Abstract
The cystic fibrosis (CF) field is the beneficiary of five species of animal models that lack functional cystic fibrosis transmembrane conductance regulator (CFTR) channel. These models are rapidly informing mechanisms of disease pathogenesis and CFTR function regardless of how faithfully a given organ reproduces the human CF phenotype. New approaches of genetic engineering with RNA-guided nucleases are rapidly expanding both the potential types of models available and the approaches to correct the CFTR defect. The application of new CRISPR/Cas9 genome editing techniques are similarly increasing capabilities for in vitro modeling of CFTR functions in cell lines and primary cells using air-liquid interface cultures and organoids. Gene editing of CFTR mutations in somatic stem cells and induced pluripotent stem cells is also transforming gene therapy approaches for CF. This short review evaluates several areas that are key to building animal and cell systems capable of modeling CF disease and testing potential treatments.
Keywords: animal models, cellular systems, CF lung disease, pancreatic disease, gene editing
Introduction
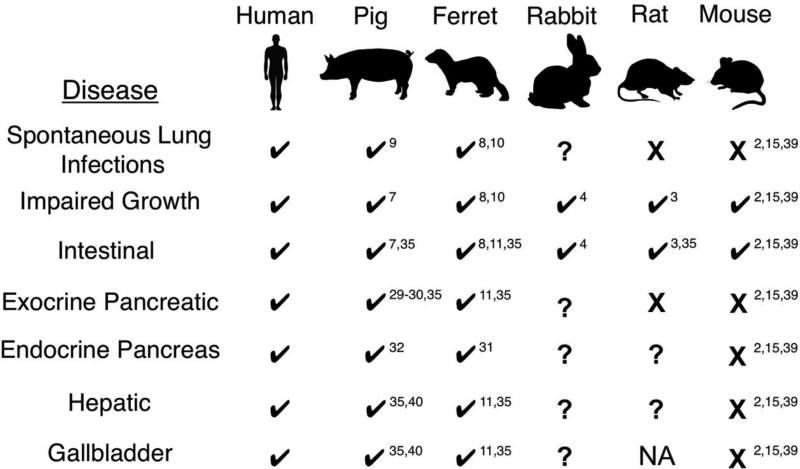
Animal models of disease are indispensable for understanding disease pathogenesis and critical for developing new therapies. The cystic fibrosis (CF) field is unusually fortunate in this regard with multiple species that offer unique advantages (Figure 1). While the CF mouse lacks a spontaneous lung and pancreatic phenotype, it has provided a wealth of insight into the biological underpinnings of CFTR-deficient epithelia in other organs such as the intestine, and lessons learned from CF mice have laid a rich foundation for other CF animal models (1, 2). The CF ferret and pig recapitulate the full spectrum of the CF phenotype observed in human patients, from inflammatory and infectious lung disease to in utero meconium ileus and CF-related diabetes (CFRD). However, the ferret and pig models are resource intensive and pose some challenges that limit broad utilization by the field. The CF rat acquires gut obstruction at weaning and while it remains unclear if the CF rat lung will become spontaneously infected, the tracheal surface has a reduced airway surface liquid layer (ASL) but normal mucociliary clearance at birth (3). The rabbit is the newest addition to the Noah’s Ark of CF models (4), however, other than gut obstruction at weaning, the phenotype remains unclear and the model is awaiting publication in a peer-reviewed forum. CRISPR/Cas9-targeting technologies used to disrupt the CFTR gene in rabbits also has significant potential for generating in vitro models of epithelia for the study of cellular processes impacted by CFTR. This brief review will discuss salient features of both in vitro and in vivo CF model systems.
Figure 1.
Organ disease presence or absence in various CFTR-knockout (CF) animal models. Organs affected in humans with CF are listed on the left. Check marks below each CF model species indicates disease presence, while question marks denote disease has yet to be evaluated. Organs marked by an X indicate lack of overt disease. NA, not applicable as rats do not have a gallbladder. Superscript denotes references for the relevant studies in the literature.
Animal models of CF
The CFTR-knockout pig and ferret were first generated nearly 10 years ago (5, 6). These species were chosen because of their conserved lung cell biology with humans (Table 1). For example, ferrets and pigs contain submucosal glands (SMGs) throughout the cartilaginous airways, while in mice and rats SMGs are limited to the trachea and rabbits lack SMGs. Organs affected by the lack of CFTR in pigs and ferrets include the lung, pancreas, intestine, liver, gallbladder, and vas deferens (7–11). We will focus this portion of the review on several of these organs and compare across the most described model species.
Table 1.
Cellular anatomy of the airways in CF animal models.
| Species | Submucosal Glands (Airway Level) |
Secretory Cell Type (Proximal Airways) |
Secretory Cell Type (Distal Airways) |
Respiratory Bronchioles |
|---|---|---|---|---|
| Mouse | Proximal Trachea | Club | Club | Absent |
| Rat | Trachea | Serous | Club | Absent |
| Rabbit | Absent | Club | Club | Absent |
| Ferret | Trachea / Bronchial | Goblet | Club | Several generations |
| Pig | Trachea / Bronchial | Goblet | Club | Single generation |
| Human | Trachea / Bronchial | Goblet | Club | Several generations |
The information in this table was largely extracted from the following book (12).
