Abstract
Glycans and glycoconjugates are involved in regulating a vast array of cellular and molecular processes. Despite the importance of glycans in biology and disease, characterization of glycans remains difficult due to their structural complexity and diversity. Mass spectrometry (MS)-based techniques have emerged as the premier analytical tools for characterizing glycans. However, traditional MS-based strategies struggle to distinguish the large number of coexisting isomeric glycans that are indistinguishable by mass alone. Because of this, ion mobility spectrometry coupled to MS (IM-MS) has received considerable attention as an analytical tool for improving glycan characterization due to the capability of IM to resolve isomeric glycans prior to MS measurements. In this review, we present recent improvements in IM-MS instrumentation and methods for the structural characterization of isomeric glycans. In addition, we highlight recent applications of IM-MS that illustrate the enormous potential of this technology in a variety of research areas, including glycomics, glycoproteomics, and glycobiology.
Keywords: Ion mobility, Mass spectrometry, Glycans, Glycoconjugates, Isomers, Glycomics
Graphic abstract
Introduction
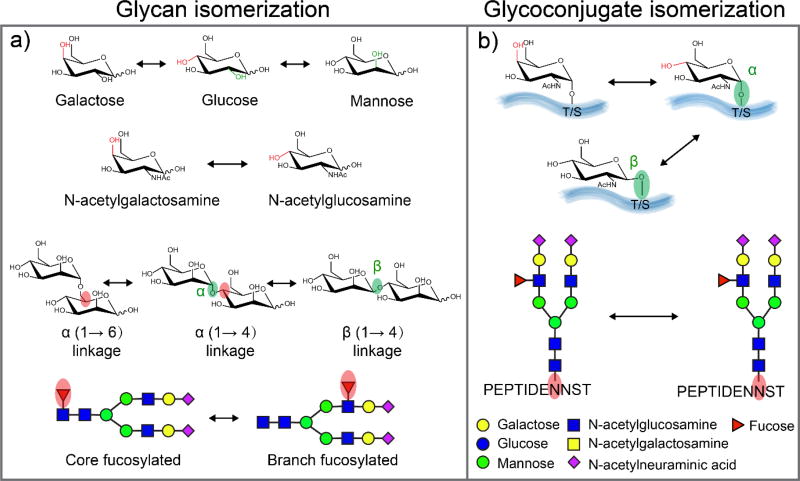
As one of the most abundant and complex protein post-translational modifications (PTMs), glycosylation is associated with many key biological processes including cell adhesion, molecular trafficking, receptor activation, and signal transduction [1]. The analysis of glycans and glycoconjugates is challenging due to the large diversity of structures resulting from the non-template driven biosynthesis [2,3]. In addition, many of the monosaccharides that compose larger glycans are structural isomers, and they can be connected via either α- or β-stereochemistry at multiple linkage positions, resulting in many glycan isomers (Figure 1a). Isomeric glycans can also have a variety of connectivities to numerous sites on other classes of molecules such as proteins, contributing to their strucutral complexity (Figure 1b).
Figure 1.
The isomerization of glycan and glycoconjugates. a) The building blocks (monosaccharides) that compose larger glycans are structural isomers (Hexose: galactose, glucose, mannose, N-acetylhexosamine: N-acetylgalactosamine, N-acetylglucosamine); monosaccharides can be connected either α- or β-stereochemistry at multiple potential linkage position; fucose could be either attached to N-glycan core or branches. b) Epimeric glycoconjugates results from alternative configurations (α- or β-) at the anomeric linkages or the presence of epimeric glycan monomers (galactose or glucose), scheme modified from reference [43]; two isomeric N-glycopeptides differ in the site of N-glycan attachment.
Separation and detailed structural characterization of glycan or glycoconjugate isomers is crucial for understanding their roles in various biological processes. Benefiting from speed and sensitivity of analysis, liquid chromatography (LC)-MS and capillary electrophoresis (CE)-MS have emerged as powerful techniques for glycan characterization [4–6]. Despite recent improvements to nearly all aspects of MS-based analyical workflows for glycan and glycoconjugate characterization, it remains challenging to achieve complete structural elucidation due to the complexity of glycans and lack of standard reference databases. Therefore, new techniques and methods that enhance the differentiation of glycan and glycoconjugate isomers would be highly desirable.
Although it has been two decades since IM-MS was orginally used to separate glycan isomers, recent technological advancements have sparked increased interest in IM-MS for glycan and glycoconjugate analysis [7]. Unlike other commonly used separation techniques such as LC and CE, IM-MS is a post-ionization gas-phase technique that separates ions based on differences in shape and charge as they travel through a buffer gas under the influence of an electric field [8,9]. The time it takes for an analyte ion to travel through the IM cell can be used to calculate rotationally averaged collision cross section (CCS) which provides an additional parameter that can be used to identify compounds as well as information about molecular conformation [8–10].
Initial applications of IM to carbohydrate analysis focused on distinguishing small isomeric carbohydrate standards [11,12]. Due to the advancement and commercialization of IM-MS instrumentation, a growing number of labs continue to demonstrate that IM-MS is a fast, sensitive, and effective method for resolving carbohydrate isomers. For example, IM-MS has been used to separate a variety of isomeric species, including connectivity and configurational isomers [13]. Furthermore, studies have been extended to more complex systems such as N- and O-glycans and intact glycopeptides [14–20]. Here, we discuss the latest developments in IM-MS methods and technology that have allowed for enhanced sepration and structural characterization of glycans and glycoconjugates and discuss advances necessary for IM to become more widely used in glycomics and glycoproteomics workflows.
