Abstract
Background
To define urine or serum biomarkers in predicting renal function recovery after liver transplantation (LT).
Methods
Adults listed for LT (02/11 – 07/14) and with modified diet for renal disease-6 (MDRD-6) <60 mL/min provided urine/blood samples at baseline and serially until LT for biomarkers in serum (pg/ml) and urine (pg/mg creatinine).
Results
Of 271 LT listed patients (mean age 57 yrs., 63% males, median listing MELD 17.5), one year AKI probability was 49%, with odds of 1.3, 3.0, 4.6, and 8.5 fold for listing MELD 16–20, 21–25, 26–30, and >30, compared to MELD <16. 37 died over 1 year from listing, with 2 fold increased odds with AKI. Among 67 patients with MDRD<60, only urinary epidermal growth factor (EGF) was different comparing AKI (increase in serum creatinine ≥.3 mg/dL from baseline within past 3 months) vs. no AKI (2254 vs. 4253, P=0.003). Differences between acute tubular necrosis (ATN) and hepatorenal syndrome could not be ascertained, for small sample of 3 patients with ATN. Analyzing 15 of 43 receiving LT and MDRD-6 <30 prior to LT, biomarkers were not different comparing five patients recovering renal function (MDRD-6 >50 mL/min) at six months vs. 10 without recovery.
Conclusions
AKI is common among LT listed patients, with a negative impact on transplant free survival. Serum and urine biomarkers are not associated with recovery of renal function after LT. Multicenter studies are suggested to a) develop strategies to reduce development of AKI and b) deriving novel biomarkers using to accurately predict renal recovery after LT.
Keywords: Biomarkers, Simultaneous Liver Kidney, AKI, Cirrhosis, ESRD
INTRODUCTION
Acute kidney injury (AKI) occurs commonly in patients with cirrhosis with rates of about 19–49% [1–8], and negatively impacts patient survival before and after liver transplantation (LT) [1, 5, 6, 9–12]. AKI in patients with cirrhosis occurs commonly due to volume loss; hepatorenal syndrome (HRS) due to vasoconstriction and reduced renal blood flow; and acute tubular necrosis (ATN) due to prolonged pre-renal factors, sepsis, or nephrotoxic insults [8, 11, 13–15]. To our knowledge, there are no studies describing AKI among patients with cirrhosis after being listed for liver transplantation (LT).
Routine clinical and laboratory evaluation is often unable to accurately differentiate hepatorenal syndrome (HRS) from intra-renal causes, and renal biopsy is invasive with a potential for complications [7, 8, 16]. AKI secondary to ATN may often require simultaneous liver kidney (SLK) transplantation for renal function recovery. In contrast, AKI due to HRS, usually recovers after liver transplantation (LT) alone. Imperfect criteria for allocating simultaneous liver kidney (SLK) transplantation in the setting of AKI, [17, 18] combined with introduction of MELD score for LT listing [19], have resulted in over 300% increase in SLK transplantation [18, 20]. Given the scarcity of donor kidneys [21], there is a need for biomarkers or models for accurate prediction of renal recovery after LT alone for optimal allocation of donor kidneys.
Biomarkers of renal injury such as neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), interlukin-18 (IL-18), human endothelin-1 (HE-1), uromodulin (UMOD), epidermal growth factor (EGF), fatty acid binding protein (FABP) and many others have been shown to predict recovery of renal function in patients with AKI without liver disease, development of AKI during LT, and differentiating HRS from ATN [10, 22, 23]. However, there are limited data on efficacy of these biomarkers in predicting recovery of renal function after LT alone. We prospectively recruited patients with liver cirrhosis listed for LT with specific aims to a) examine the probability of development of AKI and its impact on waitlist mortality and b) association of levels of serum and urine biomarkers of renal injury with type of AKI before LT and with renal function recovery after LT.
METHODS
Study Population
This is a prospective-retrospective cohort study of adult patients with liver cirrhosis listed for LT between 02/2011 and 07/2014 (Figure 1). Patients with prior liver or kidney transplant were excluded. The prospective cohort included cirrhosis patients listed for LT between April 2013 and July 2014, and were recruited after informed consent in an ongoing prospective longitudinal study to define urine and serum biomarkers in predicting recovery of renal function after LT. The retrospective cohort included patients listed between February 2011 and the recruitment of prospective study cohort starting April 2013. Both cohorts were followed until the data cut-off date of December 2015. Medical charts were reviewed to obtain prospective follow up data on the retrospective cohort. The study was approved by our institutional review board. Study was conducted adhering to the Declaration of Helsinki. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the ‘Declaration of Istanbul on Organ Trafficking and Transplant Tourism’.
Figure 1.
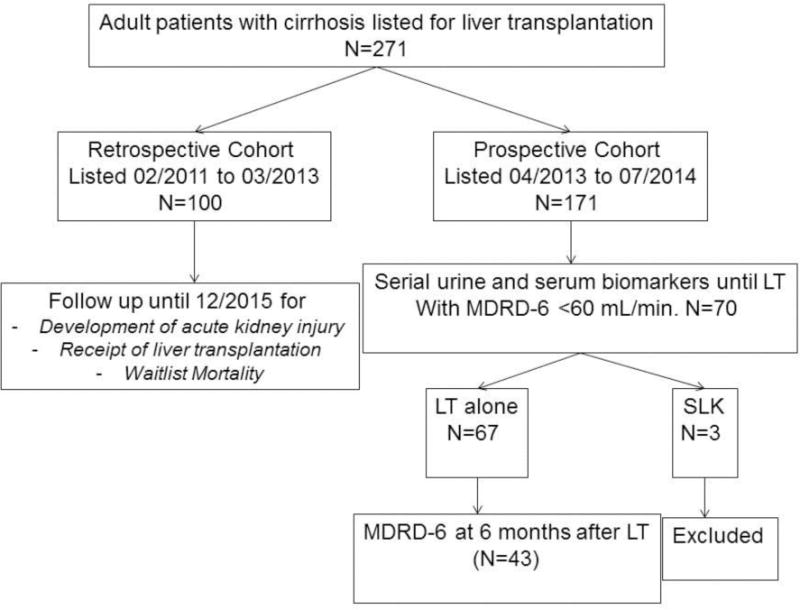
Study Design and Population
LT: Liver Transplant; AKI: Acute Kidney Injury
Study Outcomes
One year probability of AKI and of patient survival after LT listing.
Collection of Urine and Serum Samples
A subgroup of patients with MDRD-6 <60 mL/min. of the prospective cohort were consented to provide 50 mL urine and 10 mL blood samples at recruitment and then every future encounter in outpatient or during inpatient admission, until LT or removal from the waitlist. Patients receiving LT alone were followed for six months to measure their renal function and assess recovery of renal function. Patients receiving SLK transplantation were excluded from the analysis (Figure 1). Please see Supplementary material methods section for details on data collection, definitions, measurement of serum and urine biomarkers, and statistical analyses approach.