Lung disease
CFTR-knockout mice were engineered soon after the discovery of the gene (13, 14) and while they have an intestinal phenotype, it is generally accepted that CF mice do not spontaneously develop lung infections like CF humans (2, 15). Nonetheless, certain strains of CF mice have an elevated proinflammatory response to Pseudomonas-laden beads agar bead instilled in the lung when compared to control animals (16); however, this abnormal response is thought to stem from CFTR function within the hematopoietic compartment since bone marrow transplants between CF→WT and WT→CF can either produce or reverse the phenotype, respectively (17). The lack of substantial and spontaneous lung disease in CF mice has prompted the creation of alternative mouse models that exhibited the classic muco-obstructive and inflammatory pathology observed in human CF lungs. Targeted overexpression of a subunit of the epithelial sodium channel (β-ENaC) in mouse airways produced a model strikingly similar to CF lung disease, including reduced mucociliary transport (MCT), airway obstruction with mucus, neutrophil dominant immune responses, increased susceptibility to bacterial infections, and shortened lifespan (18, 19). The need for animal models that fully reproduce the human CF lung phenotype prompted the creation of CF ferrets and pigs. CFTR-knockout ferrets and pigs both acquire spontaneous lung infections after birth, generally within weeks to months (8, 9). The CF ferret is particularly susceptible to bacterial colonization within the first few weeks of life and must be reared on antibiotics to survive this period (8, 10). However, once the ferret lung is mature (~1 month of age), CF lung disease progresses more slowly following removal of antibiotics over several months (10). Colonizing bacterial flora in both models are quite diverse but Staphylococcus, Streptococcus, Enterococcus, and other enteric bacteria predominate (9, 10). Mucus secretion is exuberant and remains unusually adherent to tissue structures, both at the submucosal gland duct and along epithelial surfaces (8–11, 20), and mucociliary clearance is severely impaired (20–22). Studies of lung inflammation in both species reveal two seemingly distinct patterns. In the newborn CF pig, lungs have normal levels of neutrophils and IL-8 on the bronchoalveolar lavage (BAL) fluid (7). By contrast, the lungs of CF ferrets born sterilely versus natural birth exhibit dysregulated neutrophil and IL-8 centered inflammation to bacterial exposure during birth as compared to non-CF animals (21) and this same theme progresses in older infected CF ferrets (23). The similarity of both transgenic models to the human lung phenotype is enabling the rapid translation of therapies that target the fundamental defect in CF lung disease, including approaches to alter the airway surface pH (24, 25) and gene therapy (26–28).
Pancreatic disease and CFRD
In contrast to CF mice and rats, which do not develop exocrine pancreatic disease (2, 3), CF pigs and ferrets have a significant pancreatic phenotype (11, 29, 30). Disease progression in the pancreas differs between the two species, initiating in utero in CF pigs (29) and after birth in CF ferrets (8, 11). These pancreatic phenotypes have enabled a better understanding of the pathogenesis of CFRD. Both CF ferret and pig are born with glycemic abnormalities characterized by altered or impaired insulin secretion (31, 32) and abnormalities in insulin secretion are also observed in isolated CF ferret islets (31). Recent studies in the CF ferret model have determined that early exocrine-mediated inflammation reduces beta cell mass and impairs insulin secretion very early in life and that a fibrogenic-to-adipogenic transition is linked to a resurgence of islets with improved glycemic status (33). This phenomenon of early-onset impaired glucose tolerance followed by recovery has also been recently observed in 2-4-year-old CF children (34). Similar to CF humans, older CF ferrets bifurcate into two groups that have either normal or CFRD phenotypes. These findings demonstrate that CFRD is primarily an insulin-deficient disease that emerges early in life and that it develops in well-defined phases characterized by periods of intense inflammation and subsequent pancreatic remodeling. Improved understanding of these processes may empower the early diagnosis and treatment of CF patients at risk for CFRD later in life.
Hepatic disease
The incidence of CF liver disease (CFLD) is 5–10% and contributes 2.5% to the overall mortality of CF patients (35, 36). This also begins early in life with a mean age of diagnosis of 10 years (37) and there is no current effective therapy that averts hepatic failure. Ursodeoxycholic acid (UDCA, ursodiol) is recommended and does appear to help, but many patients still progress to complete liver failure and require transplantation (36, 38). Whereas CF mice do not develop liver disease (although there is an abnormal response to chronic cholate administration) (39), both CF pig and ferret models develop liver and gallbladder disease, although the onset of gallbladder disease is much earlier in CF pigs (7, 11, 35). While bile pH in the gallbladder is significantly more acidic in newborn CF pig (40), it is unchanged in newborn CF ferrets despite the lack of cAMP-mediated chloride conductance (41). Interestingly, newborn CF ferrets are born with elevated alanine aminotransferase (ALT) and total bilirubin levels, suggestive of liver disease (8). Despite the low incidence (0.3%) of clinically apparent liver disease in CF infants (42), 53% demonstrate abnormally elevated liver function tests including ALT and this typically resolves by 2–3 years of age (43). However, this rise in liver function enzymes during the CF neonatal period was not associated with neonatal cholestasis in CF infants (43) or CF ferrets (8). Furthermore, serum ALT and bilirubin levels are normalized by UDCA treatment during the neonatal period CF ferrets (8), as has also been shown to be the case in CF infants (44). Like the pancreas, such studies are beginning uncover early phases of self-resolving disease pathology that may impact liver function later in life. Further investigation of hepatic disease in these larger models may lead to improved methods of treatment of a fairly neglected, but significant, organ affected in CF.
