneously.
Figure 3.
Production of H2O2 by arginolytic strains. (A) H2O2 produced during growth in different broth media, (B) pyruvate oxidase activity, (C) lactate oxidase activity, (D) L-arginine oxidase activity, and (E) NADH oxidase activity. (*) indicates statistically significant difference between the arginolytic strain and DL1 in a given growth condition (p<0.05).
To determine the primary metabolic pathways for the production of H2O2 by the commensal streptococci, the activity of various H2O2-generating oxidases was assayed as detailed in the methods section (Figure 3B–E). The strains S. parasanguinis A1, S. gordonii A10, S. australis A13, S. sanguinis A42, S. cristatus A52 and S. cristatus A55 presented with higher levels of pyruvate oxidase activity, compared to the reference strain of S. gordonii, DL1 (p<0.001). S. gordonii A10 and A11, and S. cristatus A52 and A55 also produced significant quantities of lactate oxidase, which during the conversion of lactate to pyruvate generates H2O2. All strains produced very low levels of L-arginine oxidase activity and NADH oxidase activity under the growth conditions tested.
S. mutans UA159 inhibits the growth of arginolytic bacteria when inoculated first
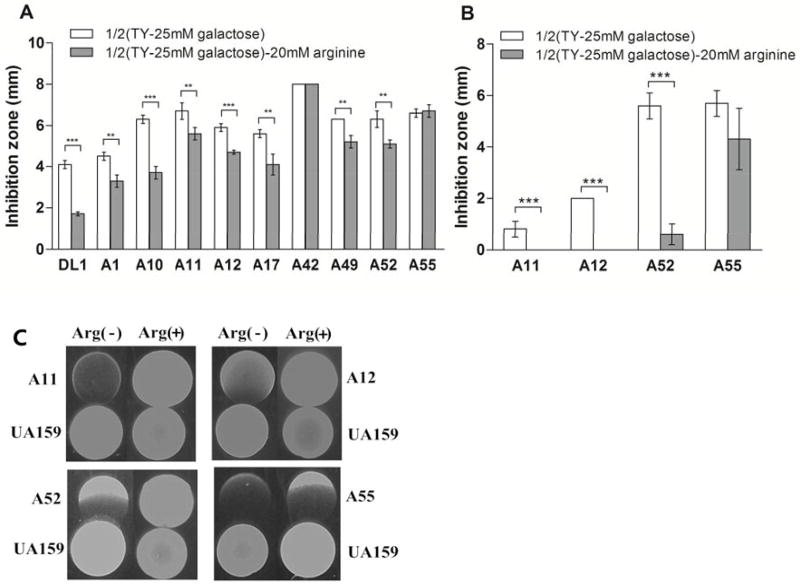
S. mutans UA159 could inhibit the growth of all the arginolytic strains if inoculated first, albeit to differing degrees. Strains of S. cristatus and S. intermedius appered to be more sensitive to inhibition by UA159 compared to S. parasanguinis, S. gordonii and S. australis (Tables 1 and 3). Table 3 and Figure 4 show that the antagonistic capacity of UA159 on the arginolytic strains was also affected by the carbohydrate source, richness of the medium, and presence of arginine or oxygen. Table 3 shows that oxygen favors the antagonistic capacity of S. mutans against the arginolytic strains, which is consistent with the observation that growth in an aerobic conditions induces mutacin gene expression [Ahn et al., 2007]. Arginine was shown to significantly enhance the resistance of arginolytic strains to inhibition by S. mutans during growth in either aerobic or anaerobic conditions. Significantly smaller zones of inhibition were elicited by S. mutans against the arginolytic strains during aerobic growth in the presence of arginine, as compared to growth without arginine (p<0.01), with the exception of S. sanguinis A42 and S. cristatus A55 (p>0.05). Figure 4C shows no zones of inhibition of S. mutans with S. gordonii A11, or with S. australis A12 or S. cristatus A52 during anaerobic growth in the presence of arginine (p<0.001).
Figure 4.