RESULTS
Study Population
A total of 271 patients (median age 56 years, 63% males, 83% Caucasians, median MDRD-6 and MELD score at listing of 66 mL/min and 18 respectively) meeting eligibility criteria for the study were analyzed (Table 1). Pre-existent CKD was present in 64 (24%) patients, which was due to diabetes in 31 patients. Common causes of liver cirrhosis were hepatitis C virus infection, non-alcoholic steatohepatitis, and alcohol use in 100 (37%), 72 (27%), and 36 (13%) patients respectively. Of 100 patients with HCV infection, 44 had received treatment for this disease. A total of 230 (85%) patients had decompensated disease with either presence of ascites or hepatic encephalopathy or varices (Table 1).
Table 1.
Baseline characteristics at time of listing for liver transplantation
| Variable | Total (N=271) | No AKI (N=164) | AKI (N=107) | P | |
|---|---|---|---|---|---|
| Age in years Median (IQR) | 56 (50–62) | 57 (51–62) | 56 (48–61) | 0.064 | |
|
| |||||
| Males N (%) | 170 (63) | 106 (65) | 64 (60) | 0.42 | |
|
| |||||
| Ethnicity N (%) | Caucasian | 226 (83) | 140 (85) | 86 (80) | |
| African American | 33 (12) | 15 (10) | 17 (16) | 0.09 | |
| Hispanic | 6 (2) | 2 (1) | 4 (4) | ||
| Others | 6 (2) | 6 (4) | 0 (0) | ||
|
| |||||
| Body mass index Median (IQR) | 28 (25–32) | 27 (25–32) | 28 (25–33) | 0.25 | |
|
| |||||
| Liver disease etiology N (%) | Hepatitis C | 100 (37) | 62 (38) | 38 (35) | |
| NASH | 72 (27) | 40 (24) | 32 (30) | 0.82 | |
| Alcohol | 36 (13) | 23 (14) | 13 (12) | ||
| Other | 63 (23) | 39 (24) | 24 (23) | ||
|
| |||||
| Beta blockers | 143 (53) | 88 (54) | 55 (51) | 0.72 | |
|
| |||||
| Statins | 12 (4.4) | 6 (3.7) | 6 (5.6) | 0.45 | |
|
| |||||
| None | 91 (33) | 67 (41) | 25 (23) | ||
| Ascites | Mild to moderate | 84 (31) | 50 (30) | 34 (32) | 0.006 |
| Refractory | 95 (36) | 47 (29) | 48 (45) | ||
|
| |||||
| Hepatic encephalopathy | 168 (62) | 91 (56) | 77 (72) | 0.008 | |
|
| |||||
| Varices | 170 (63) | 101 (62) | 69 (64) | 0.63 | |
|
| |||||
| Pre-existing HCC N (%) | 56 (21) | 41 (25) | 15 (14) | 0.029 | |
|
| |||||
| Pre-existing CKD N (%) | 64 (24) | 27 (16) | 37 (35) | 0.0006 | |
|
| |||||
| Platelets ×109/L Median (IQR) | 73 (55–114) | 72 (52–105) | 77 (59–127) | 0.38 | |
|
| |||||
| Serum sodium (mEq/L) Median (IQR) | 136 (134–139) | 137 (134–139) | 135 (132–138) | 0.01 | |
|
| |||||
| Listing MELD score Median (IQR) | 18 (14–22) | 17 (14–19) | 19 (15–25) | 0.0003 | |
|
| |||||
| Listing MDRD-6 mL/min Median (IQR) | 66 (49–91) | 76 (57–101) | 56 (37–73) | <0.0001 | |
AKI: Acute kidney injury; IQR: Interquartile range; NASH: Non-Alcoholic Steatohepatitis; HCC: Hepatocellular Carcinoma; CKD: Chronic Kidney Disease; MELD=Model for End-stage Liver Disease; MDRD: Modified Diet in Renal Disease HR: Hazard Ratio; CI: Confidence Interval; CKD: Chronic Kidney Disease; MELD: Model for End-stage Liver Disease
Of the prospective cohort, 70 patients (mean age 58 yrs., 54% males, 84% Caucasians) with MDRD-6 <60 (median 37 mL/min) were recruited to provide serum and urine samples for biomarkers measurements (Supplementary Figure 1 and Supplementary Table 1). About half of these patients had underlying CKD (10% on hemodialysis, due to associated comorbidities of diabetes mellitus in 49% and hypertension in 46% (Table 1). Of 7 patients on hemodialysis at the time of inclusion into the study, 3 were receiving this for ESRD (all these receiving SLK transplantation and were excluded from the analysis on biomarkers), and remaining four were initiated on dialysis for AKI.
A total of 242 samples were collected from 70 patients from the time of recruitment until removal from the transplant list, with 131 serum samples from 69 patients and 111 urine samples from 65 patients (Supplementary Figure 1). A total of 35 and 37 patients provided only the baseline serum or urine sample, and 22 and 18 patients provided two serum or urine samples. The remaining patients provided more than two samples with maximum of five serum samples by each of the two patients and six urine samples by one patient (Supplementary Figure 1).
Probability of Acute Kidney Injury
Over a median (interquartile range) follow up period of 1.43 (0.85–2.17) years, 107 of 271 (39.5%) patients developed first episode of AKI, with one year probability of 49% (Figure 2A). Similar probabilities at listing MELD score <16 (N=95), 16–20 (N=100), 21–25 (N=33), 26–30 (N=19), and >30 (N=24) were 31.9%, 42.8%, 73.7%, 72.8%, and 91.7% respectively (Log Rank P<0.0001, Supplementary Figure 2). Of 107 first AKI episodes, volume responsive pre-renal was the most common etiology of AKI in 61 (57%) patients followed by ATN in 28 (27%), HRS in 10 (9%), and miscellaneous causes in 8 (7%) patients including post-renal etiology in two patients (Supplementary Figure 3). Patients with AKI (N=107) compared to 164 patients without AKI were more likely to have pre-existing CKD, more likely to have refractory ascites and hepatic hepatic encephalopathy, less likely to have hepatocellular carcinoma (HCC), and had lower listing MDRD-6 and serum sodium, and higher MELD score (Table 1). The two groups were no different on decompensated disease (82 vs. 89%, P=0.15) and on varices and platelet count (Table 1). The proportion of patients receiving treatment for HCV infection was also similar in the two groups at 44% (27 of 62) vs. 45% (17 of 38), P=0.91.
Figure 2.
