Abstract
A large cohort of HCC patients from several collaborating Turkish institutions was examined for the tumor parameters of maximum diameter (MTD), portal vein thrombosis(PVT) and alpha-fetoprotein (AFP) levels. A relationship was found between MTD and blood platelet levels. Patients with large ≥5cm tumors who had normal platelet levels had significantly larger tumors, higher percent PVT and significantly lower blood total bilirubin and liver cirrhosis than similar ≥5cm tumor patients having thrombocytopenia. Comparison of patients with and without PVT, showed significantly larger tumors, greater multifocality, blood AFP and C-reactive protein levels and interestingly, lower HDL levels in the patients with PVT. Fifty-eight % of the total cohort had AFP levels ≤100 IU/ml (and 42.1% had values ≤20 IU/ml). These patients had significantly smaller tumors, less tumor multifocality and percent PVT, lower total bilirubin and less cirrhosis. There was considerable geographic heterogeneity within Turkey in the patterns of HCC presentation, with areas of higher and lower HBV, HDV, cirrhosis and tumor aggressiveness parameters. Turkish patients thus have distinct patterns of presentation, but the biological relationships between MTD and both platelets and bilirubin levels are similar to the relationships that have been reported in other ethnic patient groups.
Keywords: Albumin, HCC, Turkey, AFP, tumor mass, aggressiveness
Introduction
The classification of tumors and prognostication for patients with hepatocellular carcinoma (HCC) has generally been considered to be a reflection of 2 sets of separate factors, since the first such report by Okuda (1). These are liver factors and tumor factors, which are separate yet likely related (2,3). All modern classification schemata incorporate parameters from both sets of factors (4,5). However, there are many characteristics that distinguish HCC patients in various parts of the world. We report here for the first time, on a large HCC database from several collaborating institutions in Turkey, which is a Mediterranean country that constitutes a land bridge between Europe and Asia, and we focus on 3 parameters of tumor behavior, namely maximum tumor diameter (MTD), portal vein invasion (PVT) and alpha-fetoprotein (AFP) levels, and the correlates of each parameter.
Methods
Patient data
We analyzed a database of 1332 prospectively-accrued HCC patients who had full baseline tumor parameter data, including CT scan information on HCC size, number of tumor nodules and presence or absence of PVT and plasma AFP levels; complete blood count; routine blood liver function tests, (total bilirubin, GGTP, ALKP, albumin, transaminases) and patient demographics. Diagnosis was made either via tumor biopsy or according to international guidelines (6,7). Database management conformed to legislation on privacy and this study conforms to the ethical guidelines of the Declaration of Helsinki and approval for this retrospective study on de-identified HCC patients was obtained by the Institutional Review Board.
Statistical analysis
Mean and SD for continuous variables, and relative frequency for categorical variables, were used as indices of centrality and dispersion of the distribution. For categorical variables, the Chi-square and z test for proportions were used.
The Pearson’s correlation was used to measure the association between two continuous variables and the Wilcoxon rank-sum (Mann-Whitney) test, to test the difference between two categories, and the Kruskal-Wallis rank test to test the difference among categories. Linear regression model was used to evaluate the associations between maximum tumor diameter on single variables examined. The final multiple linear regression were obtained with the backward stepwise method and the variables that showed associations with p<0.10 were left in the models.
When testing the null hypothesis of no association, the probability level of α error, two tailed, was 0.05. All the statistical computations were made using STATA 10.0 Statistical Software (StataCorp. 2007. Stata Statistical Software: release 10. College Station, TX: StataCorp LP, Statistical Software (StataCorp. 2007. Stata Statistical Software: release 10. College Station, TX: StataCorp LP,
Results
Patient Characteristics of total cohort
Table 1 shows the descriptive characteristics of the total patient cohort. 81.11 were male and 81.42% had cirrhosis. The predominant etiological factor was hepatitis B (HBV) in 60.86%, followed by hepatitis C (HCV) in 20.72% of patients. The mean maximum tumor diameter (MTD) was 5.89cm, with approximately a third of patients having tumors <3.5cm, 3.5–6.5cm and >6.5cm. The mean alpha-fetoprotein (AFP) level was 5686.54 IU/mL, and 42.13% of patients had normal AFP laboratory values and 41.97% of patients had AFP values >100 IU/mL. The mean albumin was low at 3.09 g/dL and the mean total bilirubin was elevated at 2.96 mg/dL.
Table 1.