In vitro models to study CFTR function and CF pathogenesis
Here we briefly review in vitro cell models that have been used and developed to study CFTR function in light of recent developments in gene editing techniques. It is acknowledged that polarized in vitro models of epithelia are critical to better understand the role of CFTR. These models, however, show limitations since they rely on comparing a CF condition to a non-CF condition, thus introducing genetic background variability and restricted access to few CFTR variants. In addition, manipulation of CFTR gene expression by conventional methods remains very challenging in these polarized systems.
The recent revolution in genome editing has brought a rise in the use of techniques such as transcription activator-like effector nucleases (TALENs), zinc-finger nucleases (ZFNs) and clustered regularly interspaced short palindromic repeats-Cas9 (CRISPR-Cas9) system for CF research (45, 46). Based on the type II CRISPR system described in Streptococcus pyogenesCRISPR-Cas9 consists of RNA endonuclease (Cas9) that cleaves DNA, and a single guide RNA molecule complementary to a target sequence flanked by proto- spacer adjacent motifs (47). CRISPR-Cas9 has created the opportunity to simply manipulate CFTR gene expression in established epithelial cell models, resulting in insertions or deletions of the targeted locus, or precise replacement of mutations when a homologous donor template is supplied. Although, early CRISPR- Cas9 systems displayed off-target effects, new versions show highly specific target gene binding and editing (45, 48).
Typically, cell lines derived from adenocarcinomas like Calu-3 (submucosal airway glands) and T84 (colon) cells are widely used because of their high level of CFTR expression, its apical localization when cultured on Transwell® filters, and their ability to develop a significant transepithelial resistance. Standardization of the culture protocol made primary human airway epithelial cells (HAECs) one of the favored in vitro models for CF research (49). Primary HAEC cultures are established by expanding progenitor cells isolated from biopsies (polyps, nasal and bronchial brushes, dissected bronchi) of donors and patients, which are then seeded on Transwell® filters and exposed to an air-liquid interface for several weeks. Under these conditions, progenitor cells differentiate into a pseudostratified epithelium that recapitulates the typical mucociliary structure of the airway epithelium (49). Pharmacological correction of CFTR function in primary nasal HAECs can be used as a biomarker in personalized CF treatment. These cultures, which can regenerate after injury, also represent an excellent model to investigate the mechanisms of differentiation and repair in vitro (50, 51). The CRISPR-Cas9 system has already been applied to both model systems to efficiently knockdown CFTR (52, 53).
Among other in vitro-based assays to monitor CFTR function, organoids have become a model of choice for CF research. Organoids are progenitor cells grown in a 3D-environment in the presence of conditioned medium or supporting cells that organize similarly to in vivo organs (with an internal lumen). Representative organoids of the intestinal and respiratory systems have been generated and these models represent robust systems for screening of drugs that may act alone or in combination to correct CFTR channels harboring different mutations (54, 55). The feasibility of using CRISPR-Cas9 to correct defective CFTR in intestinal organoids has been demonstrated (56), indicating that this approach can be applied to other organ-derived 3D structures exhibiting the CF phenotype. Somatic progenitor cells from other CFTR-expressing tissues may be an interesting alternative (57), as seen in human mesenchymal cultures from periodontal ligament stem cells, which are easy to isolate and propagate in vitro.
New development of in vitro models to study tissue regeneration and disease modeling came with the discovery of human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) (58, 59). iPSCs are obtained by reprogramming somatic cells into an ESC state, and then differentiating them into specific human tissues (60). Although differentiation of iPSCs is challenging, their derivation toward an airway epithelium has already been reported, making iPSCs a limitless source of cells (61–63). This system, when used in conjunction with organoid culture (64), offers the possibility to obtain a CF model that can be patient- specific, carrying rare CFTR mutations. The CRISPR-Cas9 approach has been also used to efficiently target correction of F508del-CFTR patient-derived iPSCs differentiated to proximal airway cells (48).
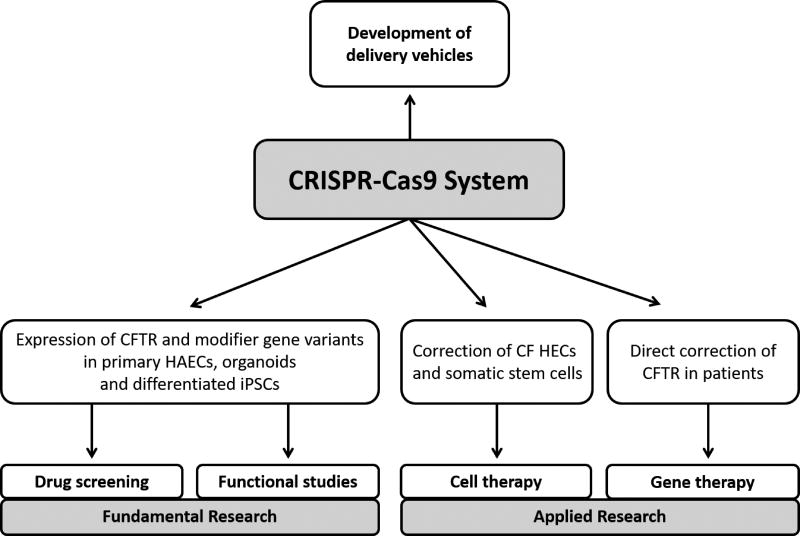
All these reports pave the way for in vitro manipulation of CFTR expression in non-CF and CF epithelia with their isogenic controls, and open new perspectives for disease modeling (Figure 2). Introducing rare CFTR mutations and/or generating organ-like tissues will allow for the identification of more specific molecules for drug development and test their efficiency according to the cellular environment where CFTR is natively expressed. Understanding the influence of modifier genes or interactors on CFTR function will be feasible, as well as genetically manipulating alternative signaling pathways that contribute to epithelial homeostasis. Finally, CRISPR-Cas9 may accelerate correction of CFTR mutations in the context of cell and gene therapy.