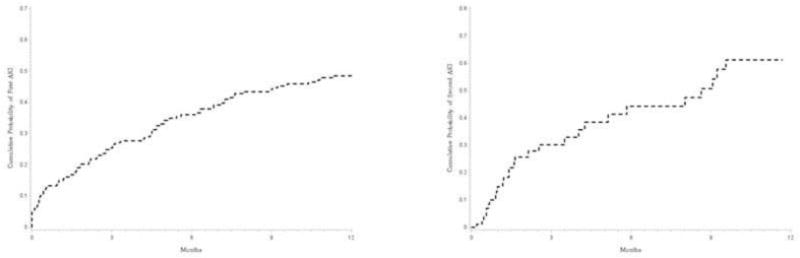
A) Cumulative probability of development of acute kidney injury at one year from listing for liver transplantation. The results show that the cumulative probability over one year for development of acute kidney injury is about 49% from the time of listing for liver transplantation. B) Cumulative probability of development of second episode of acute kidney injury (AKI) at one year from listing among transplant free survivors of patients with first episode of AKI. Among 45 transplant free survivors of 107 patients with first AKI episode, the cumulative probability of the second episode was about 60% at one year from the time of listing for liver transplantation.
Predictors of Acute Kidney Injury
On a cox proportional hazard regression analysis model, MELD score and serum sodium at listing independently predicted development of AKI at one year, with 3 point increase in MELD score increasing the risk by 39% (Supplementary Table 2). Cox regression model built with MELD score as a categorical variable, showed AKI risk at one year from listing to increase linearly by 335%, 399%, and 1223% respectively for listing MELD score of 21–25, 26–30, and >30 respectively, compared to MELD score <15 (Supplementary Table 2).
Impact of Acute Kidney Injury on Waitlist Mortality
A total of 73 (27%) patients died while waiting for LT, higher among patients with AKI (33.6 vs. 12.2%, P=0.0001). Of 73 deaths on waitlist, 37 occurred within first year from listing (21 among patients with AKI). One year probability of survival from listing for LT was about 86%, lower in patients with AKI (72.2% vs. 83%, P=0.025, Figure 3). After controlling for demographics (age and gender), diabetes, obesity, and listing MELD score, development of AKI increased risk for waitlist mortality at one year from listing by over two-fold: HR (95% CI) of 2.27 (1.28–4.02, P=0.005, Supplementary Table 3). Within the group of 107 patients with AKI, cox proportion hazard regression analysis was built again. Recurrent AKI was not a predictor of waitlist mortality, 0.89 (0.39–2.02, P=0.79). Significant predictors were MELD at listing 1.07 (1.03–1.12, P=0.002) and obesity 1.82 (1.09–3.01, P=0.02).
Figure 3.
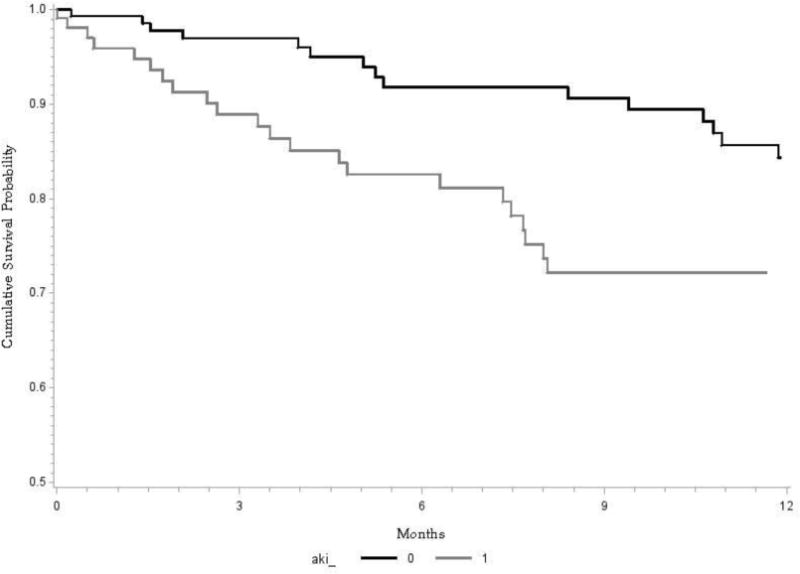
Kaplan Meier curve showing survival at one year from the time of listing for liver transplantation: Comparison of patients with and without acute kidney injury (AKI). Overall survival was about 86%, significantly lower among patients with AKI compared to those not developing AKI (72.2 vs. 83%, Log Rank P=0.025).
A total of 145 of 271 (54%) patients were transplanted during the study period, with 8 (2.96% of both the cohorts and 5.5% of all LT) receiving SLK. Proportion of patients receiving LT was similar comparing patients with and without AKI (49% vs. 53%, P=0.47, Supplemental Figure 4). MDRD-6 was consistently lower among patients with AKI at listing, and then at 3 months, 6 months, and 12 months from listing, compared to patients without AKI However, patients surviving the AKI episode without LT return their MDRD-6 to baseline (median MDRD-6 around 60–65 mL/min, Supplementary Figure 5).
Recurrent Acute Kidney Injury
A total of 147 episodes of AKI developed in 107 patients, 27 with two AKI episodes, 12 with three episodes, and one patient had more than three episodes (Supplementary Figure 3). The etiology of recurrent AKI was similar to the proportion of etiologies in the first episode (Supplementary Figure 3). Of 107 patients with first AKI episode, 2nd AKI occurred in 26 (24%) patients among LT free survivors, with one year probability of 60% (Figure 2B). Respective cumulative probabilities for second episode of AKI were 25%, 30%, and 21% among patients with listing MELD <21, 21–25, and >25 compared to MELD<20.
Biomarker Analysis
a) MDRD-6 >30 vs. ≤30 mL/min
Of 122 samples, 34 samples with MDRD-6 ≤30 (median 23 mL/min) compared to 88 samples with MDRD-6 >30 (median 51 mL/min) had significantly higher serum HE-1 and NGAL levels (Supplementary Table 4 and Figure 4 A–B). On urine biomarkers analysis, EGF and IL-18 were significantly lower for samples with MDRD-6 ≤30 (Supplementary Table 4 and Figure 4 C–D). Other biomarkers were not significantly different comparing the two strata of MDRD-6 (Supplementary Table 4).
Figure 4.