Characteristics of HCC patients in the total cohort.
| Variables * | Values (n = 1332) |
|---|---|
| Gender (%) | |
| Females | 18.89 |
| Males | 81.11 |
| Age (yr) | 62.16±11.36 |
| Cirrhosis (%) | 81.42 |
| Cigarettes (%) | 51.02 |
| Alcohol (%) | 15.37 |
| HbsAg +ve (%) | 60.86 |
| HCV +ve (%) | 20.72 |
| MTD (cm) | 5.89±4.03 |
| MTD (%) | |
| <3.5 cm | 31.78 |
| ≥3.5/<6.5 cm | 34.79 |
| >6.5 cm | 33.43 |
| PVT (%) | 28.55 |
| AFP (IU/mL) | 5686.54±36413.94 |
| LDL (mg/dL) | 99.57±91.84 |
| Triglycerides (mg/dL) | 105.73±62.18 |
| HDL (mg/dL) | 36.55±18.29 |
| Creatinine (mg/dL) | 1.10±2.18 |
| HbA1c (mmol/mol) | 5.96±2.01 |
| Total Protein (g/dl) | 6.90±7.77 |
| Albumin (g/dL) | 3.09±0.75 |
| INR | 1.43±3.52 |
| CRP (mg/L) | 17.52±31.75 |
| ALKP (U/L) | 216.68±270.10 |
| GGTP (U/L) | 162.32±179.42 |
| AST (U/L) | 141.04±449.32 |
| ALT (U/L) | 79.89±171.85 |
| Total Bilirubin (mg/dL) | 2.96±4.69 |
| Platelet counts (103/μL) (%) | |
| <125 | 30.95 |
| ≥125 | 69.05 |
| AFP (IU/mL) (%) | |
| ≤20 | 42.13 |
| >20/≤100 | 15.91 |
| >100/≤1000 | 20.42 |
| >1000 | 21.55 |
| CRP (mg/L) (%) | |
| >10 | 35.84 |
| ≤10 | 64.16 |
All values: M±SD or No. of Patients (%)
Abbreviations: GGTP, gamma glutamyl transpeptidae; ALKP, Alkaline phosphatase; AFP, Alpha-fetoprotein; MTD, Maximum Tumor Diameter; HDL: High Density Lipoprotein Cholesterol; LDL: Low Density Lipoprotein Cholesterol; HbA1c, Glycated hemoglobin; HCT, Hematocrit; PT, P INR, International Normalized Ratio; CRP, C-Reactive Protein.
Maximum Tumor Diameter (MTD)
The MTD was then further examined. Patients were divided in 3 size terciles and the means and distributions of the associated peripheral blood platelet counts were then calculated. Fig 1 shows that the mean platelet counts increased with increase in MTD tercile, as has been reported in other cohorts (8,9). Patients with the largest MTD tumors had normal platelet counts and thus lesser degrees of fibrosis, as noted previously (10). Characteristics of HCC patients with small and large MTD tumors were then further characterized, after merging terciles II and III, due to the small patient numbers in tercile III. Patient groups with either MTD<5cm or MTD ≥5cm tumors were then each dichotomized according to blood platelet counts of <125 or >125 × 103/μL (Table 2), as low blood platelets are a cirrhosis surrogate (11). In the small tumor group, there was little difference in tumor characteristics between the platelet subgroups, although albumin and bilirubin levels were significantly worse in the low platelet subgroup, as expected. By contrast, in the larger tumor group, AFP was significantly higher and PVT% of patients was significantly higher in the high platelet subgroup compared to the low platelet subgroup. In the large MTD subgroup with higher platelets with larger tumors, cirrhosis was significantly less, as was total bilirubin. Thus, platelet dichotomization selects for a patient phenotype with better liver function but more advanced HCC. A linear regression model of MTD showed several significant single variables (Table 3). However, in the Final Multiple Linear Regression model, only platelet counts and presence of PVT were significant.
Fig 1.
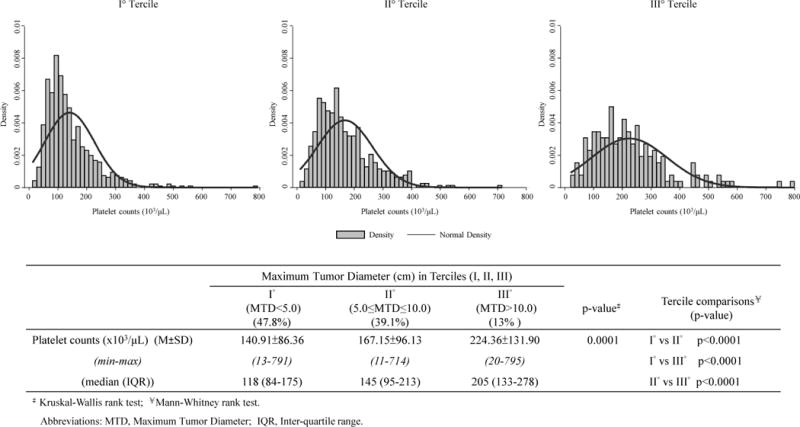
Peripheral blood platelet counts (103/μL) among Maximum Tumor Diameter (cm) terciles.
Table 2.