Growth inhibition zones of S. mutans UA159 on arginolytic strains in diluted TY (TY-D-25mM galactose) agar plates with or without 20 mM arginine. Mean average of inhibition zones ± standard deviation (millimeter). (A) aerobic growth condition, (B) anaerobic growth condition, and (C) growth inhibition zones in anaerobic growth condition. Statistically significant differences between growth conditions within the same strain is shown as (**) p<0.01 or (***) p<0.001.
DISCUSSION
The application of new molecular methodologies, such as next-generation sequencing, to the study of the human oral microbiome has revealed remarkable diversity in the microbiota in oral biofilms [Mager et al., 2003; Corby et al., 2005; Aas et al., 2008; Dewhirst et al., 2010; Gross et al., 2010; Crielaard et al., 2011; Burne et al., 2012]. Inherent to the ecological view of any natural environment is that bacterial populations evolve competitive and cooperative strategies to survive and persist. For example, antagonism between beneficial commensals and cariogenic bacteria is an example of a strategy used by bacteria to survive in supragingival biofilms, and is also a major factor that shapes the composition and ecology of biofilms. However, the evolutionary mechanisms that select for and maintain traits that benefit certain bacteria and populations in the oral cavity, ultimately affecting dental health, are not well characterized. This study investigated the antagonistic potential of a spectrum of low-passage clinical isolates of commensal arginolytic strains against and by the strongly cariogenic and well-characterized human isolate S. mutans UA159. Although previous studies have demonstrated the antagonistic capacity of laboratory strains of S. gordonii and S. sanguinis against S. mutans [Kreth et al., 2005; Kreth et al., 2008; Zhu and Kreth, 2012], to our knowledge, this study is the first to focus on a diverse group of clinical isolates of known arginine metabolic capacity to compete with a cariogenic bacterium. Not only was there a great degree of heterogeneity in the capacity of strains to compete with S. mutans, but also the order in which the organisms were established and environmental factors greatly influenced the outcomes of interbacterial interactions. S. mutans UA159 consistently inhibited the growth of arginolytic bacteria when it was allowed to become established prior to inoculation of the commensal organisms. However, some arginolytic bacteria could also inhibit the growth of UA159 when they were inoculated first or simultaneously with UA159. Importantly, most of the arginolytic bacteria studied here, such as S. sanguinis, S. parasanguinis, S. gordonii, and S. cristatus are pioneer colonizers of the tooth surfaces [Hojo et al., 2009; Wu and Xie, 2010; Liang et al., 2011] and therefore have the potential to become established on the teeth prior to acquisition of S. mutans. In fact, early colonization of S. sanguinis in the oral cavity of infants was shown to delay the colonization of mutans streptococci [Caufield et al., 2000], which in turn has been associated with lower caries prevalence in the primary [Kohler et al., 1988] and permanent dentition [Kohler and Andreen, 2012]. Thus, early establishment on tooth surfaces of strongly arginolytic strains with high capacities to compete with S. mutans in oral biofilms may be a potent deterrent to the initiation of carious lesions.
Long-term persistence of an organism in a complex ecosystem requires effective mechanisms to prevent being dominated or eliminated by competitors [Hibbing et al., 2010]. In the context of dental caries, lesions begin to form on tooth surfaces when the cycles of acidification outweigh those of alkalization of oral biofilms; driving the eventual emergence of a more aciduric microflora and lowers biofilm pH values levels that results in a net loss of tooth mineral [Burne and Marquis, 2000; Huang et al., 2012c]. Metabolic activities by oral bacteria that help to promote a more neutral pH and to prevent the outgrowth of cariogenic species should have a strong anti-caries effect. Importantly, ammonia production from arginine metabolism is a typical example of a strategy used by many abundant oral streptococci to survive acidification of oral biofilms and to compete with bacteria in oral biofilms that can better tolerate acidic conditions. A microflora that is both robustly arginolytic and capable of interfering with the colonization, growth or expression of virulence attributes of S. mutans should be beneficial to the host.