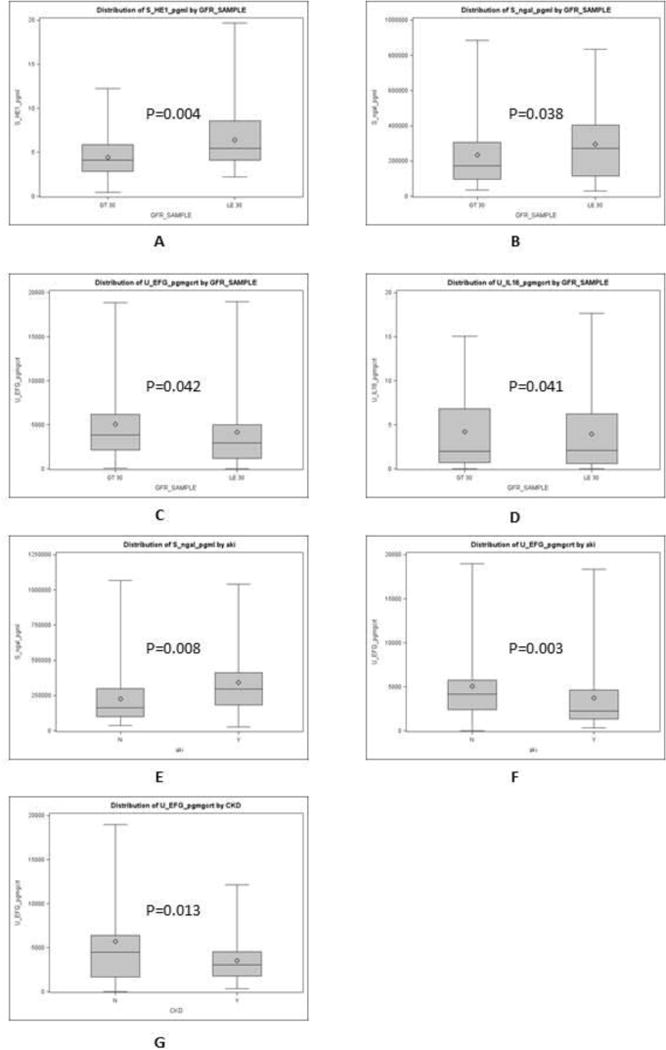
Box whisker plots comparing samples with modified diet for renal disease-6 (MDRD-6) >30 mL/min vs. samples with MDRD-6 ≤30 mL/min for A) serum human endothelin-1 (pg/mL), B) serum neutrophil gelatinase associated lipocalin or NGAL, C) urinary endothelial growth factor, D) urinary interleukin-18 or IL-18; comparing samples without acute kidney injury (AKI) at the time of sample collection vs. samples with AKI for E) serum human endothelin-1, F) serum neutrophil gelatinase associated lipocalin or NGAL; comparing samples without chronic kidney disease (CKD) vs. samples with CKD for G) urinary endothelial growth factor.
b) AKI vs. no AKI
Of 134 samples, 37 samples with adjudication of AKI at the time of sample collection compared to 97 samples without AKI had significantly higher serum NGAL and a trend for higher HE-1 levels (Table 2 and Figure 4 E–F). On urine biomarkers analysis, only EGF was significantly different with lower levels for samples with AKI (Table 2 and Figure 4G). Other biomarkers were not significantly different comparing the two strata of MDRD-6 (Table 2).
Table 2.
Biomarkers levels in serum (pg/ml) and in urine (pg/mg creatinine) comparing samples without vs. with acute kidney injury (AKI) at the time of sample collection.
| No AKI (N=97) | AKI (N=37) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Median | 1st quartile | 3rd quartile | N | Median | 1st quartile | 3rd quartile | P | |
| Serum Biomarkers | |||||||||
| NGAL | 94 | 162,965 | 99,213.04 | 299,049.92 | 37 | 297,345.92 | 182,276.04 | 411,097.83 | 0.0083 |
| HE-1 | 94 | 4.26 | 2.64 | 6.06 | 37 | 4.84 | 2.97 | 7.84 | 0.0985 |
| Urine Biomarkers (pg/mg creatinine) | |||||||||
| Albumin | 79 | 3,336,779 | 764,194 | 9,085,347 | 32 | 7,311,11 | 2,181,58 | 13,661,532 | 0.113 |
| B-2 microglobulin | 79 | 36,874 | 6,789 | 190,199 | 32 | 19,058 | 5,886 | 138,822 | 0.767 |
| Cystatin C | 79 | 22,754 | 8,844 | 78,219 | 32 | 20,086 | 7,971 | 51,811 | 0.714 |
| EGF | 79 | 4,253 | 2,517 | 6,938 | 32 | 2,254 | 1,350 | 4,651 | 0.003 |
| NGAL | 79 | 42,340 | 12,827 | 154,712 | 32 | 68,020 | 26,406 | 188,569 | 0.154 |
| Osteopontin | 79 | 423,158 | 187,445 | 808,137 | 32 | 576,900 | 151,498 | 937,021 | 0.527 |
| Uromodulin | 79 | 2,555,037 | 965,133 | 5,236,253 | 32 | 1,838,723 | 1,116,396 | 4,254,726 | 0.768 |
| Interleukin-18 | 79 | 5.87 | 1.19 | 26.79 | 32 | 3.22 | 0.73 | 7.86 | 0.117 |
| KIM-1 | 79 | 1,071 | 287 | 2,612 | 32 | 1,472 | 488 | 3,833 | 0.304 |
| HE-1 | 78 | 0.56 | 0.20 | 1.26 | 31 | 0.40 | 0.14 | 2.38 | 0.434 |
| FABP-2 | 44 | 0.19 | 0.03 | 1.69 | 18 | 0.13 | 0.05 | 0.64 | 0.896 |
| MDRD-6 | 85 | 49 | 37 | 68 | 37 | 28 | 22 | 44 | <0.001 |
NGAL: Neutrophil gelatinase-associated lipocalin; EGF: Epidermal growth factor; KIM: Kidney injury molecule; HE: Human endothelin; FABP: Fatty acid binding protein; MDRD: Modified diet for renal disease
c) Type of AKI
Of 37 samples with AKI, cause of AKI was pre-renal in 22, HRS in 12, and ATN in three patients. Compared to pre-renal AKI, serum NGAL was higher in ATN and serum HE-1 was higher in HRS (Table 3). However, statistical comparison could not be derived for comparing ATN and HRS with only 3 observations with AKI due to ATN (Table 3).
Table 3.