Comparisons amongst HCC patients in tumor diameter groups, dichotomized by platelets levels.
| Parameter* | Maximum Tumor Diameter
|
|||||
|---|---|---|---|---|---|---|
| MTD<5.0cm
|
p-value# | MTD≥5.0 cm
|
p-value# | |||
| Platelets<125 (24.7%) | Platelets≥125 (23.1%) | Platelets<125 (16.9%) | Platelets≥125 (35.2%) | |||
| Platelet counts (103/μL) | 83.74±24.36 | 201.98±87.02 | <0.0001 | 83.07±25.51 | 228.89±101.83 | <0.0001 |
| Hemoglobin (g/dL) | 12.00±2.27 | 12.75±2.25 | 0.0001 | 12.03±2.10 | 12.57±2.19 | 0.004 |
| GGTP (U/L) | 131.30±168.25 | 166.40±192.89 | 0.17 | 123.29±114.89 | 200.31±191.84 | <0.0001 |
| ALKP (U/L) | 162.73±137.63 | 216.78±256.68 | 0.23 | 185.69±136.80 | 251.02±243.25 | 0.009 |
| Total Bilirubin (mg/dL) | 2.76±3.22 | 2.21±3.90 | <0.0001 | 3.32±3.99 | 2.56±4.48 | <0.0001 |
| Albumin (g/dL) | 3.00±0.75 | 3.29±0.76 | <0.0001 | 2.89±0.67 | 3.12±0.74 | 0.0002 |
| AFP (IU/mL) | 1384.09±7792.46 | 2441.81±14956.60 | 0.12 | 5058.27±19926.07 | 10152.25±45830.54 | 0.02 |
| MTD (cm) | 3.01±1.00 | 3.03±0.97 | 0.81 | 8.30±3.69 | 9.28±3.88 | 0.0002 |
| Nodules number (%) | 0.72 ^ | 0.13 ^ | ||||
| 1 | 237 (72.70) | 220 (71.43) | 149 (68.04) | 283 (61.26) | ||
| 2–3 | 89 (27.30) | 88 (28.57) | 70 (31.96) | 176 (38.10) | ||
| >3 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (0.65) | ||
| PV Thrombosis (%) | 45 (13.89) | 49 (17.56) | 0.21 ^ | 77 (35.32) | 194 (44.29) | 0.03 ^ |
| Cirrhosis (%) | 307 (93.31) | 207 (68.09) | <0.001 ^ | 216 (96.43) | 345 (74.03) | <0.001 ^ |
| Etiology (%) | ||||||
| HBV | 132 (43.71) | 124 (45.42) | 0.68 ^ | 100 (52.08) | 193 (46.17) | 0.17 ^ |
| HCV | 69 (21.70) | 53 (17.91) | 0.24 ^ | 47 (21.46) | 67 (14.92) | 0.03 ^ |
| HDV | 43 (13.61) | 16 (5.52) | 0.001 ^ | 20 (9.22) | 35 (7.97) | 0.59 ^ |
All values: Means±Standard Deviation as continuous; Frequences and percentage (%) as categorical.
Wilcoxon rank-sum (Mann-Whitney) test;
Chi-square test.
Abbreviations: GGTP, gamma glutamyl transpeptidae; ALKP, Alkaline phosphatase; AFP, Alpha-fetoprotein; MTD, Maximum Tumor Diameter; PVT, Portal Vein Thrombosis.
Table 3.
Linear regression model of MTD (cm), on single variables (A).
Final Multiple Linear regression model, in stepwise method, of MTD (cm), on all variables included together in the model (B).
| Parameter | β | se(β) | p-value | 95% C.I. |
|---|---|---|---|---|
| (A) | ||||
| Platelet counts (103/μL) | 0.012 | 0.001 | <0.001 | 0.010 to 0.05 |
| Hemoglobin (g/dL) | −0.076 | 0.068 | 0.265 | −0.209 to 0.057 |
| GGTP (U/L) | 0.002 | 0.001 | 0.010 | 0.0005 to 0.0038 |
| ALKP (U/L) | 0.002 | 0.001 | 0.013 | 0.0004 to 0.0030 |
| Total Bilirubin (mg/dL) | 0.011 | 0.038 | 0.776 | −0.065 to 0.086 |
| Albumin (g/dL) | −0.068 | 0.198 | 0.731 | −0.458 to 0.321 |
| AFP (IU/mL) | 0.00002 | 0.000005 | 0.001 | 0.000006 to 0.000025 |
| Nodules number (%) | 0.335 | 0.297 | 0.259 | −0.248 to 0.917 |
| PV Thrombosis (%) | 2.516 | 0.309 | <0.001 | 1.909 to 3.123 |
| Cirrhosis (%) | −0.763 | 0.375 | 0.042 | −1.498 to −0.028 |
| (B) | ||||
| Platelet counts (103/μL) | 0.011 | 0.001 | <0.001 | 0.009 to 0.014 |
| PV Thrombosis (%) | 2.210 | 0.296 | <0.001 | 1.630 to 2.790 |
Abbreviations: β: coefficient; se(β): standard error of coefficient; GGTP, gamma glutamyl transpeptidae; ALKP, Alkaline phosphatase; AFP, Alpha-fetoprotein; MTD, Maximum Tumor Diameter; PVT, Portal Vein Thrombosis.
Portal vein thrombosis (PVT) and AFP levels
Patients were next examined according to presence or absence of PVT (Table 4). Patients who were PVT positive had significantly larger tumors (MTD) and higher platelet levels, significantly higher AFP and C-reactive protein (CRP) levels, and higher total bilirubin, ALKP, GGTP levels. Interestingly, levels of ‘good’ HDL cholesterol were significantly lower in the PVT positive group. To our knowledge, this has not been previously reported.