In this study, the antagonistic capacity of the arginolytic strains on S. mutans UA159 varied significantly between different species, between isolates of the same taxa, and between strains isolated from subjects of different caries status. The agar-based inhibition assay used in this study is routinely used to assess the ability of S. mutans to inhibit the growth of commensals as mutacins are optimally produced in colonies on agar plates. The assay is also consistent, reliable and suitable for screening of a large number of clinical strains. A wide spectrum of antagonistic capacity against S. mutans was observed among strains of S. intermedius, S. sanguinis, S. parasanguinis, S. gordonii, S. australis and S. cristatus. More specifically, strains of S. intermedius showed no antagonistic capacity against S. mutans, whereas strains of S. australis showed the highest capacity to inhibit the growth of S. mutans when inoculated first or simultaneously on the different media tested. Furthermore, two strains of S. sanguinis isolated from a CF subject, most similar in 16S rRNA gene sequence to SK36 and ChDC B357, also presented different antagonistic capacities against S. mutans. A strain of S. gordonii (A8, 16S rRNA gene sequence most similar to str. Challis substr. CH1) isolated from a CF subject presented greater inhibitory effect on S. mutans compared to a similar strain (A7) isolated from a CA subject. In fact, strains isolated from CF subjects generally displayed stronger antagonistic capacity against S. mutans than those from CA subjects. This observation provides support for the hypothesis that there is significant heterogeneity in the phenotypic capabilities across and within species of oral bacteria that can impact their influence on oral health and caries development [Burne et al., 2012; Palmer et al., 2013]. According to Hibbing [Hibbing et al., 2010], the combination of rapid growth rates and large population sizes results in the introduction of many unique mutations in bacterial species, which can mediate competition even between genetically identical individuals in a population. These data emphasize the need to expand efforts on comprehensive comparative genomics of abundant commensals associated with CA and CF individuals and to use this information to establish whether there are genotypes or clearly definable genetic differences that can allow for the discrimination of commensals that are beneficial from those that are merely not harmful, or that may be overtly harmful; such as the low-pH streptococci described initially by van Houte and co-workers [van Houte et al., 1996]. Thus, we plan to obtain complete genome sequences for a large collection of commensal streptococci that display a spectrum in potentially beneficial phenotypic behaviors.
Mutacin production by S. mutans, ADS expression and H2O2 production by commensal oral streptococci, and a variety of other factors that may influence the persistence of these species in oral biofilms are subject to regulation by enviromental inputs and other signals [Lemos et al., 2005; Ahn et al., 2007; Lemos and Burne, 2008; Kreth et al., 2009; Kreth et al., 2011; Burne et al., 2012; Huang et al., 2015]. The findings presented here clearly show that nutrient source and availability, oxygen and other factors (e.g. factors susceptible to proteinase K) profoundly affect the competition between commensal/beneficial streptococci and a caries pathogen. During fasting periods, galactose is one of the more abundant carbohydrates secreted into the oral cavity [Caldwell and Pigman, 1966] and certain commensal streptococci have a higher capacity to transport and metabolize galactose than most S. mutans isolates [Zeng et al., 2012]. Periodic ingestion of dietary carbohydrates provides other carbohydrates to oral bacteria, such as glucose, which leads to rapid acid production by S. mutans, but can also leads to repression of ADS gene expression via carbohydrate catabolite repression (CCR). Moreover, H2O2 production may also be down-regulated by CCR [Zheng et al., 2011a]. Our findings revealed greater inhibition of growth of S. mutans by arginolytic strains and higher levels of H2O2 production when cells were grown with galactose as the primary carbohydrate source, compared to glucose. Other factors favoring the antagonistic capacity of, and H2O2 production by, arginolytic bacteria included decreased availability of nutrients in tryptone and/or yeast extract, and the presence of arginine or oxygen. Particularly, arginine has been recently shown to influence the architecture and physicochemical properties of the biofilm matrix formed in mixed cultures of S. mutans UA159 and S. gordonii DL1 [He et al., 2016]. Arginine decreased the expression of gtfB, encoding an enzyme involved in water-insoluble glucan synthesis, and a bacteriocin (SMU.150), while increasing the expression of spxB of S. gordonii, which produces H2O2 from pyruvate.