Figure 2.
Uses of CRISPR-Cas9 in CF research and therapies. Delivery of CRISPR-Cas9 System by lentivirus or other innovative vehicles will ease gene editing of polarized epithelia in vitro and in patients.
Utility of CFTR Humanized Animal Models
In vitro modeling described above provides advantages as the cells can be of human origin and readily manipulated, while animal models come with their own cellular and organ physiology, which may differ from humans. Insertion of full human genes into a relevant animal species is an attractive amalgamation of respective strengths for each system that can be particularly beneficial for the testing of human-specific elements of gene function, regulation, or response to potential therapeutics for a disease or condition. This approach often involves the insertion of large genomic sequences that include promoters, untranslated flanking regions, and introns, which most often exceed the carrying capacity of commonly used plasmid and viral systems. Thus, bacteria artificial chromosomes (BAC) or yeast artificial chromosomes (YAC) are most often used. To deliver BAC or YAC DNA to the germline of mice, three different techniques can be used: pronuclear injection of purified DNA (65), lipofection of the DNA into embryonic stem cells (66), or yeast spheroblast fusion with embryonic stem cells (67). Among the three methods, microinjection of isolated BAC or YAC DNA into the zygote is the most common method to generate transgenic animals.
Using these techniques, researchers have created various mouse models to investigate human diseases such as neurodegenerative conditions, cancers, and other genetic disorders. Overexpression of human amyloid precursor protein (APP) and mutated APP in mice via YAC-mediated insertion has been achieved by multiple groups to study Alzheimer’s disease (68, 69). Other YAC-based mouse models with mutated genes associated with Alzheimer’s disease have also been generated (70–72). Transgenic mice expressing the full-length human Huntington’s disease gene have been generated using YAC-mediated introduction of the coding sequence (73). The resulting phenotype validates that YAC128 HD transgenic mice faithfully recapitulate many human pathological features (74), thus providing an excellent model of this disease. Various other neurological disorders such as epilepsy (75), Down’s Syndrome (76, 77), and Parkinson’s disease are benefiting from the availability of humanized murine models. Humanized transgenic mouse models are similarly proving invaluable in cancer and immunology research. Chimeric mice carrying human immunoglobulin heavy chain and c-myc develop B-cell lymphomas (78, 79) and other transgenic mice expressing human immunoglobulins produced human-specific antibodies when challenged with antigens of medical interest (80, 81). Besides neurological disorders and cancers, a YAC-based human hemoglobin transgenic mouse model represents a useful tool for Sickle cell disease studies (82, 83).
Both BAC and YAC technology have been used to generate models of cystic fibrosis. A pig model of cystic fibrosis was generated using BACs carrying mutated porcine CFTR through homologous recombination in somatic cells followed by nuclear transfer (84) and YAC DNA was used to generate a mouse expressing human CFTR (85). More recently, BAC DNA was utilized to generate a mouse model that expresses human CFTR (86). This model is currently being modified using CRISPR/Cas9 technology to generate specific mutations seen in human CF patients. The availability of mouse and other larger animal models expressing human CFTR provides the opportunity to unravel additional pathophysiological details and provides a robust platform to test novel therapeutics for many diseases, including cystic fibrosis.
Summary and future directions
The study of biologic systems requires the selection of a faithful model to which perturbations can be investigated, quantified, and related to disease processes. In CF, this is complicated by the multi-system nature of this disease. In recent years, the advances in genetic engineering of relevant species that fully recapitulate the human disease have matured alongside the development of highly sophisticated in vitro model systems that can compartmentalize various microenvironments. Together these arms provide unprecedented flexibility in experimental design, allowing scientists to ask questions previously thought inaccessible in the laboratory. Relating these studies in the laboratory to experimentally driven clinical trials in CF subjects, will greatly enhance the field’s understanding of clinically important targets for therapy. These innovations are revealing more about the pathogenesis of CF than ever thought possible and are redirecting efforts along new lines of thought.
Supplementary Material
Acknowledgments
Funding : This work was supported by the National Institutes of Health P30 DK54759 pilot grant (to BHR), R24HL123482 and DK096518 (to JFE), the Cystic Fibrosis Foundation ROSEN17I0 and ROSEN17XX0 (to BHR), and the Swiss CF Foundation, Foundation ABCF, Vaincre la Mucoviscidose, and the Swiss National Science Foundation (to MC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest : None of the authors have a conflict of interest relevant to this publication.