Table 4.
Characteristics of HCC patients between Portal Vein Thrombosis categories.
| Variables * | Portal Vein Thrombosis
|
||
|---|---|---|---|
| Negative | Positive | p# | |
| Sex (M) (%) | 959 (80.86) | 393 (82.91) | 0.33 ^ |
| Age (yrs) | 62.46±11.15 | 61.18±11.98 | 0.03 |
| Cigarettes smoke (%) | 316 (50.32) | 160 (54.42) | 0.24 ^ |
| Alcohol (%) | 97 (16.39) | 47 (16.43) | 0.99 ^ |
| Cirrhosis (%) | 917 (78.71) | 383 (84.36) | 0.01 ^ |
| HbsAg(+) (%) | 691 (60.14) | 293 (65.99) | 0.03 ^ |
| HCV(+) (%) | 229 (19.93) | 85 (19.14) | 0.72 ^ |
| Glucose (mg/dL) | 121.62±56.36 | 115.55±44.31 | 0.35 |
| Total Cholesterol (mg/dL) | 151.31±50.08 | 152.61±52.60 | 0.67 |
| LDL (mg/dL) | 95.25±41.90 | 101.43±44.76 | 0.11 |
| HDL (mg/dL) | 37.27±16.67 | 34.14±20.67 | 0.003 |
| Triglycerides (mg/dL) | 105.94±61.41 | 105.09±59.17 | 0.96 |
| MTD (cm) | 5.08±3.55 | 8.07±4.55 | <0.0001 |
| Nodules number (%) | <0.001 | ||
| 1 | 845 (72.28) | 215 (49.77) | |
| 2–3 | 322 (27.54) | 216 (50.00) | |
| >3 | 2 (0.17) | 1 (0.23) | |
| Hemoglobin (g/dL) | 12.48±2.25 | 12.07±2.24 | 0.001 |
| Hct (%) | 37.29±6.75 | 36.05±6.67 | 0.0005 |
| Platelet counts (103/μL) | 153.74±95.34 | 176.71±110.25 | 0.0002 |
| Ferritin (ng/mL) | 262.90±510.74 | 282.80±366.39 | 0.002 |
| Creatinine (mg/dL) | 1.01±0.73 | 1.20±4.02 | 0.31 |
| HbA1c (mmol/mol) | 6.21±1.70 | 6.18±1.80 | 0.73 |
| Total Protein (g/dl) | 6.94±9.41 | 6.83±4.69 | 0.03 |
| Albumin (g/dL) | 3.12±0.75 | 2.97±0.69 | 0.0003 |
| PT (%) | 14.63±4.47 | 14.82±5.60 | 0.81 |
| INR | 1.47±4.58 | 1.35±0.53 | 0.23 |
| CRP (mg/L) | 12.32±22.85 | 21.17±34.01 | <0.0001 |
| AFP (IU/mL) | 2910.73±16801.27 | 9890.79±44862.11 | <0.0001 |
| ALKP (U/L) | 194.60±186.56 | 248.68±262.28 | 0.002 |
| GGTP (U/L) | 150.33±172.01 | 188.37±181.97 | <0.0001 |
| AST (U/L) | 113.59±216.89 | 113.24±106.04 | 0.005 |
| ALT (U/L) | 81.69±183.25 | 62.15±53.90 | 0.71 |
| Total Bilirubin (mg/dL) | 2.33±3.49 | 3.07±4.50 | 0.01 |
All values: Means±Standard Deviation as continuous; Frequences and percentage (%) as categorical.
Wilcoxon rank-sum (Mann-Whitney) test;
Chi-square test.
Abbreviations: GGTP, gamma glutamyl transpeptidae; ALKP, Alkaline phosphatase; AFP, Alpha-fetoprotein; MTD, Maximum Tumor Diameter; HDL: High Density Lipoprotein Cholesterol; LDL: Low Density Lipoprotein Cholesterol; HbA1c, Glycated hemoglobin; HCT, Hematocrit; PT, Prothrombin Time; INR, International Normalized Ratio; CRP, C-Reactive Protein.
Patients were next divided into 3 groups according to blood AFP levels of <100, 100–1000 and >1000 IU/mL (Table 5) and their tumor and non-tumor parameters were examined. As expected, MTD and PVT% significantly increased with increase in AFP group. There was also a trend to tumor multifocality with increase in AFP group. There were also increases in ALKP, GGTP and total bilirubin levels with increase in AFP group, and a statistically significant increase in platelet counts, likely reflective of the increase in MTDs.
Table 5.