Our findings expand our knowledge of the effects of arginine metabolism on the ecology of oral biofilms, and clearly demonstrate that there is a profound heterogeneity among commensal streptococci in their arginolytic and antagonism capacities, which cannot be discerned simply by 16S rRNA gene sequence comparisons. Certain commensals may have a particularly beneficial impact on the ecology of oral biofilms by synergistically moderating plaque pH and antagonizing the growth and virulence of caries pathogens, and perhaps periodontal pathogens, through H2O2 production and other metabolic mechanisms. Future studies should evaluate the ability of a select subset of commensal strains to antagonize other caries pathogens (e.g. Scardovia, Lactobacilli). Of significant clinical relevance, our approach coupled with analysis of the genomes of these organisms can guide the formulation of probiotic preparations composed of beneficial commensals for control of oral diseases.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Dental and Craniofacial Research K23 DE023579 and R01 DE25832. R.A.B. designed the study. M.M.N. performed the clinical examinations for recruitment of research subjects and collected dental plaque samples. X.H., C.M.B., and S.J.A. performed the biochemical and molecular biology assays of the study. M.J. performed the statistical analysis. X.H., R.A.B. and M.M.N. evaluated and interpreted the data, and wrote the manuscript.
R.A.B. and M.M.N. hold a provisional patent through the University of Florida on methodologies to identify arginolytic oral isolates that might eventually be incorporated into probiotic formulations to promote oral health. R.A.B. and M.M.N have received research funding in the past from the Colgate-Palmolive Company, which is presently marketing toothpastes that contain arginine as an active ingredient.
Footnotes
DECLARATION OF INTERESTS
The authors have no other potential conflicts of interest.
Contributor Information
Xuelian Huang, Division of General Dentistry, Eastman Institute for Oral Health, University of Rochester, Rochester, NY, USA.
Christopher M. Browngardt, Department of Oral Biology, College of Dentistry, University of Florida, Gainesville, FL, USA
Min Jiang, Department of Epidemiology and Biostatistics, West China School of Public Health, Sichuan University, Chengdu, Sichuan, China.
Sang-Joon Ahn, Department of Oral Biology, College of Dentistry, University of Florida, Gainesville, FL, USA.
Robert A. Burne, Department of Oral Biology, College of Dentistry, University of Florida, Gainesville, FL, USA.
Marcelle M. Nascimento, Department of Restorative Dental Sciences, Division of Operative Dentistry, College of Dentistry, University of Florida, Gainesville, FL, USA.
References
- Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, Leys EJ, Paster BJ. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 2008;46:1407–1417. doi: 10.1128/JCM.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SJ, Wen ZT, Burne RA. Effects of oxygen on virulence traits of Streptococcus mutans. J Bacteriol. 2007;189:8519–8527. doi: 10.1128/JB.01180-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne RA, Marquis RE. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett. 2000;193:1–6. doi: 10.1111/j.1574-6968.2000.tb09393.x. [DOI] [PubMed] [Google Scholar]
- Burne RA, Zeng L, Ahn SJ, Palmer SR, Liu Y, Lefebure T, Stanhope MJ, Nascimento MM. Progress dissecting the oral microbiome in caries and health. Adv Dent Res. 2012;24:77–80. doi: 10.1177/0022034512449462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell RC, Pigman W. Changes in protein and glycoprotein concentrations in human submaxillary saliva under various stimulatory conditions. Arch Oral Biol. 1966;11:437–450. doi: 10.1016/0003-9969(66)90108-7. [DOI] [PubMed] [Google Scholar]