References
- 1.Keiser NW, Engelhardt JF. New animal models of cystic fibrosis: what are they teaching us? Curr Opin Pulm Med. 2011;17(6):478–83. doi: 10.1097/MCP.0b013e32834b14c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guilbault C, Saeed Z, Downey GP, Radzioch D. Cystic fibrosis mouse models. Am J Respir Cell Mol Biol. 2007;36(1):1–7. doi: 10.1165/rcmb.2006-0184TR. [DOI] [PubMed] [Google Scholar]
- 3.Tuggle KL, Birket SE, Cui X, Hong J, Warren J, Reid L, et al. Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PLoS One. 2014;9(3):e91253. doi: 10.1371/journal.pone.0091253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J, Rajagopolan C, Hou X, Chen E, Boucher RC, Sun F. Rabbit models for cystic fibrosis. Pediatr Pulmonol. 2016;51:A158–9. [Google Scholar]
- 5.Rogers CS, Hao Y, Rokhlina T, Samuel M, Stoltz DA, Li Y, et al. Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J Clin Invest. 2008;118(4):1571–7. doi: 10.1172/JCI34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun X, Yan Z, Yi Y, Li Z, Lei D, Rogers CS, et al. Adeno-associated virus-targeted disruption of the CFTR gene in cloned ferrets. J Clin Invest. 2008;118(4):1578–83. doi: 10.1172/JCI34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321(5897):1837–41. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho HJ, et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest. 2010;120(9):3149–60. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2(29):29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun X, Olivier AK, Liang B, Yi Y, Sui H, Evans TI, et al. Lung phenotype of juvenile and adult cystic fibrosis transmembrane conductance regulator-knockout ferrets. Am J Respir Cell Mol Biol. 2014;50(3):502–12. doi: 10.1165/rcmb.2013-0261OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X, Olivier AK, Yi Y, Pope CE, Hayden HS, Liang B, et al. Gastrointestinal pathology in juvenile and adult CFTR-knockout ferrets. The American journal of pathology. 2014;184(5):1309–22. doi: 10.1016/j.ajpath.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parent RA. Comparative biology of the normal lung. Amsterdam ; Boston: Elsevier/AP, Academic Press is an imprint of Elsevier; 2015. [Google Scholar]
- 13.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, et al. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257(5073):1083–8. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 14.Dorin JR, Dickinson P, Alton EW, Smith SN, Geddes DM, Stevenson BJ, et al. Cystic fibrosis in the mouse by targeted insertional mutagenesis. Nature. 1992;359(6392):211–5. doi: 10.1038/359211a0. [DOI] [PubMed] [Google Scholar]
- 15.Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79(1 Suppl):S193–214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- 16.Heeckeren A, Walenga R, Konstan MW, Bonfield T, Davis PB, Ferkol T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Invest. 1997;100(11):2810–5. doi: 10.1172/JCI119828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Heeckeren AM, Davis PB. Examining the contribution of resident and migratory cells in mediating the exaggerated inflammatory response in cystic fibrosis lung infections with mucoid P. aeruginosa. Pediatr Pulmonol. 2004;38(S27):274. [Google Scholar]
- 18.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10(5):487–93. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 19.Zhou-Suckow Z, Duerr J, Hagner M, Agrawal R, Mall MA. Airway mucus, inflammation and remodeling: emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res. 2017;367(3):537–50. doi: 10.1007/s00441-016-2562-z. [DOI] [PubMed] [Google Scholar]
- 20.Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science. 2014;345(6198):818–22. doi: 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keiser NW, Birket SE, Evans IA, Tyler SR, Crooke AK, Sun X, et al. Defective innate immunity and hyperinflammation in newborn cystic fibrosis transmembrane conductance regulator-knockout ferret lungs. Am J Respir Cell Mol Biol. 2015;52(6):683–94. doi: 10.1165/rcmb.2014-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoegger MJ, Awadalla M, Namati E, Itani OA, Fischer AJ, Tucker AJ, et al. Assessing mucociliary transport of single particles in vivo shows variable speed and preference for the ventral trachea in newborn pigs. Proc Natl Acad Sci U S A. 2014;111(6):2355–60. doi: 10.1073/pnas.1323633111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng X, Alfoldi J, Gori K, Eisfeld AJ, Tyler SR, Tisoncik-Go J, et al. The draft genome sequence of the ferret (Mustela putorius furo) facilitates study of human respiratory disease. Nature biotechnology. 2014;32(12):1250–5. doi: 10.1038/nbt.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abou Alaiwa MH, Launspach JL, Sheets KA, Rivera JA, Gansemer ND, Taft PJ, et al. Repurposing tromethamine as inhaled therapy to treat CF airway disease. JCI Insight. 2016;1(8) doi: 10.1172/jci.insight.87535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah VS, Meyerholz DK, Tang XX, Reznikov L, Abou Alaiwa M, Ernst SE, et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science. 