Improving IM-Based Isomer Separations
Although many proof-of-principle experiments have shown the potential of IM to separate glycan isomers, baseline separation of isomeric glycans is difficult as they often have minor differences in CCS. This is especially problematic as studies are expanded to larger glycans and glycoconjugates because minor changes in the glycan composition often result in subtle differences in the overall structure. Furthermore, improved glycan separation will be crucial for extending the applications of IM-MS technology to large-scale studies of glycans and glycoproteins in complex mixtures from biological systems (i.e., glycomics and glycoproteomics). Thus, various analytical workflows that include IM separation have been developed to enhance the separation of isomeric glycans.
One of major factors that has limited the utility of IM-MS for many applications is that many instrument platforms lack the mobility resolution necessary to resolve isomeric species that have minor differences in CCS. The development of high resolution IM instrumentation using structures for lossless ion manipulations (SLIM) technology has shown great potential to enable separations of a variety of isomeric species [21]. Recently, a novel instrument was developed capable of ultralong pathlength travelling wave ion mobility (TWIM) separations on a serpentine-shaped SLIM device that has a 30-fold increase in IM resolution compared to traditional drift tube IM and TWIM instruments [22]. In addition to providing baseline separation of isomers lacto-N-hexaose and lacto-N-neohexaose, high resolution SLIM IM-MS revealed a new conformation of lacto-N-neohexaose. This suggests that SLIM-based IM separations will provide a level of conformational information about glycans that was previously inaccessible.
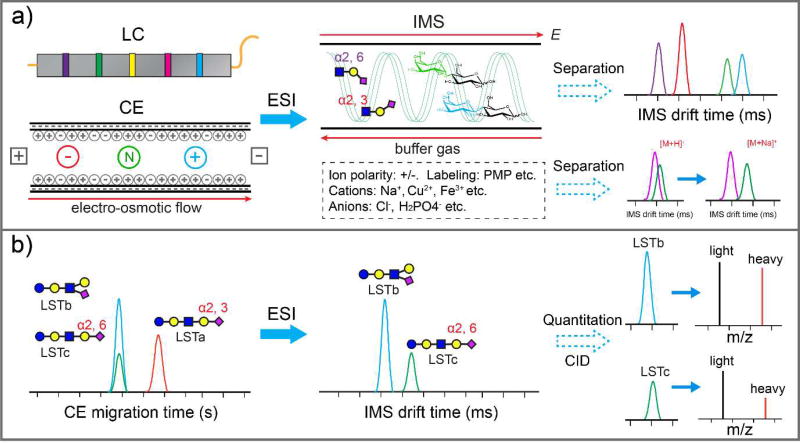
An alternative approach to increase isomer separation by improving IM-MS instrumentation is to optimize the charge state or polarity of the glycan ions to yield optimal separation of isomeric species. Numerous studies have demonstrated that mobility separations of glycan isomers can be optimized by manipulating the ion charge state or charge polarity [23,24]. Because of this it is important to consider a variety of charge carriers, such as metal cations and anions, and ionization methods for improving mobility separations (Figure 2a) [25–29]. Furthermore, it was demonstrated that electron transfer reactions with group II metal-coordinated carbohydrates improve separation of isomeric species, suggesting the potential for ion-ion reactions in the gas-phase for differentiation of isomeric oligosaccharides [30].
Figure 2.
Coupling IM with LC or CE. a) After LC or CE separation, analytes were ionized by ESI and subject to another dimension of separation afforded by IM based on their shape and charge through a buffer gas under a weak electric field (E). Sample preparation or gas-phase chemistry could be manipulated to improve glycan isomer separation. b) CE-ESI-TWIM-MS/MS analysis of a mixture of aminoxyTMT6-128 (light) and aminoxyTMT6-131 (heavy) differentially labeled sialyllacto-N-tetraose a, b, c (LSTa, LSTb, LSTc). CE was able to separate LSTa with LSTb/c, but was unable to resolve LSTb and LSTc. Benefiting from another dimension of separation afforded by TWIM, baseline separation between LSTb and LSTc was achieved, which enables quantitative analysis of each isomer following MS/MS [38].
As the detection and the characterization of glycans is often hindered by the lack of a chromophore and their poor ionization properties in either spectroscopic or mass-spectrometric detection, a wide variety of glycan labeling reagents have been developed, which provide the opportunity to manipulate the conformation of the glycans in the gas-phase. Although 1-phenyl-3-methyl-5-pyrazolone (PMP) was originally developed to enhance UV detection, a recent study showed increased separation of structural isomers after PMP derivatization [31]. Reacting with cis-diols on carbohydrates, boronic acid (BA) derivatization has also been shown to have great potential in improving isobaric carbohydrate differentiation as an ion mobility shift strategy [32]. Besides covalent binding, non-covalent binding such as crown ethers for peptides and metal cations for carbohydrates is another promising approach to enhance separations [24,33,34]. Recently, non-covalent complexes between monosaccharides and combinations of metal cations, peptides, and amino acids were demonstrated to improve differentiation of 8 pairs of enantiomeric glucose isomers [35]. Although there is no universal strategy to use sample preparation or gas-phase chemistry to improve glycan isomer separation, the strategies discussed above are important considerations for achieving optimal separation.