- Caufield PW, Dasanayake AP, Li Y, Pan Y, Hsu J, Hardin JM. Natural history of Streptococcus sanguinis in the oral cavity of infants: evidence for a discrete window of infectivity. Infect Immun. 2000;68:4018–4023. doi: 10.1128/iai.68.7.4018-4023.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corby PM, Lyons-Weiler J, Bretz WA, Hart TC, Aas JA, Boumenna T, Goss J, Corby AL, Junior HM, Weyant RJ, Paster BJ. Microbial risk indicators of early childhood caries. J Clin Microbiol. 2005;43:5753–5759. doi: 10.1128/JCM.43.11.5753-5759.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crielaard W, Zaura E, Schuller AA, Huse SM, Montijn RC, Keijser BJ. Exploring the oral microbiota of children at various developmental stages of their dentition in the relation to their oral health. BMC Med Genomics. 2011;4:22. doi: 10.1186/1755-8794-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran TM, Ma YS, Rutherford GC, Marquis RE. Turning on and turning off the arginine deiminase system in oral streptococci. Can J Microbiol. 1998;44:1078–1085. doi: 10.1139/cjm-44-11-1078. [DOI] [PubMed] [Google Scholar]
- De Jong MH, Van Der Hoeven JS, Van Os JH. Growth of micro-organisms from supragingival dental plaque on saliva agar. J Dent Res. 1986;65:85–88. doi: 10.1177/00220345860650021601. [DOI] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Chen YYM, Burne RA. Control of expression of the arginine deiminase operon of Streptococcus gordonii by CcpA and Flp. J Bacteriol. 2004;186:2511–2514. doi: 10.1128/JB.186.8.2511-2514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Chen YYM, Snyder JA, Burne RA. Isolation and molecular analysis of the gene cluster for the arginine deiminase system from Streptococcus gordonii DL1. Appl Environ Microbiol. 2002;68:5549–5553. doi: 10.1128/AEM.68.11.5549-5553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler ML, Ingram-Smith CJ, Smith KS. Direct detection of the acetate-forming activity of the enzyme acetate kinase. J Vis Exp. 2011;58:e3474. doi: 10.3791/3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross EL, Leys EJ, Gasparovich SR, Firestone ND, Schwartzbaum JA, Janies DA, Asnani K, Griffen AL. Bacterial 16S sequence analysis of severe caries in young permanent teeth. J Clin Microbiol. 2010;48:4121–4128. doi: 10.1128/JCM.01232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Hwang G, Liu Y, Gao L, Kilpatrick-Liverman L, Santarpia P, Zhou X, Koo H. L-arginine modifies the exopolysaccharides matrix and thwarts Streptococcus mutans outgrowth within mixed-species oral biofilms. J Bacteriol. 2016;198(19):2651–2661. doi: 10.1128/JB.00021-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbing ME, Fuqua C, Parsek MR, Peterson SB. Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol. 2010;8:15–25. doi: 10.1038/nrmicro2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo K, Nagaoka S, Ohshima T, Maeda N. Bacterial interactions in dental biofilm development. J Dent Res. 2009;88:982–990. doi: 10.1177/0022034509346811. [DOI] [PubMed] [Google Scholar]
- Huang X, Cheng L, Exterkate RAM, Liu M, Zhou X, Li J, ten Cate JM. Effect of pH on Galla chinensis extract's stability and anti-caries properties in vitro. Arch Oral Biol. 2012a;57:1093–1099. doi: 10.1016/j.archoralbio.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Huang X, Exterkate RAM, ten Cate JM. Factors associated with alkali production from arginine in dental biofilms. J Dent Res. 2012b;91:1130–1134. doi: 10.1177/0022034512461652. [DOI] [PubMed] [Google Scholar]