Comparisons among AFP groups in HCC patients.
| Parameter* | AFP<100 | 100≤AFP≤1000 | AFP>1000 | p-value ψ | Comparisons p-value # | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| (n=1027) | (n=364) | (n=382) | |||||
| (a) | (b) | (c) | (b)vs(a) | (c)vs(a) | (c)vs(b) | ||
| Platelet counts (103/μL) | 148.96±96.31 | 157.14±91.55 | 182.82±106.50 | 0.0001 | 0.06 | <0.0001 | 0.0009 |
| Hemoglobin (g/dL) | 12.36±2.31 | 12.26±2.19 | 12.02±2.33 | 0.04 | 0.70 | 0.02 | 0.06 |
| GGTP (U/L) | 135.73±165.25 | 175.91±162.20 | 203.10±193.38 | 0.0001 | <0.0001 | <0.0001 | 0.17 |
| ALKP (U/L) | 201.94±302.30 | 222.41±213.92 | 240.38±239.29 | 0.0001 | 0.003 | 0.0001 | 0.58 |
| Total Bilirubin (mg/dL) | 2.52±3.76 | 3.68±6.09 | 3.21±4.91 | 0.002 | 0.002 | 0.007 | 0.70 |
| Albumin (g/dL) | 3.17±0.76 | 3.02±0.74 | 2.95±0.71 | 0.0001 | 0.005 | <0.0001 | 0.20 |
| AFP (IU/mL) | 18.10±22.08 | 433.71±296.65 | 25931.36±75120.01 | 0.0001 | <0.0001 | <0.0001 | <0.0001 |
| MTD (cm) | 5.30±3.71 | 6.29±4.09 | 7.54±4.35 | 0.0001 | <0.0001 | <0.0001 | 0.0001 |
| Nodules number (%) | 0.04 ^ | ||||||
| 1 | 633 (70.65) | 212 (66.04) | 202 (61.77) | 0.13 § | 0.004 § | 0.26 § | |
| 2–3 | 262 (29.24) | 109 (33.96) | 124 (37.92) | 0.12 § | 0.005 § | 0.29 § | |
| >3 | 1 (0.11) | 0 (0.00) | 1 (0.31) | 0.32 § | 0.55 § | 0.32 § | |
| PV Thrombosis (%) | 184 (21.03) | 105 (34.09) | 145 (44.48) | <0.001 ^ | <0.0001 § | <0.0001 § | 0.007 § |
| Cirrhosis (%) | 803 (79.11) | 304 (83.98) | 325 (85.08) | 0.01 ^ | 0.04 § | 0.007 § | 0.68 § |
All values: Means±Standard Deviation as continuous; Frequences and percentage (%) as categorical.
Kruskal-Wallis rank test;
Wilcoxon rank-sum (Mann-Whitney) test;
Chi-square test;
Test z for proportions.
Abbreviations: GGTP, gamma glutamyl transpeptidae; ALKP, Alkaline phosphatase; AFP, Alpha-fetoprotein; MTD, Maximum Tumor Diameter; PVT, Portal Vein Thrombosis.
We then examined the associations for MTD and platelets and for PVT and MTD (Fig 2, upper and lower charts, respectively). We found a significant correlation for MTD and platelets (r=0.3348, p<0.0001). Box plots were then created for MTD and PVT categories. There were significant differences in the MTDs between PVT positive and negative patients, p<0.0001.
Fig 2.
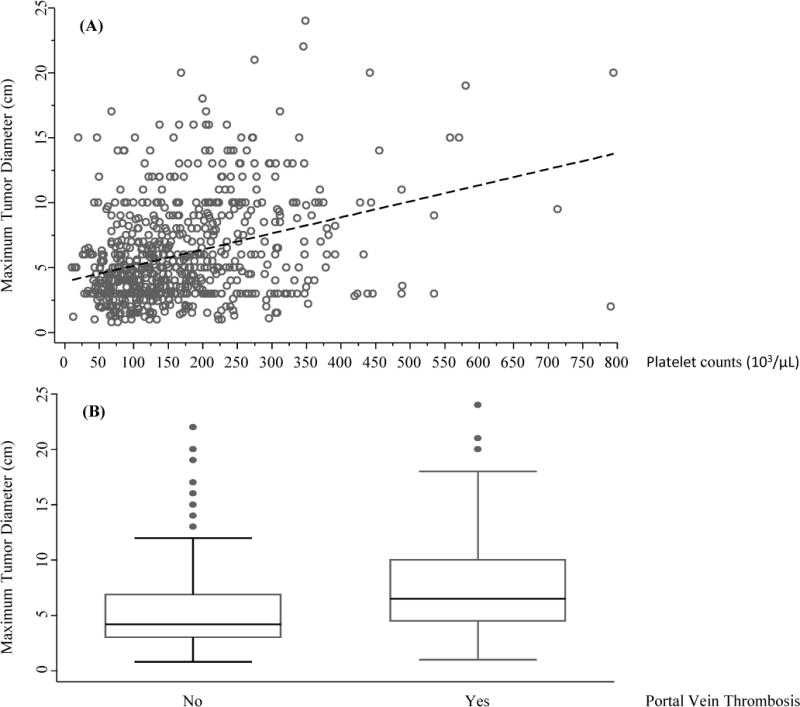
(A). Scatter plots between Maximum Tumor Diameter (cm) and Platelet counts (103/μL). Pearson’s correlation r = 0.3348; p<0.0001
(B). Box plots of Maximum Tumor Diameter (cm) between Portal Vein Thrombosis categories. Wilcoxon rank-sum (Mann-Whitney) test p<0.0001
Turkish Geography and HCC
As this database was composed of patients from several geographic sites throughout Turkey, we were curious as to whether there might be any regional differences in the patterns of HCC presentation or the patients. Table 6 shows patient groups from the locations that contributed most patients, and several marked differences were observed. MTD differed by locale, with the highest mean of 7.0cm being in Mersin and the lowest mean MTDs of 5.03 and 5.33cm being in Ankara and Hatay. There were also large differences in PVT%, being 40.74% in Mersin (highest MTD also) and the lowest in Hatay with 20.69% (also smallest MTD). Multifocality with >1 tumor nodule was highest in Diyabakir (53.49% of patients) and lowest in Mersin (17.02% of patients). Mean AFP levels were