2016;351(6272):503–7. doi: 10.1126/science.aad5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan Z, Stewart ZA, Sinn PL, Olsen JC, Hu J, McCray PB, Jr, et al. Ferret and pig models of cystic fibrosis: prospects and promise for gene therapy. Human gene therapy Clinical development. 2015;26(1):38–49. doi: 10.1089/humc.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steines B, Dickey DD, Bergen J, Excoffon KJ, Weinstein JR, Li X, et al. CFTR gene transfer with AAV improves early cystic fibrosis pig phenotypes. JCI Insight. 2016;1(14):e88728. doi: 10.1172/jci.insight.88728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooney AL, Abou Alaiwa MH, Shah VS, Bouzek DC, Stroik MR, Powers LS, et al. Lentiviral-mediated phenotypic correction of cystic fibrosis pigs. JCI Insight. 2016;1(14) doi: 10.1172/jci.insight.88730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu-El-Haija M, Ramachandran S, Meyerholz DK, Abu-El-Haija M, Griffin M, Giriyappa RL, et al. Pancreatic damage in fetal and newborn cystic fibrosis pigs involves the activation of inflammatory and remodeling pathways. The American journal of pathology. 2012;181(2):499–507. doi: 10.1016/j.ajpath.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abu-El-Haija M, Sinkora M, Meyerholz DK, Welsh MJ, McCray PB, Jr, Butler J, et al. An activated immune and inflammatory response targets the pancreas of newborn pigs with cystic fibrosis. Pancreatology. 2011;11(5):506–15. doi: 10.1159/000332582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olivier AK, Yi Y, Sun X, Sui H, Liang B, Hu S, et al. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest. 2012;122(10):3755–68. doi: 10.1172/JCI60610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uc A, Olivier AK, Griffin MA, Meyerholz DK, Yao J, Abu-El-Haija M, et al. Glycaemic regulation and insulin secretion are abnormal in cystic fibrosis pigs despite sparing of islet cell mass. Clin Sci (Lond) 2015;128(2):131–42. doi: 10.1042/CS20140059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi Y, Sun X, Gibson-Corley K, Xie W, Liang B, He N, et al. A Transient Metabolic Recovery from Early Life Glucose Intolerance in Cystic Fibrosis Ferrets Occurs During Pancreatic Remodeling. Endocrinology. 2016;157(5):1852–65. doi: 10.1210/en.2015-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi Y, Norris AW, Wang K, Sun X, Uc A, Moran A, et al. Abnormal Glucose Tolerance in Infants and Young Children with Cystic Fibrosis. Am J Respir Crit Care Med. 2016;194(8):974–80. doi: 10.1164/rccm.201512-2518OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olivier AK, Gibson-Corley KN, Meyerholz DK. Animal models of gastrointestinal and liver diseases. Animal models of cystic fibrosis: gastrointestinal, pancreatic, and hepatobiliary disease and pathophysiology. Am J Physiol Gastrointest Liver Physiol. 2015;308(6):G459–71. doi: 10.1152/ajpgi.00146.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colombo C, Battezzati PM, Crosignani A, Morabito A, Costantini D, Padoan R, et al. Liver disease in cystic fibrosis: A prospective study on incidence, risk factors, and outcome. Hepatology. 2002;36(6):1374–82. doi: 10.1053/jhep.2002.37136. [DOI] [PubMed] [Google Scholar]
- 37.Lamireau T, Monnereau S, Martin S, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. J Hepatol. 2004;41(6):920–5. doi: 10.1016/j.jhep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros. 2011;10(Suppl 2):S29–36. doi: 10.1016/S1569-1993(11)60006-4. [DOI] [PubMed] [Google Scholar]
- 39.Wilke M, Buijs-Offerman RM, Aarbiou J, Colledge WH, Sheppard DN, Touqui L, et al. Mouse models of cystic fibrosis: phenotypic analysis and research applications. J Cyst Fibros. 2011;10(Suppl 2):S152–71. doi: 10.1016/S1569-1993(11)60020-9. [DOI] [PubMed] [Google Scholar]
- 40.Uc A, Giriyappa R, Meyerholz DK, Griffin M, Ostedgaard LS, Tang XX, et al. Pancreatic and biliary secretion are both altered in cystic fibrosis pigs. Am J Physiol Gastrointest Liver Physiol. 2012;303(8):G961–8. doi: 10.1152/ajpgi.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher JT, Tyler SR, Zhang Y, Lee BJ, Liu X, Sun X, et al. Bioelectric characterization of epithelia from neonatal CFTR knockout ferrets. Am J Respir Cell Mol Biol. 2013;49(5):837–44. doi: 10.1165/rcmb.2012-0433OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scott-Jupp R, Lama M, Tanner MS. Prevalence of liver disease in cystic fibrosis. Arch Dis Child. 1991;66(6):698–701. doi: 10.1136/adc.66.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindblad A, Glaumann H, Strandvik B. Natural history of liver disease in cystic fibrosis. Hepatology. 1999;30(5):1151–8. doi: 10.1002/hep.510300527. [DOI] [PubMed] [Google Scholar]
- 44.Scher H, Bishop WP, McCray PB., Jr Ursodeoxycholic acid improves cholestasis in infants with cystic fibrosis. Ann Pharmacother. 1997;31(9):1003–5. doi: 10.1177/106002809703100909. [DOI] [PubMed] [Google Scholar]
- 45.Harrison PT, Sanz DJ, Hollywood JA. Impact of gene editing on the study of cystic fibrosis. Human Genetics. 2016;135(9):983–92. doi: 10.1007/s00439-016-1693-3. [DOI] [PubMed] [Google Scholar]