Coupling IM with orthogonal separation techniques
In addition to improving IM-MS technology, it is important to consider the enhanced analytical capability by coupling IM-MS with a variety of orthogonal separation techniques. Because mobility separations typically occur on the order of milliseconds it is possible to couple IM between LC [36,37] or CE [38] and mass spectrometry for glycan analysis (Figure 2a). The combination of orthogonal separation methods has been demonstrated to offer improved characterization of isomeric glycans. For example, the combination of hydrophilic interaction chromatography (HILIC) and TWIM was used for separation of isomeric pectic oligosaccharides [37]. In addition, a straightforward reversed-phase LC-IM-MS platform was developed for integrated proteomic and glycomic studies [39]. Furthermore, the combination of CE with TWIM-CID-MS/MS provided improved separation and quantitation of aminoxy tandem mass tag (aminoxyTMT)-labeled human milk oligosaccharides (HMOs) (Figure 2b) [38].
Coupling IM with MS-based fragmentation and spectroscopic techniques
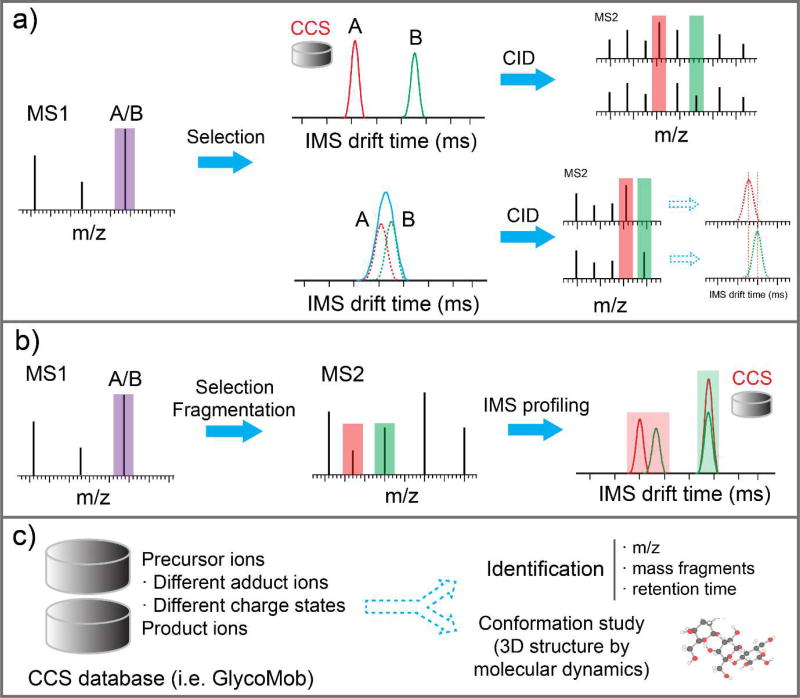
The development of instrumentation that couples IM with a variety of fragmentation techniques has been crucial for improving the identification of glycan and glycopeptide isomers. It is important to note that coupling IM with MS/MS is mutually beneficial for both techniques. That is, ion mobility separations can be used to deconvolute complex fragmentation spectra that arise from coeluting isomeric species (Figure 3a). In addition, fragmentation spectra can be used to deconvolute CCS distributions of partially resolved isomeric species (Figure 3a).
Figure 3.
IM-MS analysis of precursor ions and their fragments. a) Two scenarios exist for co-eluted isomers A/B for IM-MS analysis after being selected by quadrupole. Scenario one: A and B could be baseline-separated by IM. The mobility-selected ions could be subject to CID separately and signature product ions could be obtained for each †isomer. Scenario two: A and B could not be completely resolved by IM. Signature product ions were obtained for unresolved species. Drift time profiles of these signature products ions were extracted from total drift time profiles to differentiate A and B. b) Co-eluted isomers A/B were selected by quadrupole for MS/MS and the drift time profiles could be obtained for all product ions. Those product ions that are indicative of the isomeric structures of the analyte could be distinguished by IM and be used to differentiate the isomers. c) The CCS of both precursor ions and product ions could be measured and implemented into a CCS database. The CCS values could be used as an additional parameter for glycan identification besides the commonly used m/z, mass fragments, and retention time. Furthermore, conformational study could be conducted by molecular dynamics to acquire the 3D structures of glycans.
An increasing number of studies have demonstrated that it is beneficial to perform ion mobility experiments on both precursor and fragment ions of isomeric species (Figure 3b). For example, IM analysis of precursor and fragmentation ions was used to differentiate Lewis and blood group epitopes [40]. Furthermore, it was recently demonstrated that IM analysis of MS/MS derived fragments of glycans can be used to differentiate anomeric glycosidic linkages [41]. Another promising approach capable of performing mobility separation of precursor and fragment ions is tandem IM (IM-CID-IM) in which two mobility regions are separated by a region where ions can be mobility selected and collisionally activated [42]. IM-IM-MS was recently used to distinguish underivatized carbohydrate isomers based on differences in mobilities of fragments ions. By probing ion mobility profiles of product ions, IM was also used to distinguish α2,3 or α2,6 sialic-acid linkage [15,43].