Biomarkers levels in serum (pg/ml) and in urine (pg/mg creatinine) samples comparing types of acute kidney injury (AKI): pre-renal volume responsive vs. acute tubular necrosis (ATN) vs. hepatorenal syndrome (HRS).
| Pre-renal AKI (N=22) | ATN (N=3) | HRS (N=12) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median | 1st quartile | 3rd quartile | N | Median | 1st quartile | 3rd quartile | N | Median | 1st quartile | 3rd quartile | |
| Serum Biomarkers | ||||||||||||
| NGAL | 22 | 238,095 | 163,881 | 357,090 | 3 | 382,541 | 339,747 | 836,177 | 12 | 296,290 | 182,991 | 587,019 |
| HE-1 | 22 | 4.35 | 2.85 | 7.43 | 3 | 2.88 | 2.65 | 19.66 | 12 | 6.61 | 4.61 | 9.47 |
| Urine Biomarkers (pg/mg creatinine) | ||||||||||||
| Albumin | 19 | 3,895,366 | 1,583,940 | 13,132,606 | 2 | 16,522,643 | 14,649,559 | 18,395,725 | 11 | 7,657,418 | 1,127,192 | 13,173,988 |
| B-2 microglobulin | 19 | 29,273 | 4,228 | 143,138 | 2 | 886,027 | 13,946 | 1,758,108 | 11 | 18,364 | 6,081 | 134,507 |
| Cystatin C | 19 | 20,934 | 8,417 | 37,098 | 2 | 93,026 | 9,606 | 176,446 | 11 | 15,231 | 6,961 | 329,438 |
| EGF | 19 | 2,943 | 1,765 | 4,983 | 2 | 1,316 | 1,040 | 1,591 | 11 | 1,328 | 913 | 4,837 |
| NGAL | 19 | 47,893 | 38,437 | 207,814 | 2 | 607,849 | 598,718 | 616,980 | 11 | 67,078 | 9,266 | 149,689 |
| Osteopontin | 19 | 760,531 | 429,939 | 1,096,634 | 2 | 166,367 | 96,446 | 236,289 | 11 | 153,846 | 87,863 | 580,297 |
| Uromodulin | 19 | 2,803,694 | 1,697,890 | 4,951,781 | 2 | 1,147,485 | 893,124 | 1,401,845 | 11 | 1,138,358 | 823,544 | 2,638,723 |
| Interleukin-18 | 19 | 3.36 | 0.74 | 8.55 | 2 | 4.96 | 3.09 | 6.83 | 11 | 2.74 | 0.43 | 9.36 |
| KIM-1 | 19 | 1,511 | 507 | 4,036 | 2 | 3,607 | 3,167 | 4,047 | 11 | 934 | 149 | 1,896 |
| HE-1 | 19 | 0.31 | 0.09 | 2.38 | 2 | 1.50 | 0.40 | 2.60 | 10 | 0.39 | 0.17 | 1.24 |
| FABP-2 | 9 | 0.08 | 0.03 | 0.64 | 2 | 0.10 | 0.07 | 0.14 | 7 | 0.48 | 0.05 | 11.81 |
| MDRD-6 | 22 | 35 | 27 | 51 | 3 | 19 | 13 | 19 | 12 | 23 | 18 | 30 |
NGAL: Neutrophil gelatonase-associated lipocalin; EGF: Epidermal growth factor; KIM: Kidney injury molecule; HE: Human endothelin; FABP: Fatty acid binding protein; MDRD: Modified diet for renal disease
d) CKD vs. no CKD
Of 134 samples, 62 samples from patients with CKD (median MDRD-6 of 34 mL/min) differed only for urinary EGF, compared to samples obtained from patients without CKD (median MDRD-6 of 51 mL/min) (Supplementary Table 5 and Figure 4B). Further, samples from CKD patients tended to have higher serum NGAL and urinary albumin (Supplementary Table 5 and Figure 4A, 4C).
e) Recovery of Renal Function after Liver Transplantation Alone
Of 70 patients recruited into the study, 46 were transplanted until the end of the data collection. Of these, 43 received liver alone (mea age 57 years, 53% males, 84% Caucasians, baseline MDRD-6 of 40 mL/min) and three patients received SLK transplantation (Table 1). About 44% of transplanted patients had underlying CKD, with pre-transplant hemodialysis in one patient (Table 1). Of 43 patients receiving LT alone, 24 patients provided sample within a month prior to LT, and these patients did not differ at baseline from all the transplanted patients (Table 1).
To examine association of serum and urinary biomarkers with recovery of renal function, we focused on 15 patients receiving liver alone with MDRD-6 <30 at the last sample obtained within a month prior to LT. Of these, 5 patients recovered renal function and 10 patients did not recover their renal function, with recovery defined as return of MDRD-6 to >50 at six months after LT. None of the biomarkers in serum and urine on the latest sample available prior to LT were different comparing patients with and without recovery of renal function. There was a trend for higher UMOD among patients showing recovery of renal function compared to patients not recovering renal function at six months after LT (Supplementary Figure 6 A). Other details on pre-transplant variables and post-transplant use of calcineurin inhibitor are provided in Supplementary Table 5.
DISCUSSION
The frequency of AKI in patients with cirrhosis varies between 19–49%, similar to the current study [1–6]. Differences across studies may be due to study population and follow up time. In this study, probability of occurrence of AKI at one year from the time of listing for LT was 49%. In a retrospective study on 82 patients with Child’s class C cirrhosis, AKI occurred in about 23% patients over one year period [24]. In another study, the incidence of AKI was 18% at one year among hospitalized patients with cirrhosis [25]. AKI in cirrhosis is most commonly due to volume responsive prerenal injury followed by ATN and type 1 HRS, similar to what we found in the current study [7, 8, 11, 14, 15].
Biomarkers such as NGAL, KIM-1, IL-18, and FABBP-2 can diagnose AKI earlier, differentiate ATN from HRS, and predict outcomes [22, 23, 26–31]. Of these, serum NGAL and urinary IL-18 were different in this study. Our study population with MDRD-6 <60 and presence of underlying CKD probably explain our findings [23, 31–33]. Although, the levels were higher in ATN compared to HRS in this study, small sample size limited statistical difference.
Few studies have previously shown association of risk of development of AKI with the Child Turcotte Pugh stage [3, 34]. There is only one study showing association of AKI occurrence with the baseline MELD score [6]. However, this prospective study examined for in hospital AKI, unlike our study examining one year probability of AKI among LT listed patients. In another prospective study on 92 cirrhosis patients, 82 episodes of AKI occurred. Of 49 patients developing AKI in this study, 33 (67%) developed a second episode of AKI [35], similar to our study. Studies have negative impact of AKI on the outcome of cirrhosis patients and on need for hospitalization, intensive unit care, dialysis, and use of hospital resources, as observed in the current study [1, 5, 6, 9–12]. Small number of patients with documented use of statins in this study limited the analysis of the impact of these drugs on the survival. Of note, use of beta blockers was not associated with the development of AKI risk in this study.