- Huang X, Liu M, Li J, Zhou X, ten Cate JM. Chemical composition of Galla chinensis extract and the effect of its main component(s) on the prevention of enamel demineralization in vitro. Int J Oral Sci. 2012c;4:146–151. doi: 10.1038/ijos.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Palmer SR, Ahn S-J, Richards VP, Williams ML, Nascimento MM, Burne RA. A highly arginolytic Streptococcus species that potently antagonizes Streptococcus mutans. Appl Environ Microbiol. 2016;82:2187–2201. doi: 10.1128/AEM.03887-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Schulte RM, Burne RA, Nascimento MM. Characterization of the arginolytic microflora provides insights into pH homeostasis in human oral biofilms. Caries Res. 2015;49:165–176. doi: 10.1159/000365296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberg I. Prevention and dental caries. J Prev Dent. 1978;5:9–17. [PubMed] [Google Scholar]
- Kohler B, Andreen I. Mutans streptococci and caries prevalence in children after early maternal caries prevention: a follow-up at 19 years of age. Caries Res. 2012;46:474–480. doi: 10.1159/000339665. [DOI] [PubMed] [Google Scholar]
- Kohler B, Andreen I, Jonsson B. The earlier the colonization by mutans streptococci, the higher the caries prevalence at 4 years of age. Oral Microbiol Immunol. 1988;3:14–17. doi: 10.1111/j.1399-302x.1988.tb00598.x. [DOI] [PubMed] [Google Scholar]
- Kreth J, Merritt J, Qi F. Bacterial and host interactions of oral streptococci. DNA Cell Biol. 2009;28:397–403. doi: 10.1089/dna.2009.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth J, Merritt J, Qi F, Dong X, Shi W. Antagonistic, synergistic, and counteroffensive strategies for streptococcal interspecies interactions. In: Kolenbrander P, editor. Oral Microbial Communities. Washington, DC: ASM Press; 2011. pp. 331–343. [Google Scholar]
- Kreth J, Merritt J, Shi W, Qi F. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J Bacteriol. 2005;187:7193–7203. doi: 10.1128/JB.187.21.7193-7203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth J, Zhang Y, Herzberg MC. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J Bacteriol. 2008;190:4632–4640. doi: 10.1128/JB.00276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos J, Abranches J, Burne R. Responses of cariogenic streptococci to environmental stresses. Current Issues Mol Biol. 2005;7:95–107. [PubMed] [Google Scholar]
- Lemos JA, Burne RA. A model of efficiency: stress tolerance by Streptococcus mutans. Microbiology. 2008;154:3247–3255. doi: 10.1099/mic.0.2008/023770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JA, Quivey RG, Koo H, Abranches J. Streptococcus mutans: a new gram-positive paradigm? Microbiology. 2013;159:436–445. doi: 10.1099/mic.0.066134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Chen Y-YM, Ruiz T, Wu H. New cell surface protein involved in biofilm formation by Streptococcus parasanguinis. Infect Immun. 2011;79:3239–3248. doi: 10.1128/IAI.00029-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Tong H, Dong X. Function of the pyruvate oxidase-lactate oxidase cascade in interspecies competition between Streptococcus oligofermentans and Streptococcus mutans. Appl Environ Microbiol. 2012;78:2120–2127. doi: 10.1128/AEM.07539-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Dong Y, Chen YYM, Burne RA. Environmental and growth phase regulation of the Streptococcus gordonii arginine deiminase genes. Appl Environ Microbiol. 2008;74:5023–5030. doi: 10.1128/AEM.00556-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager DL, Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Distribution of selected bacterial species on intraoral surfaces. J Clin Periodontol. 2003;30:644–654. doi: 10.1034/j.1600-051x.2003.00376.x. [DOI] [PubMed] [Google Scholar]
- Margolis HC, Duckworth JH, Moreno EC. Composition of pooled resting plaque fluid from caries-free and caries-susceptible individuals. J Dent Res. 1988;67:1468–1475. doi: 10.1177/00220345880670120601. [DOI] [PubMed] [Google Scholar]
- Marquis R. Oxygen metabolism, oxidative stress and acid-base physiology of dental plaque biofilms. J Ind Microbiol. 1995;15:198–207. doi: 10.1007/BF01569826. [DOI] [PubMed] [Google Scholar]