In addition to coupling IM with collision-based fragmentation methods, IM has recently been combined with a variety of fragmentation and spectroscopic techniques, including electron activated dissociation (ExD) [44], UV photodissociation [45,46], and cryogenic IR spectroscopy [47,48]. The combination of selected accumulation-trapped IM (SA-TIMS) with Fourier transform ion cyclotron resonance (FTICR) mass spectrometers makes it possible to perform ExD on mobility-selected ions [44]. It was recently demonstrated that coupling IM with cryogenic IR spectroscopy can be used to identify isomeric glycosaminoglycans that are partially resolved by IM [49].
Collision Cross Section Databases
Another benefit IM-MS provides is the ability to measure CCS values which can be implemented into databases and used as additional criteria for structural identification (Figure 3c) [43,50]. Because CCS values are an additional parameter to improve the identification of glycan and glycopeptides, several groups have compiled CCS databases of glycans and glycopeptides that have potential to aide in glycan identifications [25,28,51–53]. For example, GlycoMob is an online database of >900 CCS values of glycans and their fragments. In addition, a database containing glycopeptide CCS values was recently presented that has the potential to aide in identification of unique glycoforms [54].
Accurate glycan identification based on CCS values is still in its early stage with several challenges remaining before this approach could be used routinely for glycan identification. One of the challenges lies in the shortage of available CCS values resulting from the difficulty in synthesizing glycan standards especially for these complex N- or O-glycans [55]. Another limitation of CCS databases is that CCS values are not intrinsic properties of ions in the same way as m/z values. CCS values depend on a variety of factors such as ionization conditions, buffer gas, instrument parameters, and the calibration method if not measured in a linear drift tube. Because of this, the development of robust standard operating procedures, quality controls, and calibration methods is necessary for CCS databases to be used effectively. Despite the challenges listed above, implementation of CCS databases into analytical workflows to improve glycan and glycoconjugate identification will likely be a crucial step for expanding the role and utility of IM-MS in the glycosciences. With increasing amounts of IM-MS data acquired each day, especially after coupling with LC, powerful bioinformatics tools that enable easy data acquisition, analysis and processing in a high-throughput and rapid way is key to advancing the IM-MS enhanced glycomics workflow development. Continuous efforts into platform development to support the storage of IM-MS data is also highly needed to facilitate the database query for the glycoscience community. Such platform should have the capability to support the complexity of IM-MS data, including the precursor ion and fragment ion m/z information, CCS information and LC information such as PGC-LC retention time reference [56], as well as the connections among these important parameters and information. Furthermore, the advancement of instrumentation with higher mobility resolution and CCS measurement accuracy will certainly improve the effectiveness and accuracy of IM-MS assisted glycomics workflow in a substantial manner.
Improved characterization of intact glycoconjugates
Although most examples mentioned above illustrate the utility of IM-MS for separating free or released glycans, an emerging application of IM-MS is the structural characterization of glycans bound to other classes of molecules such as proteins, peptides, and lipids (i.e., glycoconjugates). The analysis of IM-MS can reveal information about the macro- and micro- heterogeneity of glycosylation of glycoconjugates. For example, IM can separate intact glycopeptides that differ only in the glycosylation sites [16]. Furthermore, IM-MS analysis of glycopeptide fragments was demonstrated to be an effective strategy to distinguish α2,3 versus α2,6 sialic acid linkages on intact glycopeptides [15,16]. In addition to glycopeptides, IM-MS was recently used to separate glycolipid isomers [57]. It is also important to note that as IM-MS technologies evolve, they have the potential to be extended to characterize glycosylation of larger systems such as intact glycoproteins, antibodies, and virus capsids. For example, IM-MS and collision induced unfolding was used to provide qualitative information about glycosylation levels for intact antibodies [58].
Improved characterization of biological samples
One of the most promising applications of IM-MS is the characterization of glycans and glycoconjugates in complex mixtures from biological systems. Several studies have shown the benefit of adding IM into MS-based workflows for the analysis of biological samples [20,59]. For example, incorporation of field asymmetric ion mobility spectrometry (FAIMS) into a bottom-up proteomic workflow increased the number of glycopeptides identified from flagellin from Campylobacter jejuni 11168 [20]. It is important to note that IM separation of isomeric and isobaric species prior to MS analysis has been shown to improve quantification accuracy of both peptides and glycans [38,60]. In addition to separating isomers, IM can also be used to extract glycan and glycopeptide regions from interference ions or other molecular species [39,61–63]. Being able to separate various molecular species, IM has the potential to aide multi-omics studies, which could greatly simplify sample preparation procedures and provide more detailed information about molecular and cellular processes [64,65]. Due to the recent improvements in technology and methods described here, the application of IM-MS will likely continue to be extended beyond proof-of-principle experiments performed on glycan standards to complex mixtures from a variety of biological samples.
Conclusions
IM-MS continues to emerge as a powerful method for characterizing the enormous structural diversity and complexity of glycans and glycoconjugates. Recent advances in IM-MS instrumentation and methods have positioned this technique to enable discoveries in the glycosciences. In the future, the development of hybrid methods that couples high-resolution IM with orthogonal separation techniques and MS-based fragmentation and spectroscopic techniques will likely provide the analytical capability to extend the application of IM-MS to more complex biological systems in order to unravel the role of glycans and glycoconjugates in biology and disease.
Highlights.
IM-MS is a powerful tool for glycan and glycoconjugate isomer analysis.
Coupling IM with orthogonal separation techniques along with gas-phase manipulations greatly enhance isomer separation.
Coupling IM with MS-based fragmentation and spectroscopic techniques improves structure characterization.