We also examined other biomarkers, which have not been evaluated earlier in patients with cirrhosis such as HE-1, EGF, UMOD or the data are scanty on their assessment such as cystatin C, β2M, OPN, and albumin [36, 37]. HE-1 is a peptide released from endothelial cells resulting in renal vasoconstriction [38]. HE-1 increases in plasma and in urine in HRS patients [39]. In our study, serum and urinary HE-1 levels were highest in HRS followed by ATN and levels were lowest in pre-renal AKI, similar to earlier reports. Cystatin-C, a low molecular weight protein exclusively eliminated by glomerular filtration, has been used for GFR calculation [40]. In one study, plasma cystatin C levels predicted sustained AKI and its outcomes among patients in the intensive care setting [41]. In the current study, there were no differences on urinary cystatin-C levels based on AKI. p2M, a small molecule freely filtered by glomerulus and reabsorbed by proximal tubules, and its serum levels increase with a decline in GFR [42]. In this study, there were no differences on urinary p2M levels as levels may be confounded with infections and inflammatory state. [43] OPN is an inflammatory cytokine, and its serum levels predict onset of AKI and its outcome [44]. In the current study, OPN urinary levels were no different for AKI, likely due to confounding with underlying infections and presence of CKD [45]. Albuminuria is well described as a marker for CKD and for diabetic nephropathy. In the current study, there were no differences on urinary albumin levels based on degree of GFR decline and for AKI.
None of the biomarkers were associated with renal recovery after LT, except for UMOD. In an earlier study, combined model including elevated OPN and tissue inhibitor of metalloproteinase-1, age <57 years, and absence of diabetes was 82% accurate in predicting renal recovery after LT, and this combined model was more accurate compared to models including only biomarkers levels or only clinical variables [46].
Physicians in clinical practice should be extra careful in using diuretics and counseling patients on measures for preventing AKI. This becomes more relevant in the background of shortage of donor kidneys and lack of evidence based guidelines for allocation of SLK transplantation [47, 48]. Whether transjugular intrahepatic portosystemic shunt would be a feasible preventive strategy among cirrhosis patients who have had an episode of AKI remains a testable hypothesis [49].
Analysis of a large homogeneous prospective cohort of cirrhosis patients listed for LT, robust clinical and biomarker data, and use of updated definition of AKI with removal of upper ceiling of 1.5 mg/dL for serum creatinine are strengths of our study. However, our study has certain limitations including data from a single center, possibility of missing some of the AKI episodes in this retrospective cohort, lack of information on the intravenous albumin use which can confound the MDRD-6 values, and adjudicating cause of AKI based on clinical and laboratory data. Further, information on intra-operative and post-transplant variables which could affect renal function at six months after LT was lacking.
In conclusion, AKI occurs frequently among patients with cirrhosis listed for LT. Development of AKI is associated with increased waitlist mortality and increased use of hospital resources. Patients with AKI who survive without need for transplantation have about 60% probability of developing second episode of AKI within a year from the first episode of AKI. None of the pretransplant biomarkers were associated with recovery of renal function after LT.
We suggest developing larger multicenter studies as a basis for deriving an accurate model combining clinical variables with various biomarkers, to predict recovery of renal function after LT alone. Given encouraging data on the ability of UMOD in predicting renal recovery after LT, studies are suggested to examine proteomics and metabolomics approach on urine samples to explore other biomarkers which could be useful in accurate differentiation of HRS from ATN and in predicting of recovery of renal function after LT.
Supplementary Material
Table 4.
Biomarkers levels in serum (pg/ml) and in urine (pg/mg creatinine) samples taken within one month prior to liver transplantation (LT) alone among patients with pre-transplant MDRD <30 mL/min: comparing without vs. with recovery of renal function at six months after LT (MDRD-6 >50 mL/min)
| No Recovery (N=10) | Recovery (N=5) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Median | 1st quartile | 3rd quartile | N | Median | 1st quartile | 3rd quartile | P | |
| Serum Biomarkers | |||||||||
| NGAL | 7 | 183,707 | 113,572 | 299,018 | 5 | 411,098 | 293,614 | 663,551 | 0.220 |
| HE-1 | 7 | 6.19 | 4.13 | 7.84 | 5 | 9.11 | 4.84 | 9.82 | 0.529 |
| Urine Biomarkers (pg/mg creatinine) | |||||||||
| Albumin | 7 | 9,070,194 | 3,046,796 | 40,272,481 | 5 | 6,964,801 | 2,830,440 | 11,851,111 | 0.723 |
| B-2 microglobulin | 7 | 15,253 | 8,417 | 6,036,864 | 5 | 18,364 | 3,467 | 122,286 | 0.723 |
| Cystatin C | 7 | 175,206 | 13,967 | 396,264 | 5 | 39,080 | 15,231 | 186,934 | 0.906 |
| EGF | 7 | 4,684 | 2,848 | 4,920 | 5 | 1,151 | 344 | 1,328 | 0.302 |
| NGAL | 7 | 59,420 | 28,723 | 113,169 | 5 | 149,689 | 68,962 | 179,275 | 0.416 |
| Osteopontin | 7 | 540,697 | 304,619 | 1,092,702 | 5 | 129,306 | 87,863 | 153,846 | 0.215 |
| Uromodulin | 7 | 5,248,257 | 2,762,422 | 9,280,816 | 5 | 823,544 | 265,520 | 1,823,135 | 0.104 |
| Interleukin-18 | 7 | 4.90 | 1.99 | 7.59 | 5 | 6.67 | 2.74 | 8.55 | 0.723 |
| KIM-1 | 7 | 2,340 | 1,022 | 3,346 | 5 | 1,838 | 813 | 6,458 | 0.906 |
| HE-1 | 7 | 2.07 | 0.19 | 4.13 | 5 | 0.34 | 0.14 | 0.45 | 0.416 |
| FABP-2 | 7 | 33.82 | 1.39 | 66.24 | 5 | 0.50 | 0.31 | 6.16 | 0.299 |
| MDRD-6 Pre Transplant | 7 | 28 | 16 | 40 | 5 | 21 | 19 | 27 | 0.636 |
NGAL: Neutrophil gelatinase-associated lipocalin; EGF: Epidermal growth factor; KIM: Kidney injury molecule; HE: Human endothelin; FABP: Fatty acid binding protein; MDRD: Modified diet for renal disease
Acknowledgments
We greatly acknowledge the support from the O’Brien Center of Acute Kidney Injury at the UAB for conducting this study and coordinating with the laboratory measurements of biomarkers. Satish Rao gratefully acknowledges the assistance provided for this work by the NIH NIDDK UAB/UCSD O’Brien Core Center for Acute Kidney Injury Research Grant (NIH 1P30 DK 079337). We would also like to acknowledge on support of our work by the grant UL1TR001417 by the National Center for Advancing Translational Sciences of the National Institute of Health.
Footnotes
Disclosure: None of the authors have any financial or any other conflict to disclose.