- Merritt J, Qi F. The mutacins of Streptococcus mutans: regulation and ecology. Mol Oral Microbiol. 2012;27:57–69. doi: 10.1111/j.2041-1014.2011.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento MM, Burne RA. Caries prevention by arginine metabolism in oral biofilms: translating science into clinical success. Current Oral Health Reports. 2014;1:79–85. [Google Scholar]
- Nascimento MM, Gordan VV, Garvan CW, Browngardt CM, Burne RA. Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiol Immunol. 2009;24:89–95. doi: 10.1111/j.1399-302X.2008.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento MM, Liu Y, Kalra R, Perez S, Adewumi A, Xu X, Burne RA. Arginine metabolism may confer caries resistance in children. J Dent Res. 2012;91:691. doi: 10.1177/0022034513487907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Zhang Z, Huang I-H, Wu C, Merritt J, Shi W, Qi F. Genes involved in the repression of mutacin I production in Streptococcus mutans. Microbiology. 2009;155:551–556. doi: 10.1099/mic.0.021303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SR, Miller JH, Abranches J, Zeng L, Lefebure T, Richards VP, Lemos JA, Stanhope MJ, Burne RA. Phenotypic heterogeneity of genomically-diverse isolates of Streptococcus mutans. Plos One. 2013;8:e61358. doi: 10.1371/journal.pone.0061358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissons CH, Cutress TW. pH changes during simultaneous metabolism of urea and carbohydrate by human salivary bacteria in vitro. Arch Oral Biol. 1988;33:579–587. doi: 10.1016/0003-9969(88)90133-1. [DOI] [PubMed] [Google Scholar]
- Stephan RM. Intra-oral hydrogen-ion concentration associated with dental caries activity. J Dent Res. 1944;23:257–266. [Google Scholar]
- Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- Tanzer J, Livingston J, Thompson A. The microbiology of primary dental caries in humans. J Dent Educ. 2001;65:1028–1037. [PubMed] [Google Scholar]
- Tong H, Chen W, Merritt J, Qi F, Shi W, Dong X. Streptococcus oligofermentans inhibits Streptococcus mutans through conversion of lactic acid into inhibitory H2O2: a possible counteroffensive strategy for interspecies competition. Mol Microbiol. 2007;63:872–880. doi: 10.1111/j.1365-2958.2006.05546.x. [DOI] [PubMed] [Google Scholar]
- Turtola LO, Luoma H. Plaque pH in caries-active and inactive subjects modified by sucrose and fluoride, with and without bicarbonate-phosphate. Scand J Dent Res. 1972;80:334–343. doi: 10.1111/j.1600-0722.1972.tb00299.x. [DOI] [PubMed] [Google Scholar]
- van Houte J, Lopman J, Kent R. The final pH of bacteria comprising the predominant flora on sound and carious human root and enamel surfaces. J Dent Res. 1996;75:1008–1014. doi: 10.1177/00220345960750040201. [DOI] [PubMed] [Google Scholar]
- Wu J, Xie H. Role of arginine deiminase of Streptococcus cristatus in Porphyromonas gingivalis colonization. Antimicrob Agents Chemother. 2010;54:4694–4698. doi: 10.1128/AAC.00284-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Martino NC, Burne RA. Two gene clusters coordinate galactose and lactose metabolism in Streptococcus gordonii. Appl Environ Microbiol. 2012;78:5597–5605. doi: 10.1128/AEM.01393-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Chen Z, Itzek A, Ashby M, Kreth J. Catabolite control protein A controls hydrogen peroxide production and cell death in Streptococcus sanguinis. J Bacteriol. 2011a;193:516–526. doi: 10.1128/JB.01131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Itzek A, Chen Z, Kreth J. Environmental influences on competitive hydrogen peroxide production in Streptococcus gordonii. Appl Environ Microbiol. 2011b;77:4318–4328. doi: 10.1128/AEM.00309-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Kreth J. The role of hydrogen peroxide in environmental adaptation of oral microbial communities. Oxid Med Cell Longev. 2012;2012:717843. doi: 10.1155/2012/717843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.