CCS database provides another dimension of information for confident structural identification.
IM-MS contributes to improved characterization of complex biological samples.
Acknowledgments
This research was supported in part by the National Institutes of Health (NIH) grants R21AG055377, R01 DK071801, and National Science Foundation (NSF) grant CHE-1710140. This research was supported by the National Institutes of Health, under Ruth L. Kirschstein National Research Service Award T32 HL 007936 from the National Heart Lung and Blood Institute to the University of Wisconsin-Madison Cardiovascular Research Center (MSG Postdoctoral Fellowship). LL acknowledges a Vilas Distinguished Achievement Professorship and Janis Apinis Professorship with funding provided by the Wisconsin Alumni Research Foundation and University of Wisconsin-Madison School of Pharmacy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
• of special interest
• • of outstanding interest
- 1.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Rakus JF, Mahal LK. New technologies for glycomic analysis: toward a systematic understanding of the glycome. Annual review of analytical chemistry. 2011;4:367–392. doi: 10.1146/annurev-anchem-061010-113951. [DOI] [PubMed] [Google Scholar]
- 3.Zhu M, Bendiak B, Clowers B, Hill HH. Ion mobility-mass spectrometry analysis of isomeric carbohydrate precursor ions. Anal Bioanal Chem. 2009;394:1853–1867. doi: 10.1007/s00216-009-2865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pabst M, Altmann F. Glycan analysis by modern instrumental methods. Proteomics. 2011;11:631–643. doi: 10.1002/pmic.201000517. [DOI] [PubMed] [Google Scholar]
- 5.Mariño K, Bones J, Kattla JJ, Rudd PM. A systematic approach to protein glycosylation analysis: a path through the maze. Nat Chem Biol. 2010;6:713–723. doi: 10.1038/nchembio.437. [DOI] [PubMed] [Google Scholar]
- 6.Zaia J. Mass spectrometry and the emerging field of glycomics. Chem Biol. 2008;15:881–892. doi: 10.1016/j.chembiol.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray C, Thomas B, Upton R, Migas L, Eyers C, Barran P, Flitsch S. Applications of ion mobility mass spectrometry for high throughput, high resolution glycan analysis. Biochimica et Biophysica Acta (BBA)-General Subjects. 2016;1860:1688–1709. doi: 10.1016/j.bbagen.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Bohrer BC, Merenbloom SI, Koeniger SL, Hilderbrand AE, Clemmer DE. Biomolecule analysis by ion mobility spectrometry. Annu. Rev. Anal. Chem. 2008;1:293–327. doi: 10.1146/annurev.anchem.1.031207.113001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanu AB, Dwivedi P, Tam M, Matz L, Hill HH. Ion mobility–mass spectrometry. J Mass Spectrom. 2008;43:1–22. doi: 10.1002/jms.1383. [DOI] [PubMed] [Google Scholar]
- 10.Jurneczko E, Barran PE. How useful is ion mobility mass spectrometry for structural biology? The relationship between protein crystal structures and their collision cross sections in the gas phase. Analyst. 2011;136:20–28. doi: 10.1039/c0an00373e. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, Wyttenbach T, Bowers MT. Gas phase structures of sodiated oligosaccharides by ion mobility/ion chromatography methods. International journal of mass spectrometry and ion processes. 1997;167:605–614. [Google Scholar]
- 12.Liu Y, Clemmer DE. Characterizing oligosaccharides using injected-ion mobility/mass spectrometry. Anal Chem. 1997;69:2504–2509. doi: 10.1021/ac9701344. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann J, Hahm H, Seeberger P, Pagel K. Identification of carbohydrate anomers using ion mobility-mass spectrometry. Nature. 2015;526:241–244. doi: 10.1038/nature15388. [DOI] [PubMed] [Google Scholar]
- 14.Harvey DJ, Scarff CA, Edgeworth M, Struwe WB, Pagel K, Thalassinos K, Crispin M, Scrivens J. Travelling - wave ion mobility and negative ion fragmentation of high - mannose N - glycans. J Mass Spectrom. 2016;51:219–235. doi: 10.1002/jms.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guttman M, Lee KK. Site-Specific Mapping of Sialic Acid Linkage Isomers by Ion Mobility Spectrometry. Anal Chem. 2016;88:5212–5217. doi: 10.1021/acs.analchem.6b00265. • This study demonstrates the ability of IM to differentiate sialic acid linkage isomers on both N- and O-linked glycopeptides, which indicates great potential of IM in elucidating glycan structures at a site-specific level after being incorporated in the conventional glycoproteomics platforms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinneburg H, Hofmann J, Struwe WB, Thader A, Altmann F, Silva DV, Seeberger PH, Pagel K, Kolarich D. Distinguishing N-acetylneuraminic acid linkage isomers on glycopeptides by ion mobility-mass spectrometry. Chemical Communications. 2016;52:4381–4384. doi: 10.1039/c6cc01114d. • • IM separation of isomeric N-glycopeptides differing in the N-glycan attachment site is demonstrated. Fragment ion mobilities are used to differentiate glycan product ions containing α2,3 and α2,6 NeuAc linkages. This approach is used to differentiate α2,3 from α2,6 sialylated glycopeptides obtained from complex mixtures using two forms of a-1 proteinase inhibitor (A1PI) produced in different cell types. [DOI] [PubMed] [Google Scholar]