References
- 1.Angeli P, Rodriguez E, Piano S, Ariza X, Morando F, Sola E, Romano A, Garcia E, Pavesi M, Risso A, Gerbes A, Willars C, Bernardi M, Arroyo V, Gines P, Consortium CSIoE-C Acute kidney injury and acute-on-chronic liver failure classifications in prognosis assessment of patients with acute decompensation of cirrhosis. Gut. 2015;64:1616–1622. doi: 10.1136/gutjnl-2014-307526. [DOI] [PubMed] [Google Scholar]
- 2.Montoliu S, Balleste B, Planas R, Alvarez MA, Rivera M, Miquel M, Masnou H, Cirera I, Morillas RM, Coll S, Sala M, Garcia-Retortillo M, Canete N, Sola R. Incidence and prognosis of different types of functional renal failure in cirrhotic patients with ascites. Clin Gastroenterol Hepatol. 2010;8:616–622. doi: 10.1016/j.cgh.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 3.Prakash J, Mahapatra AK, Ghosh B, Arora P, Jain AK. Clinical spectrum of renal disorders in patients with cirrhosis of liver. Ren Fail. 2011;33:40–46. doi: 10.3109/0886022X.2010.541582. [DOI] [PubMed] [Google Scholar]
- 4.Piano S, Rosi S, Maresio G, Fasolato S, Cavallin M, Romano A, Morando F, Gola E, Frigo AC, Gatta A, Angeli P. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol. 2013;59:482–489. doi: 10.1016/j.jhep.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 5.Fagundes C, Barreto R, Guevara M, Garcia E, Sola E, Rodriguez E, Graupera I, Ariza X, Pereira G, Alfaro I, Cardenas A, Fernandez J, Poch E, Gines P. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol. 2013;59:474–481. doi: 10.1016/j.jhep.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 6.Wong F, O’Leary JG, Reddy KR, Patton H, Kamath PS, Fallon MB, Garcia-Tsao G, Subramanian RM, Malik R, Maliakkal B, Thacker LR, Bajaj JS, North American Consortium for Study of End-Stage Liver D New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology. 2013;145:1280–1288 e1281. doi: 10.1053/j.gastro.2013.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gines P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279–1290. doi: 10.1056/NEJMra0809139. [DOI] [PubMed] [Google Scholar]
- 8.Russ KB, Stevens TM, Singal AK. Acute Kidney Injury in Patients with Cirrhosis. J Clin Transl Hepatol. 2015;3:195–204. doi: 10.14218/JCTH.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bucsics T, Mandorfer M, Schwabl P, Bota S, Sieghart W, Ferlitsch A, Trauner M, Peck-Radosavljevic M, Reiberger T. Impact of acute kidney injury on prognosis of patients with liver cirrhosis and ascites: A retrospective cohort study. J Gastroenterol Hepatol. 2015;30:1657–1665. doi: 10.1111/jgh.13002. [DOI] [PubMed] [Google Scholar]
- 10.Belcher JM, Garcia-Tsao G, Sanyal AJ, Bhogal H, Lim JK, Ansari N, Coca SG, Parikh CR, Consortium T-A Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013;57:753–762. doi: 10.1002/hep.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.du Cheyron D, Bouchet B, Parienti JJ, Ramakers M, Charbonneau P. The attributable mortality of acute renal failure in critically ill patients with liver cirrhosis. Intensive Care Med. 2005;31:1693–1699. doi: 10.1007/s00134-005-2842-7. [DOI] [PubMed] [Google Scholar]
- 12.Cholongitas E, Calvaruso V, Senzolo M, Patch D, Shaw S, O’Beirne J, Burroughs AK. RIFLE classification as predictive factor of mortality in patients with cirrhosis admitted to intensive care unit. J Gastroenterol Hepatol. 2009;24:1639–1647. doi: 10.1111/j.1440-1746.2009.05908.x. [DOI] [PubMed] [Google Scholar]
- 13.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 14.Schrier RW, Shchekochikhin D, Gines P. Renal failure in cirrhosis: prerenal azotemia, hepatorenal syndrome and acute tubular necrosis. Nephrol Dial Transplant. 2012;27:2625–2628. doi: 10.1093/ndt/gfs067. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064–2077. doi: 10.1002/hep.22605. [DOI] [PubMed] [Google Scholar]
- 16.Tanriover B, Mejia A, Weinstein J, Foster SV, Ghalib R, Mubarak A, Chen SS. Analysis of Kidney Function and Biopsy Results in Liver Failure Patients With Renal Dysfunction: A New Look to Combined Liver Kidney Allocation in the Post-MELD Era. Transplantation. 2008;86:1548–1553. doi: 10.1097/TP.0b013e31818b22cc. [DOI] [PubMed] [Google Scholar]
- 17.Charlton MR, Wall WJ, Ojo AO, Gines P, Textor S, Shihab FS, Marotta P, Cantarovich M, Eason JD, Wiesner RH, Ramsay MA, Garcia-Valdecasas JC, Neuberger JM, Feng S, Davis CL, Gonwa TA, International Liver Transplantation Society Expert P Report of the first international liver transplantation society expert panel consensus conference on renal insufficiency in liver transplantation. Liver Transpl. 2009;15:S1–34. doi: 10.1002/lt.21877. [DOI] [PubMed] [Google Scholar]
- 18.Eason JD, Gonwa TA, Davis CL, Sung RS, Gerber D, Bloom RD. Proceedings of Consensus Conference on Simultaneous Liver Kidney Transplantation (SLK) Am J Transplant. 2008;8:2243–2251. doi: 10.1111/j.1600-6143.2008.02416.x. [DOI] [PubMed] [Google Scholar]
- 19.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 20.Singal AK, Salameh H, Kuo YF, Wiesner RH. Evolving frequency and outcomes of simultaneous liver kidney transplants based on liver disease etiology. Transplantation. 2014;98:216–221. doi: 10.1097/TP.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 21.Schold JD, Meier-Kriesche HU, Duncan RP, Reed AI. Deceased donor kidney and liver transplantation to nonresident aliens in the United States. Transplantation. 2007;84:1548–1556. doi: 10.1097/01.tp.0000296289.69158.a7. [DOI] [PubMed] [Google Scholar]
- 22.Siew ED, Ware LB, Ikizler TA. Biological markers of acute kidney injury. J Am Soc Nephrol. 2011;22:810–820. doi: 10.1681/ASN.2010080796. [DOI] [PubMed] [Google Scholar]
- 23.Belcher JM, Garcia-Tsao G, Sanyal AJ, Thiessen-Philbrook H, Peixoto AJ, Perazella MA, Ansari N, Lim J, Coca SG, Parikh CR, Consortium T-A Urinary biomarkers and progression of AKI in patients with cirrhosis. Clin J Am Soc Nephrol. 2014;9:1857–1867. doi: 10.2215/CJN.09430913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu CC, Yeung LK, Tsai WS, Tseng CF, Chu P, Huang TY, Lin YF, Lu KC. Incidence and factors predictive of acute renal failure in patients with advanced liver cirrhosis. Clin Nephrol. 2006;65:28–33. doi: 10.5414/cnp65028. [DOI] [PubMed] [Google Scholar]