highest in Mersin (10109 ng/mL) and lowest in Mardin and Ankara (2885 and 3254 ng/mL respectively). There was also large regional difference in underlying liver disease. Cirrhosis was present in 88.29% of patients in Diyabakir, but only 62.92% of patients in Hatay. Furthermore, there were big regional differences in HBV, HCV and HDV. HBV was 73.95% in Diyabakir, but only 40.24% in Hatay. Conversely, HCV incidence in these HCC patients was highest in Hatay (30.49%) and lowest in Diyabakir and Mardin (8.37 and 7.81%, respectively). By contrast, HDV was 17.13% in Diyabakir, but less than 10% elsewhere. Thus, Mersin patients had the largest tumors, highest incidence of PVT and highest AFP levels. HBV was highest in Diyabakir (Mardin a close second) and HCV was highest in Hatay. Mersin patients not only had largest MTD, but also highest total bilirubin levels (4.63mg/dL) and the lowest bilirubin levels were in Adana (2.20mg/dL).
Table 6.
Comparisons HCC patient by Birth Place.
| Parameter* | Birth Place
|
p-value# | |||||
|---|---|---|---|---|---|---|---|
|
Adana (n=213) |
Ankara (n=45) |
Diyabakir (n=222) |
Hatay (n=90) |
Mardin (n=65) |
Mersin (n=105) |
||
| Sex (Males) (%) | 173 (81.22) | 36 (80.00) | 188 (84.68) | 68 (75.56) | 55 (84.62) | 84 (80.00) | 0.51 |
| Age (yr) | 63.33±11.28 | 62.96±9.02 | 58.57±11.94 | 64.31±12.62 | 59.35±12.72 | 62.87±9.27 | 0.0001 |
| Cirrhosis (%) | 160 (76.19) | 37 (82.22) | 196 (88.29) | 56 (62.92) | 53 (81.54) | 88 (85.44) | <0.001 |
| Etiology (%) | <0.001 ^ | ||||||
| HBV(−) & HCV(−) | 40 (20.51) | 11 (25.58) | 38 (17.67) | 24 (29.27) | 13 (20.31) | 25 (26.60) | |
| HBV(+) & HCV(−) | 120 (61.54) | 25 (58.14) | 159 (73.95) | 33 (40.24) | 46 (71.88) | 50 (53.19) | |
| HBV(−) & HCV(+) | 35 (17.95) | 7 (16.28) | 18 (8.37) | 25 (30.49) | 5 (7.81) | 19 (20.21) | |
| HDV(+) | 10 (5.08) | 0 (0.00) | 37 (17.13) | 4 (4.88) | 6 (9.68) | 5 (5.00) | <0.001 |
| Platelet counts (103/μL) | 175.33±111.09 | 133.09±72.95 | 161.07±95.23 | 164.34±110.62 | 187.41±129.35 | 157.67±101.54 | 0.21 |
| Hemoglobin (g/dL) | 12.14±2.15 | 11.95±2.65 | 12.26±2.30 | 11.31±2.07 | 12.10±2.27 | 11.45±2.28 | 0.002 |
| GGTP (U/L) | 121.03±155.33 | 295.00±130.11 | 185.87±162.03 | 121.28±113.40 | 216.14±261.51 | 138.66±181.97 | 0.0001 |
| AST (U/L) | 85.57±182.56 | 160.00±104.65 | 128.59±142.77 | 85.90±65.42 | 119.74±122.60 | 413.13±1420.63 | 0.0001 |
| ALKP (U/L) | 160.97±119.41 | 223.00±97.58 | 205.33±164.69 | 216.04±237.91 | 269.09±269.01 | 271.43±640.20 | 0.01 |
| Total Bilirubin (mg/dL) | 2.20±2.91 | 2.99±16.56 | 2.58±3.61 | 2.41±3.88 | 3.47±5.86 | 4.63±6.95 | 0.32 |
| AFP (IU/mL) | 5857.67±23424.97 | 3253.74±15819.15 | 8508.09±53108.07 | 9469.25±39870.72 | 2884.87±8652.05 | 10108.97±46954.41 | 0.08 |
| MTD (cm) | 6.39±4.29 | 5.03±3.27 | 6.19±3.89 | 5.33±3.66 | 5.78±3.42 | 7.0±3.65 | 0.03 |
| Nodules number (%) | <0.001 ^ | ||||||
| 1 | 117 (60.00) | 28 (66.67) | 99 (46.05) | 66 (77.65) | 33 (52.38) | 39 (82.98) | |
| 2–3 | 78 (40.00) | 14 (33.33) | 115 (53.49) | 19 (22.35) | 30 (47.62) | 8 (17.02) | |
| >3 | 0 (0.00) | 0 (0.00) | 1 (0.47) | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| PV Thrombosis (%) | 61 (29.61) | 17 (41.46) | 73 (33.80) | 18 (20.69) | 22 (35.48) | 11 (40.74) | 0.11 ^ |
All values: Means±Standard Deviation as continuous; Frequences and percentage (%) as categorical.