- 17.Creese AJ, Cooper HJ. Separation and identification of isomeric glycopeptides by high field asymmetric waveform ion mobility spectrometry. Anal Chem. 2012;84:2597–2601. doi: 10.1021/ac203321y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell JL, Baba T, Liu C, Lane CS, Le Blanc JY, Hager JW. Analyzing Glycopeptide Isomers by Combining Differential Mobility Spectrometry with Electron-and Collision-Based Tandem Mass Spectrometry. J Am Soc Mass Spectrom. 2017:1–8. doi: 10.1007/s13361-017-1663-6. [DOI] [PubMed] [Google Scholar]
- 19.Zhu F, Trinidad JC, Clemmer DE. Glycopeptide site heterogeneity and structural diversity determined by combined lectin affinity chromatography/IMS/CID/MS techniques. J Am Soc Mass Spectrom. 2015;26:1092–1102. doi: 10.1007/s13361-015-1110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ulasi GN, Creese AJ, Hui SX, Penn CW, Cooper HJ. Comprehensive mapping of O - glycosylation in flagellin from Campylobacter jejuni 11168: A multienzyme differential ion mobility mass spectrometry approach. Proteomics. 2015;15:2733–2745. doi: 10.1002/pmic.201400533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibrahim YM, Hamid AM, Deng L, Garimella SV, Webb IK, Baker ES, Smith RD. New frontiers for mass spectrometry based upon structures for lossless ion manipulations. Analyst. 2017;142:1010–1021. doi: 10.1039/c7an00031f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng L, Webb IK, Garimella SV, Hamid AM, Zheng X, Norheim RV, Prost SA, Anderson GA, Sandoval JA, Baker ES, et al. Serpentine Ultralong Path with Extended Routing (SUPER) High Resolution Traveling Wave Ion Mobility-MS using Structures for Lossless Ion Manipulations. Anal Chem. 2017;89:4628–4634. doi: 10.1021/acs.analchem.7b00185. • • Structures for lossless ion manipulations (SLIM) is used to obtain extended ion mobility separation pathlengths and a 30-fold increase in IM resolution compared to conventional IM instrumentation. This high-resolution method is used to reveal a new conformational feature for lacto-N-neohexaose. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Struwe W, Baldauf C, Hofmann J, Rudd P, Pagel K. Ion mobility separation of deprotonated oligosaccharide isomers–evidence for gas-phase charge migration. Chemical Communications. 2016;52:12353–12356. doi: 10.1039/c6cc06247d. [DOI] [PubMed] [Google Scholar]
- 24.Zheng X, Zhang X, Schocker NS, Renslow RS, Orton DJ, Khamsi J, Ashmus RA, Almeida IC, Tang K, Costello CE, et al. Enhancing glycan isomer separations with metal ions and positive and negative polarity ion mobility spectrometry-mass spectrometry analyses. Anal Bioanal Chem. 2017;409:467. doi: 10.1007/s00216-016-9866-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Struwe W, Benesch J, Harvey D, Pagel K. Collision cross sections of high-mannose N-glycans in commonly observed adduct states–identification of gas-phase conformers unique to [M– H] – ions. Analyst. 2015;140:6799–6803. doi: 10.1039/c5an01092f. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, Dodds ED. Ion-neutral collisional cross sections of carbohydrate isomers as divalent cation adducts and their electron transfer products. Analyst. 2015;140:6912–6921. doi: 10.1039/c5an01093d. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann W, Hofmann J, Pagel K. Energy-resolved ion mobility-mass spectrometry—a concept to improve the separation of isomeric carbohydrates. J Am Soc Mass Spectrom. 2014;25:471–479. doi: 10.1007/s13361-013-0780-0. [DOI] [PubMed] [Google Scholar]
- 28.Huang Y, Dodds ED. Ion mobility studies of carbohydrates as group I adducts: isomer specific collisional cross section dependence on metal ion radius. Anal Chem. 2013;85:9728–9735. doi: 10.1021/ac402133f. [DOI] [PubMed] [Google Scholar]
- 29.Zhu F, Glover MS, Shi H, Trinidad JC, Clemmer DE. Populations of metal-glycan structures influence MS fragmentation patterns. J Am Soc Mass Spectrom. 2015;26:25–35. doi: 10.1007/s13361-014-1000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y, Dodds ED. Discrimination of isomeric carbohydrates as the electron transfer products of group II cation adducts by ion mobility spectrometry and tandem mass spectrometry. Anal Chem. 2015;87:5664–5668. doi: 10.1021/acs.analchem.5b00759. [DOI] [PubMed] [Google Scholar]
- 31.Yang H, Shi L, Zhuang X, Su R, Wan D, Song F, Li J, Liu S. Identification of structurally closely related monosaccharide and disaccharide isomers by PMP labeling in conjunction with IM-MS/MS. Sci Rep. 2016;6 doi: 10.1038/srep28079. srep28079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fenn LS, McLean JA. Enhanced carbohydrate structural selectivity in ion mobility-mass spectrometry analyses by boronic acid derivatization. Chemical Communications. 2008:5505–5507. doi: 10.1039/b810421b. [DOI] [PubMed] [Google Scholar]