- 46.Barrangou R. Cas9 Targeting and the CRISPR Revolution. Science. 2014;344(6185):707–8. doi: 10.1126/science.1252964. [DOI] [PubMed] [Google Scholar]
- 47.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Firth AL, Menon T, Parker GS, Qualls SJ, Lewis BM, Ke E, et al. Functional Gene Correction for Cystic Fibrosis in Lung Epithelial Cells Generated from Patient iPSCs. Cell Reports. 2015;12(9):1385–90. doi: 10.1016/j.celrep.2015.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Randell SH, Fulcher ML, O'Neal W, Olsen JC. Primary epithelial cell models for cystic fibrosis research. Methods Mol Biol. 2011;742:285–310. doi: 10.1007/978-1-61779-120-8_18. [DOI] [PubMed] [Google Scholar]
- 50.Crespin S, Bacchetta M, Huang S, Dudez T, Wiszniewski L, Chanson M. Approaches to study differentiation and repair of human airway epithelial cells. Methods Mol Biol. 2011;742:173–85. doi: 10.1007/978-1-61779-120-8_10. [DOI] [PubMed] [Google Scholar]
- 51.Wansleeben C, Barkauskas CE, Rock JR, Hogan BLM. Stem cells of the adult lung: their development and role in homeostasis, regeneration, and disease. Wires Dev Biol. 2013;2(1):131–48. doi: 10.1002/wdev.58. [DOI] [PubMed] [Google Scholar]
- 52.Bellec J, Bacchetta M, Losa D, Anegon I, Chanson M, Nguyen TH. CFTR Inactivation by Lentiviral Vector-mediated RNA Interference and CRISPR-Cas9 Genome Editing in Human Airway Epithelial Cells. Current Gene Therapy. 2015;15(5):447–59. doi: 10.2174/1566523215666150812115939. [DOI] [PubMed] [Google Scholar]
- 53.Chung WY, Song M, Park J, Namkung W, Lee J, Kim H, et al. Generation of Delta F508-CFTR T84 cell lines by CRISPR/Cas9-mediated genome editing. Biotechnol Lett. 2016;38(12):2023–34. doi: 10.1007/s10529-016-2190-4. [DOI] [PubMed] [Google Scholar]
- 54.Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nature Medicine. 2013;19(7):939. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 55.Tan Q, Choi KM, Sicard D, Tschumperlin DJ. Human airway organoid engineering as a step toward lung regeneration and disease modeling. Biomaterials. 2017;113:118–32. doi: 10.1016/j.biomaterials.2016.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, et al. Functional Repair of CFTR by CRISPR/Cas9 in Intestinal Stem Cell Organoids of Cystic Fibrosis Patients. Cell Stem Cell. 2013;13(6):653–8. doi: 10.1016/j.stem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Cianci E, Recchiuti A, Trubiani O, Diomede F, Marchisio M, Miscia S, et al. Human Periodontal Stem Cells Release Specialized Proresolving Mediators and Carry Immunomodulatory and Prohealing Properties Regulated by Lipoxins. Stem Cells Transl Med. 2016;5(1):20–32. doi: 10.5966/sctm.2015-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 59.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 60.Martin U. Pluripotent stem cells for disease modeling and drug screening: new perspectives for treatment of cystic fibrosis? Mol Cell Pediatr. 2015;2(1):15. doi: 10.1186/s40348-015-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong AP, Chin S, Xia S, Garner J, Bear CE, Rossant J. Efficient generation of functional CFTR-expressing airway epithelial cells from human pluripotent stem cells. Nature Protocols. 2015;10(3) doi: 10.1038/nprot.2015.021. [DOI] [PubMed] [Google Scholar]
- 62.Firth AL, Dargitz CT, Qualls SJ, Menon T, Wright R, Singer O, et al. Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(17):E1723–E30. doi: 10.1073/pnas.1403470111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mou HM, Zhao R, Sherwood R, Ahfeldt T, Lapey A, Wain J, et al. Generation of Multipotent Lung and Airway Progenitors from Mouse ESCs and Patient-Specific Cystic Fibrosis iPSCs. Cell Stem Cell. 2012;10(4):385–97. doi: 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCauley KB, Hawkins F, Serra M, Thomas DC, Jacob A, Kotton DN. Efficient Derivation of Functional Human Airway Epithelium from Pluripotent Stem Cells via Temporal Regulation of Wnt Signaling. Cell Stem Cell. 2017;20(6):844. doi: 10.1016/j.stem.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schedl A, Montoliu L, Kelsey G, Schutz G. A yeast artificial chromosome covering the tyrosinase gene confers copy number-dependent expression in transgenic mice. Nature. 1993;362(6417):258–61. doi: 10.1038/362258a0. [DOI] [PubMed] [Google Scholar]
- 66.Strauss WM, Dausman J, Beard C, Johnson C, Lawrence JB, Jaenisch R. Germ line transmission of a yeast artificial chromosome spanning the murine alpha 1(I) collagen locus. Science. 1993;259(5103):1904–7. doi: 10.1126/science.8096090. [DOI] [PubMed] [Google Scholar]
- 67.Jakobovits A, Moore AL, Green LL, Vergara GJ, Maynard-Currie CE, Austin HA, et al. Germ-line transmission and expression of a human-derived yeast artificial chromosome. Nature. 1993;362(6417):255–8. doi: 10.1038/362255a0. [DOI] [PubMed] [Google Scholar]
- 68.Lamb BT, Sisodia SS, Lawler AM, Slunt HH, Kitt CA, Kearns WG, et al. Introduction and expression of the 400 kilobase amyloid precursor protein gene in transgenic mice [corrected] Nature genetics. 1993;5(1):22–30. doi: 10.1038/ng0993-22. [DOI] [PubMed] [Google Scholar]