Kruskal-Wallis rank test;
Chi-square test.
Abbreviations: GGTP, gamma glutamyl transpeptidae; AST, aspartate aminotransaminase; ALKP, Alkaline phosphatase; AFP, Alpha-fetoprotein; MTD, Maximum Tumor Diameter; PVT, Portal Vein Thrombosis.
Discussion
Tumor characteristics that are generally considered for HCC patients are predominantly maximum tumor size or MTD and portal vein thrombosis or PVT, and to a lesser extent, AFP (due to its variability). Larger tumors have a worse prognosis in cancer in general as well as HCC (12). However, 40% of this cohort of HCC patients had AFP of <20 IU/ml. This is similar to findings elsewhere (13) and doubtless contributes to the uncertainty concerning use of blood AFP levels as a screening tool (14). Platelets have been previously found to be both a harbinger of HCC in patients that are pre-disposed, as well as a cirrhosis surrogate and to be associated with tumor size (15, 11, 9). In this cohort, 80.4% of patients had cirrhosis and 30.9% had thrombocytopenia (Table 1). Furthermore, there was a significant difference in platelet levels between patients having smaller versus larger MTD tumors, p=0.0001 (Fig 1) and this was also seen in the significant Pearson’s correlation (Fig 2). In a regression analysis, both platelets and presence of PVT were significant for MTD (Table 3).
PVT positivity was present in 28.55% of the total cohort (Table 1). When PVT positive and negative patients were compared (Table 4), the PVT positive patients were found to have significantly larger tumors (MTD), as also seen in Fig 2, and tumor multifocality and AFP levels, but also higher total bilirubin levels. Whether this is due to more aggressive tumors causing parenchymal destruction, or due to an increased PVT in patients with worse cirrhosis, is not addressed here, except that there was less cirrhosis in the PVT positive group. Remarkably however, there were significantly higher cardio-protective HDL levels in the PVT negative group. We think this is a first report of this association, as we are not aware of this being reported elsewhere.
Patients were trichotomized into 3 groups based on their AFP levels of <100, 100–1,000 and >1000 IU/ml (Table 5). The majority of patients had levels <100 IU/ml (Tables 1 and 5). Patients in the high AFP group also had significantly larger tumors (MTD) and tumor multifocality and PVT % as well as platelet numbers. Patients with higher AFP also had worse liver function, as judged by total bilirubin, GGT and ALKP levels and lower albumin and higher cirrhosis %, but the differences were not significant between the 2 elevated AFP groups.
An advantage of this multi-institutional study was the unusual possibility to compare HCC presentation in differing parts of a large country (Table 6). The Table only includes the groups that contributed the largest patient numbers, predominantly in the center and east of Turkey. However, there were some remarkable differences. Firstly, the incidence of HDV was mainly in patients from Diyabakir and Mardin. Secondly, there was a considerable range of presence of cirrhosis, from 85.4% in Mersin to 62.9% in Hatay. Thirdly, tumor characteristics showed considerable heterogeneity. Thus, mean MTD was 7cm in Mersin, but 5cm in Ankara; PVT was over 40% in Mersin and Ankara, yet only 20% in Hatay; tumor multifocality was 53% in Diyabakir, yet only 17% in Mersin.
In Turkey, the prevalence of inactive HBV carrier patients is more common than many countries and these patients are usually ignored in terms of treatment. But, some of them may have severe fibrosis or cirrhosis. These patients may develop HCC over time. This could explain why HBV-related HCC patients have more common cirrhosis than other etiology-associated HCC patients in our cohort. In the Diyarbakır region, HDV infection is extremely prevalent (Table 6). Dual infection may cause cirrhosis more commonly.
In the Hatay region, HCV infection is more common than in other regions of Turkey. The high prevalence may be related to inappropriate dental treatments. It seems this problem is being solved by dental awareness and added hygiene. However, it is difficult to explain why HCV– related HCC patients in Hatay had less cirrhosis. The average MTD of Hatay patients was the second lowest (above Ankara), possibly because of the mix of HBV plus HCV etiology patients in Hatay.