- 33.Hilderbrand AE, Myung S, Clemmer DE. Exploring crown ethers as shift reagents for ion mobility spectrometry. Anal Chem. 2006;78:6792–6800. doi: 10.1021/ac060439v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bohrer BC, Clemmer DE. Shift reagents for multidimensional ion mobility spectrometry-mass spectrometry analysis of complex peptide mixtures: evaluation of 18-Crown-6 ether complexes. Anal Chem. 2011;83:5377–5385. doi: 10.1021/ac200892r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaye M, Nagy G, Clemmer D, Pohl N. Multidimensional analysis of 16 glucose isomers by ion mobility spectrometry. Anal Chem. 2016;88:2335–2344. doi: 10.1021/acs.analchem.5b04280. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi Y, Nishima W, Re S, Sugita Y. Confident identification of isomeric N - glycan structures by combined ion mobility mass spectrometry and hydrophilic interaction liquid chromatography. Rapid Commun Mass Spectrom. 2012;26:2877–2884. doi: 10.1002/rcm.6412. [DOI] [PubMed] [Google Scholar]
- 37.Leijdekkers AG, Huang J-H, Bakx EJ, Gruppen H, Schols HA. Identification of novel isomeric pectic oligosaccharides using hydrophilic interaction chromatography coupled to traveling-wave ion mobility mass spectrometry. Carbohydr Res. 2015;404:1–8. doi: 10.1016/j.carres.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Zhong X, Chen Z, Snovida S, Liu Y, Rogers JC, Li L. Capillary electrophoresis15 electrospray ionization-mass spectrometry for quantitative analysis of glycans labeled with multiplex carbonyl-reactive tandem mass tags. Anal Chem. 2015;87:6527–6534. doi: 10.1021/acs.analchem.5b01835. • Combination of CE with IM is used to achieve separation of three oligosaccharide isomers from human milk, which cannot be separated by either CE or IM alone. Improved quantitation of isomers is also achieved by combining CE and IM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lareau NM, May JC, McLean JA. Non-derivatized glycan analysis by reverse phase liquid chromatography and ion mobility-mass spectrometry. Analyst. 2015;140:3335–3338. doi: 10.1039/c5an00152h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofmann J, Stuckmann A, Crispin M, Harvey DJ, Pagel K, Struwe WB. Identification of Lewis and Blood Group Carbohydrate Epitopes by Ion Mobility-Tandem-Mass Spectrometry Fingerprinting. Anal Chem. 2017;89:2318–2325. doi: 10.1021/acs.analchem.6b03853. [DOI] [PubMed] [Google Scholar]
- 41.Gray CJ, Schindler B, Migas LG, Picmanova M, Allouche AR, Green AP, Mandal S, Motawia MS, Sánchez-Párez R, Bjarnholt N, et al. Bottom-up elucidation of glycosidic bond stereochemistry. Anal Chem. 2017;89:4540–4549. doi: 10.1021/acs.analchem.6b04998. • This study demonstrates that “memory” of glycan anomeric configuration is retained after fragmentation. [DOI] [PubMed] [Google Scholar]
- 42.Gaye M, Kurulugama R, Clemmer D. Investigating carbohydrate isomers by IMS-CID-IMS-MS: precursor and fragment ion cross-sections. Analyst. 2015;140:6922–6932. doi: 10.1039/c5an00840a. • Multidimensional IM (IM-CID-IM-MS) is used to distinguish carbohydrate isomers based on fragment mobilities for species difficult to distinguish based on precursor mobilities. [DOI] [PubMed] [Google Scholar]
- 43.Both P, Green A, Gray C, Šardzík R, Voglmeir J, Fontana C, Austeri M, Rejzek M, Richardson D, Field R, et al. Discrimination of epimeric glycans and glycopeptides using IM-MS and its potential for carbohydrate sequencing. Nat Chem. 2014;6:65–74. doi: 10.1038/nchem.1817. [DOI] [PubMed] [Google Scholar]
- 44.Pu Y, Ridgeway ME, Glaskin RS, Park MA, Costello CE, Lin C. Separation and identification of isomeric glycans by selected accumulation-trapped ion mobility spectrometry-electron activated dissociation tandem mass spectrometry. Anal Chem. 2016;88:3440–3443. doi: 10.1021/acs.analchem.6b00041. • SA-TIMS is coupled to FTICR-MS to perform ExD fragmentation on mobility selected ions, which facilitates the separation and identification of glycan linkage isomers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrison KA, Clowers BH. Differential Fragmentation of Mobility-Selected Glycans via Ultraviolet Photodissociation and Ion Mobility-Mass Spectrometry. J Am Soc Mass Spectrom. 2017;6:1236–1241. doi: 10.1007/s13361-017-1621-3. [DOI] [PubMed] [Google Scholar]
- 46.Lee S, Valentine SJ, Reilly JP, Clemmer DE. Analyzing a mixture of disaccharides by IMS-VUVPD-MS. Int J Mass spectrom. 2012;309:161–167. doi: 10.1016/j.ijms.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masellis C, Khanal N, Kamrath MZ, Clemmer DE, Rizzo TR. Cryogenic Vibrational Spectroscopy Provides Unique Fingerprints for Glycan Identification. J Am Soc Mass Spectrom. 2017:1–6. doi: 10.1007/s13361-017-1728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hernandez O, Isenberg S, Steinmetz V, Glish GL, Maitre P. Probing mobility-selected saccharide isomers: selective ion–molecule reactions and wavelength-specific IR activation. The Journal of Physical Chemistry A. 2015;119:6057–6064. doi: 10.1021/jp511975f. [DOI] [PubMed] [Google Scholar]