- 69.Pearson BE, Choi TK. Expression of the human beta-amyloid precursor protein gene from a yeast artificial chromosome in transgenic mice. Proc Natl Acad Sci U S A. 1993;90(22):10578–82. doi: 10.1073/pnas.90.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loring JF, Paszty C, Rose A, McIntosh TK, Murai H, Pierce JE, et al. Rational design of an animal model for Alzheimer's disease: introduction of multiple human genomic transgenes to reproduce AD pathology in a rodent. Neurobiology of aging. 1996;17(2):173–82. doi: 10.1016/0197-4580(95)02076-4. [DOI] [PubMed] [Google Scholar]
- 71.Lamb BT, Call LM, Slunt HH, Bardel KA, Lawler AM, Eckman CB, et al. Altered metabolism of familial Alzheimer's disease-linked amyloid precursor protein variants in yeast artificial chromosome transgenic mice. Human molecular genetics. 1997;6(9):1535–41. doi: 10.1093/hmg/6.9.1535. [DOI] [PubMed] [Google Scholar]
- 72.Lamb BT, Bardel KA, Kulnane LS, Anderson JJ, Holtz G, Wagner SL, et al. Amyloid production and deposition in mutant amyloid precursor protein and presenilin-1 yeast artificial chromosome transgenic mice. Nature neuroscience. 1999;2(8):695–7. doi: 10.1038/11154. [DOI] [PubMed] [Google Scholar]
- 73.Van Raamsdonk JM, Warby SC, Hayden MR. Selective degeneration in YAC mouse models of Huntington disease. Brain research bulletin. 2007;72(2–3):124–31. doi: 10.1016/j.brainresbull.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 74.Fan J, Gladding CM, Wang L, Zhang LY, Kaufman AM, Milnerwood AJ, et al. P38 MAPK is involved in enhanced NMDA receptor-dependent excitotoxicity in YAC transgenic mouse model of Huntington disease. Neurobiology of disease. 2012;45(3):999–1009. doi: 10.1016/j.nbd.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 75.Musumeci SA, Calabrese G, Bonaccorso CM, D'Antoni S, Brouwer JR, Bakker CE, et al. Audiogenic seizure susceptibility is reduced in fragile X knockout mice after introduction of FMR1 transgenes. Experimental neurology. 2007;203(1):233–40. doi: 10.1016/j.expneurol.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 76.Rachidi M, Lopes C, Vayssettes C, Smith DJ, Rubin EM, Delabar JM. New cerebellar phenotypes in YAC transgenic mouse in vivo library of human Down syndrome critical region-1. Biochemical and biophysical research communications. 2007;364(3):488–94. doi: 10.1016/j.bbrc.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 77.Seo H, Isacson O. The hAPP-YAC transgenic model has elevated UPS activity in the frontal cortex similar to Alzheimer's disease and Down's syndrome. Journal of neurochemistry. 2010;114(6):1819–26. doi: 10.1111/j.1471-4159.2010.06902.x. [DOI] [PubMed] [Google Scholar]
- 78.Butzler C, Zou X, Popov AV, Bruggemann M. Rapid induction of B-cell lymphomas in mice carrying a human IgH/c-mycYAC. Oncogene. 1997;14(11):1383–8. doi: 10.1038/sj.onc.1200968. [DOI] [PubMed] [Google Scholar]
- 79.Palomo C, Zou X, Nicholson IC, Butzler C, Bruggemann M. B-cell tumorigenesis in mice carrying a yeast artificial chromosome-based immunoglobulin heavy/c-myc translocus is independent of the heavy chain intron enhancer (Emu) Cancer research. 1999;59(21):5625–8. [PubMed] [Google Scholar]
- 80.Green LL, Hardy MC, Maynard-Currie CE, Tsuda H, Louie DM, Mendez MJ, et al. Antigen-specific human monoclonal antibodies from mice engineered with human Ig heavy and light chain YACs. Nature genetics. 1994;7(1):13–21. doi: 10.1038/ng0594-13. [DOI] [PubMed] [Google Scholar]
- 81.Pruzina S, Williams GT, Kaneva G, Davies SL, Martin-Lopez A, Bruggemann M, et al. Human monoclonal antibodies to HIV-1 gp140 from mice bearing YAC-based human immunoglobulin transloci. Protein engineering, design & selection : PEDS. 2011;24(10):791–9. doi: 10.1093/protein/gzr038. [DOI] [PubMed] [Google Scholar]
- 82.Chang JC, Lu R, Lin C, Xu SM, Kan YW, Porcu S, et al. Transgenic knockout mice exclusively expressing human hemoglobin S after transfer of a 240-kb betas-globin yeast artificial chromosome: A mouse model of sickle cell anemia. Proc Natl Acad Sci U S A. 1998;95(25):14886–90. doi: 10.1073/pnas.95.25.14886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ye L, Chang JC, Lu R, Kan YW. High oxygen environment during pregnancy rescues sickle cell anemia mice from prenatal death. Blood cells, molecules & diseases. 2008;41(1):67–72. doi: 10.1016/j.bcmd.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 84.Klymiuk N, Mundhenk L, Kraehe K, Wuensch A, Plog S, Emrich D, et al. Sequential targeting of CFTR by BAC vectors generates a novel pig model of cystic fibrosis. Journal of molecular medicine. 2012;90(5):597–608. doi: 10.1007/s00109-011-0839-y. [DOI] [PubMed] [Google Scholar]
- 85.Manson AL, Trezise AE, MacVinish LJ, Kasschau KD, Birchall N, Episkopou V, et al. Complementation of null CF mice with a human CFTR YAC transgene. The EMBO journal. 1997;16(14):4238–49. doi: 10.1093/emboj/16.14.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gawenis LR, Walker NM, Strubberg AM, Harris A, Clarke LL. Generation of BAC transgenic mice expressing human CFTR. Pediatr Pulmonol. 2015;50(12):A86. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.