The relationship between cirrhosis and platelet counts is an interesting and complex issue. HBV and HCV can replicate in the platelets and decrease platelet survival. This replication is being seen more commonly in HCV infection than other causes, and is related to cirrhosis. Furthermore, the relationship of thrombocytopenia is likely to the degree of cirrhotic fibrosis, and measures of that severity were not available for this cohort. Platelet counts may also be related to Child-Pugh score (CPS) which indicates liver functional capacity. Patients with high CPS may have lower platelet counts, because thrombopoetin which promotes bone morrow is synthesized in the liver.
This study of a large HCC population at presentation shows certain unusual features, such as high HBV, presence of HDV, large range of cirrhosis and in tumor characteristics. Yet the underlying biology, such as the relationship of MTD to platelets and to PVT, are similar to other reports. A drawback is the absence of survival data, in part due to many patients being from far-flung and rural areas. However, we show the fascinating variability in HCC presentation in a large country and how biological principles in the relationship amongst parameters can be reproduced here.
Acknowledgments
This work is supported in part by NIH grant CA 82723 (B.I.C)
Abbreviations
- HCC
hepatocellular carcinoma
- PVT
portal vein thrombosis
- AFP
alpha-fetoprotein
- GGTP
gamma glutamyl transpeptidase
- ALKP
alkaline phosphatase
- AST
aspartate aminotransferase; prothrombin time
- INR
international normalized ratio
- Hb
haemoglobin
- Alb
albumin
- MTD
maximum tumor diameter
- CT
computerized axial tomography scan
Footnotes
Disclosure statement: The authors declare no conflict of interest.
References
- 1.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Carr BI, Guerra V. A hepatocellular carcinoma aggressiveness index and its relationship to liver enzyme levels. Oncology. 2016;90:215–20. doi: 10.1159/000444394. [DOI] [PubMed] [Google Scholar]
- 3.Carr BI, Guerra V, Giannini EG, Farinati F, et al. A liver index and its relationship to in dices of HCC aggressiveness. J Integr Oncol. 2016 Oct;5(4) doi: 10.4172/2329-6771.1000178. pii: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sirivatanauksorn Y, Tovikkai C. Comparison of staging systems of hepatocellular carcinoma. HPB Surg. 2011;2011:818217. doi: 10.1155/2011/818217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen ZH, Hong YF, Lin J, Li X, et al. Validation and ranking of seven staging systems of hepatocellular carcinoma. Oncol Lett. 2017;14:705–714. doi: 10.3892/ol.2017.6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kudo M, Matsui O, Izumi N, Iijima H, et al. Liver Cancer Study Group of Japan.Surveillance and diagnostic algorithm for hepatocellular carcinoma proposed by the Liver Cancer Study Group of Japan: 2014 update. Oncology. 2014;87(Suppl 1):7–21. doi: 10.1159/000368141. [DOI] [PubMed] [Google Scholar]
- 7.Terzi E, Salvatore V, Negrini G, Piscaglia F. Ongoing challenges in the diagnosis of hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2016;10:451–63. doi: 10.1586/17474124.2016.1124758. [DOI] [PubMed] [Google Scholar]
- 8.Carr BI, Guerra V. HCC and its microenvironment. Hepatogastroenterology. 2013;60:1433–7. doi: 10.5754/hge121028. [DOI] [PubMed] [Google Scholar]
- 9.Carr BI, Guerra V, Pancoska P. Thrombocytopenia in relation to tumor size in patients with hepatocellular carcinoma. Oncology. 2012;83:339–45. doi: 10.1159/000342431. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita Y, Takahashi M, Baba Y, Kanazawa S. Hepatocellular carcinoma with or without cirrhosis: a comparison of CT and angiographic presentations in the United States and Japan. Abdom Imaging. 1993;18:168–75. doi: 10.1007/BF00198057. [DOI] [PubMed] [Google Scholar]
- 11.Lu SN, Wang JH, Liu SL, Hung CH, et al. Thrombocytopenia as a surrogate for cirrhosis and a marker for the identification of patients at high-risk for hepatocellular carcinoma. Cancer. 2006;107:2212–22. doi: 10.1002/cncr.22242. [DOI] [PubMed] [Google Scholar]
- 12.Huang WJ, Jeng YM, Lai HS, Sheu FY, et al. Tumor size is a major determinant of prognosis of resected stage I hepatocellular carcinoma. Langenbecks Arch Surg. 2015;400:725–34. doi: 10.1007/s00423-015-1329-4. [DOI] [PubMed] [Google Scholar]
- 13.Silva JP, Gorman RA, Berger NG, Tsai S, et al. The prognostic utility of baseline alpha-fetoprotein for hepatocellular carcinoma patients. J Surg Oncol. 2017 Jul 25; doi: 10.1002/jso.24742. [Epub] [DOI] [PubMed] [Google Scholar]
- 14.Cabibbo G, Craxì A. Epidemiology, risk factors and surveillance of hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2010;14:352–5. [PubMed] [Google Scholar]
- 15.Colombo M, Sangiovanni A. Etiology, natural history and treatment of hepatocellular carcinoma. Antiviral Res. 2003;60:145–50. doi: 10.1016/j.antiviral.2003.08.010. [DOI] [PubMed] [Google Scholar]