- 25.Tandon P, James MT, Abraldes JG, Karvellas CJ, Ye F, Pannu N. Relevance of New Definitions to Incidence and Prognosis of Acute Kidney Injury in Hospitalized Patients with Cirrhosis: A Retrospective Population-Based Cohort Study. PLoS One. 2016;11:e0160394. doi: 10.1371/journal.pone.0160394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J, Devarajan P, Barasch J. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 28.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Li NS, Lv LS, Huang JH, Tang H, Chen JX, Ma HJ, Wu XM, Lou TQ. A comparison of the performances of an artificial neural network and a regression model for GFR estimation. Am J Kidney Dis. 2013;62:1109–1115. doi: 10.1053/j.ajkd.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Yokoyama T, Kamijo-Ikemori A, Sugaya T, Hoshino S, Yasuda T, Kimura K. Urinary excretion of liver type fatty acid binding protein accurately reflects the degree of tubulointerstitial damage. Am J Pathol. 2009;174:2096–2106. doi: 10.2353/ajpath.2009.080780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francoz C, Nadim MK, Durand F. Kidney biomarkers in cirrhosis. J Hepatol. 2016;65:809–824. doi: 10.1016/j.jhep.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Fagundes C, Pepin MN, Guevara M, Barreto R, Casals G, Sola E, Pereira G, Rodriguez E, Garcia E, Prado V, Poch E, Jimenez W, Fernandez J, Arroyo V, Gines P. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol. 2012;57:267–273. doi: 10.1016/j.jhep.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Xie Y, Shao X, Ni Z, Mou S. L-FABP: A novel biomarker of kidney disease. Clin Chim Acta. 2015;445:85–90. doi: 10.1016/j.cca.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Chen YW, Wu CJ, Chang CW, Lee SY, Sun FJ, Chen HH. Renal function in patients with liver cirrhosis. Nephron Clin Pract. 2011;118:c195–203. doi: 10.1159/000321384. [DOI] [PubMed] [Google Scholar]
- 35.Tsien CD, Rabie R, Wong F. Acute kidney injury in decompensated cirrhosis. Gut. 2013;62:131–137. doi: 10.1136/gutjnl-2011-301255. [DOI] [PubMed] [Google Scholar]
- 36.Belcher JM, Sanyal AJ, Peixoto AJ, Perazella MA, Lim J, Thiessen-Philbrook H, Ansari N, Coca SG, Garcia-Tsao G, Parikh CR, Consortium T-A Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology. 2014;60:622–632. doi: 10.1002/hep.26980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ariza X, Sola E, Elia C, Barreto R, Moreira R, Morales-Ruiz M, Graupera I, Rodriguez E, Huelin P, Sole C, Fernandez J, Jimenez W, Arroyo V, Gines P. Analysis of a urinary biomarker panel for clinical outcomes assessment in cirrhosis. PLoS One. 2015;10:e0128145. doi: 10.1371/journal.pone.0128145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 39.Moore K, Wendon J, Frazer M, Karani J, Williams R, Badr K. Plasma endothelin immunoreactivity in liver disease and the hepatorenal syndrome. N Engl J Med. 1992;327:1774–1778. doi: 10.1056/NEJM199212173272502. [DOI] [PubMed] [Google Scholar]
- 40.Mindikoglu AL, Dowling TC, Weir MR, Seliger SL, Christenson RH, Magder LS. Performance of chronic kidney disease epidemiology collaboration creatinine-cystatin C equation for estimating kidney function in cirrhosis. Hepatology. 2014;59:1532–1542. doi: 10.1002/hep.26556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nejat M, Pickering JW, Walker RJ, Endre ZH. Rapid detection of acute kidney injury by plasma cystatin C in the intensive care unit. Nephrol Dial Transplant. 2010;25:3283–3289. doi: 10.1093/ndt/gfq176. [DOI] [PubMed] [Google Scholar]
- 42.Bianchi C, Donadio C, Tramonti G, Consani C, Lorusso P, Bonino C, Lunghi F. Uninephrectomy increases kidney beta2-microglobulin: can it play a role in the progression of kidney damage? Ren Fail. 2001;23:507–516. doi: 10.1081/jdi-100104733. [DOI] [PubMed] [Google Scholar]
- 43.Shinkai S, Chaves PH, Fujiwara Y, Watanabe S, Shibata H, Yoshida H, Suzuki T. Beta2-microglobulin for risk stratification of total mortality in the elderly population: comparison with cystatin C and C-reactive protein. Arch Intern Med. 2008;168:200–206. doi: 10.1001/archinternmed.2007.64. [DOI] [PubMed] [Google Scholar]
- 44.Nejat M, Pickering JW, Walker RJ, Westhuyzen J, Shaw GM, Frampton CM, Endre ZH. Urinary cystatin C is diagnostic of acute kidney injury and sepsis, and predicts mortality in the intensive care unit. Crit Care. 2010;14:R85. doi: 10.1186/cc9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Malki AL. Assessment of urinary osteopontin in association with podocyte for early predication of nephropathy in diabetic patients. Dis Markers. 2014;2014:493736. doi: 10.1155/2014/493736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levitsky J, Baker TB, Jie C, Ahya S, Levin M, Friedewald J, Al-Saden P, Salomon DR, Abecassis MM. Plasma protein biomarkers enhance the clinical prediction of kidney injury recovery in patients undergoing liver transplantation. Hepatology. 2014;60:2017–2026. doi: 10.1002/hep.27346. [DOI] [PubMed] [Google Scholar]
- 47.Singal AK, Salameh H, Kuo YF, Wiesner RH. Evolving Frequency and Outcomes of Simultaneous Liver Kidney Transplants Based on Liver Disease Etiology. Transplantation. 2014;98:216–221. doi: 10.1097/TP.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 48.Formica RN, Aeder M, Boyle G, Kucheryavaya A, Stewart D, Hirose R, Mulligan D. Simultaneous Liver-Kidney Allocation Policy: A Proposal to Optimize Appropriate Utilization of Scarce Resources. Am J Transplant. 2016;16:758–766. doi: 10.1111/ajt.13631. [DOI] [PubMed] [Google Scholar]
- 49.Bai M, Qi XS, Yang ZP, Yang M, Fan DM, Han GH. TIPS improves liver transplantation-free survival in cirrhotic patients with refractory ascites: an updated meta-analysis. World J Gastroenterol. 2014;20:2704–2714. doi: 10.3748/wjg.v20.i10.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