- 49.Khanal N, Masellis C, Kamrath MZ, Clemmer DE, Rizzo TR. Glycosaminoglycan analysis by cryogenic messenger-tagging IR spectroscopy combined with IMS-MS. Anal Chem. 2017 doi: 10.1021/acs.analchem.7b01467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.May JC, Morris CB, McLean JA. Ion Mobility Collision Cross Section Compendium. Anal Chem. 2016;89:1032–1044. doi: 10.1021/acs.analchem.6b04905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pagel K, Harvey DJ. Ion mobility–mass spectrometry of complex carbohydrates: collision cross sections of sodiated N-linked glycans. Anal Chem. 2013;85:5138–5145. doi: 10.1021/ac400403d. [DOI] [PubMed] [Google Scholar]
- 52.Hofmann J, Struwe WB, Scarff CA, Scrivens JH, Harvey DJ, Pagel K. Estimating collision cross sections of negatively charged N-glycans using traveling wave ion mobility-mass spectrometry. Anal Chem. 2014;86:10789–10795. doi: 10.1021/ac5028353. [DOI] [PubMed] [Google Scholar]
- 53.Glaskin RS, Khatri K, Wang Q, Zaia J, Costello CE. Construction of a Database of Collision Cross Section Values for Glycopeptides, Glycans, and Peptides Determined by IM-MS. Anal Chem. 2017;89:4452–4460. doi: 10.1021/acs.analchem.6b04146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Struwe WB, Pagel K, Justin L, Benesch P, Harvey DJ, Campbell MP. GlycoMob: an ion mobility-mass spectrometry collision cross section database for glycomics. Glycoconjugate J. 2016;33:399. doi: 10.1007/s10719-015-9613-7. • CCS database containing over 900 CCSs values of glycans, oligosaccharide standards and their fragments with various adducts in both positive and negative mode. [DOI] [PubMed] [Google Scholar]
- 55.Wiederschain GY. Essentials of glycobiology. Biochemistry (Moscow) 2009;74:1056-1056. [Google Scholar]
- 56.Pabst M, Bondili JS, Stadlmann J, Mach L, Altmann F. Mass+ retention time=structure: a strategy for the analysis of N-glycans by carbon LC-ESI-MS and its application to fibrin N-glycans. Anal Chem. 2007;79:5051–5057. doi: 10.1021/ac070363i. [DOI] [PubMed] [Google Scholar]
- 57.Wojcik R, Webb IK, Deng L, Garimella SV, Prost SA, Ibrahim YM, Baker ES, Smith RD. Lipid and glycolipid isomer analyses using ultra-high resolution ion mobility spectrometry separations. International journal of molecular sciences. 2017;18:183. doi: 10.3390/ijms18010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tian Y, Han L, Buckner AC, Ruotolo BT. Collision induced unfolding of intact antibodies: rapid characterization of disulfide bonding patterns, glycosylation, and structures. Anal Chem. 2015;87:11509–11515. doi: 10.1021/acs.analchem.5b03291. [DOI] [PubMed] [Google Scholar]
- 59.Sarbu M, Robu AC, Ghiulai RM, Vukelić Ze, Clemmer DE, Zamfir AD. Electrospray ionization ion mobility mass spectrometry of human brain gangliosides. Anal Chem. 2016;88:5166–5178. doi: 10.1021/acs.analchem.6b00155. [DOI] [PubMed] [Google Scholar]
- 60.Sturm RM, Lietz CB, Li L. Improved isobaric tandem mass tag quantification by ion mobility mass spectrometry. Rapid Commun Mass Spectrom. 2014;28:1051–1060. doi: 10.1002/rcm.6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harvey DJ, Scarff CA, Edgeworth M, Crispin M, Scanlan CN, Sobott F, Allman S, Baruah K, Pritchard L, Scrivens JH. Travelling wave ion mobility and negative ion fragmentation for the structural determination of N - linked glycans. Electrophoresis. 2013;34:2368–2378. doi: 10.1002/elps.201200669. [DOI] [PubMed] [Google Scholar]
- 62.Harvey DJ, Sobott F, Crispin M, Wrobel A, Bonomelli C, Vasiljevic S, Scanlan CN, Scarff CA, Thalassinos K, Scrivens JH. Ion mobility mass spectrometry for extracting spectra of N-glycans directly from incubation mixtures following glycan release: application to glycans from engineered glycoforms of intact, folded HIV gp120. J Am Soc Mass Spectrom. 2011;22:568–581. doi: 10.1007/s13361-010-0053-0. [DOI] [PubMed] [Google Scholar]
- 63.Harvey DJ, Crispin M, Bonomelli C, Scrivens JH. Ion Mobility Mass Spectrometry for Ion Recovery and Clean-Up of MS and MS/MS Spectra Obtained from Low Abundance Viral Samples. J Am Soc Mass Spectrom. 2015;26:1754–1767. doi: 10.1007/s13361-015-1163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fenn LS, McLean JA. Structural separations by ion mobility-MS for glycomics and glycoproteomics. Mass Spectrometry of Glycoproteins: Methods and Protocols. 2013:171–194. doi: 10.1007/978-1-62703-146-2_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fenn LS, McLean JA. Simultaneous glycoproteomics on the basis of structure using ion mobility-mass spectrometry. Molecular BioSystems. 2009;5:1298–1302. doi: 10.1039/b909745g. [DOI] [PubMed] [Google Scholar]